Drug–Drug Interactions of Selective Serotonin Reuptake Inhibitors: A Pharmacovigilance Study on Real-World Evidence from the EudraVigilance Database
Abstract
1. Introduction
2. Results
2.1. Descriptive Analysis
2.2. Disproportionality Analysis
2.2.1. All DDIs
2.2.2. Drug Inhibition
2.2.3. Potentiating Drug Interaction
2.2.4. Unspecified DDIs
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Study Design
4.2. Material
4.3. Descriptive Analysis
4.4. Disproportionality Analysis
4.5. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Purtle, J.; Nelson, K.L.; Counts, N.Z.; Yudell, M. Population-Based Approaches to Mental Health: History, Strategies, and Evidence. Annu. Rev. Public Health 2020, 41, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Ma, W.; Tong, Y.; Zheng, J. Temporal and Spatial Trend Analysis of All-Cause Depression Burden Based on Global Burden of Disease (GBD) 2019 Study. Sci. Rep. 2024, 14, 12346. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; McCarty, M.F.; O’Keefe, J.H. Coenzyme Q10 for the Treatment of Heart Failure: A Review of the Literature. Open Hear. 2015, 2, e000326. [Google Scholar] [CrossRef]
- Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 31 July 2024).
- Hohls, J.K.; König, H.H.; Quirke, E.; Hajek, A. Anxiety, Depression and Quality of Life—A Systematic Review of Evidence from Longitudinal Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 12022. [Google Scholar] [CrossRef] [PubMed]
- Paljärvi, T.; Tiihonen, J.; Lähteenvuo, M.; Tanskanen, A.; Fazel, S.; Taipale, H. Psychotic Depression and Deaths Due to Suicide. J. Affect. Disord. 2023, 321, 28–32. [Google Scholar] [CrossRef]
- American Psychological Association. Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts: Guideline Development Panel for the Treatment of Depressive Disorders Approved by Apa Council of Representatives; American Psychological Association: Washington, DC, USA, 2019. [Google Scholar]
- American Psychological Association. Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts; American Psychological Association: Washington, DC, USA, 2019; Available online: https://www.apa.org/depression-guideline (accessed on 2 August 2024).
- NICE. Depression in Adults: Treatment and Management NICE Guideline; NICE: Manchester, UK, 2022; Available online: www.nice.org.uk/guidance/ng222 (accessed on 3 August 2024).
- Canadian Network for Mood and Anxiety Treatments. Clinicians Guidelines: 2016 Depression Guidelines; CANMAT: Vancouver, BC, Canada, 2016; Available online: https://www.canmat.org/2019/03/17/2016-depression-guidelines/ (accessed on 3 August 2024).
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Baumann, P. Pharmacokinetic-Pharmacodynamic Relationship of the Selective Serotonin Reuptake Inhibitors. Clin. Pharmacokinet. 1996, 31, 444–469. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.V.E.; Wilson, C.A.; Ayre, K.; Robertson, L.; South, E.; Molyneaux, E.; Trevillion, K.; Howard, L.M.; Khalifeh, H. Antidepressant Treatment for Postnatal Depression. Cochrane Database Syst. Rev. 2021, 2021, CD013560. [Google Scholar] [CrossRef]
- Bruggeman, C.; O’Day, C.S. Selective Serotonin Reuptake Inhibitor Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- EMA. CHMP Prozac—Annex I List of the Names, Pharmaceutical Forms, Strengths of the Medicinal Products, Route of Administration, Marketing Authorisation Holders in the Member States; EMA: Amsterdam, The Netherland, 2006; Available online: https://www.ema.europa.eu/en/documents/referral/prozac-article-6-12-referral-annex-i-ii-iii_en.pdf (accessed on 16 September 2024).
- EMA. Zoloft—Annex I List of the Names, Pharmaceutical Forms, Strengths of the Medicinal Products, Route of Administration, Marketing Authorisation Holders in the Member States; EMA: Amsterdam, The Netherland; Available online: https://www.ema.europa.eu/en/documents/referral/zoloft-article-30-referral-annex-i-ii-iii-iv_en.pdf (accessed on 16 September 2024).
- EMA. Seroxat—Annex 1 List of the Invented Names, Pharmaceutical Forms, Strengths of the Medicinal Products, Route of Administration and Marketing Authorisation Holders in the Member States; EMA: Amsterdam, The Netherlands, 2005; Available online: https://www.ema.europa.eu/en/documents/referral/paroxetine-article-31-referral-annex-i-ii-iii-iv_en.pdf (accessed on 16 September 2024).
- Rafaniello, C.; Sullo, M.G.; Carnovale, C.; Pozzi, M.; Stelitano, B.; Radice, S.; Bernardini, R.; Rossi, F.; Clementi, E.; Capuano, A. We Really Need Clear Guidelines and Recommendations for Safer and Proper Use of Aripiprazole and Risperidone in a Pediatric Population: Real-World Analysis of EudraVigilance Database. Front. Psychiatry 2020, 11, 550201. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). EudraVigilance. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/pharmacovigilance-research-development/eudravigilance (accessed on 3 August 2024).
- Sabella, D. Improving Mental Health Awareness. Am. J. Nurs. 2021, 121, 66–69. [Google Scholar] [CrossRef]
- Chadwick, R. Mental Health Awareness. Bioethics 2023, 37, 423. [Google Scholar] [CrossRef] [PubMed]
- Luberenga, I.; Kasujja, R.; Vasanthan, L.T.; Nyende, A.; Tumwebaze, E.; Henry Joseph, L.J. Mental Health Awareness Programmes to Promote Mental Well-Being at the Workplace among Workforce in the Low-Income and Middle-Income Countries: A Scoping Review Protocol. BMJ Open 2023, 13, e073012. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Eyre, O.; Patel, V.; Brent, D. Depression in Young People. Lancet 2022, 400, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Karyotaki, E.; Eckshtain, D.; Ng, M.Y.; Corteselli, K.A.; Noma, H.; Quero, S.; Weisz, J.R. Psychotherapy for Depression Across Different Age Groups: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2020, 77, 694–702. [Google Scholar] [CrossRef]
- Kautzky, A.; Bartova, L.; Fugger, G.; Dold, M.; Souery, D.; Montgomery, S.; Zohar, J.; Mendlewicz, J.; Fabbri, C.; Serretti, A.; et al. Age as a Moderating Factor of Treatment Resistance in Depression. Eur. Psychiatry 2023, 66, e35. [Google Scholar] [CrossRef]
- Cuijpers, P.; Franco, P.; Ciharova, M.; Miguel, C.; Segre, L.; Quero, S.; Karyotaki, E. Psychological Treatment of Perinatal Depression: A Meta-Analysis. Psychol. Med. 2023, 53, 2596–2608. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Wang, G. Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-Analysis. J. Clin. Nurs. 2022, 31, 2665–2677. [Google Scholar] [CrossRef]
- Simon, G.E.; Moise, N.; Mohr, D.C. Management of Depression in Adults: A Review. JAMA 2024, 332, 141–152. [Google Scholar] [CrossRef]
- Swetlitz, N. Depression’s Problem with Men. AMA J. Ethics 2021, 23, E586–E589. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Moon, T.W. Pharmaceuticals in the Marine Environment: Occurrence, Fate, and Biological Effects. In Contaminants of Emerging Concern in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 11–71. [Google Scholar] [CrossRef]
- Rafiq, A.; Capolupo, M.; Addesse, G.; Valbonesi, P.; Fabbri, E. Antidepressants and Their Metabolites Primarily Affect Lysosomal Functions in the Marine Mussel, Mytilus galloprovincialis. Sci. Total Environ. 2023, 903, 166078. [Google Scholar] [CrossRef]
- Vasskog, T.; Anderssen, T.; Pedersen-Bjergaard, S.; Kallenborn, R.; Jensen, E. Occurrence of Selective Serotonin Reuptake Inhibitors in Sewage and Receiving Waters at Spitsbergen and in Norway. J. Chromatogr. A 2008, 1185, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Bajda, T.; Grela, A.; Pamuła, J.; Kuc, J.; Klimek, A.; Matusik, J.; Franus, W.; Alagarsamy, S.K.K.; Danek, T.; Gara, P. Using Zeolite Materials to Remove Pharmaceuticals from Water. Materials 2024, 17, 3848. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Fornaro, M.; Ostinelli, E.G.; Zangani, C.; Croatto, G.; Monaco, F.; Krinitski, D.; Fusar-Poli, P.; Correll, C.U. Safety of 80 Antidepressants, Antipsychotics, Anti-Attention-Deficit/Hyperactivity Medications and Mood Stabilizers in Children and Adolescents with Psychiatric Disorders: A Large Scale Systematic Meta-Review of 78 Adverse Effects. World Psychiatry 2020, 19, 214–232. [Google Scholar] [CrossRef]
- Dietz, G.P.H.; Fajemiroye, J.O.; Zeiss, R.; Malejko, K.; Connemann, B.; Gahr, M.; Durner, V.; Graf, H. Sexual Dysfunction Induced by Antidepressants—A Pharmacovigilance Study Using Data from VigiBase™. Pharmaceuticals 2024, 17, 826. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, D.; Li, J.; Ren, M.; Liu, Y.; Si, T.; Chen, Y. Selection of the Optimal Dose of Sertraline for Depression: A Dose-Response Meta-Analysis of Randomized Controlled Trials. Psychiatry Res. 2023, 327, 115391. [Google Scholar] [CrossRef]
- Yan, N.; Hu, S. The Safety and Efficacy of Escitalopram and Sertraline in Post-Stroke Depression: A Randomized Controlled Trial. BMC Psychiatry 2024, 24, 365. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the Treatment of Adults with Major Depressive Disorder in the Maintenance Phase: A Systematic Review and Network Meta-Analysis. Mol. Psychiatry 2022, 28, 402–409. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, J.; Xu, L.; Li, Y.; Li, H.; Shen, Y.; Zheng, Q.; Li, L. Analysis of Time-Course, Dose-Effect, and Influencing Factors of Antidepressants in the Treatment of Acute Adult Patients with Major Depression. Int. J. Neuropsychopharmacol. 2020, 23, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Marks, D.M.; Park, M.H.; Ham, B.J.; Han, C.; Patkar, A.A.; Masand, P.S.; Pae, C.U. Paroxetine: Safety and Tolerability Issues. Expert Opin. Drug Saf. 2008, 7, 783–794. [Google Scholar] [CrossRef]
- Bérard, A.; Iessa, N.; Chaabane, S.; Muanda, F.T.; Boukhris, T.; Zhao, J.-P. The Risk of Major Cardiac Malformations Associated with Paroxetine Use during the First Trimester of Pregnancy: A Systematic Review and Meta-Analysis. Br. J. Clin. Pharmacol. 2016, 81, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Uguz, F.; Sahingoz, M.; Gungor, B.; Aksoy, F.; Askin, R. Weight Gain and Associated Factors in Patients Using Newer Antidepressant Drugs. Gen. Hosp. Psychiatry 2015, 37, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Cuda, S.; Censani, M.; Kharofa, R.; O’Hara, V.; Conroy, R.; Williams, D.R.; Paisley, J.; Browne, A.F.; Karjoo, S.; Browne, N.T. Medication-Induced Weight Gain and Advanced Therapies for the Child with Overweight and Obesity: An Obesity Medicine Association (OMA) Clinical Practice Statement 2022. Obes. Pillars 2022, 4, 100048. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; dos Santos Rodrigues, A.P.; Petarli, G.B.; Machado, K.P.; Flores, T.R.; Batista, S.R.; Nunes, B.P. Overweight, Obesity and Risk of Multimorbidity: A Systematic Review and Meta-Analysis of Longitudinal Studies. Obes. Rev. 2023, 24, e13562. [Google Scholar] [CrossRef]
- Merlob, P.; Stahl, B.; Sulkes, J. Paroxetine during Breast-Feeding: Infant Weight Gain and Maternal Adherence to Counsel. Eur. J. Pediatr. 2004, 163, 135–139. [Google Scholar] [CrossRef]
- Acar, S.; Erol, H.; Arslan, E.K.; Uysal, N.; Karadaş, B.; Temiz, T.K.; Kaplan, Y.C. Paroxetine Overdose During Pregnancy. Forensic Sci. Res. 2021, 6, 237–239. [Google Scholar] [CrossRef]
- Stefánsdóttir, Í.H.; Ivarsson, T.; Skarphedinsson, G. Efficacy and Safety of Serotonin Reuptake Inhibitors (SSRI) and Serotonin Noradrenaline Reuptake Inhibitors (SNRI) for Children and Adolescents with Anxiety Disorders: A Systematic Review and Meta-Analysis. Nord. J. Psychiatry 2023, 77, 137–146. [Google Scholar] [CrossRef]
- Kostev, K.; Rex, J.; Eith, T.; Heilmaier, C. Which adverse effects influence the dropout rate in selective serotonin reuptake inhibitor (SSRI) treatment? Results for 50,824 patients. GMS Ger. Med. Sci. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Simal, I.; Somers, A.; Amrouch, C.; Capiau, A.; Cherubini, A.; Cruz-Jentoft, A.J.; Gudmundsson, A.; Soiza, R.L.; O’Mahony, D.; Petrovic, M. A Descriptive Analysis of Drug-Drug Interactions and Corresponding Adverse Drug Reactions in Multimorbid Older Inpatients: Findings from the SENATOR Trial. Eur. Geriatr. Med. 2024. [Google Scholar] [CrossRef]
- Sanchez, C.; Reines, E.H.; Montgomery, S.A. A Comparative Review of Escitalopram, Paroxetine, and Sertraline: Are They All Alike? Int. Clin. Psychopharmacol. 2014, 29, 185–196. [Google Scholar] [CrossRef]
- Wyska, E. Pharmacokinetic Considerations for Current State-of-the-Art Antidepressants. Expert Opin. Drug Metab. Toxicol. 2019, 15, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Preskorn, S.H. Clinically Relevant Pharmacology of Selective Serotonin Reuptake Inhibitors. Clin. Pharmacokinet. 1997, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Radosavljevic, M.; Svob Strac, D.; Jancic, J.; Samardzic, J. The Role of Pharmacogenetics in Personalizing the Antidepressant and Anxiolytic Therapy. Genes 2023, 14, 1095. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Cooper, Z.; Lu, S.; Stanley, S.; Majda, B.T.; Collins, K.R.L.; Gilkes, L.; Rodger, J.; Akkari, P.A.; Hood, S.D. Utility of Pharmacogenetic Testing to Optimise Antidepressant Pharmacotherapy in Youth: A Narrative Literature Review. Front. Pharmacol. 2023, 14, 1267294. [Google Scholar] [CrossRef] [PubMed]
- Moreau, F.; Décaudin, B.; Tod, M.; Odou, P.; Simon, N. Impact of the Use of a Drug–Drug Interaction Checker on Pharmacist Interventions Involving Well-Known Strong Interactors. Eur. J. Hosp. Pharm. 2024. [Google Scholar] [CrossRef]
- Maitland, S.; Baker, M. Serotonin Syndrome. Drug Ther. Bull. 2022, 60, 88–91. [Google Scholar] [CrossRef]
- Scotton, W.J.; Hill, L.J.; Williams, A.C.; Barnes, N.M. Serotonin Syndrome: Pathophysiology, Clinical Features, Management, and Potential Future Directions. Int. J. Tryptophan Res. 2019, 12, 1178646919873925. [Google Scholar] [CrossRef]
- Simon, L.V.; Torrico, T.J.; Keenaghan, M. Serotonin Syndrome. Pain Med. Essent. Rev. 2024, 11, 201–202. [Google Scholar]
- Rang, S.T.; Field, J.; Irving, C. Serotonin Toxicity Caused by an Interaction between Fentanyl and Paroxetine. Can. J. Anesth. 2008, 55, 521–525. [Google Scholar] [CrossRef]
- Gollapudy, S.; Kumar, V.; Dhamee, M.S. A Case of Serotonin Syndrome Precipitated by Fentanyl and Ondansetron in a Patient Receiving Paroxetine, Duloxetine, and Bupropion. J. Clin. Anesth. 2012, 24, 251–252. [Google Scholar] [CrossRef]
- Jaber, B.L.; Lobon, L.F.; Madias, N.E. The Serotonin Syndrome Complicating Co-Prescription of Paroxetine and Clarithromycin. Am. J. Med. 2006, 119, e3. [Google Scholar] [CrossRef] [PubMed]
- Hasani, R.; Sarma, J.; Kansal, S. Serotonin Syndrome Induced by Combined Use of Sertraline and Linezolid. Anesth. Essays Res. 2019, 13, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Xavier, S.; Desai, S.; Ali, S. A Case of Serotonin Syndrome Precipitated by Quetiapine in a Middle-Aged Female on Trazodone and Sertraline. Cureus 2022, 14, e27668. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.A.; Johnson, D. Serotonin Syndrome and Dextromethorphan Toxicity Caused by Drug-Drug Interaction between Fluoxetine and Bupropion-Dextromethorphan: A Case Report. J. Clin. Psychiatry 2024, 85, 54018. [Google Scholar] [CrossRef]
- Demers, J.C.; Malone, M. Serotonin Syndrome Induced by Fluvoxamine and Mirtazapine. Ann. Pharmacother. 2001, 35, 1217–1220. [Google Scholar] [CrossRef]
- Ailawadhi, S.; Sung, K.W.; Carlson, L.A.; Baer, M.R. Serotonin Syndrome Caused by Interaction between Citalopram and Fentanyl. J. Clin. Pharm. Ther. 2007, 32, 199–202. [Google Scholar] [CrossRef]
- Talarico, G.; Tosto, G.; Pietracupa, S.; Piacentini, E.; Canevelli, M.; Lenzi, G.L.; Bruno, G. Serotonin Toxicity: A Short Review of the Literature and Two Case Reports Involving Citalopram. Neurol. Sci. 2011, 32, 507–509. [Google Scholar] [CrossRef]
- Levin, T.T.; Cortes-Ladino, A.; Weiss, M.; Palomba, M.L. Life-Threatening Serotonin Toxicity due to a Citalopram-Fluconazole Drug Interaction: Case Reports and Discussion. Gen. Hosp. Psychiatry 2008, 30, 372–377. [Google Scholar] [CrossRef]
- Baptista, G.; Eiden, C.; Monguillot, P.; Philibert, C.; Jeandel, C. Serotonin Syndrome during Treatment with Low Dose of Escitalopram Associated with Miconazole Mucoadhesive Tablet: A Suspected Drug Interaction. Int. Psychogeriatr. 2012, 24, 845–847. [Google Scholar] [CrossRef]
- Dy, P.; Arcega, V.; Ghali, W.; Wolfe, W. Serotonin Syndrome Caused by Drug to Drug Interaction between Escitalopram and Dextromethorphan. Case Rep. 2017, 2017, bcr-2017. [Google Scholar] [CrossRef]
- Telles-Correia, D.; Guerreiro, D.F.; Oliveira, S.; Figueira, M.L. Differences between SSRI’s Pharmacokinetics and Pharmacodinamics. Acta Medica Port. 2007, 20, 167–174. Available online: https://www.actamedicaportuguesa.com/revista/index.php/amp/article/view/841/517 (accessed on 24 August 2024).
- Stahl, S.M. Selectivity of SSRIs: Individualising Patient Care through Rational Treatment Choices. Int. J. Psychiatry Clin. Pract. 2004, 8, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Rayner, D.; Ramaraju, H.B.; Abbas, U.; Garcia, C.; Heybati, K.; Zhou, F.; Huang, E.; Park, Y.J.; Moskalyk, M. Efficacy and Safety of Selective Serotonin Reuptake Inhibitors in COVID-19 Management: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2023, 29, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of Early Treatment with Fluvoxamine on Risk of Emergency Care and Hospitalisation among Patients with COVID-19: The TOGETHER Randomised, Platform Clinical Trial. Lancet Glob. Health 2022, 10, e42–e51. [Google Scholar] [CrossRef]
- Deng, J.; Moskalyk, M.; Zuo, Q.K.; Garcia, C.; Abbas, U.; Ramaraju, H.B.; Rayner, D.; Park, Y.J.; Heybati, K.; Zhou, F.; et al. Evaluating Fluvoxamine for the Outpatient Treatment of COVID-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2024, 34, e2501. [Google Scholar] [CrossRef]
- Bushnell, G.A.; Rynn, M.A.; Crystal, S.; Gerhar, T.; Olfson, M. Simultaneous Benzodiazepine and SSRI Initiation in Young People with Anxiety Disorders. J. Clin. Psychiatry 2021, 82, 37519. [Google Scholar] [CrossRef]
- Albert, U.; Marazziti, D.; Salvo, G.D.i.; Solia, F.; Rosso, G.; Maina, G. A Systematic Review of Evidence-Based Treatment Strategies for Obsessive-Compulsive Disorder Resistant to First-Line Pharmacotherapy. Curr. Med. Chem. 2017, 25, 5647–5661. [Google Scholar] [CrossRef]
- Levin, J.S.; Acosta, J.; Faherty, L.J. New Prescription Fills of Selective Serotonin Reuptake Inhibitors before and during the COVID-19 Pandemic in Los Angeles County, California. J. Affect. Disord. 2022, 319, 507–510. [Google Scholar] [CrossRef]
- Morgovan, C.; Dobrea, C.M.; Butuca, A.; Arseniu, A.M.; Frum, A.; Rus, L.L.; Chis, A.A.; Juncan, A.M.; Gligor, F.G.; Georgescu, C.; et al. Safety Profile of the Trastuzumab-Based ADCs: Analysis of Real-World Data Registered in EudraVigilance. Biomedicines 2024, 12, 953. [Google Scholar] [CrossRef]
- Montastruc, J.L.; Sommet, A.; Bagheri, H.; Lapeyre-Mestre, M. Benefits and Strengths of the Disproportionality Analysis for Identification of Adverse Drug Reactions in a Pharmacovigilance Database. Br. J. Clin. Pharmacol. 2011, 72, 905–908. [Google Scholar] [CrossRef]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Grundmark, B.; Holmberg, L.; Garmo, H.; Zethelius, B. Reducing the Noise in Signal Detection of Adverse Drug Reactions by Standardizing the Background: A Pilot Study on Analyses of Proportional Reporting Ratios-by-Therapeutic Area. Eur. J. Clin. Pharmacol. 2014, 70, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Morgovan, C.; Dobrea, C.M.; Chis, A.A.; Juncan, A.M.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Ardelean, S.A.; Stoicescu, L.; Ghibu, S.; et al. A Descriptive Analysis of Direct Oral Anticoagulant Drugs Dosing Errors Based on Spontaneous Reports from the EudraVigilance Database. Pharmaceuticals 2023, 16, 455. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP)—Module IX Addendum I—Methodological Aspects of Signal Detection from Spontaneous Reports of Suspected Adverse Reactions; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]


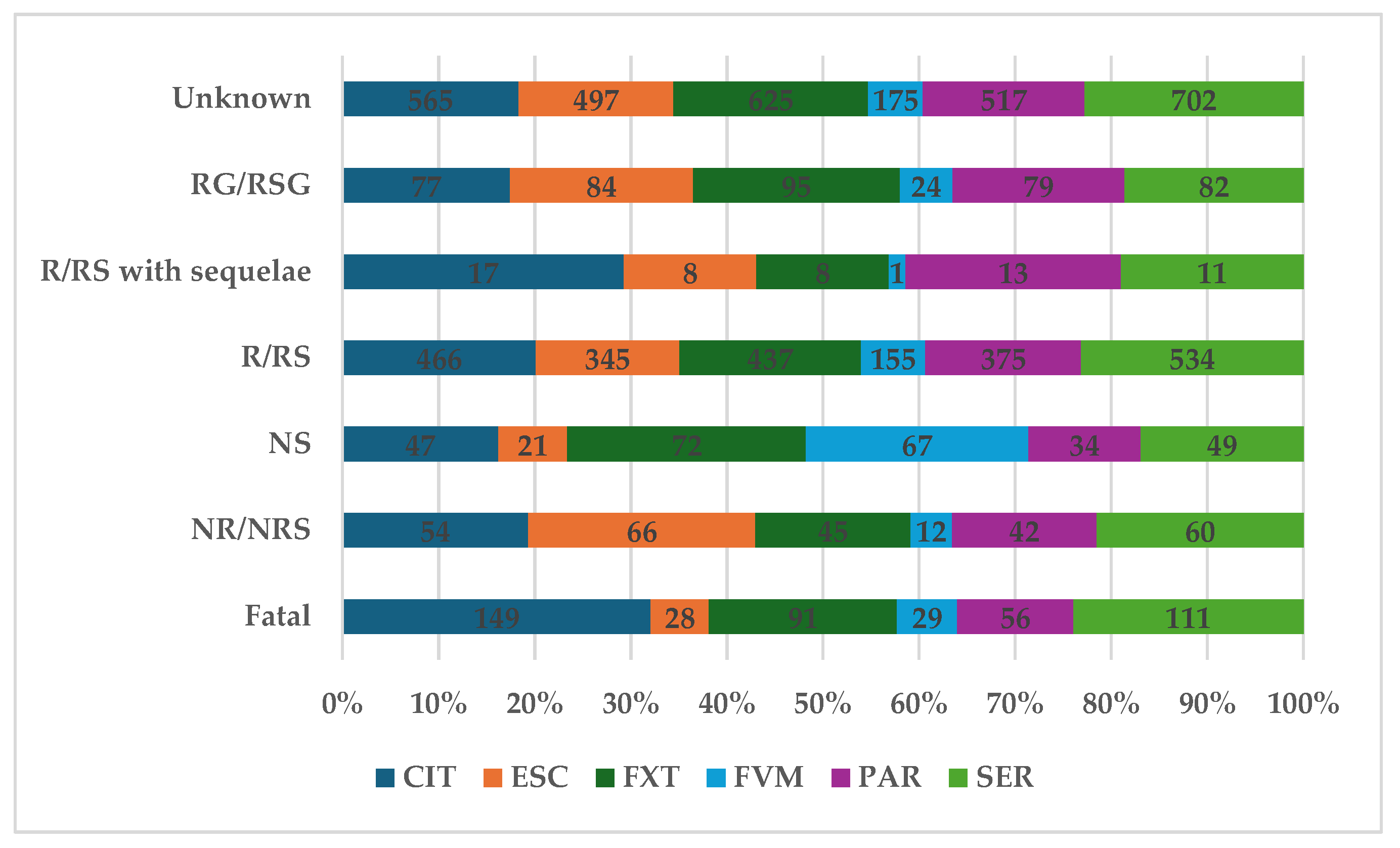
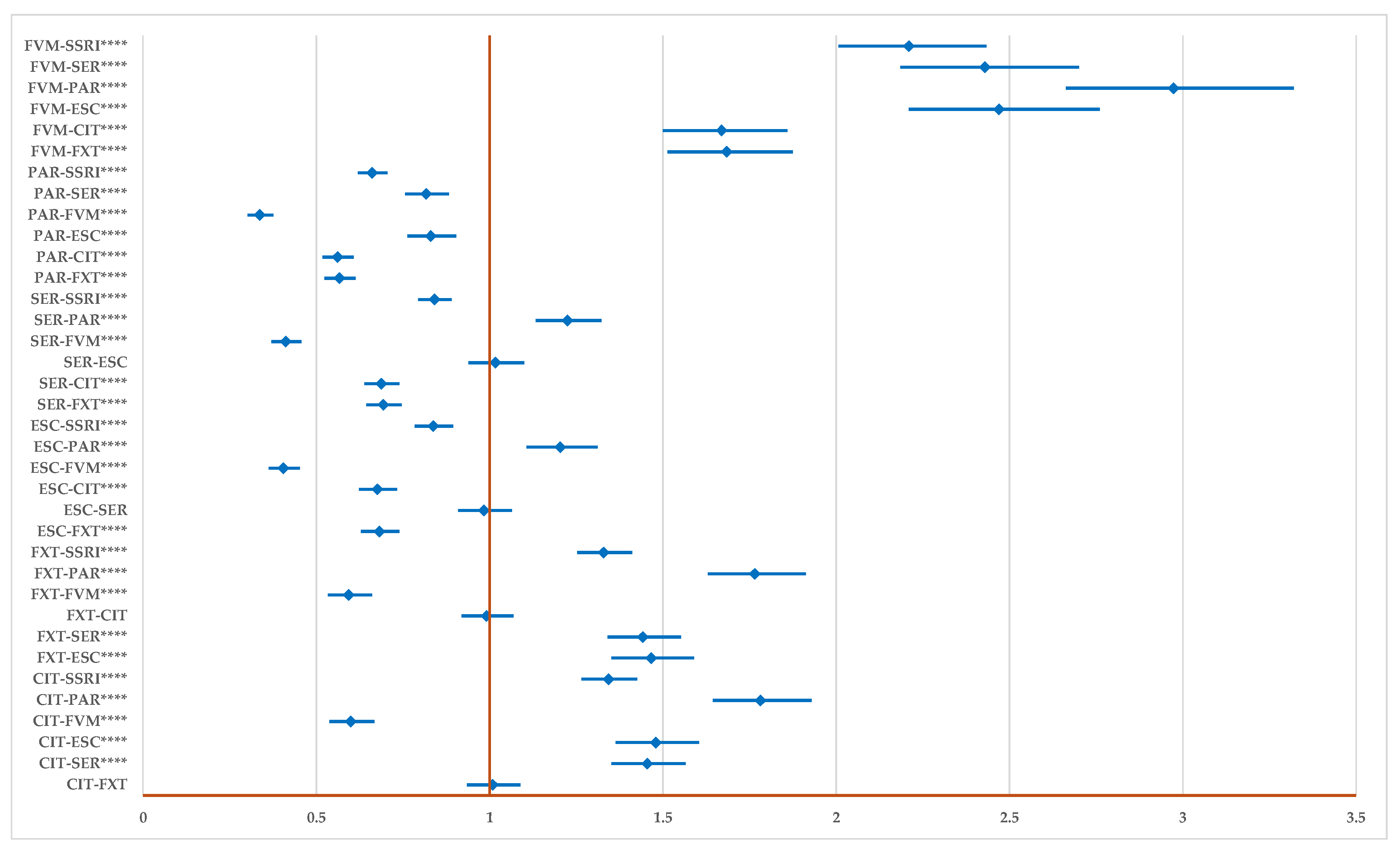


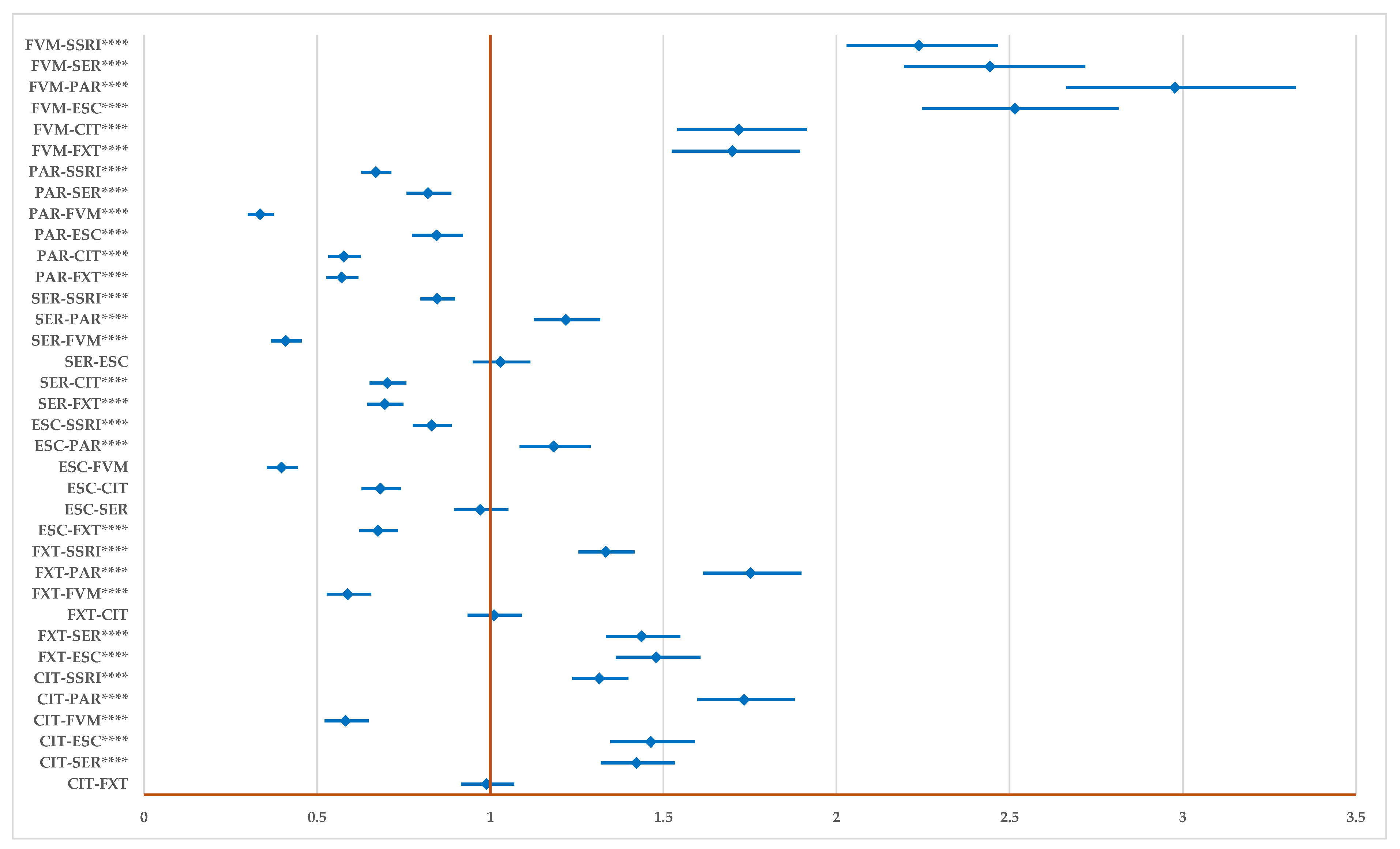
| CIT | ESC | FXT | FVM | PAR | SER | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age category | ||||||
| NS | 3595 | 4499 | 4888 | 568 | 6909 | 7625 |
| (16.10%) | (18.11%) | (22.75%) | (13.30%) | (24.23%) | (21.23%) | |
| 0–1 months | 400 | 487 | 443 | 56 | 1782 | 447 |
| (1.79%) | (1.96%) | (2.06%) | (1.31%) | (6.25%) | (1.24%) | |
| 2 months–2 years | 58 | 64 | 77 | 18 | 78 | 84 |
| (0.26%) | (0.26%) | (0.36%) | (0.42%) | (0.27%) | (0.23%) | |
| 3–11 years | 68 | 95 | 255 | 70 | 135 | 373 |
| (0.30%) | (0.38%) | (1.19%) | (1.64%) | (0.47%) | (1.04%) | |
| 12–17 years | 413 | 738 | 1754 | 322 | 522 | 2185 |
| (1.85%) | (2.97%) | (8.16%) | (7.54%) | (1.83%) | (6.08%) | |
| 18–64 years | 12,637 | 13,534 | 11,515 | 2487 | 13,574 | 18,738 |
| (56.58%) | (54.48%) | (53.58%) | (58.24%) | (47.59%) | (52.18%) | |
| 65–85 years | 4010 | 4184 | 2190 | 665 | 4413 | 5288 |
| (17.95%) | (16.84%) | (10.19%) | (15.57%) | (15.47%) | (14.72%) | |
| More than 85 years | 1155 | 1239 | 368 | 84 | 1107 | 1173 |
| (5.17%) | (4.99%) | (1.71%) | (1.97%) | (3.88%) | (3.27%) | |
| Sex category | ||||||
| Female | 13,762 | 15,573 | 13,207 | 2346 | 16,023 | 22,010 |
| (61.61%) | (62.69%) | (61.46%) | (54.94%) | (56.18%) | (61.29%) | |
| Male | 7741 | 8481 | 6144 | 1758 | 10,021 | 12,194 |
| (34.66%) | (34.14%) | (28.59%) | (41.17%) | (35.14%) | (33.95%) | |
| NS | 833 | 786 | 2139 | 166 | 2476 | 1709 |
| (3.73%) | (3.16%) | (9.95%) | (3.89%) | (8.68%) | (4.76%) | |
| Origin | ||||||
| EEA | 12,993 | 14,198 | 9133 | 1231 | 12,034 | 17,842 |
| (58.17%) | (57.16%) | (42.50%) | (28.83%) | (42.19%) | (49.68%) | |
| Non-EEA | 9343 | 10,642 | 12,356 | 3039 | 16,480 | 18,071 |
| (41.83%) | (42.84%) | (57.50%) | (71.17%) | (57.78%) | (50.32%) | |
| NS | 0 | 0 | 1 | 0 | 6 | 0 |
| (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.02%) | (0.00%) | |
| Reporter | ||||||
| HP | 17,485 | 18,263 | 16,402 | 3746 | 22,912 | 24,555 |
| (78.28%) | (73.52%) | (76.32%) | (87.73%) | (80.34%) | (68.37%) | |
| Non-HP | 4518 | 6492 | 4961 | 489 | 5399 | 10,972 |
| (20.23%) | (26.14%) | (23.09%) | (11.45%) | (18.93%) | (30.55%) | |
| NS | 333 | 85 | 127 | 35 | 209 | 386 |
| (1.49%) | (0.34%) | (0.59%) | (0.82%) | (0.73%) | (1.07%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrea, C.M.; Frum, A.; Butuca, A.; Morgovan, C.; Stoicescu, L.; Chis, A.A.; Arseniu, A.M.; Rus, L.L.; Gligor, F.G.; Vonica-Tincu, A.L. Drug–Drug Interactions of Selective Serotonin Reuptake Inhibitors: A Pharmacovigilance Study on Real-World Evidence from the EudraVigilance Database. Pharmaceuticals 2024, 17, 1278. https://doi.org/10.3390/ph17101278
Dobrea CM, Frum A, Butuca A, Morgovan C, Stoicescu L, Chis AA, Arseniu AM, Rus LL, Gligor FG, Vonica-Tincu AL. Drug–Drug Interactions of Selective Serotonin Reuptake Inhibitors: A Pharmacovigilance Study on Real-World Evidence from the EudraVigilance Database. Pharmaceuticals. 2024; 17(10):1278. https://doi.org/10.3390/ph17101278
Chicago/Turabian StyleDobrea, Carmen Maximiliana, Adina Frum, Anca Butuca, Claudiu Morgovan, Laurentiu Stoicescu, Adriana Aurelia Chis, Anca Maria Arseniu, Luca Liviu Rus, Felicia Gabriela Gligor, and Andreea Loredana Vonica-Tincu. 2024. "Drug–Drug Interactions of Selective Serotonin Reuptake Inhibitors: A Pharmacovigilance Study on Real-World Evidence from the EudraVigilance Database" Pharmaceuticals 17, no. 10: 1278. https://doi.org/10.3390/ph17101278
APA StyleDobrea, C. M., Frum, A., Butuca, A., Morgovan, C., Stoicescu, L., Chis, A. A., Arseniu, A. M., Rus, L. L., Gligor, F. G., & Vonica-Tincu, A. L. (2024). Drug–Drug Interactions of Selective Serotonin Reuptake Inhibitors: A Pharmacovigilance Study on Real-World Evidence from the EudraVigilance Database. Pharmaceuticals, 17(10), 1278. https://doi.org/10.3390/ph17101278








