Novel Tricyclic Flavonoids as Promising Anti-MRSA Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. In Vitro Assessment of Antimicrobial Susceptibility
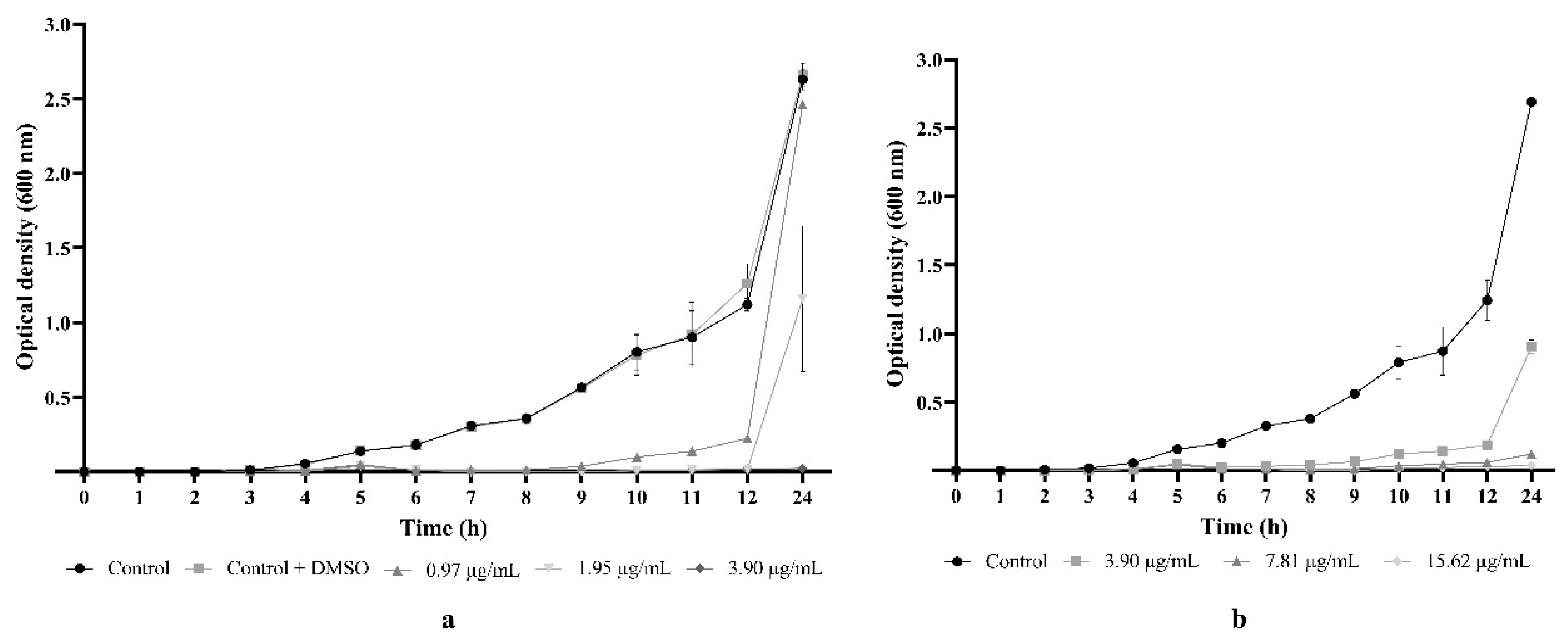
2.3. Flavonoid 5e Induced Important Bacteriostatic Effect against MRSA
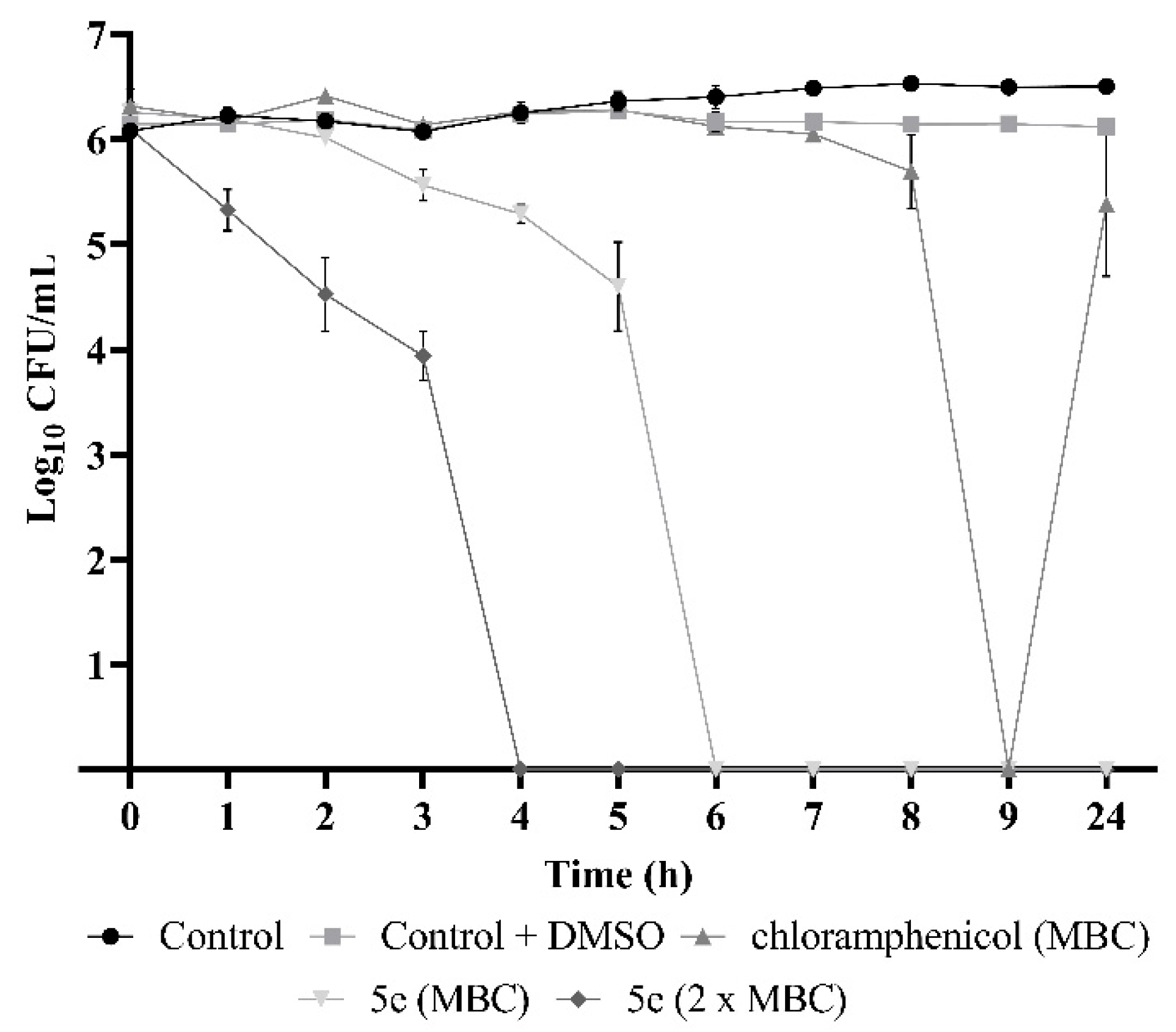
2.4. Flavonoid 5e Is a Potent Bactericidal Compound
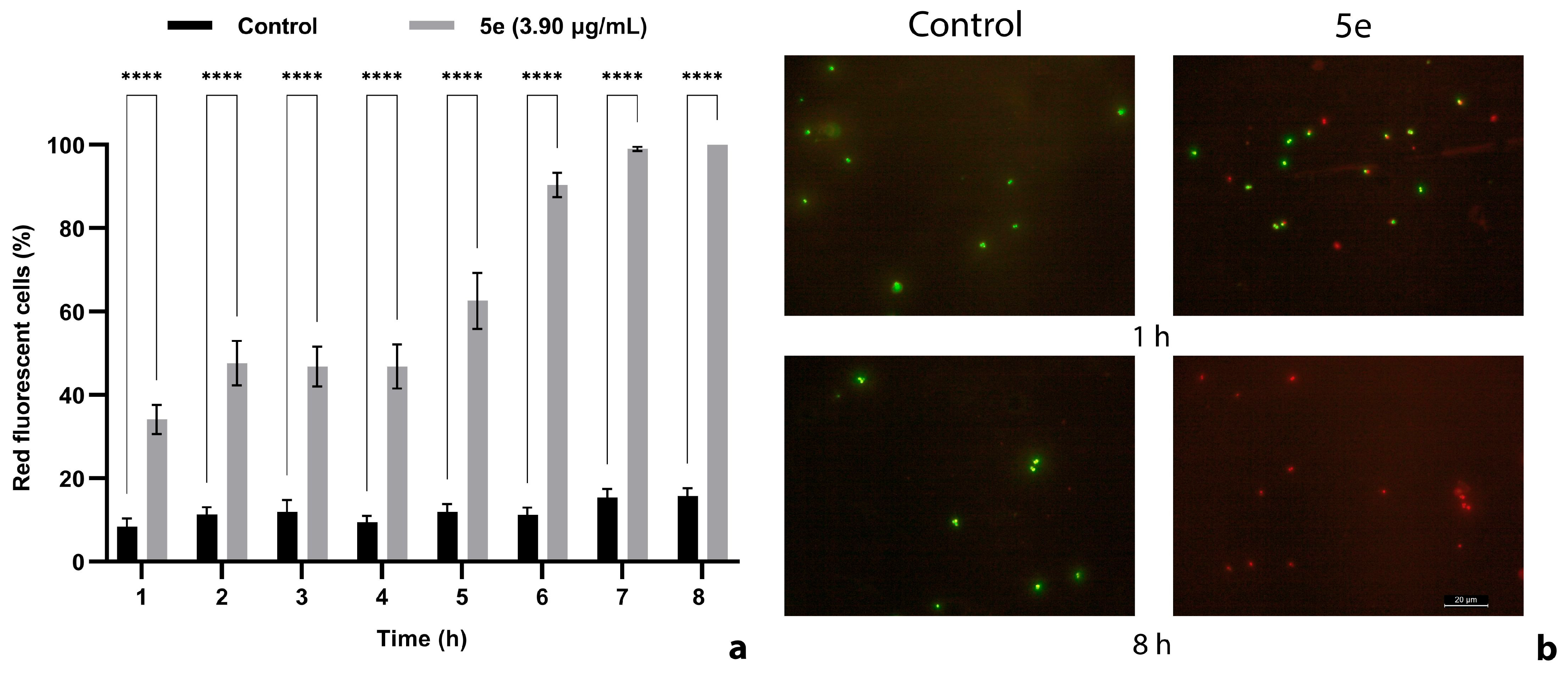
2.5. The Cell Membrane Integrity Was Impaired by 5e
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for 6-Methyl-2-phenyl-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4a)
3.1.2. 6-Methyl-2-(4-methylphenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4b)
3.1.3. 6-Methyl-2-(4-fluorophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4c)
3.1.4. 6-Methyl-2-(4-chlorophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4d)
3.1.5. 6-Methyl-2-(4-bromophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4e)
3.1.6. General Procedure for 2-N,N-Diethylamino-6-methyl-4-phenyl-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5a)
3.1.7. 2-N,N-Diethylamino-6-methyl-4-(4-methylphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5b)
3.1.8. 2-N,N-Diethylamino-6-methyl-4-(4-fluorophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5c)
3.1.9. 2-N,N-Diethylamino-6-methyl-4-(4-chlorophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5d)
3.1.10. 2-N,N-Diethylamino-6-methyl-4-(4-bromophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5e)
3.2. Microbial Strains and Culture Media
3.3. Minimum Inhibitory and Bactericidal/Fungicidal Concentration Determination
3.4. Growth Inhibition Assay
3.5. Time–Kill Assay
3.6. Assessment of Membrane Integrity by Propidium Iodide Uptake
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loewen, K.; Schreiber, Y.; Kirlew, M.; Bocking, N.; Kelly, L. Community-associated methicillin-resistant Staphylococcus aureus infection: Literature review and clinical update. Can. Fam. Physician 2017, 63, 512–520. [Google Scholar] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46 (Suppl. S5), S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thinwa, J.; Walter, E.A.; Frei, C.R. Risk factors for the development of active methicillin-resistant Staphylococcus aureus (MRSA) infection in patients colonized with MRSA at hospital admission. Am. J. Infect. Control 2016, 44, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Lynch, J.P.; Zhanel, G.G. Escalation of antimicrobial resistance among MRSA part 1: Focus on global spread. Expert Rev. Anti-Infect. Ther. 2023, 21, 99–113. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.; El Nahhas, N.; Mabrok, M.A. Methicillin-resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Bal, A.M.; Garau, J.; Gould, I.M.; Liao, C.H.; Mazzei, T.; Nimmo, G.R.; Soriano, A.; Stefani, S.; Tenover, F.C. Vancomycin in the treatment of meticillin-resistant Staphylococcus aureus (MRSA) infection: End of an era? J. Glob. Antimicrob. Resist. 2013, 1, 23–30. [Google Scholar] [CrossRef]
- Gould, I.M.; David, M.Z.; Esposito, S.; Garau, J.; Lina, G.; Mazzei, T.; Peters, G. New insights into meticillin-resistant Staphylococcus aureus (MRSA) pathogenesis, treatment and resistance. Int. J. Antimicrob. Agents 2012, 39, 96–104. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, J.A. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Moldovan, C.-V.; Savu, M.; Dussert, E.; Aboubacar, H.; Sarbu, L.G.; Matiut, S.; Cudennec, B.; Krier, F.; Ravallec, R.; Birsa, L.M.; et al. Synthetic flavonoid BrCl-Flav-an alternative solution to combat ESKAPE pathogens. Antibiotics 2022, 11, 1389. [Google Scholar] [CrossRef]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Hancock, R.E.; Bell, A. Antibiotic uptake into gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 713–720. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Xu, S.; Kang, A.; Tian, Y.; Li, X.; Qin, S.; Yang, R.; Guo, Y. Plant flavonoids with antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA). ACS Infect. Dis. 2024, 13, 3086–3097. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Yang, Z.-Q.; Liu, F.; Peng, W.-J.; Qu, S.-Q.; Li, Q.; Song, X.-B.; Zhu, K.; Shen, J.-Z. Antibacterial effect and mode of action of flavonoids from licorice against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela, M.R.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated flavonoids from Paulownia tomentosa fruits with antimicrobial potential and synergistic activity with antibiotics. Pharm. Biol. 2016, 54, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Kosalec, I. Galangin expresses bactericidal activity against multiple-resistant bacteria: MRSA, Enterococcus spp. and Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2004, 240, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Rodriguez-Guzman, R.; Manly, S.P.; Jacob, M.; Ross, S.A. Sepicanin A—A new geranyl flavanone from Artocarpus sepicanus with activity against methicillin-resistant Staphylococcus aureus (MRSA). Phytochem. Lett. 2009, 2, 141–143. [Google Scholar] [CrossRef]
- Babii, C.; Savu, M.; Motrescu, I.; Birsa, L.M.; Sarbu, L.G.; Stefan, M. Antibacterial synthetic flavonoid BrCl-Flav exhibits important anti-Candida activity by damaging cell membrane integrity. Pharmaceuticals 2021, 14, 1130. [Google Scholar] [CrossRef]
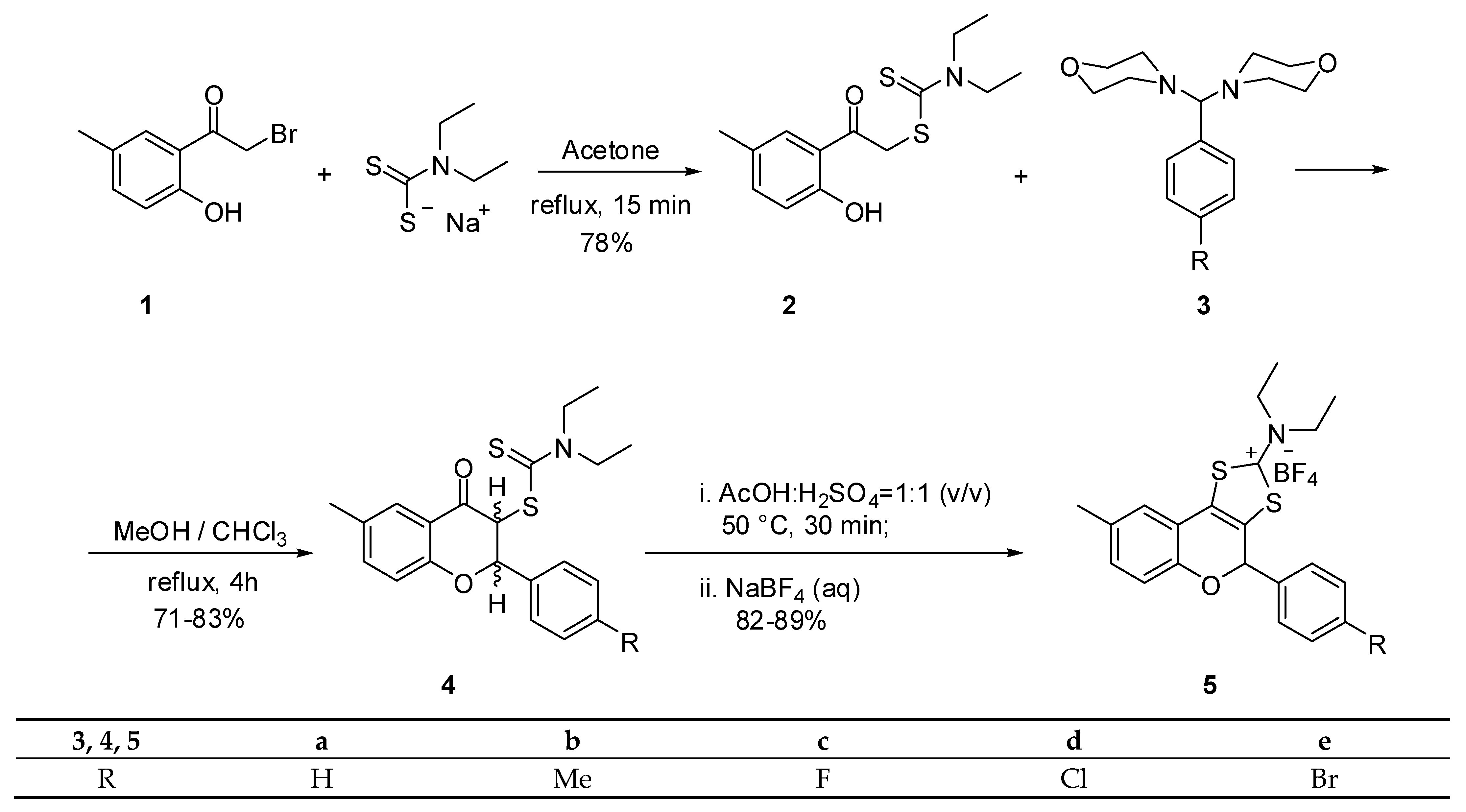

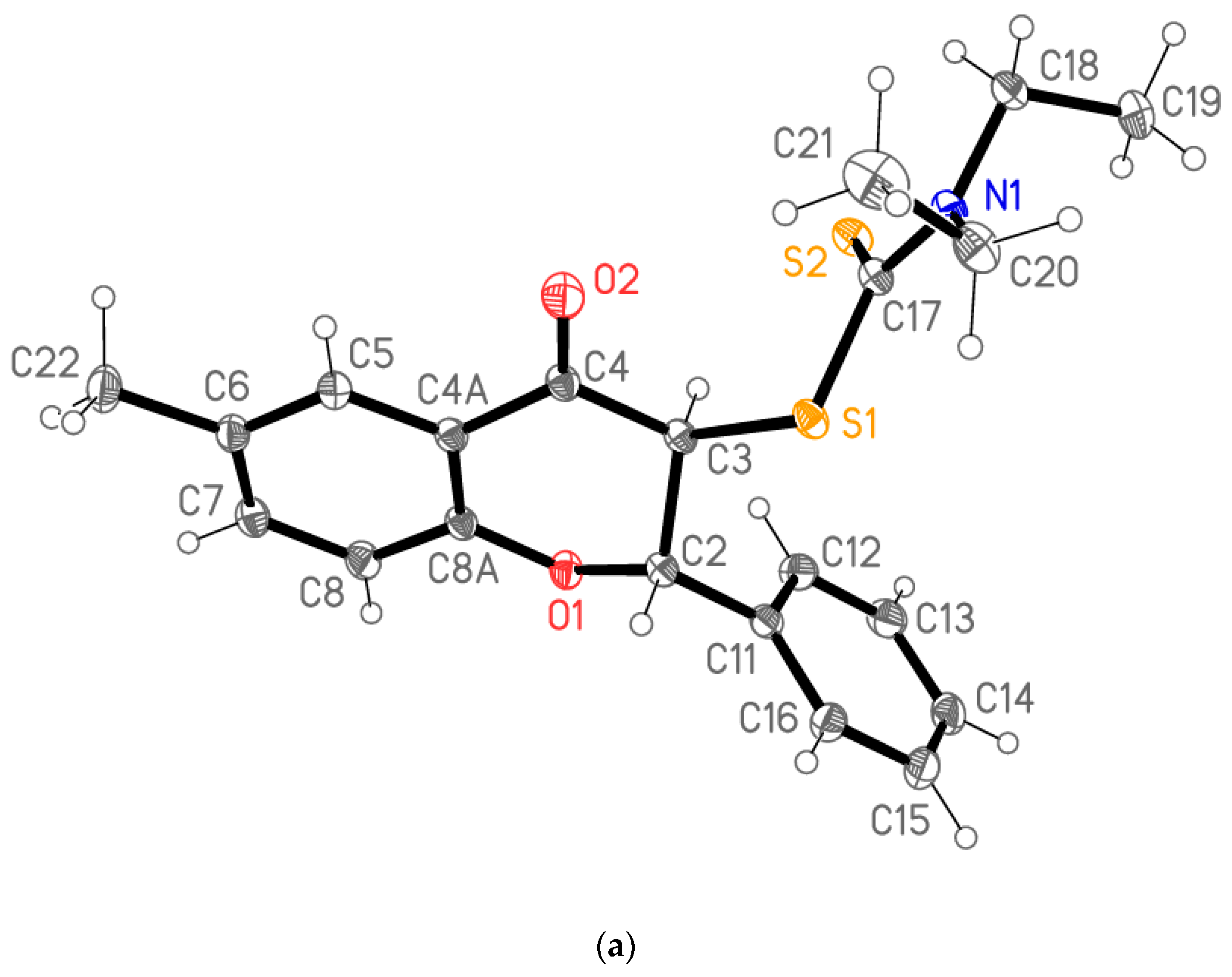
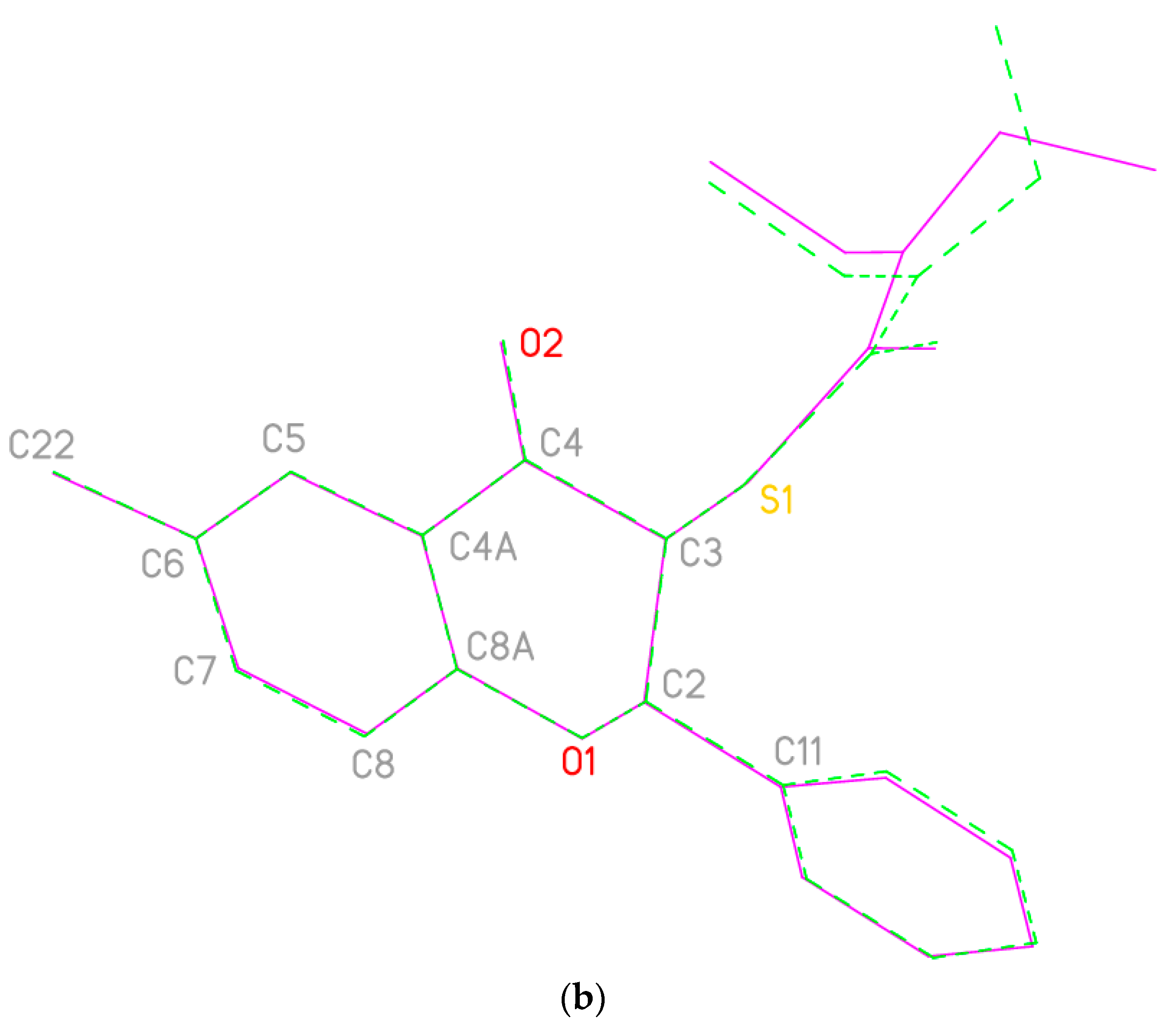
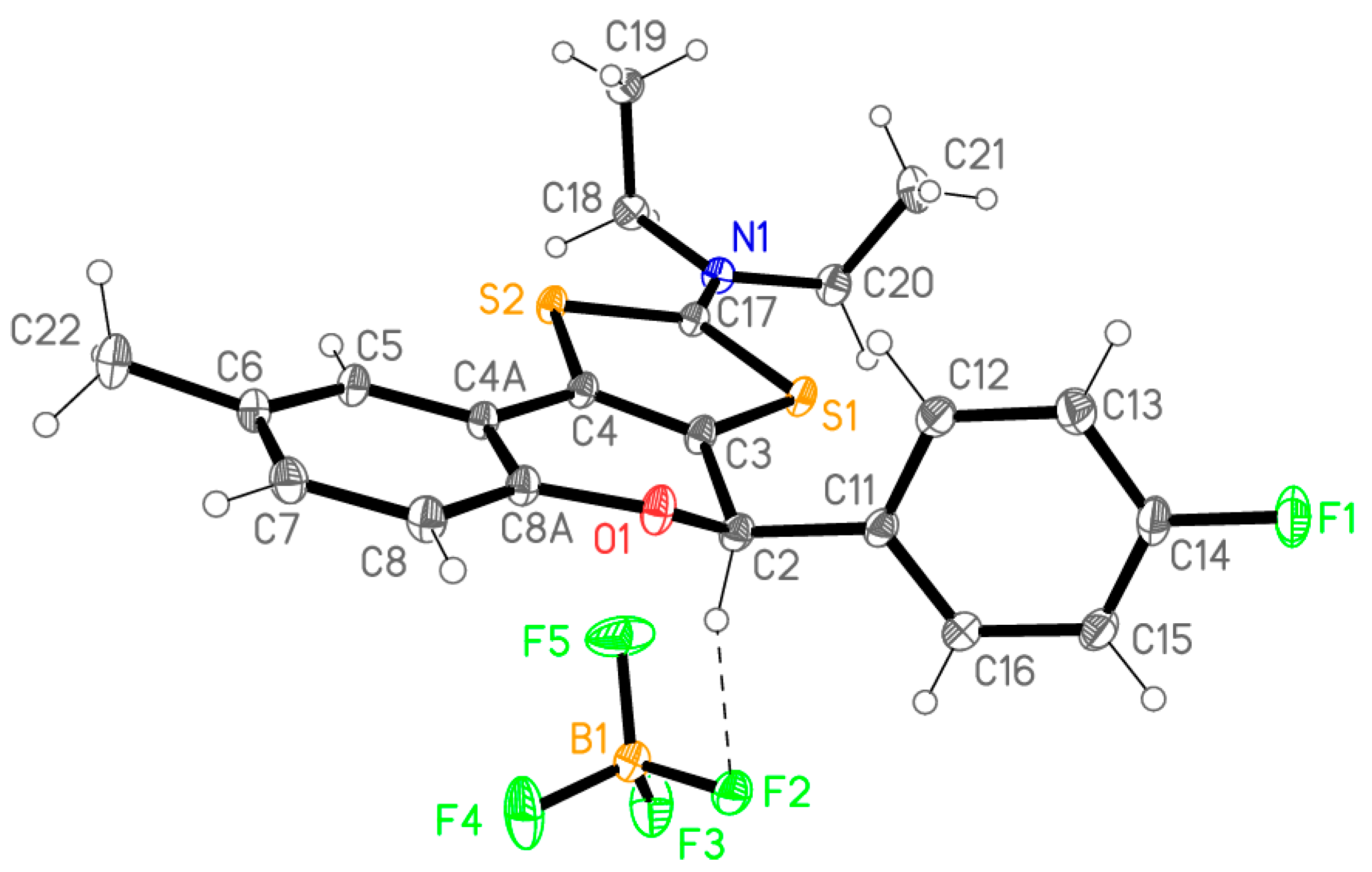
| Flavanones 4 | a | b | c | d | e |
|---|---|---|---|---|---|
| 3JH2–H3 syn (Hz) | 3.4 | 3.2 | 3.9 | 3.7 | 3.7 |
| 3JH2–H3 anti (Hz) | 7.8 | 8.1 | 8.7 | 8.3 | 8.3 |
| syn: anti ratio | 31:69 | 12:88 | 11:89 | 12:88 | 10:90 |
| Microbial Strains | 5a | 5b | 5c | 5d | 5e | Control | DMSO (%) |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 7.81 | 7.81 | 7.81 | 3.90 | 3.90 | 1.95 a/7.81 chl | 24.87 |
| S. aureus prxbio2 | 7.81 | 15.62 | 15.62 | 15.62 | 15.62 | 7.81 chl | 24.87 |
| S. aureus medbio1-2012 | 15.62 | 3.90 | 15.62 | 3.90 | 1.95 | 7.81 chl | 24.87 |
| Enterococcus faecium medbio2-2012 | 62.50 | 31.25 | 62.50 | 31.25 | 15.62 | 15.62 chl | 12.43 |
| Escherichia coli ATCC 25922 | 125 | 125 | 125 | 125 | 62.50 | 62.50 a/7.81 k | 12.43 |
| Acinetobacter pittii Cl2 | 62.50 | 62.50 | 62.50 | 62.50 | 62.50 | >250 a/0.37 cip | 6.21 |
| Pseudomonas aeruginosa PAO1 | 125 | 125 | 125 | 125 | 125 | >250 a | 12.43 |
| Candida krusei Prx | 62.50 | 31.25 | 62.50 | 31.25 | 31.25 | 62.50 f | 6.21 |
| C. albicans ATCC 10231 | 62.50 | 31.25 | 62.50 | 31.25 | 31.25 | >500 f | 6.21 |
| Microbial Strains | 5a | 5b | 5c | 5d | 5e | Control |
|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | 31.25 | 15.62 | 31.25 | 31.25 | 31.25 | 7.81 a |
| S. aureus prxbio2 | 250 | 62.50 | 250 | 250 | 62.50 | 125 chl |
| S. aureus medbio1-2012 | 62.50 | 7.81 | 31.25 | 15.62 | 3.90 | 31.25 chl |
| Enterococcus faecium medbio2-2012 | 125 | 62.50 | 62.50 | 62.50 | 31.25 | >250 chl |
| Escherichia coli ATCC 25922 | 125 | 125 | 125 | 125 | 125 | 125 a |
| Acinetobacter pittii Cl2 | 125 | 125 | 125 | 125 | 125 | >250 a |
| Pseudomonas aeruginosa PAO1 | >250 | >250 | >250 | >250 | >250 | >250 a |
| Candida krusei Prx | 125 | 31.25 | 125 | 62.50 | 31.25 | 62.50 f |
| C. albicans ATCC 10231 | 125 | 62.50 | 125 | 62.50 | 62.50 | >500 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, C.-V.; Mantea, L.-E.; Savu, M.; Jones, P.G.; Sarbu, L.G.; Stefan, M.; Birsa, M.L. Novel Tricyclic Flavonoids as Promising Anti-MRSA Agents. Pharmaceuticals 2024, 17, 1276. https://doi.org/10.3390/ph17101276
Moldovan C-V, Mantea L-E, Savu M, Jones PG, Sarbu LG, Stefan M, Birsa ML. Novel Tricyclic Flavonoids as Promising Anti-MRSA Agents. Pharmaceuticals. 2024; 17(10):1276. https://doi.org/10.3390/ph17101276
Chicago/Turabian StyleMoldovan, Cristina-Veronica, Loredana-Elena Mantea, Mihaela Savu, Peter G. Jones, Laura Gabriela Sarbu, Marius Stefan, and Mihail Lucian Birsa. 2024. "Novel Tricyclic Flavonoids as Promising Anti-MRSA Agents" Pharmaceuticals 17, no. 10: 1276. https://doi.org/10.3390/ph17101276
APA StyleMoldovan, C.-V., Mantea, L.-E., Savu, M., Jones, P. G., Sarbu, L. G., Stefan, M., & Birsa, M. L. (2024). Novel Tricyclic Flavonoids as Promising Anti-MRSA Agents. Pharmaceuticals, 17(10), 1276. https://doi.org/10.3390/ph17101276








