In Vitro Anti-Diabetic, Anti-Inflammatory, Antioxidant Activities and Toxicological Study of Optimized Psychotria malayana Jack Leaves Extract
Abstract
:1. Introduction
2. Results
2.1. Pharmacological Activity Study
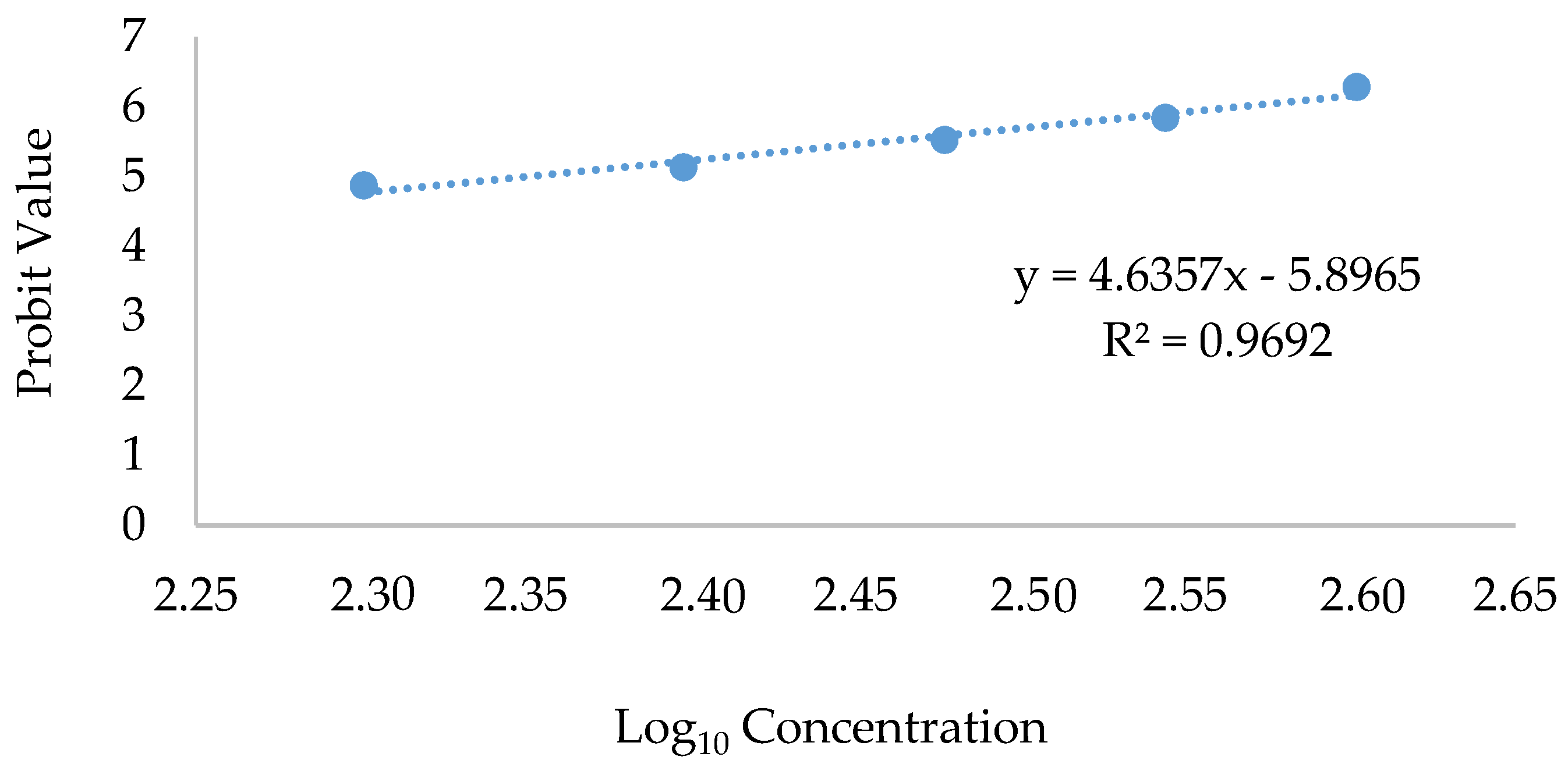
2.2. Toxicological Effect Analysis
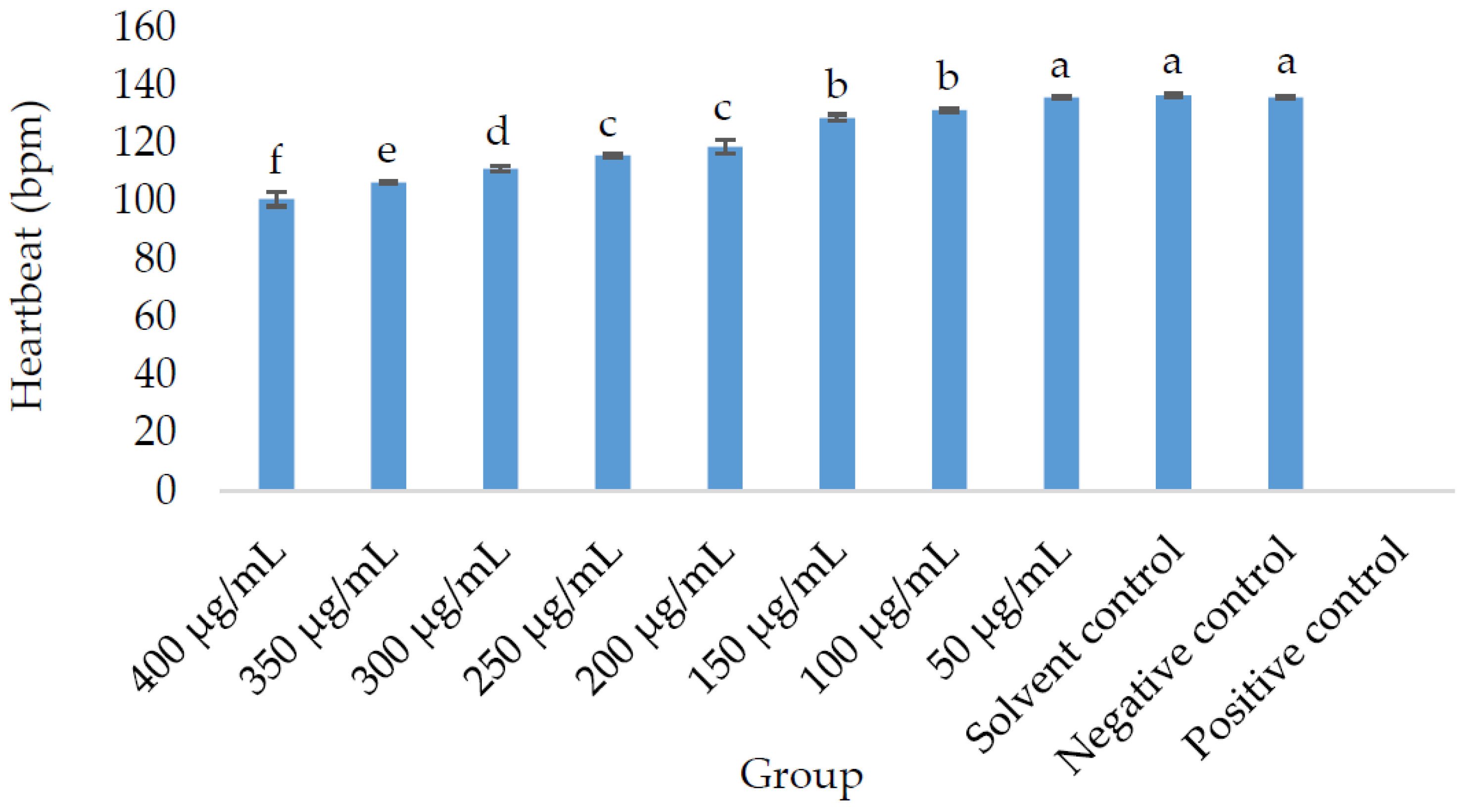
2.2.1. Morphological, Morbidity, and Mortality Outcomes in Zebrafish (Danio rerio) Embryos Treated with OE
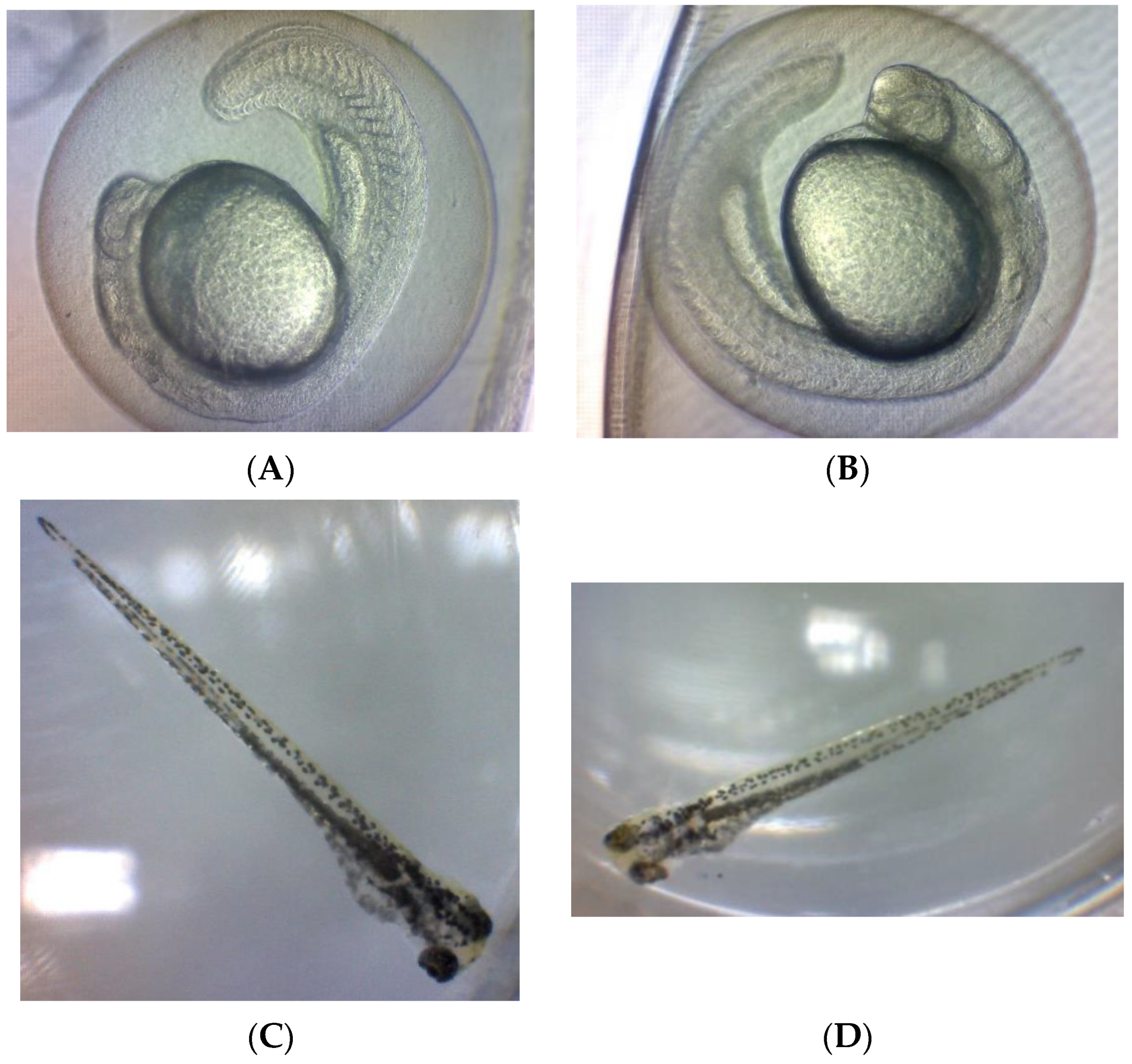
2.2.2. Teratogenic Effects of Zebrafish Embryo Exposed to OE
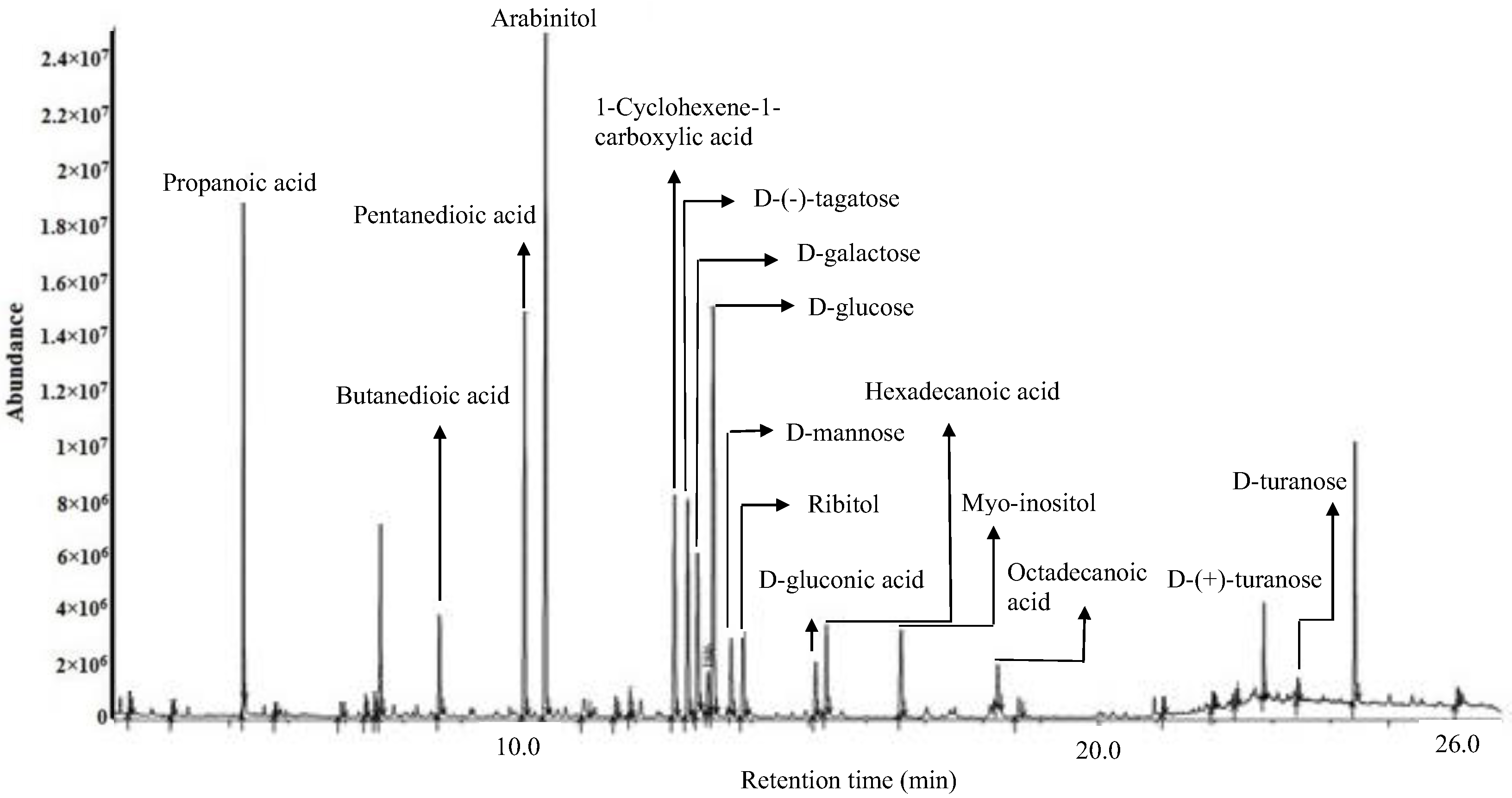
2.3. Putative Metabolites of OE Detected by GC-MS and LC-MS
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Sample and Chemicals
4.1.2. Instruments
4.1.3. Disposable Materials
4.2. Methods
4.2.1. Plant Extract Preparation
4.2.2. Pharmacological Activity
α-Glucosidase Inhibitory Activity
Soybean Lipoxygenase Inhibitory (SLOXI) Activity
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
Ferric Reducing Antioxidant Power (FRAP)
4.2.3. Toxicological Study
Toxicity Test
Maintenance of Zebrafish (Danio rerio)
The Breeding Procedure of Danio rerio and Maintenance of Embryos
Preparation of the Sample and Experimental Procedure
Microscopic Observations
Therapeutic Index
4.2.4. GC-MS Analysis
4.2.5. LC-MS Identification
4.2.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. Available online: https://idf.org/about-diabetes/what-is-diabetes/ (accessed on 2 September 2023).
- World Health Organization (WHO). Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 10 September 2023).
- Kumar, A.; Gangwar, R.; Ahmad Zargar, A.; Kumar, R.; Sharma, A. Prevalence of diabetes in India: A review of IDF Diabetes Atlas 10th edition. Curr. Diabetes Rev. 2023, 20, 2024. [Google Scholar] [CrossRef]
- International Diabetes Federation, IDF Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 2 September 2023).
- Mohamed Mahzir, K.A.; Abd Gani, S.S.; Hasanah Zaidan, U.; Halmi, M.I.E. Development of Phaleria macrocarpa (Scheff.) Boerl Fruits Using Response Surface Methodology Focused on Phenolics, Flavonoids and Antioxidant Properties. Molecules 2018, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.; Rahmawati, K.P.; Asnawati, D.; Ersalena, V.F.; Azwari, A. Characterization of Alkaloids from The Leaves of Psychotria malayana Jack of Lombok Island on The Basis of Gas Chromatography-Mass Spectroscopy. J. Pure Appl. Chem. Res. 2014, 3, 108–113. [Google Scholar] [CrossRef]
- Nipun, T.S.; Khatib, A.; Ahmed, Q.U.; Nasir, M.H.M.; Supandi, F.; Taher, M.; Saiman, M.Z. Preliminary Phytochemical Screening, In Vitro Antidiabetic, Antioxidant Activities, and Toxicity of Leaf Extracts of Psychotria malayana Jack. Plants 2021, 10, 2688. [Google Scholar] [CrossRef]
- Akmal, M.; Wadhwa, R. Alpha Glucosidase Inhibitors; StatPearls Publishing: Rockville Pike, MD, USA, 2022. [Google Scholar]
- Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Abdul Wahab, R.; Ahmed, Q.U.; Abdul Ghaffar, M.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef]
- Benchoula, K.; Khatib, A.; Jaffar, A.; Ahmed, Q.U.; Sulaiman, W.M.A.W.; Wahab, R.A.; El-Seedi, H.R. The promise of zebrafish as a model of metabolic syndrome. Exp. Anim. 2019, 68, 407–416. [Google Scholar] [CrossRef]
- Irawan, C.; Putri, I.D.; Sukiman, M.; Utami, A.; Ismail; Putri, R.; Lisandi, A.; Pratama, A.N. Antioxidant activity of DPPH, CUPRAC, and FRAP methods, as well as activity of alpha-glucosidase inhibiting enzymes from Tinospora crispa (L.) stem ultrasonic extract. Pharmacogn. J. 2022, 14, 511–520. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.N.; Gao, B.; Li, P.; Inagaki, C.; Kawabata, J. Inhibitory effect on α-glucosidase by Adhatoda vasica Nees. Food Chem. 2008, 108, 965–972. [Google Scholar] [CrossRef]
- Dobrian, A.D.; Ma, Q.; Lindsay, J.W.; Leone, K.A.; Ma, K.; Coben, J.; Galkina, E.V.; Nadler, J.L. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am. J. Physiol. 2011, 300, E410–E421. [Google Scholar] [CrossRef]
- Schneider, I.; Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 2: Medicinal plants with inhibitory activity on arachidonate 12-lipoxygenase, 15-lipoxygenase and leukotriene receptor antagonists. Phytother. Res. 2005, 19, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, H.; Attaran, N.; Jafari, Z.; Saberi, M.R.; Seyedi, S.M.; Eshghi, H.; Pordel, M.; Riazi, M.M. Design and synthesis of 4-methoxyphenylacetic acid esters as 15-lipoxygenase inhibitors and SAR comparative studies of them. Bioorg. Med. Chem. 2009, 17, 2327–2335. [Google Scholar] [CrossRef]
- Pham, A.T.; Malterud, K.E.; Paulsen, B.S.; Diallo, D.; Wangensteen, H. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimp toxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, T.Y.; Hong, J.Y.; Kim, G.J.; Oh, J.B.; Kim, M.J.; Apostolidis, E.; Lee, J.Y.; Kwon, Y.I. Anti-Obesity and Anti-Adipogenic Effects of Administration of Arginyl-Fructose-Enriched Jeju Barley (Hordeum vulgare L.) Extract in C57BL/6 Mice and in 3T3-L1 Preadipocytes Models. Molecules 2022, 27, 3248. [Google Scholar] [CrossRef] [PubMed]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Capanoglu, E.; Kamiloglu, S.; Ozkan, G.; Apak, R. Evaluation of antioxidant activity/capacity measurement methods for food products. In Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, 1st ed.; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 273–286. [Google Scholar]
- Boynes, J.W. Role of oxidative stress in development ofcomplication in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.Y.; Park, S.; Song, G.; Lim, W. Developmental toxicity of fipronil in early development of zebrafish (Danio rerio) larvae: Disrupted vascular formation with angiogenic failure and inhibited neurogenesis. J. Hazard. Mater. 2020, 385, 121531. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; He, L.; Zhang, T.; Li, C. Lentinan Impairs the Early Development of Zebrafish Embryos, Possibly by Disrupting Glucose and Lipid Metabolism. Processes 2022, 10, 120. [Google Scholar] [CrossRef]
- Hanke, N.; Staggs, L.; Schroder, P.; Litteral, J.; Fleig, S.; Kaufeld, J.; Pauli, C.; Haller, H.; Schiffer, M. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. Biomed Res. Int. 2013, 2013, 658270. [Google Scholar] [CrossRef]
- Raldúa, D.; André, M.; Babin, P.J. Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol. Appl. Pharmacol. 2008, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- McCollum, C.W.; Ducharme, N.A.; Bondesson, M.; Gustafsson, J.A. Developmental toxicity screening in zebrafish. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 67–114. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2009, 12, 1645–1654. [Google Scholar] [CrossRef]
- Bhutta, B.S.; Alghoula, F.; Berim, I. Hypoxia; StatPearls Publishing: Rockville Pike, MD, USA, 2022. [Google Scholar]
- Lajis, A.F.B. A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine. Medicina 2018, 54, 35. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, S.H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef]
- Yu, Y.L.; Li, H.L.; Wang, Y.C.; Qiao, X.G. The effect of thiram on heart development of zebrafish embryos. J. Inn. Mong. Univ. Natl. 2011, 3, 1–7. [Google Scholar]
- Hallare, A.V.; Schirling, M.; Luckenbach, T.; Kohler, H.R.; Triebskorn, R. Combined temperature and cadmium effects on developmental parameters and biomarker responses in zebrafish (Danio rerio) embryos. J. Therm. Biol. 2005, 30, 7–17. [Google Scholar] [CrossRef]
- Natrus, L.V.; Osadchuk, Y.S.; Lisakovska, O.O.; Labudzinskyi, D.O.; Klys, Y.G.; Chaikovsky, Y.B. Effect of Propionic Acid on Diabetes-Induced Impairment of Unfolded Protein Response Signaling and Astrocyte/Microglia Crosstalk in Rat Ventromedial Nucleus of the Hypothalamus. Neural Plast. 2022, 2022, 6404964. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, S.; Rezaee, F. Propionic acid counteracts the inflammation of human subcutaneous adipose tissue: A new avenue for drug development. J. Fac. Pharm. 2019, 27, 645–652. [Google Scholar] [CrossRef]
- Al-Daihan, S.; Shafi Bhat, R. Impact of Propionic Acid on Liver Damage in Rats. Int. J. Mol. Cell. Med. 2015, 4, 188–195. [Google Scholar] [PubMed]
- Lattibeaudiere, K.G.; Alexander-Lindo, R.L. Oleic Acid and Succinic Acid Synergistically Mitigate Symptoms of Type 2 Diabetes in Streptozotocin-Induced Diabetic Rats. Int. J. Endocrinol. 2022, 2022, 8744964. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, M.L.; Kuo, H.T.; Tsai, Y.C.; Lin, B.F.; Kuo, Y.H. Anti-inflammatory activities of new succinic and maleic derivatives from the fruiting body of Antrodia camphorata. J. Agric. Food Chem. 2008, 56, 7017–7022. [Google Scholar] [CrossRef] [PubMed]
- Razafindrakoto, Z.R.; Donno, D.; Tombozara, N.; Andriamaniraka, H.; Andrianjara, C.; Ramanitrahasimbola, D.; Beccaro, G.L. Antioxidant, Anti-Inflammatory, and Antidiabetic Activities of Leaves and Stems of Uapaca bojeri Bail. (EUPHORBIACEAE), an Endemic Plant of Madagascar. Pharmaceuticals 2020, 13, 71. [Google Scholar] [CrossRef]
- Hardy, R.W.; Ladenson, J.H.; Henriksen, E.J.; Holloszy, J.O.; McDonald, J.M. Palmitate stimulates glucose transport in rat adipocytes by a mechanism involving translocation of the insulin sensitive glucose transporter (GLUT4). Biochem. Biophys. Res. Commun. 1991, 177, 343–349. [Google Scholar] [CrossRef]
- Wu, D.; Liu, J.; Pang, X.; Wang, S.; Zhao, J.; Zhang, X.; Feng, L. Palmitic acid exerts pro-inflammatory effects on vascular smooth muscle cells by inducing the expression of C-reactive protein, inducible nitric oxide synthase and tumor necrosis factor-α. Int. J. Mol. Med. 2014, 34, 1706–1712. [Google Scholar] [CrossRef]
- Ding, Q.; Zhang, Z.; Ran, C.; He, S.; Yang, Y.; Du, Z.; Zhang, J.; Zhou, Z. The Hepatotoxicity of Palmitic Acid in Zebrafish Involves the Intestinal Microbiota. J. Nutr. 2018, 148, 1217–1228. [Google Scholar] [CrossRef]
- Fattore, E.; Fanelli, R. Palm oil and palmitic acid: A review on cardiovascular effects and carcinogenicity. Int. J. Food Sci. Nutr. 2013, 64, 648–659. [Google Scholar] [CrossRef]
- Ensor, M.; Banfield, A.B.; Smith, R.R.; Williams, J.; Lodder, R.A. Safety and Efficacy of D-Tagatose in Glycemic Control in Subjects with Type 2 Diabetes. J. Endocrinol. Diabetes Obes. 2015, 3, 1065. [Google Scholar]
- Kruger, C.L.; Whittaker, M.H.; Frankos, V.H. Genotoxicity tests on D-tagatose. Regul. Toxicol. Pharmacol. 1999, 29, S36–S42. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.L.; Whittaker, M.H.; Frankos, V.H.; Schroeder, R.E. Developmental toxicity study of D-tagatose in rats. Regul. Toxicol. Pharmacol. 1999, 29, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Wyss, M.; Durán Agüero, S.; Angarita Dávila, L. D-Tagatose Is a Promising Sweetener to Control Glycaemia: A New Functional Food. Biomed Res. Int. 2018, 2018, 8718053. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh-Amiri, S.; Mohammad-Alizadeh-Charandabi, S.; Abdolalipour, S.; Mirghafourvand, M. Myo-inositol supplementation for prevention of gestational diabetes mellitus in overweight and obese pregnant women: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Arefhosseini, S.; Roshanravan, N.; Asghari, S.; Tutunchi, H.; Ebrahimi-Mameghani, M. Expression of inflammatory genes, WBC-derived inflammatory biomarkers, and liver function indices: Effects of myo-inositol supplementation in obese patients with NAFLD. J. Funct. Foods 2023, 104, 105524. [Google Scholar] [CrossRef]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.P.; Lu, P.; Blaeser, A.; Lu, Q.L. Ribitol restores functionally glycosylated α-dystroglycan and improves muscle function in dystrophic FKRP-mutant mice. Nat. Commun. 2018, 9, 3448. [Google Scholar] [CrossRef]
- Stone, V.; Kudo, K.Y.; August, P.M.; Marcelino, T.B.; Matté, C. Polyols accumulated in ribose-5-phosphate isomerase deficiency increase mitochondrial superoxide production and improve antioxidant defenses in rats’ prefrontal cortex. Int. J. Dev. Neurosci. 2014, 37, 21–25. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Choi, J.S.; Chung, H.Y. Antioxidant effects of isorhamnetin 3,7-di-O-beta-D-glucopyranoside isolated from mustard leaf (Brassica juncea) in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2002, 50, 5490–5495. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, S.; Lee, H.S.; Kim, B.K.; Ohuchi, K.; Shin, K.H. Inhibitory effects of isorhamnetin-3-O-beta-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol. Pharm. Bull. 2005, 28, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Seo, K.; Ki, S.H.; Shin, S.M. Isorhamnetin Inhibits Reactive Oxygen Species-Dependent Hypoxia Inducible Factor (HIF)-1α Accumulation. Biol. Pharm. Bull. 2016, 39, 1830–1838. [Google Scholar] [CrossRef] [PubMed]
- Pengfei, L.; Tiansheng, D.; Xianglin, H.; Jianguo, W. Antioxidant properties of isolated isorhamnetin from the sea buckthorn marc. Plant Foods Hum. Nutr. 2009, 64, 141–145. [Google Scholar] [CrossRef]
- Zuo, A.; Yu, Y.; Li, J.; Xu, B.; Yu, X.; Qiu, Y.; Cao, S. Study on the relation of structure and antioxidant activity of isorhamnetin, quercetin, phloretin, silybin and phloretin isonicotinyl hydrazone. Free Radic. Antioxid. 2011, 1, 39–47. [Google Scholar] [CrossRef]
- Grdović, N.; Dinić, S.; Arambašić, J.; Mihailović, M.; Uskoković, A.; Marković, J.; Poznanović, G.; Vidović, S.; Zeković, Z.; Mujić, A.; et al. The protective effect of a mix of Lactarius deterrimus and Castanea sativa extracts on streptozotocin-induced oxidative stress and pancreatic β-cell death. Br. J. Nutr. 2012, 108, 1163–1176. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.P.; Ni, Y.F.; Du, H.Y.; Wang, R.; Li, M.J.; Wang, W.C.; Li, M.M.; Wang, X.H.; Li, L.; et al. Protective Effect of Isorhamnetin on Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation 2016, 39, 129–137. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Dong, X.; Sun, G.; Luo, Y. Protective effect of isorhamnetin on oxidative stress induced by H2O2 in H9C2 cells. China Pharmacol. Bull. 2015, 31, 853–860. [Google Scholar]
- Liang, R.; Chen, J.; Zhi, D.; Fan, Y.; Liu, W.; He, X. Effects of isorhamnetin on human liver microsomes CYPs and rat primary hepatocytes. Drug Eval. Res. 2017, 40, 627–632. [Google Scholar]
- Zhang, Z.; Chen, S.; Mei, H.; Xuan, J.; Guo, X.; Couch, L.; Dobrovolsky, V.N.; Guo, L.; Mei, N. Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci. Rep. 2015, 5, 14633. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, K. Anti-Propionibacterium acnes and the anti-inflammatory effect of Aloe ferox miller components. J. Herb. Med. 2017, 9, 53–59. [Google Scholar] [CrossRef]
- Mwale, M.; Masika, P.J. Analgesic and anti-inflammatory activities of Aloe ferox Mill. aqueous extract. Afr. J. Pharm. Pharmacol. 2010, 4, 291–297. [Google Scholar]
- Nalimu, F.; Oloro, J.; Kahwa, I.; Ogwang, P.E. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe Ferox. Future J. Pharm. Sci. 2021, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.S.; Alves, A.P.L.; Alves, G.B.C.; Cardoso, J.N.; Lopes, R.S.C.; Lopes, C.C. A new synthesis of alpha-bromo-acetophenones and its application in obtaining 2-benzoyl-benzofurans. Chem. Nova 2006, 29, 1259–1265. [Google Scholar]
- Kim, J.H.; Kim, T.J.; Kim, H.J.; Cho, C.W.; Kim, S.J.; Cho, H.S.; Kim, K.T.; Kand, J.S. A new high-performance liquid chromatographic method for the quality control of bio converted Mori Folium extracts with appropriate marker compounds related to antidiabetic. J. Anal. Sci. Technol. 2021, 12, 2. [Google Scholar] [CrossRef]
- Li, H.X.; Heo, M.; Go, Y.; Kim, Y.S.; Kim, Y.H.; Yang, S.Y.; Li, W. Coumarin and Moracin Derivatives from Mulberry Leaves (Morus alba L.) with Soluble Epoxide Hydrolase Inhibitory Activity. Molecules 2020, 25, 3967. [Google Scholar] [CrossRef]
- Schäfer, A.; Högger, P. Oligomeric procyanidins of French maritime pine bark extract (Pycnogenol) effectively inhibit alpha-glucosidase. Diabetes Res. Clin. Pract. 2007, 77, 41–46. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Song, L.; Huang, D. Profiles and α-amylase inhibition activity of proanthocyanidins in unripe Manilkara zapota (chiku). J. Agric. Food Chem. 2012, 60, 3098–3104. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Lai, S.; Liu, C.; Huang, S.; Yang, H. Structure, antioxidant and α-amylase inhibitory activities of longan pericarp proanthocyanidins. J. Funct. Foods 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Lu, Y.; Demleitner, M.F.; Song, L.; Rychlik, M.; Huang, D. Oligomeric proanthocyanidins are the active compounds in Abelmoschus esculentus Moench for its α-amylase and α-glucosidase inhibition activity. J. Funct. Foods 2016, 20, 463–471. [Google Scholar] [CrossRef]
- Mizuno, M.; Nakanishi, I.; Matsubayashi, S.; Imai, K.; Arai, T.; Matsumoto, K.I.; Fukuhara, K. Synthesis and antioxidant activity of a procyanidin B3 analogue. Bioorg. Med. Chem. Lett. 2017, 27, 1041–1044. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Prihantini, A.I.; Tachibana, S. α-Glucosidase inhibitor and antioxidant activity of procyanidin, an isolated compound from Quercus gilva Blume leaves. J. Appl. Pharm. Sci. 2022, 12, 213–218. [Google Scholar] [CrossRef]
- Shang, P.; Tang, Q.; Hu, Z.; Huang, S.; Hu, Y.; Zhu, J.; Liu, H. Procyanidin B3 alleviates intervertebral disc degeneration via interaction with the TLR4/MD-2 complex. J. Cell. Mol. Med. 2020, 24, 3701–3711. [Google Scholar] [CrossRef]
- Oizumi, Y.; Mohri, Y.; Hirota, M.; Makabe, H. Synthesis of procyanidin B3 and its anti-inflammatory activity. the effect of 4-alkoxy group of catechin electrophile in the Yb (OTf)(3)-catalyzed condensation with catechin nucleophile. J. Org. Chem. 2010, 75, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.B.R.; Nambiasan, P.N.K. Phytochemical studies on Pseudarthria viscida. J. Res. Indian Med. Yoga 1976, 11, 104–106. [Google Scholar]
- Kuppusamy, R.; Shirwaikar, A.; Sam, K.G.; Kaitheri, S.K. Antidiabetic activity of Pseudarthria viscida aqueous root extract in neonatal streptozotocin induced NIDDM rats. Braz. J. Pharmacogn. 2012, 22, 1079–1084. [Google Scholar] [CrossRef]
- Cherian, S.; Kumar, R.V.; Augusti, K.T.; Kidwai, J.R. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J. Biochem. Biophys. 1992, 29, 380–382. [Google Scholar]
- Daniel, R.S.; Mathew, B.C.; Devi, K.S.; Augusti, K.T. Antioxidant effect of two flavonoids from the bark of Ficus bengalensis Linn in hyperlipidemic rats. Indian J. Exp. Biol. 1998, 36, 902–906. [Google Scholar]
- Augusti, K.T.; Daniel, R.S.; Cherian, S.; Sheela, C.G.; Nair, C.R. Effect of leucopelargonin derivative from Ficus bengalensis Linn. on diabetic dogs. Indian J. Med. Res. 1994, 99, 82–86. [Google Scholar] [PubMed]
- Javadi, N.; Abas, F.; Abd Hamid, A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Mohamad, K.; Ooi, A.J.; Imiyabir, Z.; Chung, L.Y. Bioactivity-guided fractionation of the lipoxygenase and cyclooxygenase inhibiting constituents from Chisocheton polyandrus Merr. Fitoterapia 2012, 83, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. DPPH Microplate Protoc. 2012, 7–9. [Google Scholar]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2014, 95, 204–209. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2013; pp. 1–22. [Google Scholar]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 2002, 19, 38–48. [Google Scholar] [PubMed]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Uzir, B.F.; Nik Yusoff, N.I.; Perumal, V.; Abas, F.; Shaari, K.; Khatib, A. Identification of α-glucosidase inhibitors from Clinacanthus nutans leaf extract using liquid chromatography-mass spectrometry-based metabolomics and protein-ligand interaction with molecular docking. J. Pharm. Anal. 2019, 9, 91–99. [Google Scholar] [CrossRef]

| Sample | α-Glucosidase Inhibitory Activity, IC50 (µg/mL) | Soybean Lipoxygenase Inhibitory Activity, IC50 (µg/mL) | DPPH Scavenging, IC50 (µg/mL) | FRAP (mmol TE/mgDW) |
|---|---|---|---|---|
| Optimized extract | 2.02 ± 0.03 a | 4.92 ± 0.12 a | 13.08 ± 0.84 a | 95.44 ± 0.17 |
| Positive controls | Quercetin: 0.99 ± 0.11 b | Phenidone: 0.93 ± 0.02 b | Ascorbic acid: 2.99 ± 1.07 b | NA |
| Extract Concentration (µg/mL)/Control Group | Coagulation ofEmbryo | Non-Detachment of the Tail | Lack of Somite Formation | Lack of Heartbeat | Mortality Rate (%) |
|---|---|---|---|---|---|
| 400 | + | + | + | + | 90 |
| 350 | + | + | + | + | 80 |
| 300 | + | + | + | + | 70 |
| 250 | + | + | + | + | 55 |
| 200 | + | + | + | + | 45 |
| 150 | + | + | + | + | 35 |
| 100 | + | + | + | + | 15 |
| 50 | − | − | − | − | 0 |
| Negative Control | − | − | − | − | 0 |
| Solvent Control | − | − | − | − | 0 |
| Positive Control | + | + | + | + | 95 |
| The Extract Concentration (µg/mL)/Control Group | Yolk Size × 105 (µm2) at 24 hpf | Eye Size × 105 (µm2) at 96 hpf | Body Length (mm) at 96 hpf | Hatching Defect | Less Pigmentation | Yolk Edema | Heart Edema |
|---|---|---|---|---|---|---|---|
| 400 | 4.96 ± 0.04 f | 0.75 ± 0.02 b | NA | + | + | − | − |
| 350 | 4.96 ± 0.08 f | 0.80 ± 0.04 b | NA | + | + | − | − |
| 300 | 5.11 ± 0.05 def | 0.71 ± 0.04 b | NA | + | + | − | − |
| 250 | 5.09 ± 0.03 ef | 0.76 ± 0.01 b | NA | + | + | − | − |
| 200 | 5.18 ± 0.05 bcde | 0.77 ± 0.03 b | NA | + | + | − | − |
| 150 | 5.12± 0.05 cdef | 0.76 ± 0.01 b | NA | + | + | − | − |
| 100 | 5.23 ± 0.11 bcde | 0.75 ± 0.03 b | NA | + | + | − | − |
| 50 | 5.28 ± 0.12 bcd | 1.04 ± 0.05 a | 2.80 ± 0.00 a | _− | − | − | − |
| Solvent control | 5.31± 0.03 bc | 1.02 ± 0.02 a | 2.80 ± 0.00 a | − | − | − | − |
| Negative control | 5.33 ± 0.02 b | 1.05 ± 0.01 a | 2.73 ± 0.12 a | − | − | − | − |
| Positive control | 5.63 ± 0.04 a | 0.44 ± 0.05 c | 2.07 ± 0.12 b | + | + | + | + |
| Putative Compounds | Retention Time (min) | Area | Similarity Index (SI) | Molecular Formula |
|---|---|---|---|---|
| Propanoic acid | 3.3 | 255,716 | 97 | C3H6O2 |
| Butanedioic acid | 7.4 | 435,553 | 96 | C4H6O4 |
| Pentanedioic acid | 10.1 | 10,445,703 | 92 | C5H8O4 |
| Arabinitol | 10.5 | 12,476,129 | 94 | C5H12O5 |
| 1-cyclohexene-1-carboxylic acid | 11.7 | 548,639 | 94 | C7H10O2 |
| D-(−)-tagatose | 12.9 | 4,801,682 | 95 | C6H12O6 |
| D-galactose | 13.3 | 1,301,519 | 92 | C6H12O6 |
| D-glucose | 13.4 | 8,866,752 | 94 | C6H12O6 |
| D-mannose | 13.7 | 1,987,514 | 94 | C6H12O6 |
| Ribitol | 13.9 | 2,429,392 | 91 | C5H12O5 |
| D-gluconic acid | 15.1 | 1,660,291 | 92 | C6H12O7 |
| Hexadecanoic acid | 15.3 | 1,105,406 | 96 | C16H32O2 |
| Myo-inositol | 16.6 | 2,344,357 | 94 | C6H12O6 |
| Octadecanoic acid | 18.6 | 254,088 | 94 | C18H36O2 |
| D-(+)-turanose | 22.8 | 1,243,470 | 90 | C12H22O11 |
| D-turanose | 23.4 | 325,253 | 90 | C12H22O11 |
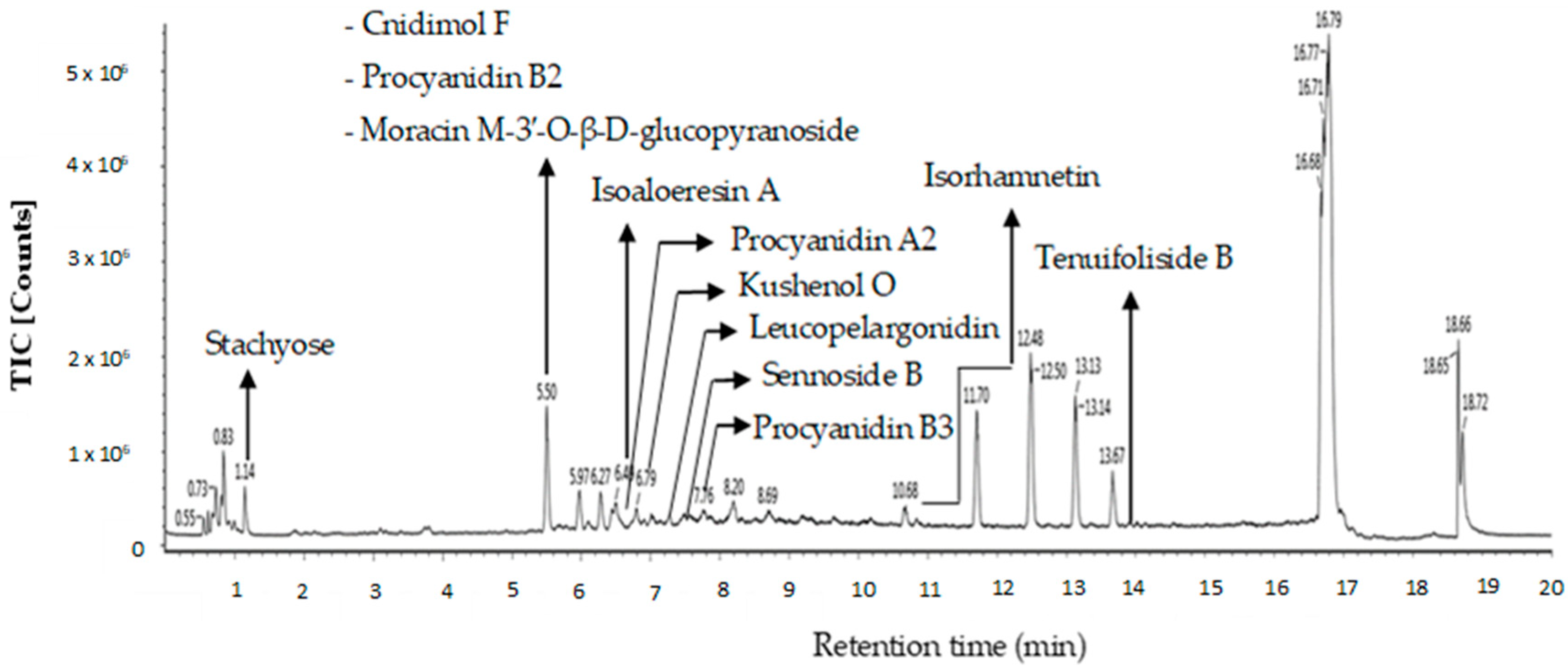
| Putative Compound | Observed RT (min) | Observed m/z | Neutral Mass (Da) | Mass Error (ppm) | Response | Adducts | Molecular Formula |
|---|---|---|---|---|---|---|---|
| Stachyose | 1.14 | 689.21 | 666.22 | 0.4 | 10,432 | +Na | C24H42O21 |
| Cnidimol F | 5.50 | 291.0849 | 290.07904 | −4.9 | 22,880 | +H | C15H14O6 |
| Procyanidin B2 | 5.50 | 579.1495 | 578.14243 | −0.4 | 284,385 | +H | C30H26O12 |
| Moracin M-3′-O-β-D-glucopyranoside | 5.50 | 427.1013 | 404.11073 | 3.2 | 21,649 | +Na | C20H20O9 |
| Isoaloeresin A | 6.49 | 563.1545 | 540.16316 | 3.7 | 32,715 | +Na | C28H28O11 |
| Procyanidin A2 | 6.51 | 577.134 | 576.12678 | 0 | 5308 | +H | C30H24O12 |
| Kushenol O | 6.80 | 601.1317 | 562.16864 | −0.2 | 10,148 | +K | C27H30O13 |
| Leucopelargonidin | 7.27 | 291.0849 | 290.07904 | −4.9 | 5102 | +H | C15H14O6 |
| Sennoside B | 7.45 | 885.1808 | 862.19564 | −4.5 | 5415 | +Na | C42H38O20 |
| Procyanidin B3 | 7.76 | 579.1496 | 578.14243 | −0.2 | 9199 | +H | C30H26O12 |
| Isorhamnetin | 10.68 | 317.0644 | 316.0583 | −3.7 | 9380 | +H | C16H12O7 |
| Tenuifoliside B | 13.91 | 691.1852 | 668.19525 | 1.1 | 7009 | +Na | C30H36O17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed Mohamad, S.N.A.; Khatib, A.; So’ad, S.Z.M.; Ahmed, Q.U.; Ibrahim, Z.; Nipun, T.S.; Humaryanto, H.; AlAjmi, M.F.; Khalifa, S.A.M.; El-Seedi, H.R. In Vitro Anti-Diabetic, Anti-Inflammatory, Antioxidant Activities and Toxicological Study of Optimized Psychotria malayana Jack Leaves Extract. Pharmaceuticals 2023, 16, 1692. https://doi.org/10.3390/ph16121692
Syed Mohamad SNA, Khatib A, So’ad SZM, Ahmed QU, Ibrahim Z, Nipun TS, Humaryanto H, AlAjmi MF, Khalifa SAM, El-Seedi HR. In Vitro Anti-Diabetic, Anti-Inflammatory, Antioxidant Activities and Toxicological Study of Optimized Psychotria malayana Jack Leaves Extract. Pharmaceuticals. 2023; 16(12):1692. https://doi.org/10.3390/ph16121692
Chicago/Turabian StyleSyed Mohamad, Sharifah Nurul Akilah, Alfi Khatib, Siti Zaiton Mat So’ad, Qamar Uddin Ahmed, Zalikha Ibrahim, Tanzina Sharmin Nipun, Humaryanto Humaryanto, Mohamed F. AlAjmi, Shaden A. M. Khalifa, and Hesham R. El-Seedi. 2023. "In Vitro Anti-Diabetic, Anti-Inflammatory, Antioxidant Activities and Toxicological Study of Optimized Psychotria malayana Jack Leaves Extract" Pharmaceuticals 16, no. 12: 1692. https://doi.org/10.3390/ph16121692
APA StyleSyed Mohamad, S. N. A., Khatib, A., So’ad, S. Z. M., Ahmed, Q. U., Ibrahim, Z., Nipun, T. S., Humaryanto, H., AlAjmi, M. F., Khalifa, S. A. M., & El-Seedi, H. R. (2023). In Vitro Anti-Diabetic, Anti-Inflammatory, Antioxidant Activities and Toxicological Study of Optimized Psychotria malayana Jack Leaves Extract. Pharmaceuticals, 16(12), 1692. https://doi.org/10.3390/ph16121692







