Combining Cisplatin with Different Radiation Qualities—Interpretation of Cytotoxic Effects In Vitro by Isobolographic Analysis
Abstract
:1. Introduction
2. Results
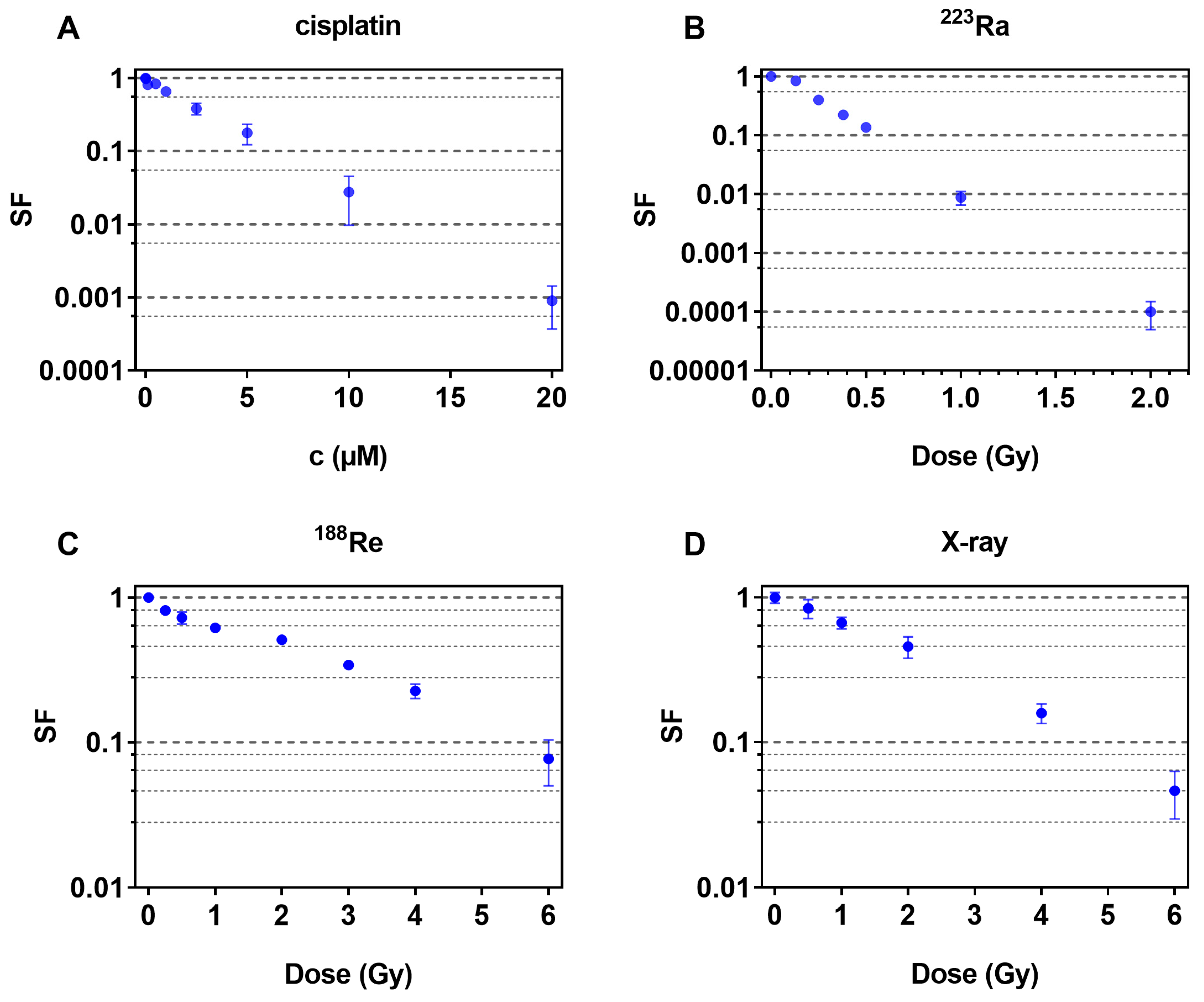
2.1. Dose–Response Curves of Single Cytotoxin Incubations
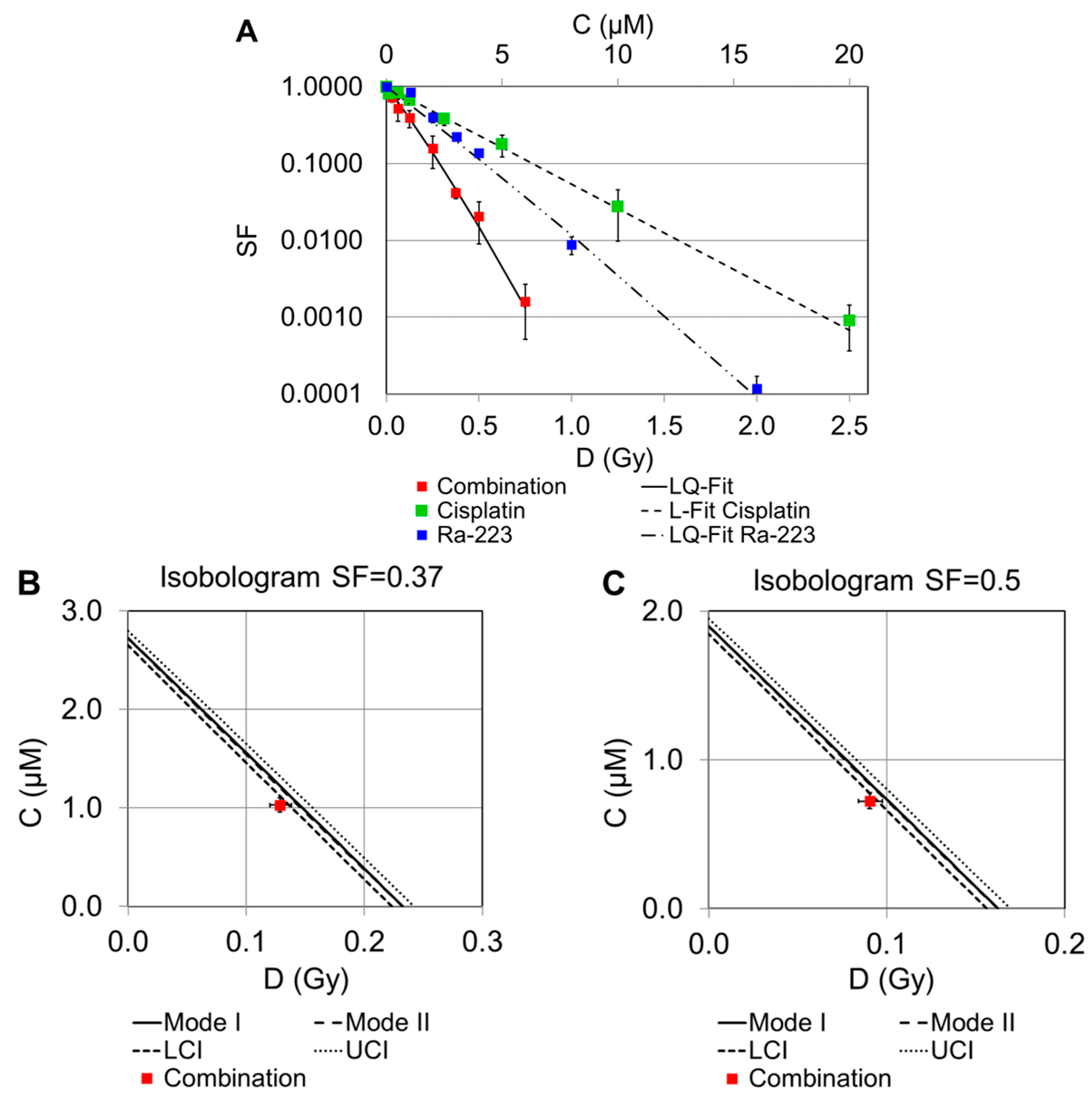
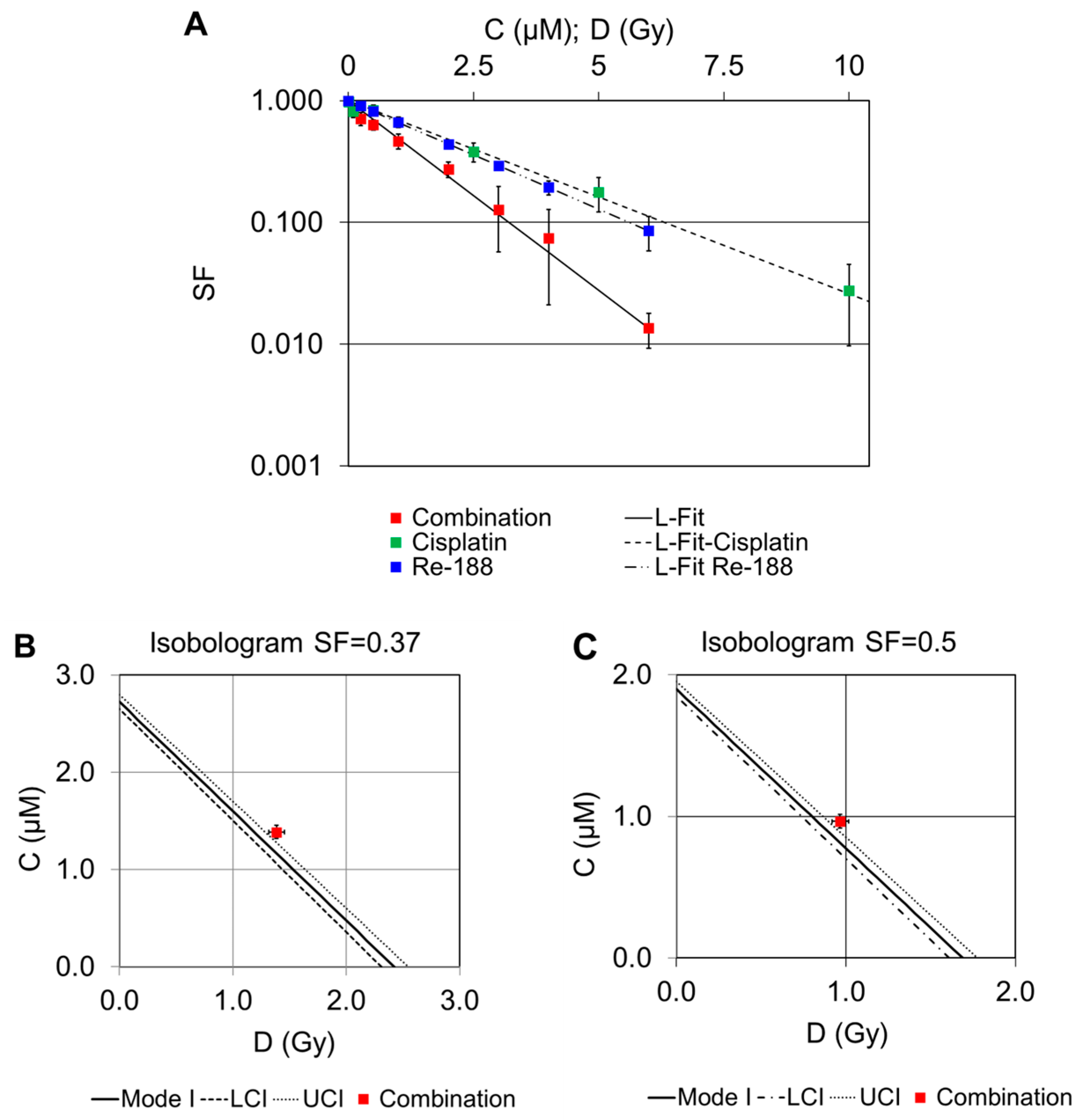
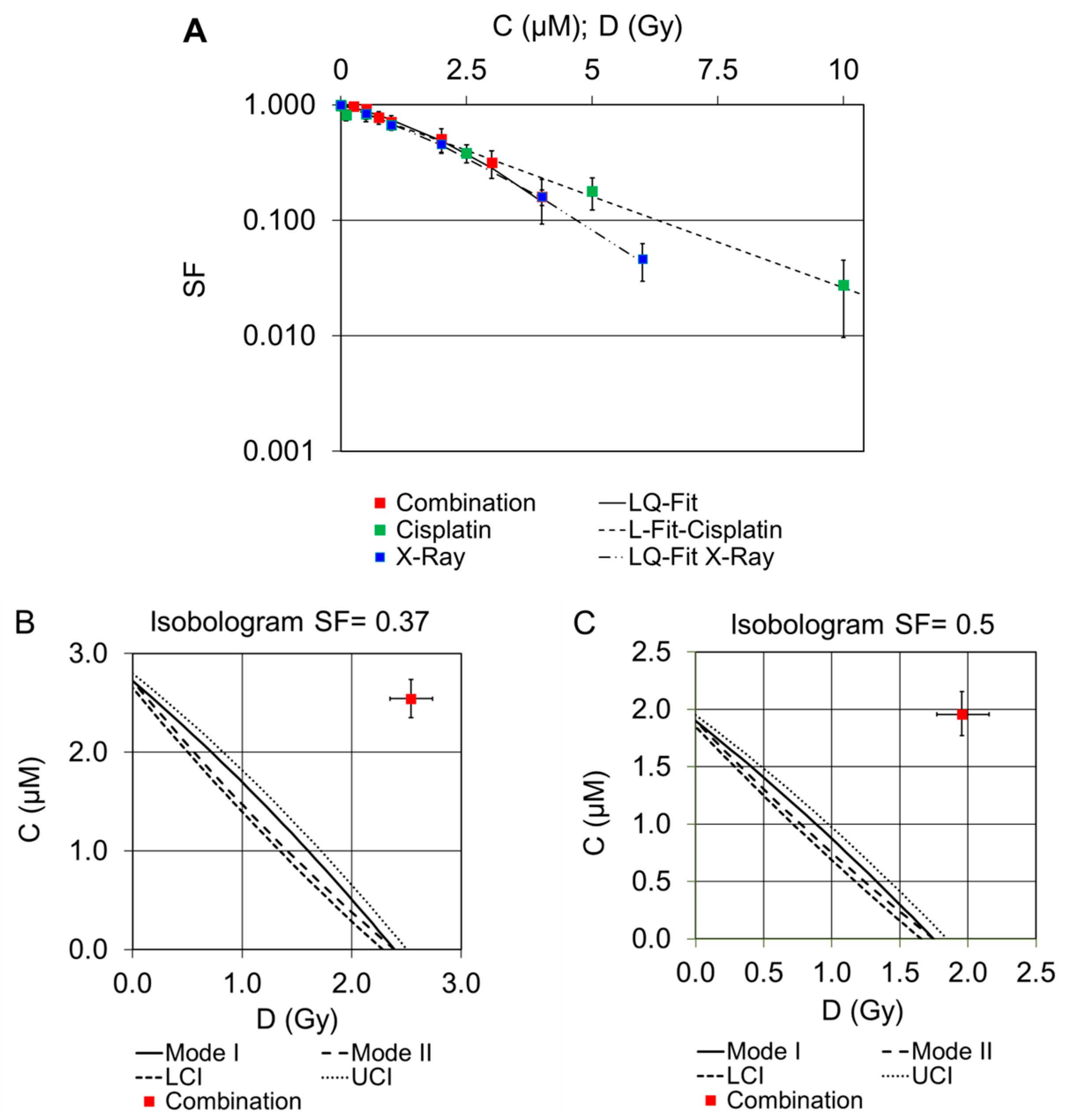
2.2. Dose–Response Curves of Combined Treatments
2.2.1. Combination of Cisplatin and 223Ra
2.2.2. Combination of Cisplatin and 188Re
2.2.3. Combination of Cisplatin and External X-rays
3. Discussion
4. Materials and Methods
4.1. Radionuclides and X-ray Irradiation
4.2. Cell Culture
4.3. Cisplatin Incubation, Irradiation Procedure, and Colony Formation Assay
4.4. Isobologram Analysis
4.5. Dosimetry
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Romani, A.M.P. Cisplatin in cancer treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Milenic, D.E.; Kim, Y.S.; Baidoo, K.E.; Wong, K.J.; Barkley, R.; Delgado, J.; Brechbiel, M.W. Exploration of a F(ab′)2 Fragment as the Targeting Agent of alpha-Radiation Therapy: A Comparison of the Therapeutic Benefit of Intraperitoneal and Intravenous Administered Radioimmunotherapy. Cancer Biother. Radiopharm. 2018, 33, 182–193. [Google Scholar] [CrossRef]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417. [Google Scholar] [CrossRef]
- Biston, M.C.; Joubert, A.; Adam, J.F.; Elleaume, H.; Bohic, S.; Charvet, A.M.; Esteve, F.; Foray, N.; Balosso, J. Cure of Fisher rats bearing radioresistant F98 glioma treated with cis-platinum and irradiated with monochromatic synchrotron X-rays. Cancer Res. 2004, 64, 2317–2323. [Google Scholar] [CrossRef]
- Candelaria, M.; Chanona-Vilchis, J.; Cetina, L.; Flores-Estrada, D.; Lopez-Graniel, C.; Gonzalez-Enciso, A.; Cantu, D.; Poitevin, A.; Rivera, L.; Hinojosa, J.; et al. Prognostic significance of pathological response after neoadjuvant chemotherapy or chemoradiation for locally advanced cervical carcinoma. Int. Semin. Surg. Oncol. 2006, 3, 3. [Google Scholar] [CrossRef]
- Caney, C.; Singh, G.; Lukka, H.; Rainbow, A.J. Combined gamma-irradiation and subsequent cisplatin treatment in human squamous carcinoma cell lines sensitive and resistant to cisplatin. Int. J. Radiat. Biol. 2004, 80, 291–299. [Google Scholar] [CrossRef]
- Gorodetsky, R.; Levy-Agababa, F.; Mou, X.; Vexler, A.M. Combination of cisplatin and radiation in cell culture: Effect of duration of exposure to drug and timing of irradiation. Int. J. Cancer 1998, 75, 635–642. [Google Scholar] [CrossRef]
- Marcu, L.G. Improving therapeutic ratio in head and neck cancer with adjuvant and cisplatin-based treatments. Biomed. Res. Int. 2013, 2013, 817279. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, S.S.; Lee, K.J.; Ju, E.J.; Park, J.; Ko, E.J.; Jung, J.; Kuroda, S.; Hong, S.M.; Hwang, J.J.; et al. Preclinical evaluation of cisplatin-incorporated bio-nanocapsules as chemo-radiotherapy for human hepatocellular carcinoma. Oncol. Rep. 2017, 38, 2259–2266. [Google Scholar] [CrossRef]
- Sisin, N.N.T.; Abdul Razak, K.; Zainal Abidin, S.; Che Mat, N.F.; Abdullah, R.; Ab Rashid, R.; Khairil Anuar, M.A.; Mohd Zainudin, N.H.; Tagiling, N.; Mat Nawi, N.; et al. Radiosensitization Effects by Bismuth Oxide Nanoparticles in Combination with Cisplatin for High Dose Rate Brachytherapy. Int. J. Nanomed. 2019, 14, 9941–9954. [Google Scholar] [CrossRef]
- Ahmed, O.; Yu, Q.; Patel, M.; Hwang, G.; Pillai, A.; Liao, C.Y.; Fung, J.; Baker, T. Yttrium-90 Radioembolization and Concomitant Systemic Gemcitabine, Cisplatin, and Capecitabine as the First-Line Therapy for Locally Advanced Intrahepatic Cholangiocarcinoma. J. Vasc. Interv. Radiol. 2023, 34, 702–709. [Google Scholar] [CrossRef]
- Chenoufi, N.; Raoul, J.L.; Lescoat, G.; Brissot, P.; Bourguet, P. In vitro demonstration of synergy between radionuclide and chemotherapy. J. Nucl. Med. 1998, 39, 900–903. [Google Scholar]
- Geldof, A.A.; de Rooij, L.; Versteegh, R.T.; Newling, D.W.; Teule, G.J. Combination 186Re-HEDP and cisplatin supra-additive treatment effects in prostate cancer cells. J. Nucl. Med. 1999, 40, 667–671. [Google Scholar]
- Timin, A.S.; Postovalova, A.S.; Karpov, T.E.; Antuganov, D.; Bukreeva, A.S.; Akhmetova, D.R.; Rogova, A.S.; Muslimov, A.R.; Rodimova, S.A.; Kuznetsova, D.S.; et al. Calcium carbonate carriers for combined chemo- and radionuclide therapy of metastatic lung cancer. J. Control. Release 2022, 344, 1–11. [Google Scholar] [CrossRef]
- Kobayashi, K.; Usami, N.; Porcel, E.; Lacombe, S.; Le Sech, C. Enhancement of radiation effect by heavy elements. Mutat. Res. 2010, 704, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Nias, A.H. Radiation and platinum drug interaction. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1985, 48, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Nadar, R.A.; Franssen, G.M.; Van Dijk, N.W.M.; Codee-van der Schilden, K.; de Weijert, M.; Oosterwijk, E.; Iafisco, M.; Margiotta, N.; Heskamp, S.; van den Beucken, J.; et al. Bone tumor-targeted delivery of theranostic 195mPt-bisphosphonate complexes promotes killing of metastatic tumor cells. Mater. Today Bio. 2021, 9, 100088. [Google Scholar] [CrossRef] [PubMed]
- Obata, H.; Tsuji, A.B.; Sudo, H.; Sugyo, A.; Minegishi, K.; Nagatsu, K.; Ogawa, M.; Zhang, M.R. In Vitro Evaluation of No-Carrier-Added Radiolabeled Cisplatin ([(189,191)Pt]cisplatin) Emitting Auger Electrons. Int. J. Mol. Sci. 2021, 22, 4622. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.D.; Bentzen, S.M.; Harari, P.M. Biologic basis for combining drugs with radiation. Semin. Radiat. Oncol. 2006, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Benzina, S.; Fischer, B.; Miternique-Grosse, A.; Dufour, P.; Denis, J.M.; Bergerat, J.P.; Gueulette, J.; Bischoff, P. Cell death induced in a human glioblastoma cell line by p(65) + Be neutrons combined with cisplatin. Life Sci. 2006, 79, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Ueda, T.; Aune, G.; Sarasin, A.; Kraemer, K.H.; Pommier, Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002, 62, 4899–4902. [Google Scholar]
- Dewey, W.C.; Ling, C.C.; Meyn, R.E. Radiation-induced apoptosis: Relevance to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 781–796. [Google Scholar] [CrossRef]
- Runge, R.; Oehme, L.; Kotzerke, J.; Freudenberg, R. The effect of dimethyl sulfoxide on the induction of DNA strand breaks in plasmid DNA and colony formation of PC Cl3 mammalian cells by alpha-, beta-, and Auger electron emitters 223Ra, 188Re, and 99mTc. EJNMMI Res. 2016, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Reissig, F.; Runge, R.; Naumann, A.; Kotzerke, J. Cisplatin—A more Efficient Drug in Combination with Radionuclides? Nuklearmedizin 2022, 61, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Akudugu, J.M.; Slabbert, J.P. Modulation of radiosensitivity in Chinese hamster lung fibroblasts by cisplatin. Can. J. Physiol. Pharmacol. 2008, 86, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Wakatsuki, M.; Ohno, T.; Nakano, T. Differences in Linear Energy Transfer Affect Cell-killing and Radiosensitizing Effects of Spread-out Carbon-ion Beams. Anticancer Res. 2020, 40, 5497–5502. [Google Scholar] [CrossRef] [PubMed]
- Sai, S.; Vares, G.; Kim, E.H.; Karasawa, K.; Wang, B.; Nenoi, M.; Horimoto, Y.; Hayashi, M. Carbon ion beam combined with cisplatin effectively disrupts triple negative breast cancer stem-like cells in vitro. Mol. Cancer 2015, 14, 166. [Google Scholar] [CrossRef]
- Benzina, S.; Altmeyer, A.; Malek, F.; Dufour, P.; Denis, J.M.; Gueulette, J.; Bischoff, P. High-LET radiation combined with oxaliplatin induce autophagy in U-87 glioblastoma cells. Cancer Lett. 2008, 264, 63–70. [Google Scholar] [CrossRef]
- Fang, X.; Sun, P.; Dong, Y.; Huang, Y.; Lu, J.J.; Kong, L. In vitro evaluation of photon and carbon ion radiotherapy in combination with cisplatin in head and neck squamous cell carcinoma cell lines. Front. Oncol. 2023, 13, 896142. [Google Scholar] [CrossRef]
- Begg, A.C. Cisplatin and radiation: Interaction probabilities and therapeutic possibilities. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 1183–1189. [Google Scholar] [CrossRef]
- Rangan, S.R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]
- Eicheler, W.; Zips, D.; Dorfler, A.; Grenman, R.; Baumann, M. Splicing mutations in TP53 in human squamous cell carcinoma lines influence immunohistochemical detection. J. Histochem. Cytochem. 2002, 50, 197–204. [Google Scholar] [CrossRef]
- Maucksch, U.; Runge, R.; Wunderlich, G.; Freudenberg, R.; Naumann, A.; Kotzerke, J. Comparison of the radiotoxicity of the (99m)Tc-labeled compounds (99m)Tc-pertechnetate, (99m)Tc-HMPAO and (99m)Tc-MIBI. Int. J. Radiat. Biol. 2016, 92, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Steel, G.G.; Peckham, M.J. Exploitable mechanisms in combined radiotherapy-chemotherapy: The concept of additivity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Redpath, J.L. Mechanisms in combination therapy: Isobologram analysis and sequencing. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1980, 38, 355–356. [Google Scholar] [CrossRef]
- Freudenberg, R.; Runge, R.; Maucksch, U.; Berger, V.; Kotzerke, J. On the dose calculation at the cellular level and its implications for the RBE of 99mTc and 123I. Med. Phys. 2014, 41, 062503. [Google Scholar] [CrossRef]
| Treatment Conditions | C50 (µM)/D50 (Gy) | C37 (µM)/D37 (Gy) |
|---|---|---|
| Cisplatin (µM) | 1.90 (1.85–1.95) | 2.72 (2.65–2.80) |
| 223Ra (Gy) | 0.163 (0.156–0.169) | 0.232 (0.223–0.242) |
| 188Re (Gy) | 1.69 (1.61–1.78) | 2.42 (2.31–2.55) |
| X-ray (Gy) | 1.75 (1.66–1.85) | 2.39 (2.28–2.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runge, R.; Reissig, F.; Herzog, N.; Oehme, L.; Brogsitter, C.; Kotzerke, J. Combining Cisplatin with Different Radiation Qualities—Interpretation of Cytotoxic Effects In Vitro by Isobolographic Analysis. Pharmaceuticals 2023, 16, 1720. https://doi.org/10.3390/ph16121720
Runge R, Reissig F, Herzog N, Oehme L, Brogsitter C, Kotzerke J. Combining Cisplatin with Different Radiation Qualities—Interpretation of Cytotoxic Effects In Vitro by Isobolographic Analysis. Pharmaceuticals. 2023; 16(12):1720. https://doi.org/10.3390/ph16121720
Chicago/Turabian StyleRunge, Roswitha, Falco Reissig, Nora Herzog, Liane Oehme, Claudia Brogsitter, and Joerg Kotzerke. 2023. "Combining Cisplatin with Different Radiation Qualities—Interpretation of Cytotoxic Effects In Vitro by Isobolographic Analysis" Pharmaceuticals 16, no. 12: 1720. https://doi.org/10.3390/ph16121720
APA StyleRunge, R., Reissig, F., Herzog, N., Oehme, L., Brogsitter, C., & Kotzerke, J. (2023). Combining Cisplatin with Different Radiation Qualities—Interpretation of Cytotoxic Effects In Vitro by Isobolographic Analysis. Pharmaceuticals, 16(12), 1720. https://doi.org/10.3390/ph16121720






