1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking
Abstract
1. Introduction
2. Results and Discussion
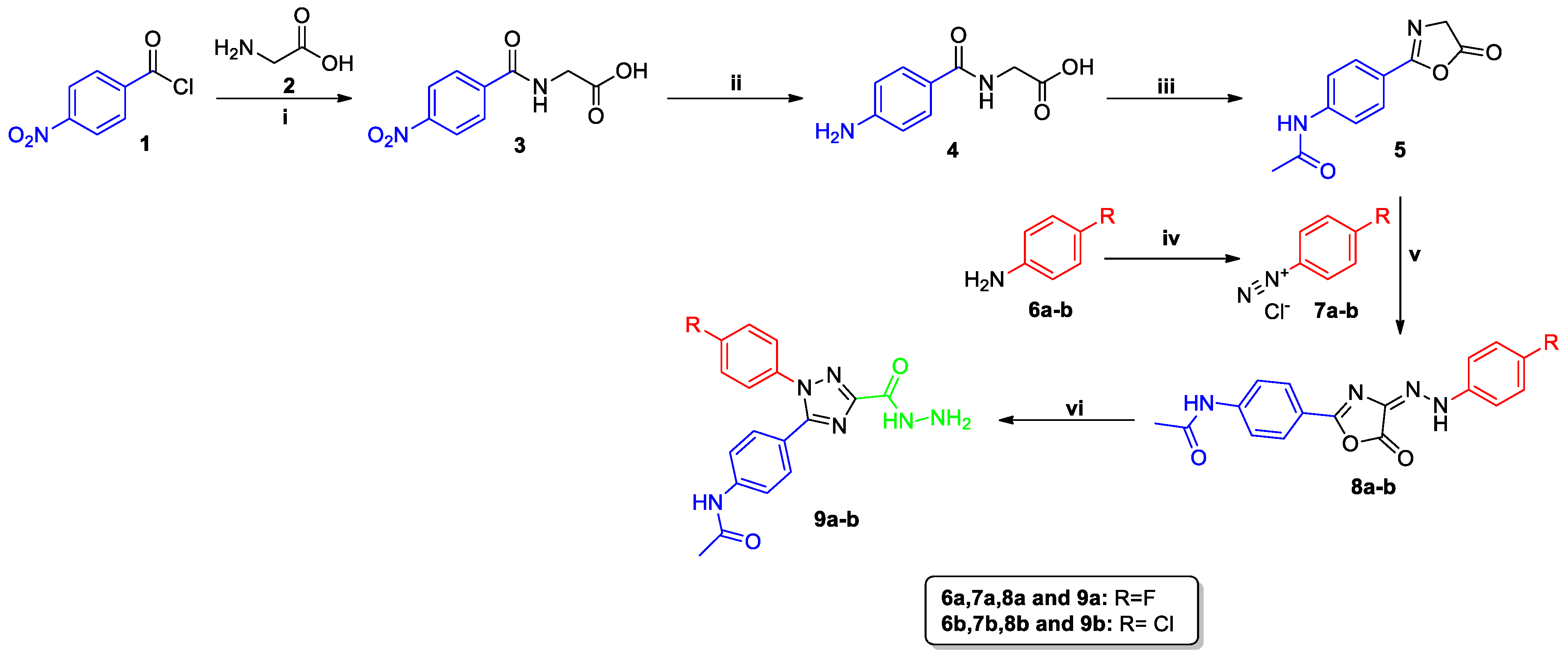
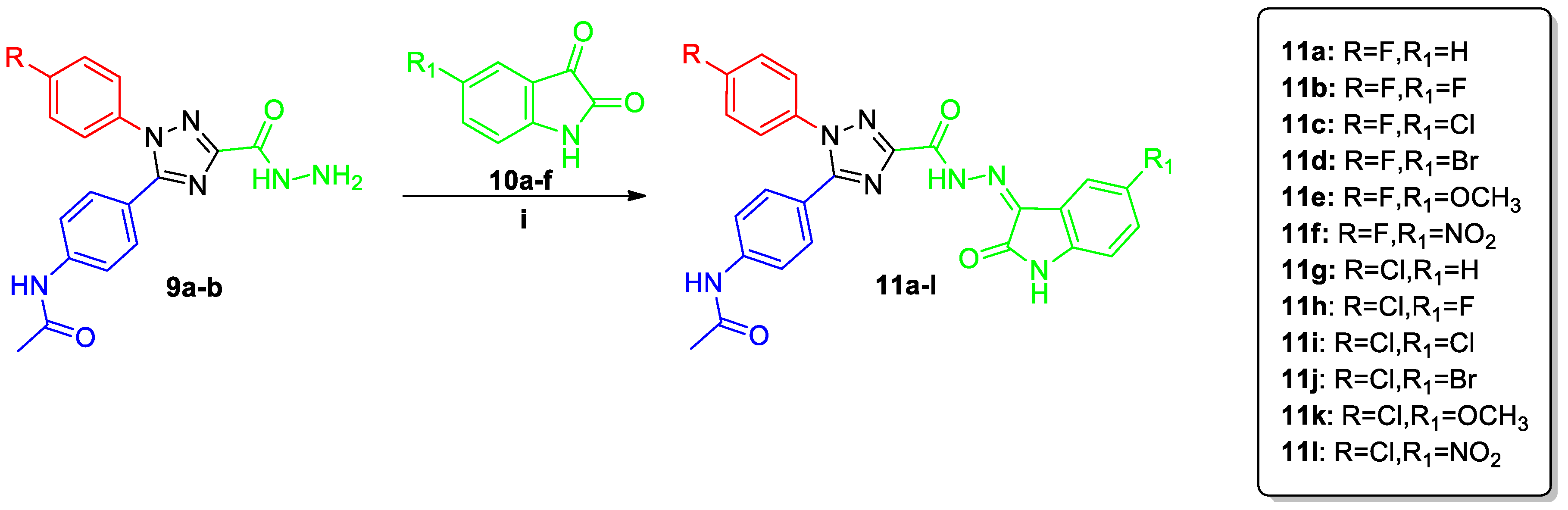
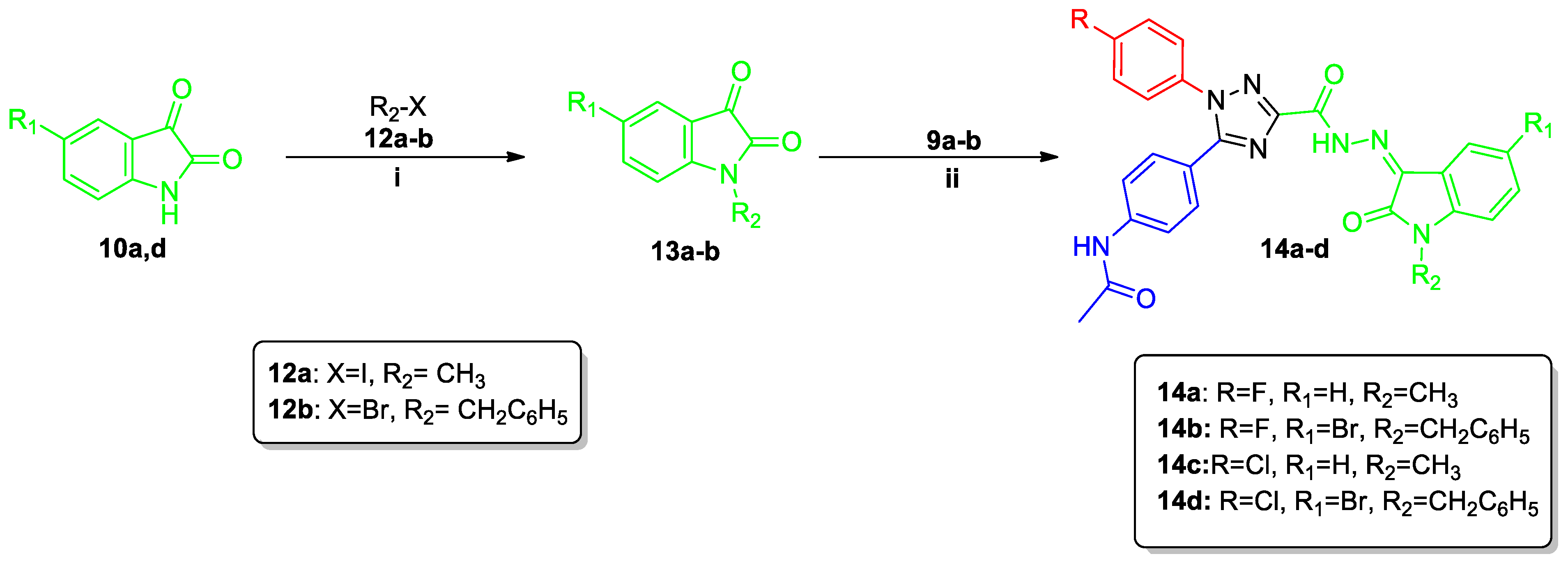
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Anti-Proliferative Activity against PANC1 and HepG2 Cell Lines
2.2.2. VEGFR-2 Inhibitory Activities
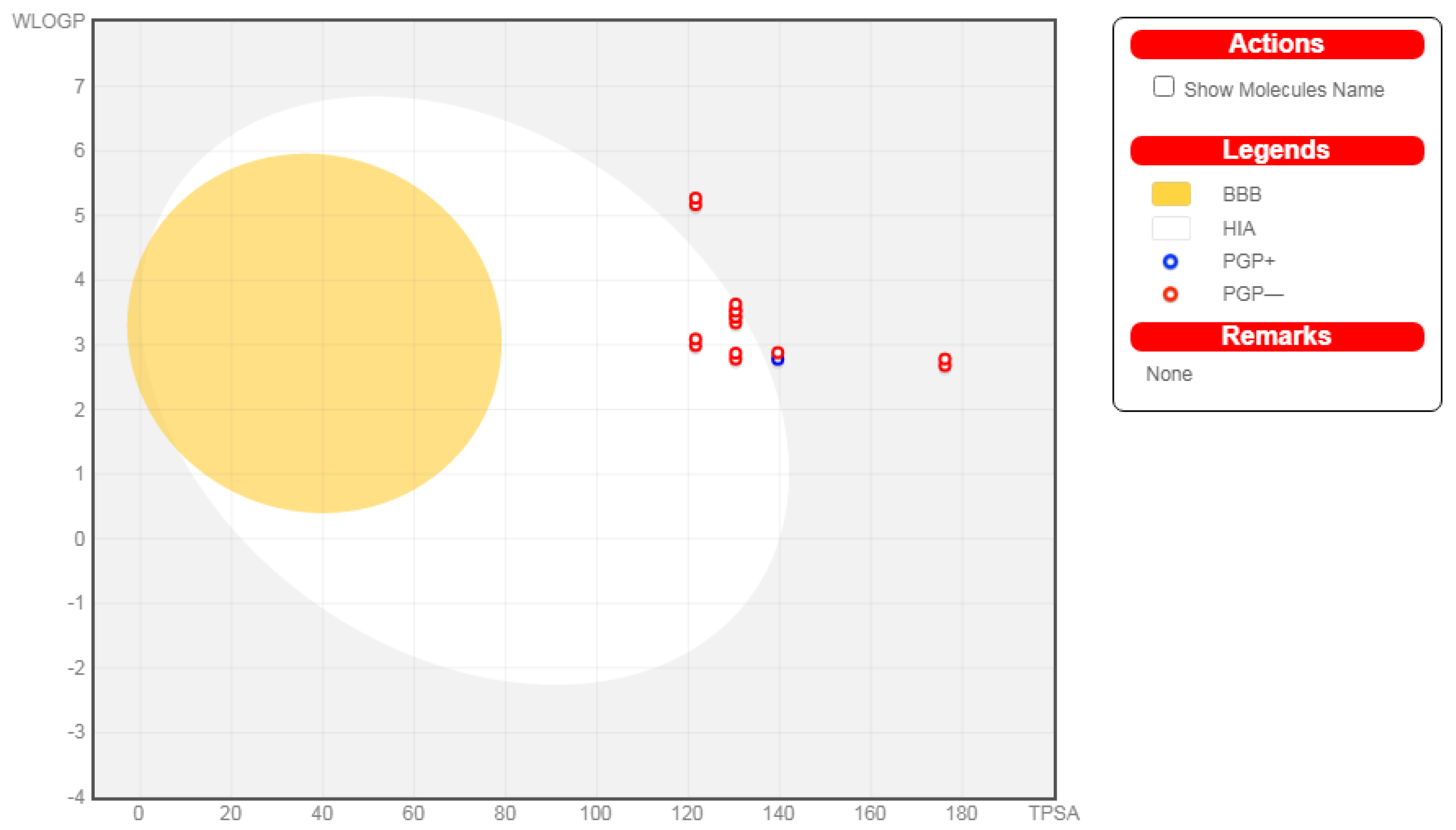
2.3. In Silico ADME Study
2.4. Molecular Modelling
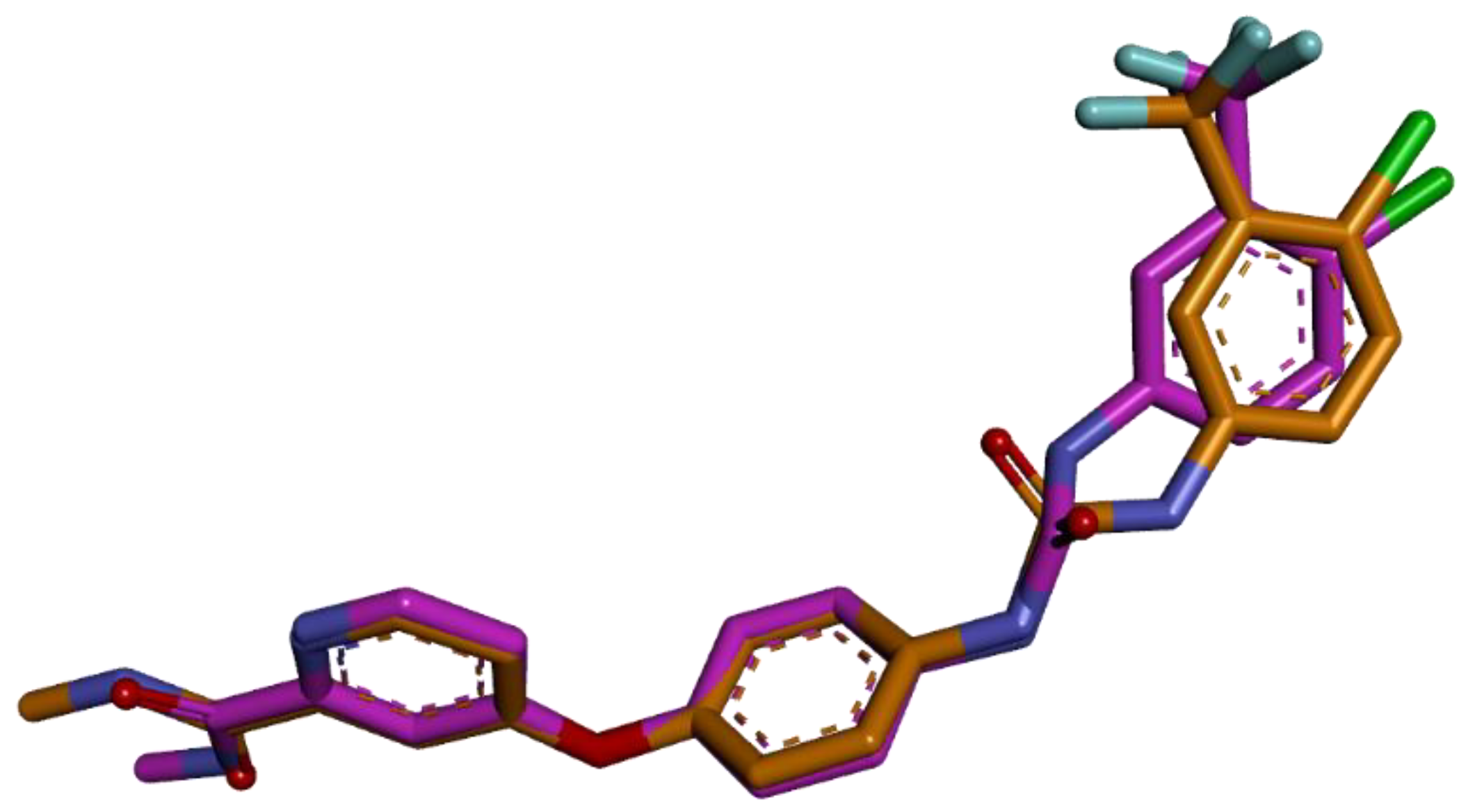
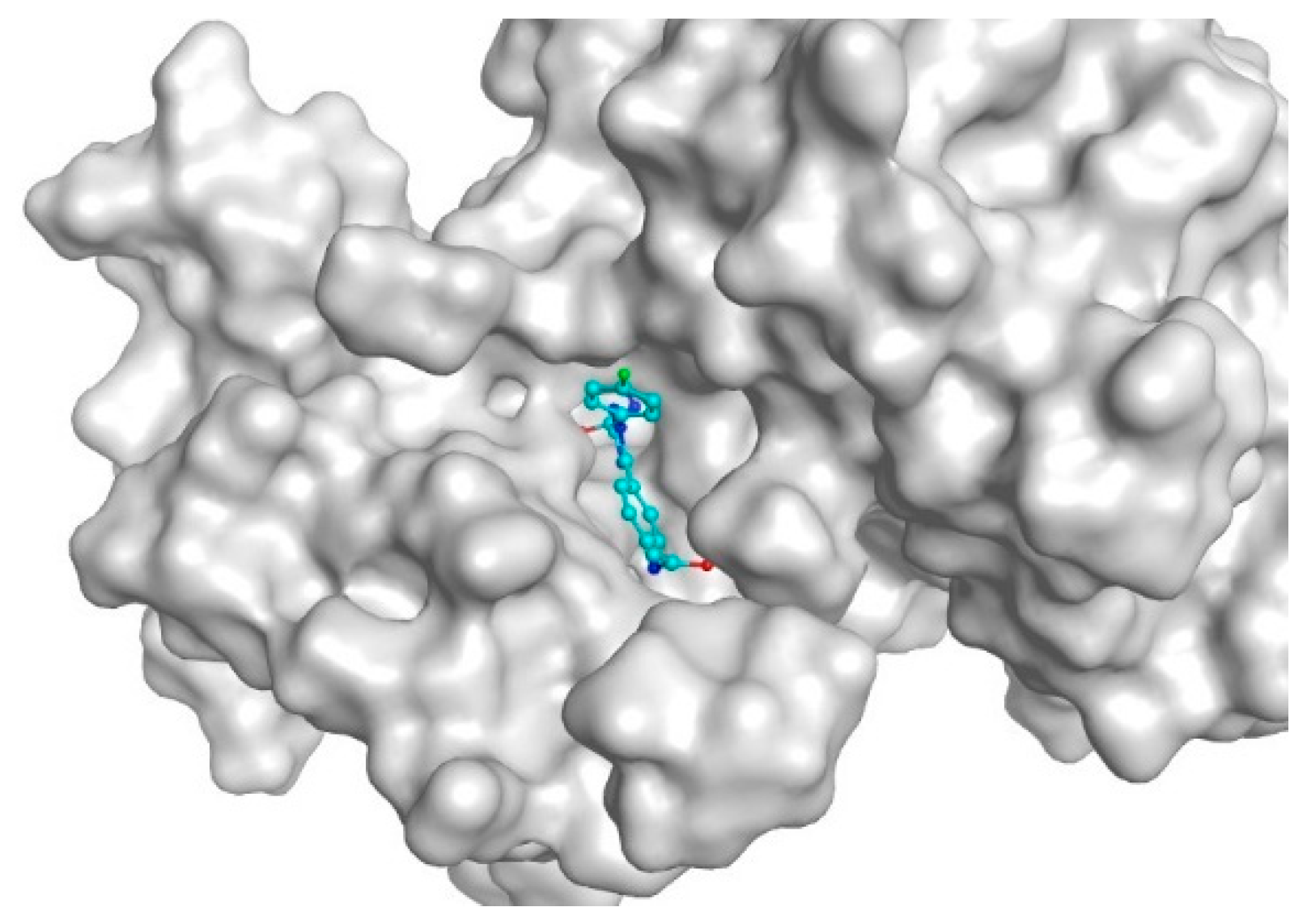
2.4.1. Molecular Docking
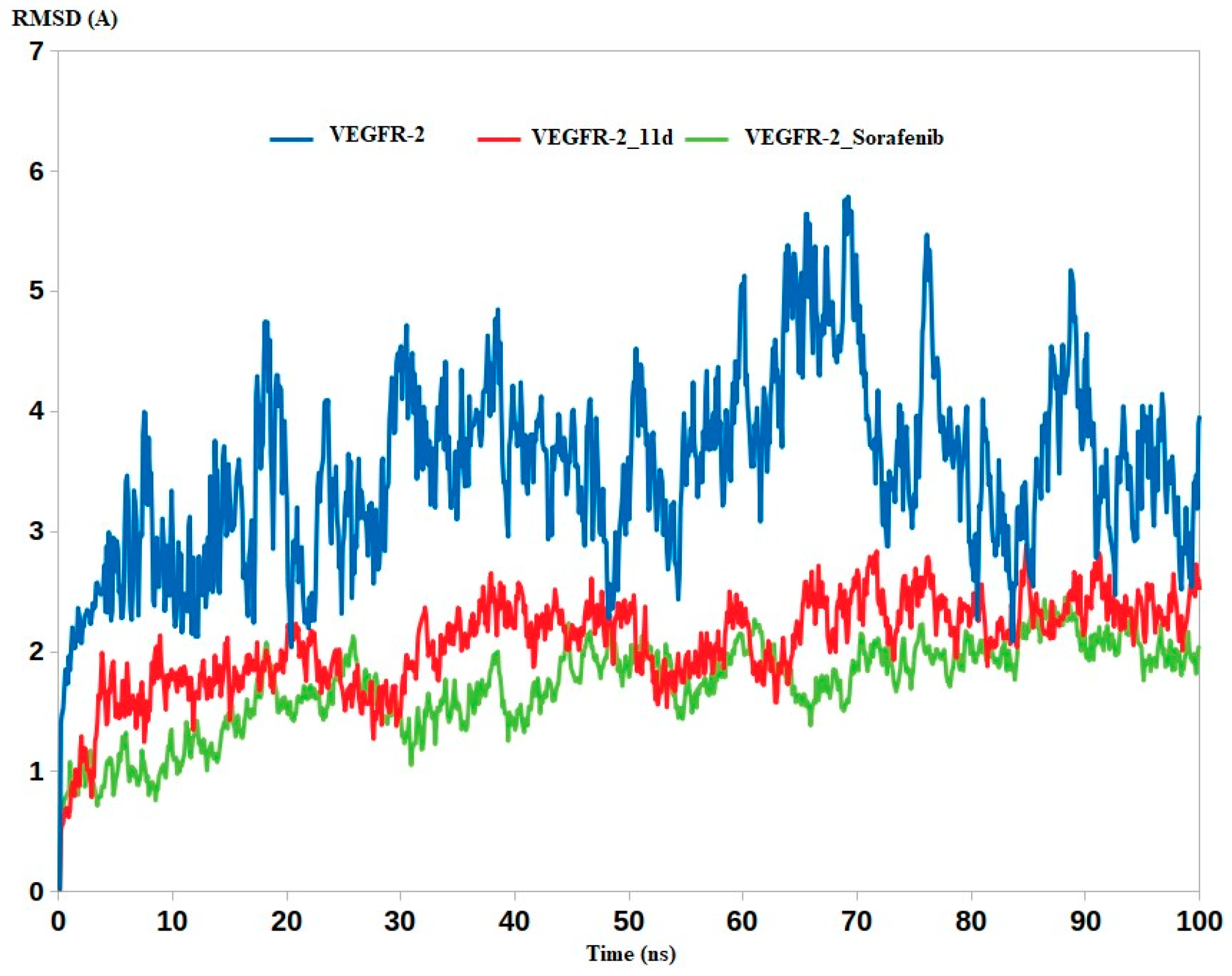
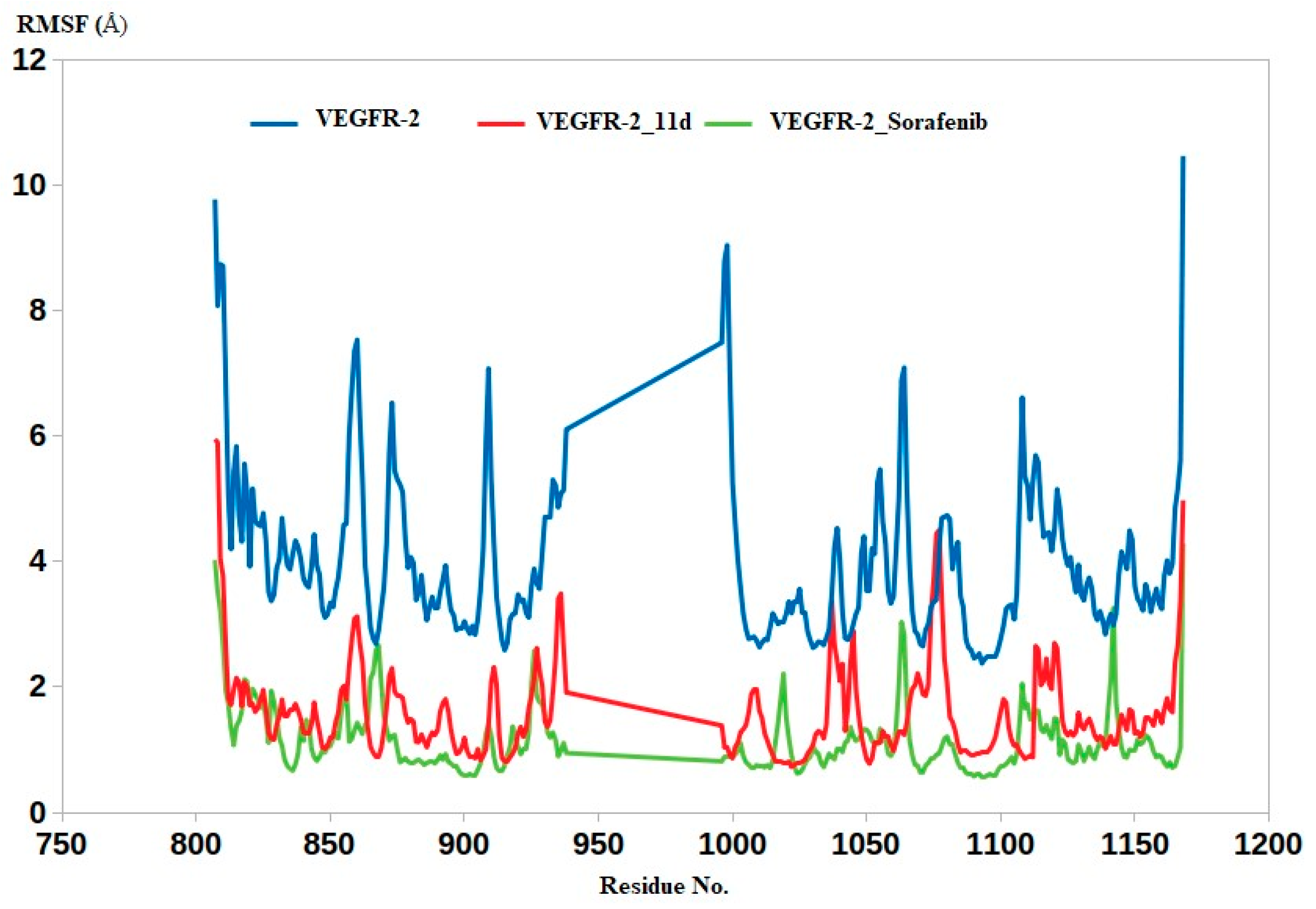
2.4.2. Molecular Dynamics
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 4-Nitrohippuric Acid (3)
3.1.2. Synthesis of 4-Aminohippuric Acid (4)
3.1.3. Synthesis of N-{4-[(4-[2-Arylhydrazin-1-ylidene]-5-oxo-4,5-dihydro-1,3-oxazol-2-yl]phenyl}acetamides (8a–b)
N-{4-[(4-[2-(4-Fluorophenyl)hydrazin-1-ylidene]-5-oxo-4,5-dihydro-1,3-oxazol-2-yl]phenyl}acetamide (8a)
N-{4-[(4-[2-(4-Chlorophenyl)hydrazin-1-ylidene]-5-oxo-4,5-dihydro-1,3-oxazol-2-yl]phenyl}acetamide (8b)
3.1.4. Synthesis of N-{4-[1-Aryl-3-(hydrazinecarbonyl)-1H-1,2,4-triazol-5-yl]phenyl}acetamide (9a–b)
N-{4-[1-(4-Fluorophenyl)-3-(hydrazinecarbonyl)-1H-1,2,4-triazol-5-yl]phenyl}acetamide (9a)
N-{4-[1-(4-Chlorophenyl)-3-(hydrazinecarbonyl)-1H-1,2,4-triazol-5-yl]phenyl}acetamide (9b)
3.1.5. Synthesis of N-[4-(1-Aryl-3-{N′-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl)phenyl]acetamides (11a–l and 14a–d)
N-{4-[1-(4-Fluorophenyl)-3-{N′-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11a)
N-[4-(3-{N′-[5-Fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-fluorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (11b)
N-[4-(3-{N′-[5-Chloro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-fluorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (11c)
N-[4-(3-{N′-[5-Bromo-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-fluorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (11d)
N-{4-[1-(4-Fluorophenyl)-3-{N′-[5-methoxy-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11e)
N-{4-[1-(4-Fluorophenyl)-3-{N′-[5-nitro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11f)
N-{4-[1-(4-Chlorophenyl)-3-{N′-[2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11g)
N-{4-[1-(4-Chlorophenyl)-3-{N′-[5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11h)
N-[4-(3-{N′-[5-Chloro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-chlorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (11i)
N-[4-(3-{N′-[5-Bromo-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-chlorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (11j)
N-{4-[1-(4-Chlorophenyl)-3-{N′-[5-methoxy-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11k)
N-{4-[1-(4-Chlorophenyl)-3-{N′-[5-nitro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (11l)
N-{4-[1-(4-Fluorophenyl)-3-{N′-[1-methyl-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (14a)
N-[4-(3-{N′-[1-Benzyl-5-bromo-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-fluorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (14b)
N-{4-[1-(4-Chlorophenyl)-3-{N′-[1-methyl-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1H-1,2,4-triazol-5-yl]phenyl}acetamide (14c)
N-[4-(3-{N′-[1-Benzyl-5-bromo-2-oxo-2,3-dihydro-1H-indol-3-ylidene]hydrazinecarbonyl}-1-(4-chlorophenyl)-1H-1,2,4-triazol-5-yl)phenyl]acetamide (14d)
3.2. Biological Evaluation
3.3. Molecular Docking
3.4. Molecular Dynamics
3.5. In Silico ADME Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in Cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Bokov, D.; Markov, A.; Jalil, A.; Shalaby, M.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal. 2022, 20, 49. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Le, X.; Cascone, T.; Jain, R.K.; Heymach, J.V. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin. Cancer Res. 2023, 29, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, A.; Melillo, G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 2012, 114, 237–267. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Yashiro, M.; Yamada, N.; Amano, R.; Noda, S.; Hirakawa, K. VEGF-A/VEGFR-2 Signaling Plays an Important Role for the Motility of Pancreas Cancer Cells. Ann. Surg. Oncol. 2012, 19, 2733–2743. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Molecular targets for the treatment of pancreatic cancer: Clinical and experimental studies. World J. Gastroenterol. 2016, 22, 776–789. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef]
- Juaid, N.; Amin, A.; Abdalla, A.; Reese, K.; Alamri, Z.; Moulay, M.; Abdu, S.; Miled, N. Anti-Hepatocellular Carcinoma Biomolecules: Molecular Targets Insights. Int. J. Mol. Sci. 2021, 22, 10774. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Llovet, J.M.; Pinyol, R.; Kelley, R.K.; El-Khoueiry, A.; Reeves, H.L.; Wang, X.W.; Gores, G.J.; Villanueva, A. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat. Cancer 2022, 3, 386–401. [Google Scholar] [CrossRef] [PubMed]
- Xelwa, N.; Candy, G.P.; Devar, J.; Omoshoro-Jones, J.; Smith, M.; Nweke, E.E. Targeting Growth Factor Signaling Pathways in Pancreatic Cancer: Towards Inhibiting Chemoresistance. Front. Oncol. 2021, 11, 683788. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Bari, S.; Surana, S.; Shirkhedkar, A.; Wakode, S.; Shelar, S.; Racharla, S.; Ugale, V.; Ghodke, M. Logical synthetic strategies and structure-activity relationship of indolin-2-one hybrids as small molecule anticancer agents: An overview. J. Mol. Struct. 2022, 1247, 131280. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Raymond, E.; Faivre, S. Sunitinib: A novel tyrosine kinase inhibitor. A brief review of its therapeutic potential in the treatment of renal carcinoma and gastrointestinal stromal tumors (GIST). Ther. Clin. Risk Manag. 2007, 3, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, H.; Izumi, N.; Aramaki, T.; Ikeda, M.; Inaba, Y.; Imanaka, K.; Okusaka, T.; Kanazawa, S.; Kaneko, S.; Kora, S.; et al. Subgroup analysis of efficacy and safety of orantinib in combination with TACE in Japanese HCC patients in a randomized phase III trial (ORIENTAL). Med. Oncol. 2019, 36, 52. [Google Scholar] [CrossRef]
- Korphaisarn, K.; Danchaivijitr, P.; Reungwetwattana, T.; Chewaskulyong, B.; Thongthieang, L.; Chindaprasirt, J.; Maneenil, K.; Sathitruangsak, C.; Vinayanuwattikun, C. Efficacy of Combination Docetaxel and Nintedanib in Advanced Non-Small Cell Lung Cancer in Thailand: A Multicenter Study. Front. Oncol. 2021, 11, 572740. [Google Scholar] [CrossRef]
- Zengin, M.; Unsal Tan, O.; Arafa, R.K.; Balkan, A. Design and synthesis of new 2-oxoquinoxalinyl-1,2,4-triazoles as antitumor VEGFR-2 inhibitors. Bioorganic Chem. 2022, 121, 105696. [Google Scholar] [CrossRef]
- Alsaedi, A.M.R.; Almehmadi, S.J.; Farghaly, T.A.; Harras, M.F.; Khalil, K.D. VEGFR2 and hepatocellular carcinoma inhibitory activities of trisubstituted triazole derivatives. J. Mol. Struct. 2022, 1250, 131832. [Google Scholar] [CrossRef]
- Elsawi, A.E.; Elbadawi, M.M.; Nocentini, A.; Almahli, H.; Giovannuzzi, S.; Shaldam, M.; Salem, R.; Ibrahim, T.M.; Abdel-Aziz, H.A.; Supuran, C.T.; et al. 1,5-Diaryl-1,2,4-triazole Ureas as New SLC-0111 Analogues Endowed with Dual Carbonic Anhydrase and VEGFR-2 Inhibitory Activities. J. Med. Chem. 2023, 66, 10558–10578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, P.L.; Gray, N.S. Targeting cancer with small molecule kinase inhibitors. Nat. Rev. Cancer 2009, 9, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.; Kenawy, A.M.; Abd El-Wahab, A.E.; Elkaeed, E.B.; Alsfouk, B.A.; Metwaly, A.M.; Eissa, I.H. Discovery of new quinoline and isatine derivatives as potential VEGFR-2 inhibitors: Design, synthesis, antiproliferative, docking and MD simulation studies. J. Biomol. Struct. Dyn. 2023, 41, 11535–11550. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.A. Drug hybridization strategies: Before or after lead identification? Expert Opin. Drug Discov. 2009, 4, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Sawdey, G.W. Rearrangement of 4-Arylazo-2-phenyloxazolin-5-ones: A New Synthesis of 1H-1,2,4-Triazoles. J. Am. Chem. Soc. 1957, 79, 1955–1956. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent anti-proliferative agents. J. Enzym. Inhib. Med. Chem. 2018, 33, 867–878. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Abo-Ashour, M.F.; Al-Warhi, T.; Elaasser, M.M.; Safwat, N.A.; Suliman, H.; Ahmed, M.F.; Al-Rashood, S.T.; Abdel-Aziz, H.A.; et al. Development of isatin-thiazolo[3,2-a]benzimidazole hybrids as novel CDK2 inhibitors with potent in vitro apoptotic anti-proliferative activity: Synthesis, biological and molecular dynamics investigations. Bioorganic Chem. 2021, 110, 104748. [Google Scholar] [CrossRef]
- Mokale, S.N.; Lokwani, D.; Shinde, D.B. Synthesis, biological activity and docking study of imidazol-5-one as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorganic Med. Chem. 2012, 20, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.C.; Farrar, C.R.; Niazy, O.; Williams, A. Structure-reactivity studies on the equilibrium reaction between phenolate ions and 2-aryloxazolin-5-ones: Data consistent with a concerted acyl-group-transfer mechanism. J. Am. Chem. Soc. 1980, 102, 6828–6837. [Google Scholar] [CrossRef]
- Abo-Ashour, M.F.; Almahli, H.; Bonardia, A.; Khalil, A.; Al-Warhi, T.; Al-Rashood, S.T.; Abdel-Aziz, H.A.; Nocentini, A.; Supuran, C.T.; Eldehna, W.M. Enaminone-based carboxylic acids as novel non-classical carbonic anhydrases inhibitors: Design, synthesis and in vitro biological assessment. J. Enzym. Inhib. Med. Chem. 2022, 37, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Friedler, L.; Smith, J.N. Comparative detoxication. 3. Hippuric acid formation in adult locusts. Biochem. J. 1954, 57, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE-Antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Eldehna, W.M.; El Hassab, M.A.; Elsayed, Z.M.; Al-Warhi, T.; Elkady, H.; Abo-Ashour, M.F.; Abourehab, M.A.S.; Eissa, I.H.; Abdel-Aziz, H.A. Design, synthesis, in vitro biological assessment and molecular modeling insights for novel 3-(naphthalen-1-yl)-4,5-dihydropyrazoles as anticancer agents with potential EGFR inhibitory activity. Sci. Rep. 2022, 12, 12821. [Google Scholar] [CrossRef]



 11a–l 11a–l | 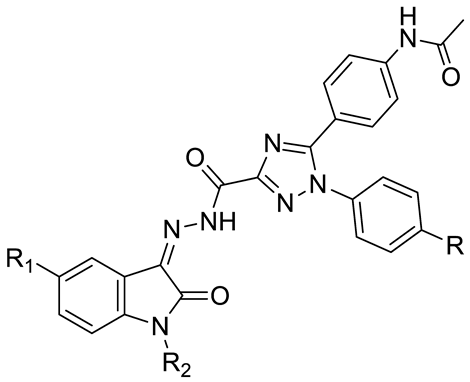 14a–d 14a–d | ||||
|---|---|---|---|---|---|
| Comp. | R | R1 | R2 | IC50 (µM) a | |
| PANC1 | HepG2 | ||||
| 11a | F | H | --- | 4.29 ± 0.1 | 2.06 ± 0.03 |
| 11b | F | F | --- | 1.74 ± 0.02 | 1.17 ± 0.03 |
| 11c | F | Cl | --- | 0.98 ± 0.06 | 2.21 ± 0.03 |
| 11d | F | Br | --- | 1.16 ± 0.02 | 0.73 ± 0.02 |
| 11e | F | OCH3 | --- | 2.22 ± 0.07 | 0.76 ± 0.02 |
| 11f | F | NO2 | --- | 0.23 ± 0.01 | 2.41 ± 0.04 |
| 11g | Cl | H | --- | 0.77 ± 0.02 | 0.71 ± 0.01 |
| 11h | Cl | F | --- | 0.22 ± 0.01 | 1.27 ± 0.04 |
| 11i | Cl | Cl | --- | 3.75 ± 0.09 | 4.49 ± 0.15 |
| 11j | Cl | Br | --- | 1.68 ± 0.06 | 1.01 ± 0.02 |
| 11k | Cl | OCH3 | --- | 1.78 ± 0.04 | 0.73 ± 0.01 |
| 11l | Cl | NO2 | --- | 0.17 ± 0.01 | 1.73 ± 0.06 |
| 14a | F | H | CH3 | 4.05 ± 0.1 | 1.63 ± 0.02 |
| 14b | F | Br | C6H5 | 0.76 ± 0.04 | 0.98 ± 0 |
| 14c | Cl | H | CH3 | 0.2 ± 0 | 0.58 ± 0.01 |
| 14d | Cl | Br | C6H5 | 0.42 ± 0.01 | 0.92 ± 0.02 |
| Dox. | 0.19 ± 0.01 | 0.43 ± 0.02 | |||
| Comp. | IC50 (µM) |
|---|---|
| 11d | 8.35 ± 0.62 |
| 11e | 11.74 ± 0.93 |
| 11g | 10.65 ± 0.71 |
| 11k | 13.22 ± 1.09 |
| 14c | 7.92 ± 0.48 |
| Comp. | VEGFR-2 IC50 (nM) a |
|---|---|
| 11d | 16.3 ± 0.42 |
| 11e | 48.4 ± 0.39 |
| 11g | 119.6 ± 1.8 |
| 11k | 41.3 ± 0.31 |
| 14c | 53.8 ± 0.61 |
| Sorafenib | 29.7 ± 0.39 |
| Compound | GI Absorption | BBB Permeant | Pgp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | Lipinski #Violations | Veber #Violations |
|---|---|---|---|---|---|---|---|---|---|---|
| 11a | High | No | No | No | Yes | Yes | No | Yes | 0 | 0 |
| 11b | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11c | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11d | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11e | Low | No | Yes | No | Yes | Yes | No | Yes | 2 | 0 |
| 11f | Low | No | No | No | No | Yes | No | Yes | 2 | 1 |
| 11g | High | No | No | No | Yes | Yes | No | Yes | 0 | 0 |
| 11h | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11i | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11j | Low | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 11k | Low | No | No | No | Yes | Yes | No | Yes | 2 | 0 |
| 11l | Low | No | No | No | No | Yes | No | Yes | 2 | 1 |
| 14a | High | No | No | No | Yes | Yes | No | Yes | 0 | 0 |
| 14b | Low | No | No | No | Yes | Yes | No | No | 1 | 0 |
| 14c | High | No | No | No | Yes | Yes | No | Yes | 1 | 0 |
| 14d | Low | No | No | No | Yes | No | No | No | 2 | 0 |
| Compound | Docking Score (kcal/mol) |
|---|---|
| 11d | −9.6 |
| 11e | −8.3 |
| 11g | −7.1 |
| 11k | −8.5 |
| 14c | −8.0 |
| Sorafenib | −9.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsawi, A.E.; Shahin, M.I.; Elbendary, H.A.; Al-Warhi, T.; Hassan, F.E.; Eldehna, W.M. 1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking. Pharmaceuticals 2024, 17, 81. https://doi.org/10.3390/ph17010081
Elsawi AE, Shahin MI, Elbendary HA, Al-Warhi T, Hassan FE, Eldehna WM. 1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking. Pharmaceuticals. 2024; 17(1):81. https://doi.org/10.3390/ph17010081
Chicago/Turabian StyleElsawi, Ahmed E., Mai I. Shahin, Hager A. Elbendary, Tarfah Al-Warhi, Fatma E. Hassan, and Wagdy M. Eldehna. 2024. "1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking" Pharmaceuticals 17, no. 1: 81. https://doi.org/10.3390/ph17010081
APA StyleElsawi, A. E., Shahin, M. I., Elbendary, H. A., Al-Warhi, T., Hassan, F. E., & Eldehna, W. M. (2024). 1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking. Pharmaceuticals, 17(1), 81. https://doi.org/10.3390/ph17010081







