Lansoprazole Ameliorates Isoniazid-Induced Liver Injury
Abstract
1. Introduction
2. Results
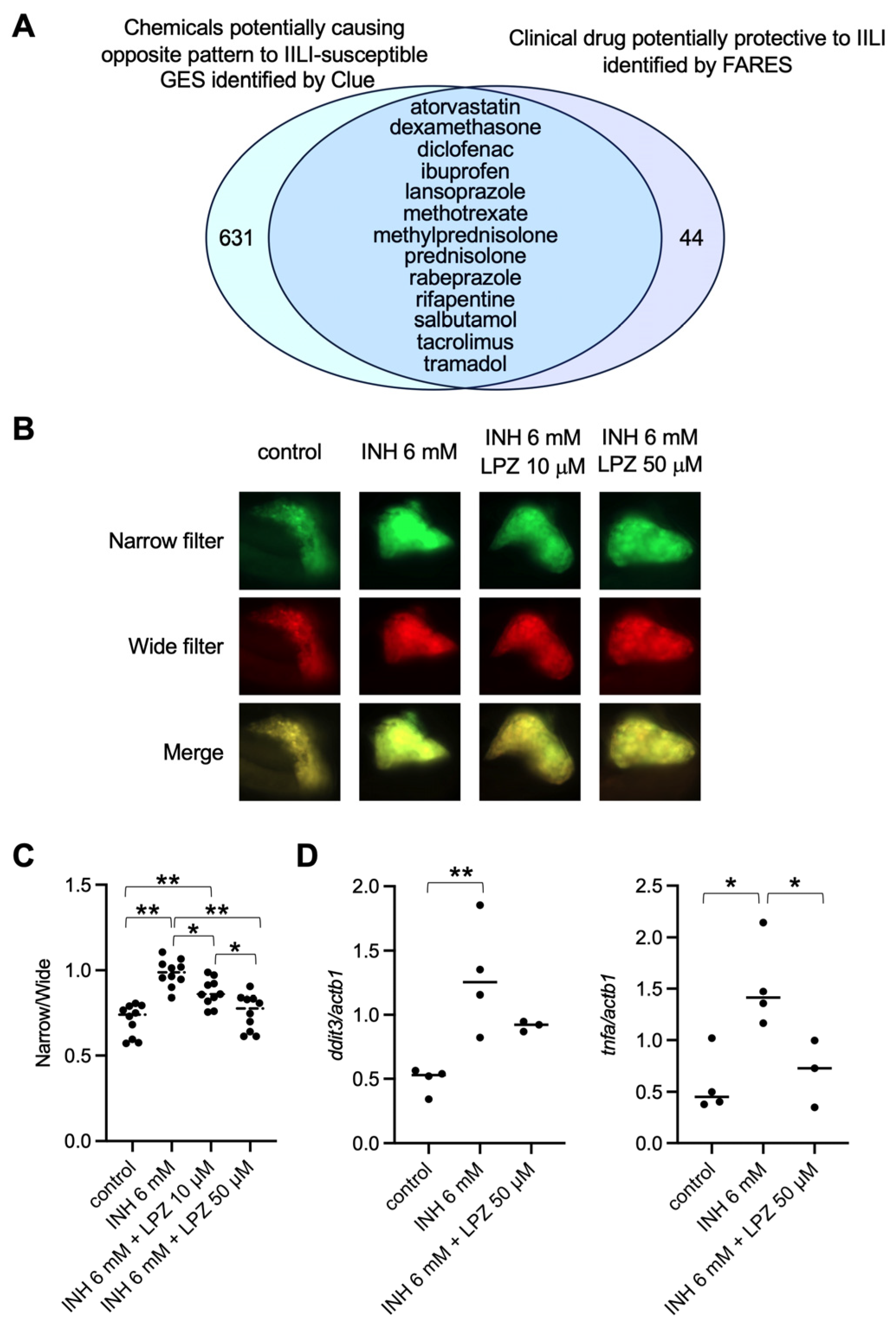
2.1. Identification of the Gene Expression Signature Associated with IILI
2.2. Lansoprazole Suppressed INH-Induced Apoptosis in Zebrafish Liver
2.3. Lansoprazole Was Protective against IILI in Patients
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Transcriptome Analysis
4.3. Bio-/Chemoinformatic Analysis
4.4. Compounds
4.5. Zebrafish Husbandry
4.6. In Vivo Fluorescence Imaging Using Tg (tagGFP-DEVD-tagRFP)
4.7. qPCR Analysis
4.8. Analysis of the Clinical Data of Patients Using Electrical Medical Records
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, s104–s109. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Guraka, A.; Shawa, I.T.; Tripathi, G.; Moritz, W.; Kermanizadeh, A. Drug induced liver injury—A 2023 update. J. Toxicol. Environ. Health Part B 2023, 26, 442–467. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Treatment of Drug-Induced Liver Injury. Biomedicines 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Hosack, T.; Damry, D.; Biswas, S. Drug-induced liver injury: A comprehensive review. Therap Adv. Gastroenterol. 2023, 16, 17562848231163410. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.; Reddy, K.M. Antitubercular Medications; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Björnsson, E.S. Hepatotoxicity by Drugs: The Most Common Implicated Agents. Int. J. Mol. Sci. 2016, 17, 224. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Devarbhavi, H.; Goel, A.; Venkatesan, R.; Eapen, C.E.; Grove, J.I.; Zafer, S.; Bjornsson, E.; Lucena, M.I.; Andrade, R.J.; et al. Genetic Risk Factors in Drug-Induced Liver Injury Due to Isoniazid-Containing Antituberculosis Drug Regimens. Clin. Pharmacol. Ther. 2021, 109, 1125–1135. [Google Scholar] [CrossRef]
- LiverTox. Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2018. [Google Scholar]
- Zhuang, X.; Li, L.; Liu, T.; Zhang, R.; Yang, P.; Wang, X.; Dai, L. Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Front. Pharmacol. 2022, 13, 1037814. [Google Scholar] [CrossRef]
- Church, R.J.; Wu, H.; Mosedale, M.; Sumner, S.J.; Pathmasiri, W.; Kurtz, C.L.; Pletcher, M.T.; Eaddy, J.S.; Pandher, K.; Singer, M.; et al. A systems biology approach utilizing a mouse diversity panel identifies genetic differences influencing isoniazid-induced microvesicular steatosis. Toxicol. Sci. 2014, 140, 481–492. [Google Scholar] [CrossRef]
- Harrill, A.H.; McAllister, K.A. New Rodent Population Models May Inform Human Health Risk Assessment and Identification of Genetic Susceptibility to Environmental Exposures. Environ. Health Perspect. 2017, 125, 086002. [Google Scholar] [CrossRef]
- AGusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; de Geus, E.J.C.; I Boomsma, D.; A Wright, F.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.-P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929. [Google Scholar] [CrossRef]
- Musa, A.; Ghoraie, L.S.; Zhang, S.-D.; Galzko, G.; Yli-Harja, O.; Dehmer, M.; Haibe-Kains, B.; Emmert-Streib, F. A review of connectivity map and computational approaches in pharmacogenomics. Brief. Bioinform. 2018, 19, 506–523. [Google Scholar] [PubMed]
- Zhao, Y.; Chen, X.; Chen, J.; Qi, X. Decoding Connectivity Map-based drug repurposing for oncotherapy. Brief. Bioinform. 2023, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Wakai, E.; Suzumura, Y.; Ikemura, K.; Mizuno, T.; Watanabe, M.; Takeuchi, K.; Nishimura, Y. An Integrated In Silico and In Vivo Approach to Identify Protective Effects of Palonosetron in Cisplatin-Induced Nephrotoxicity. Pharmaceuticals 2020, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Katoch, S.; Patial, V. Zebrafish: An emerging model system to study liver diseases and related drug discovery. J. Appl. Toxicol. 2020, 41, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Di Zeo-Sánchez, D.E.; Segovia-Zafra, A.; Matilla-Cabello, G.; Pinazo-Bandera, J.M.; Andrade, R.J.; Lucena, M.I.; Villanueva-Paz, M. Modeling drug-induced liver injury: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2022, 18, 555–573. [Google Scholar] [CrossRef]
- Shimizu, N.; Shiraishi, H.; Hanada, T. Zebrafish as a Useful Model System for Human Liver Disease. Cells 2023, 12, 2246. [Google Scholar] [CrossRef]
- De Souza Anselmo, C.; Sardela, V.F.; de Sousa, V.P.; Pereira, H.M.G. Zebrafish (Danio rerio): A valuable tool for predicting the metabolism of xenobiotics in humans? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 212, 34–46. [Google Scholar] [CrossRef]
- Jia, Z.-L.; Cen, J.; Wang, J.-B.; Zhang, F.; Xia, Q.; Wang, X.; Chen, X.-Q.; Wang, R.-C.; Hsiao, C.-D.; Liu, K.-C.; et al. Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: Activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere 2019, 227, 541–550. [Google Scholar] [CrossRef]
- Zhang, Y.; Cen, J.; Jia, Z.; Hsiao, C.D.; Xia, Q.; Wang, X.; Chen, X.; Wang, R.; Jiang, Z.; Zhang, L.; et al. Hepatotoxicity Induced by Isoniazid-Lipopolysaccharide through Endoplasmic Reticulum Stress, Autophagy, and Apoptosis Pathways in Zebrafish. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Higuchi, A.; Wakai, E.; Tada, T.; Koiwa, J.; Adachi, Y.; Shiromizu, T.; Goto, H.; Tanaka, T.; Nishimura, Y. Generation of a Transgenic Zebrafish Line for In Vivo Assessment of Hepatic Apoptosis. Pharmaceuticals 2021, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Ma, J.; Gao, H.; Zhang, Y.; Zhai, J.; Gong, J.; Song, Y.; Hu, T. Integration of metabolomics and proteomics analysis to explore the mechanism of neurotoxicity induced by receipt of isoniazid and rifampicin in mice. Neurotoxicology 2023, 94, 24–34. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Nagashima, T. Drug Repositioning and Target Finding Based on Clinical Evidence. Biol. Pharm. Bull. 2020, 43, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Nakatake, R.; Hishikawa, H.; Kotsuka, M.; Ishizaki, M.; Matsui, K.; Nishizawa, M.; Yoshizawa, K.; Kaibori, M.; Okumura, T. The Proton Pump Inhibitor Lansoprazole Has Hepatoprotective Effects in In Vitro and In Vivo Rat Models of Acute Liver Injury. Dig. Dis. Sci. 2019, 64, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Yamamoto, Y.; Yamagishi, N.; Iguchi, M.; Tamai, H.; Ito, T.; Tsuruo, Y.; Ichinose, M.; Kitano, M.; Ueyama, T. Lansoprazole prevents the progression of liver fibrosis in non-alcoholic steatohepatitis model rats. J. Pharm. Pharmacol. 2018, 70, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ueyama, T.; Nishi, T.; Yamamoto, Y.; Kawakoshi, A.; Sunami, S.; Iguchi, M.; Tamai, H.; Ueda, K.; Ito, T.; et al. Nrf2-inducing anti-oxidation stress response in the rat liver--new beneficial effect of lansoprazole. PLoS ONE 2014, 9, e97419. [Google Scholar] [CrossRef]
- Yamagishi, N.; Yamamoto, Y.; Nishi, T.; Ito, T.; Kanai, Y. Lansoprazole protects hepatic cells against cisplatin-induced oxidative stress through the p38 MAPK/ARE/Nrf2 pathway. PLoS ONE 2023, 18, e0287788. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Yang, N.; Huang, Y.; Hu, T.; Rao, C. Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 2022, 13, 1051. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Huang, S.; Jiang, X.; Xiao, W.; Wang, L.; Liu, Z.; Liu, S. Role of ER Stress in Xenobiotic-Induced Liver Diseases and Hepatotoxicity. Oxid. Med. Cell Longev. 2022, 2022, 4640161. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Anakk, S. Jekyll and Hyde: Nuclear receptors ignite and extinguish hepatic oxidative milieu. Trends Endocrinol. Metab. 2021, 32, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.-Y.; Fan, J.-T.; Hong, W.; Zhao, H.; Li, M.-H.; Jiang, L.; Fu, Y.-H.; Xing, Y.-X.; Chen, C.; Wang, J.-S. Isoniazid-induced hepatotoxicity and neurotoxicity in rats investigated by (1)H NMR based metabolomics approach. Toxicol. Lett. 2018, 295, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Yadav, A.; Dewangan, J.; Singh, S.V.; Mishra, M.; Singh, P.K.; Rath, S.K. Isoniazid prevents Nrf2 translocation by inhibiting ERK1 phosphorylation and induces oxidative stress and apoptosis. Redox Biol. 2015, 6, 80–92. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, Y.; Sun, S.; Han, T.; Li, Y.; Feng, F. Regulation of P300 and HDAC1 on endoplasmic reticulum stress in isoniazid-induced HL-7702 hepatocyte injury. J. Cell. Physiol. 2019, 234, 15299–15307. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Gao, H.; Zhai, J.; Tao, L.; Sun, J.; Song, Y.; Zhang, J. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. Int. Immunopharmacol. 2020, 85, 106634. [Google Scholar] [CrossRef]
- Desta, Z.; Soukhova, N.V.; Flockhart, D.A. Inhibition of cytochrome P450 (CYP450) isoforms by isoniazid: Potent inhibition of CYP2C19 and CYP3A. Antimicrob. Agents Chemother. 2001, 45, 382–392. [Google Scholar] [CrossRef]
- Wen, X.; Wang, J.S.; Neuvonen, P.J.; Backman, J.T. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur. J. Clin. Pharmacol. 2002, 57, 799–804. [Google Scholar] [CrossRef]
- Katsuki, H.; Hamada, A.; Nakamura, C.; Arimori, K.; Nakano, M. Role of CYP3A4 and CYP2C19 in the stereoselective metabolism of lansoprazole by human liver microsomes. Eur. J. Clin. Pharmacol. 2001, 57, 709–715. [Google Scholar] [CrossRef]
- Hermansen, A.; Regier, D.A.; Pollard, S. Developing Data Sharing Models for Health Research with Real-World Data: A Scoping Review of Patient and Public Preferences. J. Med. Syst. 2022, 46, 86. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lachmann, A.; Ma’ayan, A. Mining data and metadata from the gene expression omnibus. Biophys. Rev. 2019, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. Affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Del Carratore, F.; Jankevics, A.; Eisinga, R.; Heskes, T.; Hong, F.; Breitling, R. RankProd 2.0: A refactored bioconductor package for detecting differentially expressed features in molecular profiling datasets. Bioinformatics 2017, 33, 2774–2775. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Baldarelli, R.; Kadin, J.A.; Richardson, J.E.; Smith, C.L.; Bult, C.J. Mouse Genome Database (MGD): Knowledgebase for mouse-human comparative biology. Nucleic Acids Res. 2021, 49, D981–D987. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Westerfield, M. A Guide for the Laboratory Use of Zebrafish (Danio Rerio); University of Oregon Press: Eugene, OR, USA, 2007. [Google Scholar]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]

| Group | Term | Source | Associated Genes Found |
|---|---|---|---|
| Up | disulfide oxidoreductase activity | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | intramolecular oxidoreductase activity | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | intramolecular oxidoreductase activity, transposing S-S bonds | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | oxidoreductase activity, acting on a sulfur group of donors | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | protein disulfide isomerase activity | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | protein disulfide reductase activity | GO_MF | [PDIA3, PDIA4, PDIA6] |
| Up | binding and uptake of ligands by scavenger receptors | REACTOME | [CALR, HYOU1, SAA1] |
| Up | IRE1alpha activates chaperones | REACTOME | [DNAJB11, HYOU1, PDIA6] |
| Up | Unfolded Protein Response (UPR) | REACTOME | [CALR, DNAJB11, HYOU1, PDIA6] |
| Up | XBP1(S) activates chaperone genes | REACTOME | [DNAJB11, HYOU1, PDIA6] |
| Down | bile acid biosynthetic process | GO_BP | [CES1, CYP27A1, SLC27A5] |
| Down | bile acid metabolic process | GO_BP | [CES1, CYP27A1, SLC27A5] |
| Down | cellular response to lipoprotein particle stimulus | GO_BP | [CES1, LPL, MIA3] |
| Down | lipid storage | GO_BP | [CES1, ENPP1, HEXB, LPL] |
| Down | lipopolysaccharide-mediated signaling pathway | GO_BP | [EHHADH, LBP, NFKBIL1] |
| Down | negative regulation of cellular response to insulin stimulus | GO_BP | [ENPP1, LPL, SOCS3] |
| Down | negative regulation of tumor necrosis factor production | GO_BP | [EHHADH, IGF1, LBP, NFKBIL1] |
| Down | negative regulation of tumor necrosis factor superfamily cytokine production | GO_BP | [EHHADH, IGF1, LBP, NFKBIL1] |
| Down | neuroinflammatory response | GO_BP | [CTSC, IGF1, JUN] |
| Down | positive regulation of chemokine production | GO_BP | [EGR1, EHHADH, LBP, LPL] |
| Down | positive regulation of macrophage activation | GO_BP | [CTSC, EHHADH, LBP] |
| Down | positive regulation of pattern recognition receptor signaling pathway | GO_BP | [EHHADH, LBP, RSAD2] |
| Down | positive regulation of toll-like receptor signaling pathway | GO_BP | [EHHADH, LBP, RSAD2] |
| Down | regulation of cellular response to insulin stimulus | GO_BP | [ENPP1, LPL, SOCS3] |
| Down | regulation of DNA-templated transcription in response to stress | GO_BP | [EGR1, JUN, MIA3] |
| Down | regulation of macrophage activation | GO_BP | [CTSC, EHHADH, LBP] |
| Down | regulation of release of cytochrome c from mitochondria | GO_BP | [BCL2L11, BNIP3, IGF1] |
| Down | regulation of toll-like receptor signaling pathway | GO_BP | [EHHADH, LBP, NFKBIL1, RSAD2] |
| Down | regulation of transcription from RNA polymerase II promoter in response to stress | GO_BP | [EGR1, JUN, MIA3] |
| Down | release of cytochrome c from mitochondria | GO_BP | [BCL2L11, BNIP3, IGF1] |
| Down | response to lipoprotein particle | GO_BP | [CES1, LPL, MIA3] |
| Down | oxidoreductase activity, acting on the CH-CH group of donors | GO_MF | [PECR, RETSAT, TM7SF2] |
| Down | drug metabolism | KEGG | [CES1, GSTA5, UGT2B7, UPP2] |
| Down | PPAR signaling pathway | KEGG | [CYP27A1, EHHADH, LPL, SCD, SLC27A5] |
| Down | interferon alpha/beta signaling | REACTOME | [EGR1, RSAD2, SOCS3] |
| Characteristics | Non-LPZ (n = 226) | LPZ (n = 30) | p Value |
|---|---|---|---|
| Female | 123 (54) | 13 (43) | 0.258 |
| Age (years) | 66 [27–93] | 71 [34–86] | 0.043 |
| Body weight (kg) | 59.0 [34.5–113.9] | 55.2 [35–120] | 0.387 |
| Smoking history | 56 (25) | 7 (23) | 0.975 |
| Drinking history | 40 (18) | 4 (13) | 0.648 |
| Medical history | |||
| Heart disease | 21 (9) | 3 (10) | 0.748 |
| Hypertension | 4 (2) | 1 (3) | 0.338 |
| Type 2 diabetes disease | 7 (3) | 1 (3) | 0.664 |
| Biological parameters before INH treatment | |||
| AST (U/L) | 21 [7–81] | 19 [6–48] | 0.206 |
| ALT (U/L) | 16 [4–81] | 15 [5–69] | 0.550 |
| γ-GTP | 23 [8–439] | 25 [8–90] | 0.916 |
| T-Bil | 0.5 [0.2–3.0] | 0.5 [0.2–2.1] | 0.279 |
| ALP | 235 [97–1606] | 221 [108–583] | 0.628 |
| LDH | 189 [85–429] | 180 [100–297] | 0.194 |
| Scr (mg/dL) | 0.65 [0.36–1.1] | 0.61 [0.38–0.92] | 0.204 |
| eGFR (mL/min) | 81.1 [59.0–151] | 85.7 [63.6–126.4] | 0.113 |
| Duration of INH administration (day) | 186 [7–3385] | 250 [21–998] | 0.040 |
| Dose of INH (mg) | 250 [100–400] | 250 [100–400] | 0.464 |
| Biological parameters after INH treatment | |||
| AST (U/L) | 34 [13–2424] | 27.5 [15–179] | 0.017 |
| ALT (U/L) | 34 [4–1550] | 23.5 [8–305] | 0.013 |
| γ-GTP | 30.0 [9–551] | 24.0 [10–112] | 0.370 |
| T-Bil | 0.7 [0.2–3.6] | 0.6 [0.2–1.3] | 0.059 |
| ALP | 245 [118–2214] | 222 [100–400] | 0.108 |
| LDH | 202 [96–837] | 201.5 [115–309] | 0.754 |
| Scr (mg/dL) | 0.61 [0.4–1.83] | 0.58 [0.38–1.1] | 0.232 |
| eGFR (mL/min) | 79.7 [28.8–223] | 87.3 [46.6–155.3] | 0.184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakai, E.; Shiromizu, T.; Otaki, S.; Koiwa, J.; Tamaru, S.; Nishimura, Y. Lansoprazole Ameliorates Isoniazid-Induced Liver Injury. Pharmaceuticals 2024, 17, 82. https://doi.org/10.3390/ph17010082
Wakai E, Shiromizu T, Otaki S, Koiwa J, Tamaru S, Nishimura Y. Lansoprazole Ameliorates Isoniazid-Induced Liver Injury. Pharmaceuticals. 2024; 17(1):82. https://doi.org/10.3390/ph17010082
Chicago/Turabian StyleWakai, Eri, Takashi Shiromizu, Shota Otaki, Junko Koiwa, Satoshi Tamaru, and Yuhei Nishimura. 2024. "Lansoprazole Ameliorates Isoniazid-Induced Liver Injury" Pharmaceuticals 17, no. 1: 82. https://doi.org/10.3390/ph17010082
APA StyleWakai, E., Shiromizu, T., Otaki, S., Koiwa, J., Tamaru, S., & Nishimura, Y. (2024). Lansoprazole Ameliorates Isoniazid-Induced Liver Injury. Pharmaceuticals, 17(1), 82. https://doi.org/10.3390/ph17010082







