Radiopharmaceuticals for Treatment of Adrenocortical Carcinoma
Abstract
1. Introduction
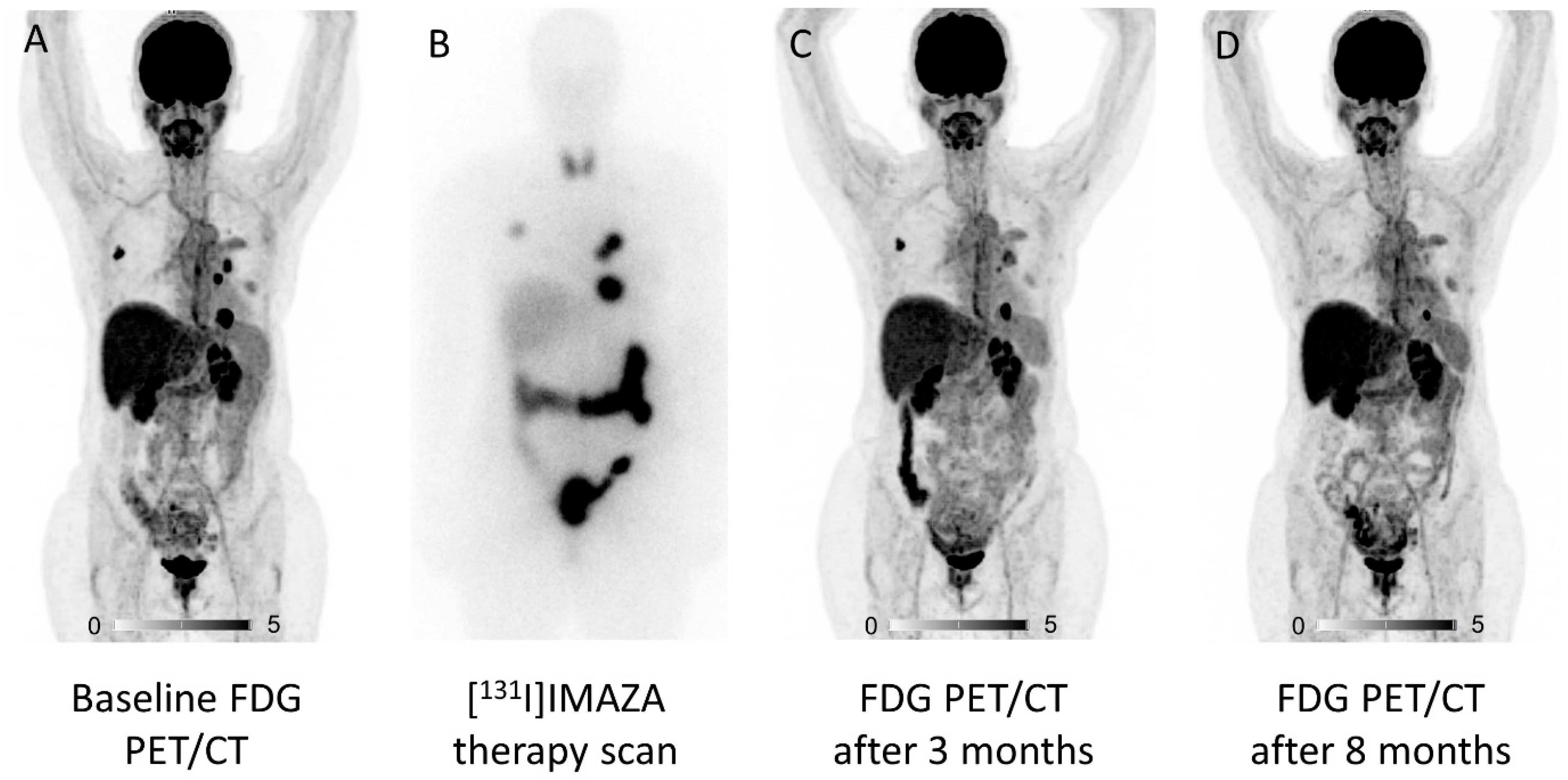
2. Molecular Imaging and Theranostic Approaches in ACC
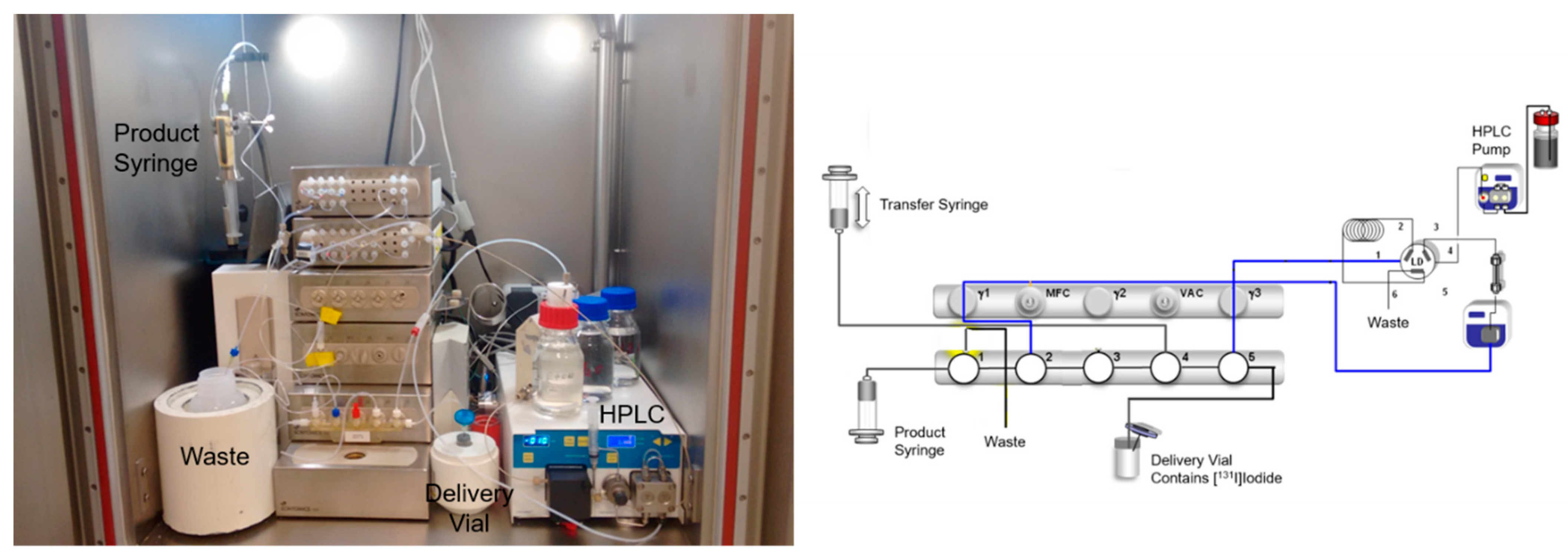
3. Radiosynthesis of [131I]IMAZA
4. Future Perspectives
5. Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerkhofs, T.M.A.; Verhoeven, R.H.A.; Van der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van de Poll-Franse, L.V.; Haak, H.R. Adrenocortical carcinoma: A population-based study on incidence and survival in the Netherlands since 1993. Eur. J. Cancer 2013, 49, 2579–2586. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. Extent of disease at presentation and outcome for adrenocortical carcinoma: Have we made progress? World J. Surg. 2006, 30, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Weigel, J.; Saeger, W.; Kroiss, M.; Wild, V.; Daffara, F.; Libe, R.; Ardito, A.; Al Ghuzlan, A.; Quinkler, M.; et al. Major prognostic role of Ki67 in localized adrenocortical carcinoma after complete resection. J. Clin. Endocrinol. Metab. 2015, 100, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.A.; Else, T.; Hughes, D.T.; Cohen, M.S.; Jolly, S.; Giordano, T.J.; Worden, F.P.; Gauger, P.G.; Hammer, G.D.; Miller, B.S. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery 2019, 165, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, Z.; Zhou, L.; Tang, Y.; Zhou, C.; Wu, K.; Zhang, F.; Zhang, F.; Wei, X.; Lu, Y.; et al. The clinical utility of ‘GRAS’ parameters in stage I-III adrenocortical carcinomas: Long-term data from a high-volume institution. Endocrine 2020, 67, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Altieri, B.; Berhane, S.; Cosentini, D.; Calabrese, A.; Haissaguerre, M.; Kastelan, D.; Fragoso, M.; Bertherat, J.; Al Ghuzlan, A.; et al. S-GRAS score for prognostic classification of adrenocortical carcinoma: An international, multicenter ENSAT study. Eur. J. Endocrinol. 2021, 186, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Libe, R.; Kroiss, M.; Allolio, B. Adrenocortical carcinoma: A clinician’s update. Nat. Rev. Endocrinol. 2011, 7, 323–335. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Fenske, W.; Weismann, D.; Agha, A.; Beuschlein, F.; Fuhrer, D.; Jurowich, C.; Quinkler, M.; Petersenn, S.; et al. Improved Survival in Patients with Stage II Adrenocortical Carcinoma Followed Up Prospectively by Specialized Centers. J. Clin. Endocr. Metab. 2010, 95, 4925–4932. [Google Scholar] [CrossRef]
- Fassnacht, M.; Johanssen, S.; Quinkler, M.; Bucsky, P.; Willenberg, H.S.; Beuschlein, F.; Terzolo, M.; Mueller, H.H.; Hahner, S.; Allolio, B.; et al. Limited Prognostic Value of the 2004 International Union Against Cancer Staging Classification for Adrenocortical Carcinomas. Cancer 2009, 115, 243–250. [Google Scholar] [CrossRef]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination chemotherapy in advanced adrenocortical carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Shen, W.T.; Clark, O.H.; Duh, Q.Y.; Kebebew, E. Risk assessment in 457 adrenal cortical carcinomas: How much does tumor size predict the likelihood of malignancy? J. Am. Coll. Surg. 2006, 202, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Kerkhofs, T.M.; Ettaieb, M.H.; Hermsen, I.G.; Haak, H.R. Developing treatment for adrenocortical carcinoma. Endocr. Relat. Cancer 2015, 22, R325–R338. [Google Scholar] [CrossRef] [PubMed]
- Terzolo, M.; Fassnacht, M. Our experience with the management of patients with non-metastatic adrenocortical carcinoma. Eur. J. Endocrinol. 2022, 187, R27–R40. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Hutter, A.M., Jr.; Kayhoe, D.E. Adrenal cortical carcinoma. Clinical features of 138 patients. Am. J. Med. 1966, 41, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, R.A.; Hickey, R.C.; Samaan, N.A. Adrenal cortical carcinoma. A study of 32 patients. Cancer 1975, 35, 549–554. [Google Scholar] [CrossRef]
- Polat, B.; Fassnacht, M.; Pfreundner, L.; Guckenberger, M.; Bratengeier, K.; Johanssen, S.; Kenn, W.; Hahner, S.; Allolio, B.; Flentje, M. Radiotherapy in adrenocortical carcinoma. Cancer 2009, 115, 2816–2823. [Google Scholar] [CrossRef]
- Hermsen, I.G.; Groenen, Y.E.; Dercksen, M.W.; Theuws, J.; Haak, H.R. Response to radiation therapy in adrenocortical carcinoma. J. Endocrinol. Investig. 2010, 33, 712–714. [Google Scholar] [CrossRef]
- Ho, J.; Turkbey, B.; Edgerly, M.; Alimchandani, M.; Quezado, M.; Camphausen, K.; Fojo, T.; Kaushal, A. Role of radiotherapy in adrenocortical carcinoma. Cancer J. 2013, 19, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Hahner, S.; Polat, B.; Koschker, A.C.; Kenn, W.; Flentje, M.; Allolio, B. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J. Clin. Endocrinol. Metab. 2006, 91, 4501–4504. [Google Scholar] [CrossRef] [PubMed]
- Sabolch, A.; Feng, M.; Griffith, K.; Hammer, G.; Doherty, G.; Ben-Josef, E. Adjuvant and definitive radiotherapy for adrenocortical carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, Z.; Shen, J.; Lian, X.; Miao, Z.; Shen, J.; Zhang, F. Efficacy of adjuvant radiotherapy for treatment of adrenocortical carcinoma: A retrospective study and an updated meta-analysis. Radiat. Oncol. 2020, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Gharzai, L.A.; Green, M.D.; Griffith, K.A.; Else, T.; Mayo, C.S.; Hesseltine, E.; Spratt, D.E.; Ben-Josef, E.; Sabolch, A.; Miller, B.S.; et al. Adjuvant Radiation Improves Recurrence-Free Survival and Overall Survival in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2019, 104, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Eppard, E.; Kurpig, S.; Bundschuh, R.A.; Schonberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. Onco Targets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Marek, G.; Capala, J. The role of nuclear medicine in modern therapy of cancer. Tumour Biol. 2012, 33, 629–640. [Google Scholar] [CrossRef]
- Han, S.J.; Kim, T.S.; Jeon, S.W.; Jeong, S.J.; Yun, M.; Rhee, Y.; Kang, E.S.; Cha, B.S.; Lee, E.J.; Lee, H.C.; et al. Analysis of adrenal masses by F-18-FDG positron emission tomography scanning. Int. J. Clin. Pr. 2007, 61, 802–809. [Google Scholar] [CrossRef]
- Leboulleux, S.; Dromain, C.; Bonniaud, G.; Auperin, A.; Caillou, B.; Lumbroso, J.; Sigal, R.; Baudin, E.; Schlumberger, M. Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: A prospective comparison with computed tomography. J. Clin. Endocr. Metab. 2006, 91, 920–925. [Google Scholar] [CrossRef]
- Wrenn, S.M.; Moore, A.L.; Shah, H.J.; Barletta, J.A.; Vaidya, A.; Kilbridge, K.L.; Doherty, G.M.; Jacene, H.A.; Nehs, M.A. Higher SUVmax on FDG-PET is associated with shorter survival in adrenocortical carcinoma. Am. J. Surg. 2023, 225, 309–314. [Google Scholar] [CrossRef]
- Werner, R.A.; Hartrampf, P.E.; Schirbel, A.; Hahner, S. Adrenal functional imaging—Which marker for which indication? Curr. Opin. Urol. 2022, 32, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Camus, B.; Cottereau, A.S.; Palmieri, L.J.; Dermine, S.; Tenenbaum, F.; Brezault, C.; Coriat, R. Indications of Peptide Receptor Radionuclide Therapy (PRRT) in Gastroenteropancreatic and Pulmonary Neuroendocrine Tumors: An Updated Review. J. Clin. Med. 2021, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.; Rapa, I.; Duregon, E.; Votta, A.; Giorcelli, J.; Buttigliero, C.; Scagliotti, G.V.; Volante, M.; Terzolo, M.; Papotti, M. Tissue Expression and Pharmacological In Vitro Analyses of mTOR and SSTR Pathways in Adrenocortical Carcinoma. Endocr. Pathol. 2017, 28, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Filice, A.; Basile, V.; Cosentini, D.; Rapa, I.; Albano, D.; Morandi, A.; Lagana, M.; Dalla Volta, A.; Bertagna, F.; et al. Treatment With Y-90/Lu-177-DOTATOC in Patients with Metastatic Adrenocortical Carcinoma Expressing Somatostatin Receptors. J. Clin. Endocr. Metab. 2020, 105, E1–E5. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.J.; Scognamiglio, T.; Liu, Y.F.; Kleiman, D.A.; Beninato, T.; Aronova, A.; Liu, H.; Jhanwar, Y.S.; Molina, A.; Tagawa, S.T.; et al. Prostate-Specific Membrane Antigen Is a Potential Antiangiogenic Target in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2016, 101, 981–987. [Google Scholar] [CrossRef][Green Version]
- Arora, S.; Damle, N.A.; Aggarwal, S.; Passah, A.; Behera, A.; Arora, G.; Bal, C.; Tripathi, M. Prostate-Specific Membrane Antigen Expression in Adrenocortical Carcinoma on 68Ga-Prostate-Specific Membrane Antigen PET/CT. Clin. Nucl. Med. 2018, 43, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef]
- Weiss, I.D.; Huff, L.M.; Evbuomwan, M.O.; Xu, X.; Dang, H.D.; Velez, D.S.; Singh, S.P.; Zhang, H.W.H.; Gardina, P.J.; Lee, J.H.; et al. Screening of cancer tissue arrays identifies CXCR4 on adrenocortical carcinoma: Correlates with expression and quantification on metastases using Cu-64-plerixafor PET. Oncotarget 2017, 8, 73387–73406. [Google Scholar] [CrossRef]
- Chifu, I.; Heinze, B.; Fuss, C.T.; Lang, K.A.; Kroiss, M.; Kircher, S.; Ronchi, C.L.; Altieri, B.; Schirbel, A.; Fassnacht, M.; et al. Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 597878. [Google Scholar] [CrossRef]
- Buck, A.K.; Haug, A.; Dreher, N.; Lambertini, A.; Higuchi, T.; Lapa, C.; Weich, A.; Pomper, M.G.; Wester, H.J.; Zehndner, A.; et al. Imaging of C-X-C Motif Chemokine Receptor 4 Expression in 690 Patients with Solid or Hematologic Neoplasms Using (68)Ga-Pentixafor PET. J. Nucl. Med. 2022, 63, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Bluemel, C.; Hahner, S.; Heinze, B.; Fassnacht, M.; Kroiss, M.; Bley, T.A.; Wester, H.J.; Kropf, S.; Lapa, C.; Schirbel, A.; et al. Investigating the Chemokine Receptor 4 as Potential Theranostic Target in Adrenocortical Cancer Patients. Clin. Nucl. Med. 2017, 42, E29–E34. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.K.; Serfling, S.E.; Lindner, T.; Hanscheid, H.; Schirbel, A.; Hahner, S.; Fassnacht, M.; Einsele, H.; Werner, R.A. CXCR4-targeted theranostics in oncology. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4133–4144. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.M.; Lang, J.; Abedinpour, F.; Zeilberger, K.; Adelmann, B.; Engelhardt, D. Different Inhibitory Effect of Etomidate and Ketoconazole on the Human Adrenal-Steroid Biosynthesis. Clin. Investig. 1993, 71, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Damani, L.A.; Mitterhauser, M.; Zolle, I.; Lin, G.; Oehler, E.; Ho, Y.P. Metabolic and Pharmacokinetic Considerations in the Design of 2-Phenyl Substituted Metyrapone Derivatives—2-Methoxyphenylmetyrapone as a Radioligand for Functional Diagnosis of Adrenal Pathology. Nucl. Med. Biol. 1995, 22, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, M.; Bonasera, T.A.; Lu, L.; Bergstrom, E.; Backlin, C.; Juhlin, C.; Langstrom, B. In vitro and in vivo primate evaluation of carbon-11-etomidate and carbon-11-metomidate as potential tracers for PET imaging of the adrenal cortex and its tumors. J. Nucl. Med. 1998, 39, 982–989. [Google Scholar] [PubMed]
- Bergstrom, M.; Juhlin, C.; Bonasera, T.A.; Sundin, A.; Rastad, J.; Akerstrom, G.; Langstrom, B. PET imaging of adrenal cortical tumors with the 11beta-hydroxylase tracer 11C-metomidate. J. Nucl. Med. 2000, 41, 275–282. [Google Scholar] [PubMed]
- Hennings, J.; Lindhe, O.; Bergstrom, M.; Langstrom, B.; Sundin, A.; Hellman, P. [(11)C]metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J. Clin. Endocr. Metab. 2006, 91, 1410–1414. [Google Scholar] [CrossRef]
- Schirbel, A.; Zolle, I.; Hammerschmidt, F.; Berger, M.L.; Schiller, D.; Kvaternik, H.; Reiners, C. [I-123/131]Iodometomidate as a radioligand for functional diagnosis of adrenal disease: Synthesis, structural requirements and biodistribution. Radiochim. Acta 2004, 92, 297–303. [Google Scholar] [CrossRef]
- Hahner, S.; Stuermer, A.; Kreissl, M.; Reiners, C.; Fassnacht, M.; Haenscheid, H.; Beuschlein, F.; Zink, M.; Lang, K.; Allolio, B.; et al. [123I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J. Clin. Endocrinol. Metab. 2008, 93, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Zolle, I.M.; Berger, M.L.; Hammerschmidt, F.; Hahner, S.; Schirbel, A.; Peric-Simov, B. New Selective Inhibitors of Steroid 11 beta-Hydroxylation in the Adrenal Cortex. Synthesis and Structure-Activity Relationship of Potent Etomidate Analogues (vol 51, pg 2244, 2008). J. Med. Chem. 2008, 51, 7652. [Google Scholar] [CrossRef]
- Hahner, S.; Kreissl, M.C.; Fassnacht, M.; Haenscheid, H.; Bock, S.; Verburg, F.A.; Knoedler, P.; Lang, K.; Reiners, C.; Buck, A.K.; et al. Functional Characterization of Adrenal Lesions Using [I-123] IMTO-SPECT/CT. J. Clin. Endocr. Metab. 2013, 98, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Hahner, S.; Kreissl, M.C.; Fassnacht, M.; Haenscheid, H.; Knoedler, P.; Lang, K.; Buck, A.K.; Reiners, C.; Allolio, B.; Schirbel, A. [I-131]Iodometomidate for Targeted Radionuclide Therapy of Advanced Adrenocortical Carcinoma. J. Clin. Endocr. Metab. 2012, 97, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Heinze, B.; Schirbel, A.; Nannen, L.; Michelmann, D.; Hartrampf, P.E.; Bluemel, C.; Schneider, M.; Herrmann, K.; Haenscheid, H.; Fassnacht, M.; et al. Novel CYP11B-ligand [I-123/131]IMAZA as promising theranostic tool for adrenocortical tumors: Comprehensive preclinical characterization and first clinical experience. Eur. J. Nucl. Med. Mol. I 2021, 49, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hahner, S.; Hartrampf, P.E.; Mihatsch, P.W.; Nauerz, M.; Heinze, B.; Hanscheid, H.; Fuss, C.T.; Werner, R.A.; Pamporaki, C.; Kroiss, M.; et al. Targeting 11-Beta Hydroxylase With [I-131]IMAZA: A Novel Approach for the Treatment of Advanced Adrenocortical Carcinoma. J. Clin. Endocr. Metab. 2022, 107, E1348–E1355. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Aslani, A.; Snowdon, G.M.; Bailey, D.L.; Schembri, G.P.; Bailey, E.A.; Pavlakis, N.; Roach, P.J. Lutetium-177 DOTATATE Production with an Automated Radiopharmaceutical Synthesis System. Asia Ocean. J. Nucl. Med. Biol. 2015, 3, 107–115. [Google Scholar]
- Schottelius, M.; Osl, T.; Poschenrieder, A.; Hoffmann, F.; Beykan, S.; Hanscheid, H.; Schirbel, A.; Buck, A.K.; Kropf, S.; Schwaiger, M.; et al. [(177)Lu]pentixather: Comprehensive Preclinical Characterization of a First CXCR4-directed Endoradiotherapeutic Agent. Theranostics 2017, 7, 2350–2362. [Google Scholar] [CrossRef]
- Hanscheid, H.; Schirbel, A.; Hartrampf, P.; Kraus, S.; Werner, R.A.; Einsele, H.; Wester, H.J.; Lassmann, M.; Kortum, M.; Buck, A.K. Biokinetics and Dosimetry of (177)Lu-Pentixather. J. Nucl. Med. 2022, 63, 754–760. [Google Scholar] [CrossRef]
- Wichmann, C.W.; Ackermann, U.; Poniger, S.; Young, K.; Nguyen, B.; Chan, G.; Sachinidis, J.; Scott, A.M. Automated radiosynthesis of [(68) Ga]Ga-PSMA-11 and [(177) Lu]Lu-PSMA-617 on the iPHASE MultiSyn module for clinical applications. J. Label. Comp. Radiopharm. 2021, 64, 140–146. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalski, K.; Schlötelburg, W.; Hartrampf, P.E.; Kosmala, A.; Buck, A.K.; Hahner, S.; Schirbel, A. Radiopharmaceuticals for Treatment of Adrenocortical Carcinoma. Pharmaceuticals 2024, 17, 25. https://doi.org/10.3390/ph17010025
Michalski K, Schlötelburg W, Hartrampf PE, Kosmala A, Buck AK, Hahner S, Schirbel A. Radiopharmaceuticals for Treatment of Adrenocortical Carcinoma. Pharmaceuticals. 2024; 17(1):25. https://doi.org/10.3390/ph17010025
Chicago/Turabian StyleMichalski, Kerstin, Wiebke Schlötelburg, Philipp E. Hartrampf, Aleksander Kosmala, Andreas K. Buck, Stefanie Hahner, and Andreas Schirbel. 2024. "Radiopharmaceuticals for Treatment of Adrenocortical Carcinoma" Pharmaceuticals 17, no. 1: 25. https://doi.org/10.3390/ph17010025
APA StyleMichalski, K., Schlötelburg, W., Hartrampf, P. E., Kosmala, A., Buck, A. K., Hahner, S., & Schirbel, A. (2024). Radiopharmaceuticals for Treatment of Adrenocortical Carcinoma. Pharmaceuticals, 17(1), 25. https://doi.org/10.3390/ph17010025





