Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish
Abstract
1. Introduction
2. Results
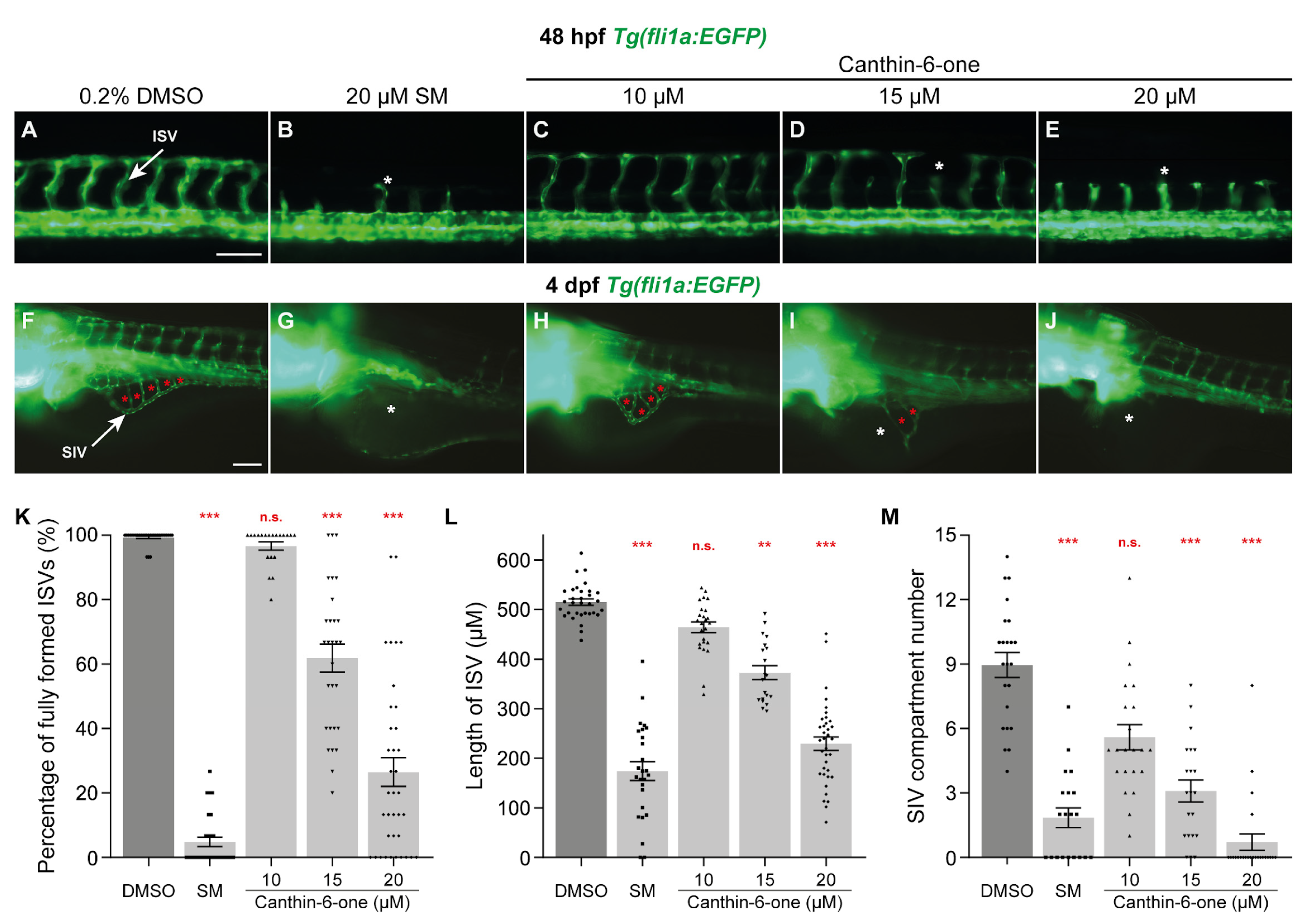
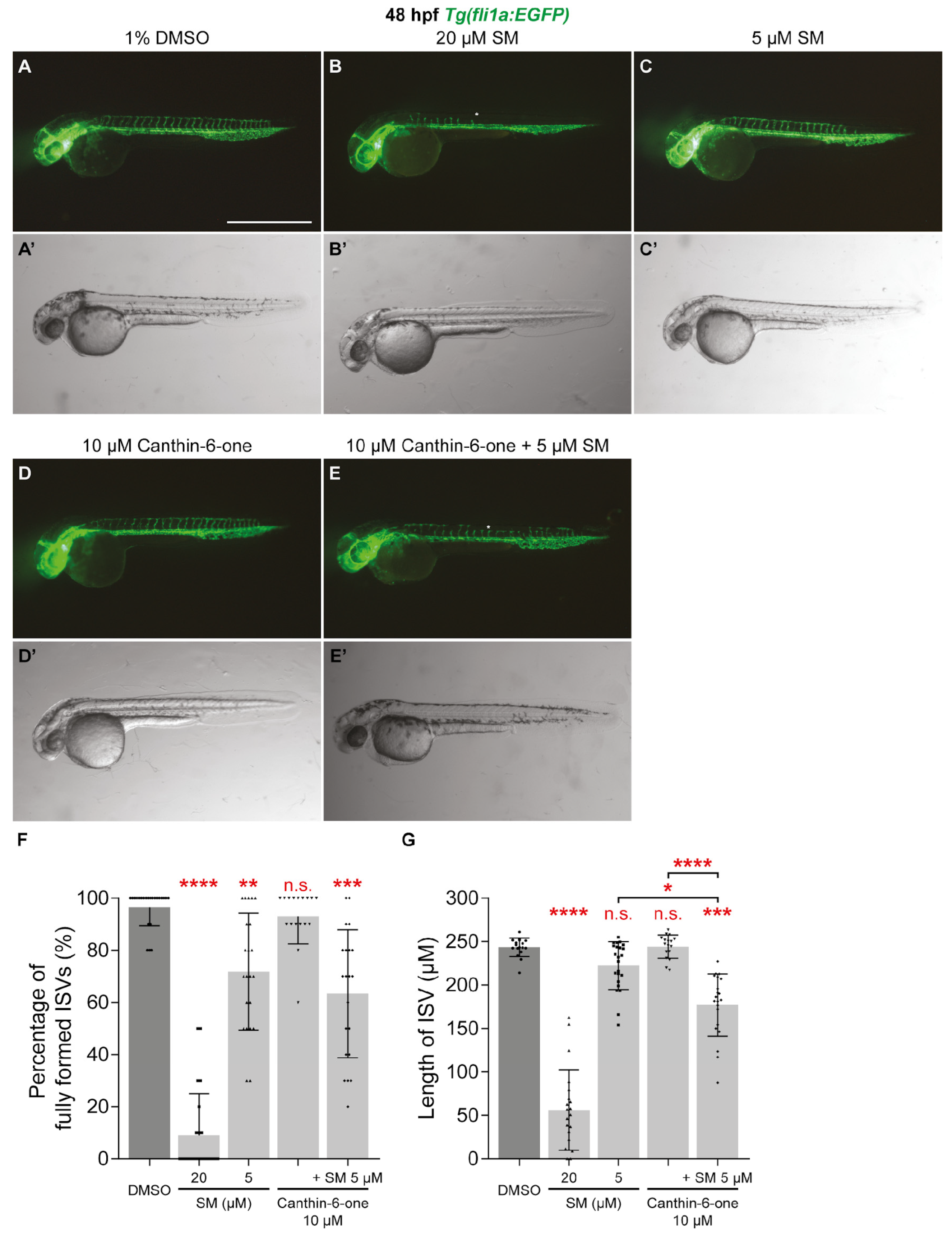
2.1. Canthin-6-One Inhibits Angiogenesis in Zebrafish
2.2. Canthin-6-One Inhibits Endothelial Cell Proliferation
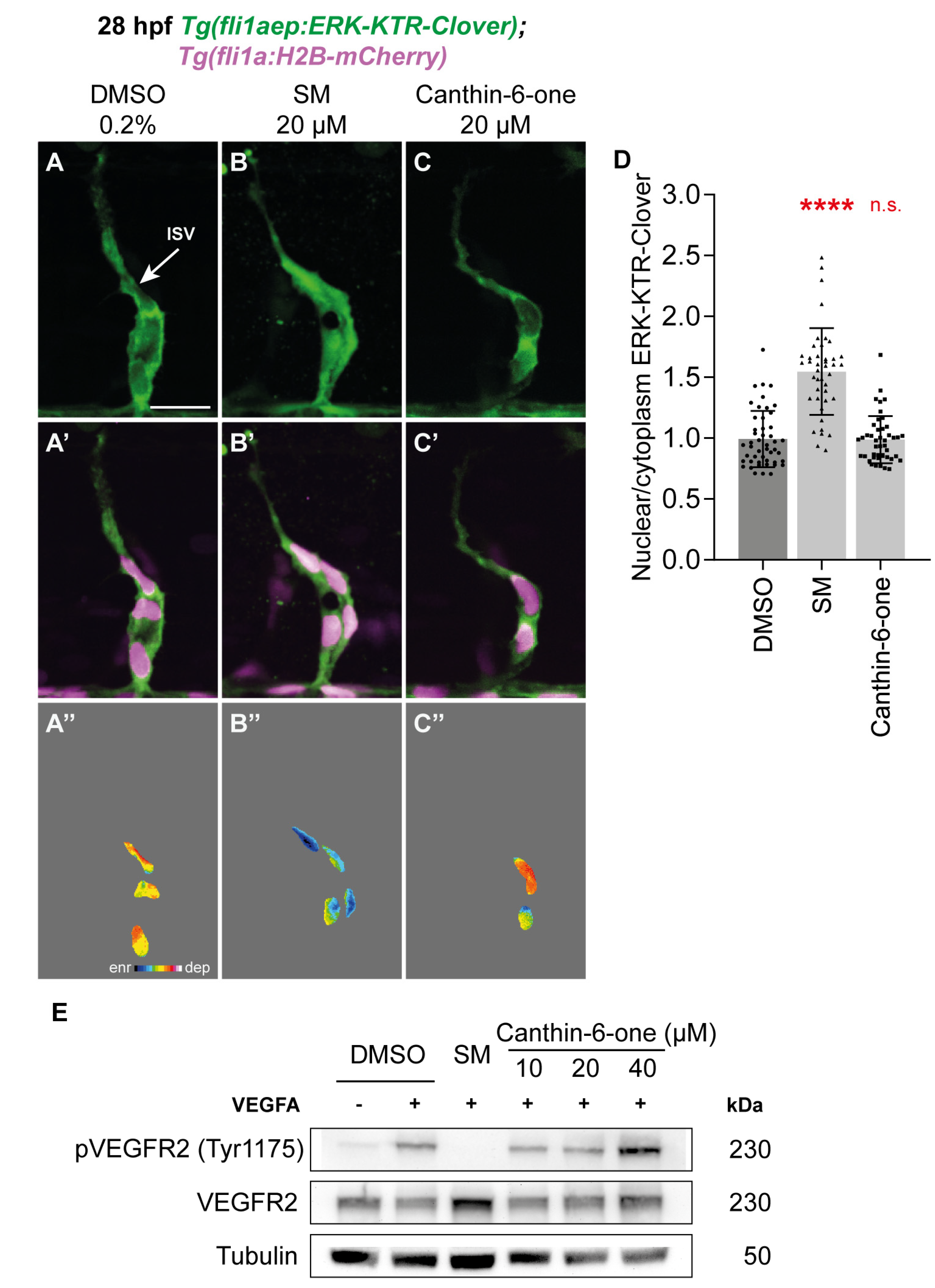
2.3. Canthin-6-One Does Not Inhibit VEGFA/VEGFR2/ERK Signalling
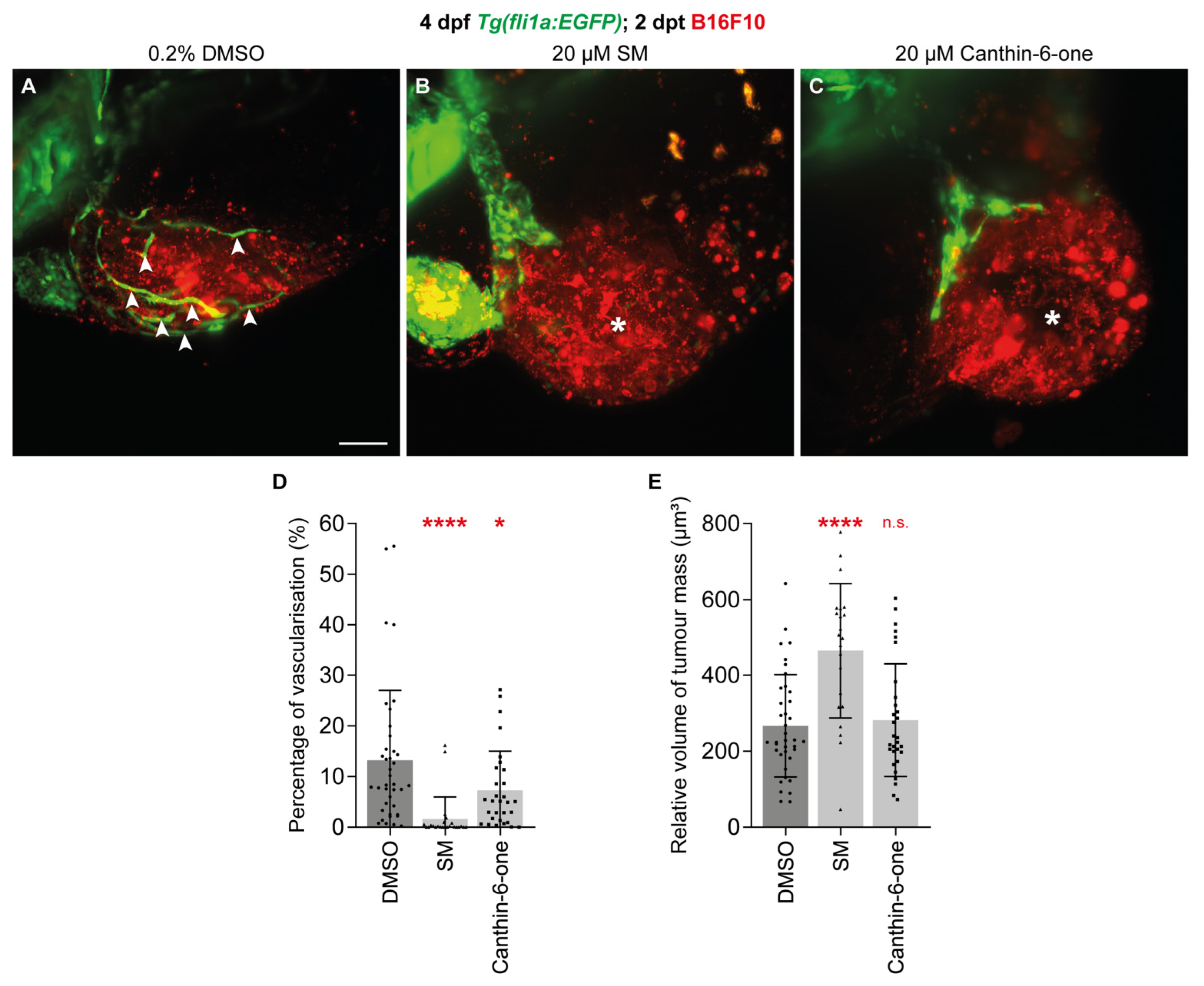
2.4. Canthin-6-One Inhibits Tumour-associated Angiogenesis and Synergises with SM
3. Discussion
4. Materials and Methods
4.1. Zebrafish Maintenance and Imaging
4.2. Chemical Administration
4.3. Quantification of Angiogenesis and Endothelial Erk Activity in Zebrafish
4.4. Cell Culture and Cell Labelling
4.5. Click-iT Cell Proliferation Assay and Cell Cycle Analysis
4.6. Western Blot and qPCR Analysis
4.7. B16F10 Xenotransplantation
4.8. Molecular Docking Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adair, T.H.; Montani, J.P. Chapter 1 Overview of Angiogenesis. In Angiogenesis; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2010. [Google Scholar]
- Aguilar-Cazares; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; de la Cruz, O.N.H.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar]
- Dudley, A.C.; Griffioen, A.W. Pathological Angiogenesis: Mechanisms and Therapeutic Strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar]
- Folkman, J. Anti-Angiogenesis: New Concept for Therapy of Solid Tumors. Ann. Surg. 1972, 175, 409–416. [Google Scholar]
- Ghalehbandi, S.; Yuzugulen, J.; Pranjol, M.Z.I.; Pourgholami, M.H. The Role of Vegf in Cancer-Induced Angiogenesis and Research Progress of Drugs Targeting Vegf. Eur. J. Pharmacol. 2023, 949, 175586. [Google Scholar] [PubMed]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic Signaling Pathways and Anti-Angiogenic Therapy for Cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar]
- Vasudev, N.S.; Reynolds, A.R. Anti-Angiogenic Therapy for Cancer: Current Progress, Unresolved Questions and Future Directions. Angiogenesis 2014, 17, 471–494. [Google Scholar] [PubMed]
- Dufies, M.; Giuliano, S.; Ambrosetti, D.; Claren, A.; Ndiaye, P.D.; Mastri, M.; Moghrabi, W.; Cooley, L.S.; Ettaiche, M.; Chamorey, E.; et al. Sunitinib Stimulates Expression of Vegfc by Tumor Cells and Promotes Lymphangiogenesis in Clear Cell Renal Cell Carcinomas. Cancer Res. 2017, 77, 1212–1226. [Google Scholar]
- Ebos, J.M.; Lee, C.R.; Cruz-Munoz, W.; Bjarnason, G.A.; Christensen, J.G.; Kerbel, R.S. Accelerated Metastasis after Short-Term Treatment with a Potent Inhibitor of Tumor Angiogenesis. Cancer Cell 2009, 15, 232–239. [Google Scholar]
- Egidi, M.J.; Krug, S.; Haybaeck, J.; Michl, P.; Griesmann, H. Anti-Angiogenic Therapy Using the Multi-Tyrosine Kinase Inhibitor Regorafenib Enhances Tumor Progression in a Transgenic Mouse Model of Ss-Cell Carcinogenesis. Br. J. Cancer 2023, 129, 1225–1237. [Google Scholar]
- Paez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Vinals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [PubMed]
- Okuda, K.S.; Lee, H.M.; Velaithan, V.; Ng, M.F.; Patel, V. Utilizing Zebrafish to Identify Anti-(Lymph)Angiogenic Compounds for Cancer Treatment: Promise and Future Challenges. Microcirculation 2016, 23, 389–405. [Google Scholar] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [PubMed]
- Goi, M.; Childs, S.J. Patterning Mechanisms of the Sub-Intestinal Venous Plexus in Zebrafish. Dev. Biol. 2016, 409, 114–128. [Google Scholar] [PubMed]
- Hen, G.; Nicenboim, J.; Mayseless, O.; Asaf, L.; Shin, M.; Busolin, G.; Hofi, R.; Almog, G.; Tiso, N.; Lawson, N.D.; et al. Venous-Derived Angioblasts Generate Organ-Specific Vessels During Zebrafish Embryonic Development. Development 2015, 142, 4266–4278. [Google Scholar]
- Isogai, S.; Horiguchi, M.; Weinstein, B.M. The Vascular Anatomy of the Developing Zebrafish: An Atlas of Embryonic and Early Larval Development. Dev. Biol. 2001, 230, 278–301. [Google Scholar]
- Isogai, S.; Lawson, N.D.; Torrealday, S.; Horiguchi, M.; Weinstein, B.M. Angiogenic Network Formation in the Developing Vertebrate Trunk. Development 2003, 130, 5281–5290. [Google Scholar]
- Koenig, A.L.; Baltrunaite, K.; Bower, N.I.; Rossi, A.; Stainier, D.Y.; Hogan, B.M.; Sumanas, S. Vegfa Signaling Promotes Zebrafish Intestinal Vasculature Development through Endothelial Cell Migration from the Posterior Cardinal Vein. Dev. Biol. 2016, 411, 115–127. [Google Scholar]
- Britto, D.D.; Wyroba, B.; Chen, W.; Lockwood, R.A.; Tran, K.B.; Shepherd, P.R.; Hall, C.J.; Crosier, K.E.; Crosier, P.S.; Astin, J.W. Macrophages Enhance Vegfa-Driven Angiogenesis in an Embryonic Zebrafish Tumour Xenograft Model. Dis. Model. Mech. 2018, 11, dmm035998. [Google Scholar]
- Fior, R.; Povoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-Cell Functional and Chemosensitive Profiling of Combinatorial Colorectal Therapy in Zebrafish Xenografts. Proc. Natl. Acad. Sci USA 2017, 114, E8234–E8243. [Google Scholar]
- Li, X.; Li, M. The Application of Zebrafish Patient-Derived Xenograft Tumor Models in the Development of Antitumor Agents. Med. Res. Rev. 2023, 43, 212–236. [Google Scholar]
- Yan, C.; Brunson, D.C.; Tang, Q.; Do, D.; Iftimia, N.A.; Moore, J.C.; Hayes, M.N.; Welker, A.M.; Garcia, E.G.; Dubash, T.D.; et al. Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish. Cell 2019, 177, 1903–1914.e14. [Google Scholar] [PubMed]
- Samat, N.; Ng, M.F.; Lee, H.M.; Ling, S.K.; Tan, P.J.; Patel, V. Canthin-6-One Isolated from Brucea Javanica Root Blocks Cancer Cells in the G2/M Phase and Synergizes with Cisplatin. Nat. Prod. Commun. 2017, 12, 8. [Google Scholar]
- Velaithan, V.; Okuda, K.S.; Ng, M.F.; Samat, N.; Leong, S.W.; Faudzi, S.M.; Abas, F.; Shaari, K.; Cheong, S.C.; Tan, P.J.; et al. Zebrafish Phenotypic Screen Identifies Novel Notch Antagonists. Investig. New Drugs 2017, 35, 166–179. [Google Scholar]
- Dai, J.; Li, N.; Wang, J.; Schneider, U. Fruitful Decades for Canthin-6-Ones from 1952 to 2015: Biosynthesis, Chemistry, and Biological Activities. Molecules 2016, 21, 493. [Google Scholar]
- Ding, J.; Sun, T.; Wu, H.; Zheng, H.; Wang, S.; Wang, D.; Shan, W.; Ling, Y.; Zhang, Y. Novel Canthin-6-One Derivatives: Design, Synthesis, and Their Antiproliferative Activities Via Inducing Apoptosis, Deoxyribonucleic Acid Damage, and Ferroptosis. ACS Omega 2023, 8, 31215–31224. [Google Scholar] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar]
- Liang, F.; Han, Y.; Gao, H.; Xin, S.; Chen, S.; Wang, N.; Qin, W.; Zhong, H.; Lin, S.; Yao, X.; et al. Kaempferol Identified by Zebrafish Assay and Fine Fractionations Strategy from Dysosma Versipellis Inhibits Angiogenesis through Vegf and Fgf Pathways. Sci. Rep. 2015, 5, 14468. [Google Scholar]
- Shin, M.; Beane, T.J.; Quillien, A.; Male, I.; Zhu, L.J.; Lawson, N.D. Vegfa Signals through Erk to Promote Angiogenesis, but Not Artery Differentiation. Development 2016, 143, 3796–3805. [Google Scholar]
- Costa, G.; Harrington, K.I.; Lovegrove, H.E.; Page, D.J.; Chakravartula, S.; Bentley, K.; Herbert, S.P. Asymmetric Division Coordinates Collective Cell Migration in Angiogenesis. Nat. Cell Biol. 2016, 18, 1292–1301. [Google Scholar]
- Okuda, K.S.; Keyser, M.S.; Gurevich, D.B.; Sturtzel, C.; Mason, E.A.; Paterson, S.; Chen, H.; Scott, M.; Condon, N.D.; Martin, P.; et al. Live-Imaging of Endothelial Erk Activity Reveals Dynamic and Sequential Signalling Events During Regenerative Angiogenesis. eLife 2021, 10, e62196. [Google Scholar]
- Regot, S.; Hughey, J.J.; Bajar, B.T.; Carrasco, S.; Covert, M.W. High-Sensitivity Measurements of Multiple Kinase Activities in Live Single Cells. Cell 2014, 157, 1724–1734. [Google Scholar] [PubMed]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer J. 2015, 21, 267–273. [Google Scholar] [PubMed]
- Gong, G.; Lin, Q.; Xu, J.; Ye, F.; Jiang, L.; Liu, W.; He, M.-F.; Feng, F.; Qu, W.; Xie, N. In Vivo Sar and Str Analyses of Alkaloids from Picrasma Quassioides Identify 1-Hydroxymethyl-8-Hydroxy-β-Carboline as a Novel Natural Angiogenesis Inhibitor. RSC Adv. 2016, 6, 9484–9494. [Google Scholar]
- Marech, I.; Leporini, C.; Ammendola, M.; Porcelli, M.; Gadaleta, C.D.; Russo, E.; De Sarro, G.; Ranieri, G. Classical and Non-Classical Proangiogenic Factors as a Target of Antiangiogenic Therapy in Tumor Microenvironment. Cancer Lett. 2016, 380, 216–226. [Google Scholar] [PubMed]
- Habeck, H.; Odenthal, J.; Walderich, B.; Maischein, H.; Schulte-Merker, S.; Consortium Tubingen Screen. Analysis of a Zebrafish Vegf Receptor Mutant Reveals Specific Disruption of Angiogenesis. Curr. Biol. 2002, 12, 1405–1412. [Google Scholar]
- Lange, M.; Ohnesorge, N.; Hoffmann, D.; Rocha, S.F.; Benedito, R.; Siekmann, A.F. Zebrafish Mutants in Vegfab Can Affect Endothelial Cell Proliferation without Altering Erk Phosphorylation and Are Phenocopied by Loss of Pi3k Signaling. Dev. Biol. 2022, 486, 26–43. [Google Scholar]
- Rossi, A.; Gauvrit, S.; Marass, M.; Pan, L.; Moens, C.B.; Stainier, D.Y.R. Regulation of Vegf Signaling by Natural and Synthetic Ligands. Blood 2016, 128, 2359–2366. [Google Scholar]
- Kubota, Y.; Oike, Y.; Satoh, S.; Tabata, Y.; Niikura, Y.; Morisada, T.; Akao, M.; Urano, T.; Ito, Y.; Miyamoto, T.; et al. Cooperative Interaction of Angiopoietin-Like Proteins 1 and 2 in Zebrafish Vascular Development. Proc. Natl. Acad. Sci. USA 2005, 102, 13502–13507. [Google Scholar]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; Grunwald, V.; Thompson, J.A.; Figlin, R.A.; Record-Study Group; et al. Efficacy of Everolimus in Advanced Renal Cell Carcinoma: A Double-Blind, Randomised, Placebo-Controlled Phase Iii Trial. Lancet 2008, 372, 449–456. [Google Scholar]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar]
- Baek, S.; Oh, T.G.; Secker, G.; Sutton, D.L.; Okuda, K.S.; Paterson, S.; Bower, N.I.; Toubia, J.; Koltowska, K.; Capon, S.J.; et al. The Alternative Splicing Regulator Nova2 Constrains Vascular Erk Signaling to Limit Specification of the Lymphatic Lineage. Dev. Cell 2019, 49, 279–292.e5. [Google Scholar]
- Okuda, K.S.; Baek, S.; Hogan, B.M. Visualization and Tools for Analysis of Zebrafish Lymphatic Development. Methods Mol. Biol. 2018, 1846, 55–70. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar]
- Okuda, K.S.; Ng, M.F.; Ruslan, N.F.; Bower, N.I.; Song, D.S.S.; Chen, H.; Baek, S.; Crosier, P.S.; Koltowska, K.; Astin, J.W.; et al. 3,4-Difluorobenzocurcumin Inhibits Vegfc-Vegfr3-Erk Signalling to Block Developmental Lymphangiogenesis in Zebrafish. Pharmaceuticals 2021, 14, 614. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and Autodocktools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. Vmd: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar]
- Cabral, T.; Mello, L.G.M.; Lima, L.H.; Polido, J.; Regatieri, C.V.; Belfort, R., Jr.; Mahajan, V.B. Retinal and Choroidal Angiogenesis: A Review of New Targets. Int. J. Retin. Vitr. 2017, 3, 31. [Google Scholar]
- Blatt, J.; Brondon, J.E.; Nieman, E.L.; Phillips, K.; Pandya, A. Repurposing of Antiangiogenic Agents for Treatment of Vascular Anomalies. Pharmacol. Ther. 2023, 250, 108520. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, M.F.; Da Silva Viana, J.; Tan, P.J.; Britto, D.D.; Choi, S.B.; Kobayashi, S.; Samat, N.; Song, D.S.S.; Ogawa, S.; Parhar, I.S.; et al. Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish. Pharmaceuticals 2024, 17, 108. https://doi.org/10.3390/ph17010108
Ng MF, Da Silva Viana J, Tan PJ, Britto DD, Choi SB, Kobayashi S, Samat N, Song DSS, Ogawa S, Parhar IS, et al. Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish. Pharmaceuticals. 2024; 17(1):108. https://doi.org/10.3390/ph17010108
Chicago/Turabian StyleNg, Mei Fong, Juliana Da Silva Viana, Pei Jean Tan, Denver D. Britto, Sy Bing Choi, Sakurako Kobayashi, Norazwana Samat, Dedrick Soon Seng Song, Satoshi Ogawa, Ishwar S. Parhar, and et al. 2024. "Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish" Pharmaceuticals 17, no. 1: 108. https://doi.org/10.3390/ph17010108
APA StyleNg, M. F., Da Silva Viana, J., Tan, P. J., Britto, D. D., Choi, S. B., Kobayashi, S., Samat, N., Song, D. S. S., Ogawa, S., Parhar, I. S., Astin, J. W., Hogan, B. M., Patel, V., & Okuda, K. S. (2024). Canthin-6-One Inhibits Developmental and Tumour-Associated Angiogenesis in Zebrafish. Pharmaceuticals, 17(1), 108. https://doi.org/10.3390/ph17010108






