Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants
Abstract
1. Introduction
2. Results
2.1. Changes in Anthropometric Profiles
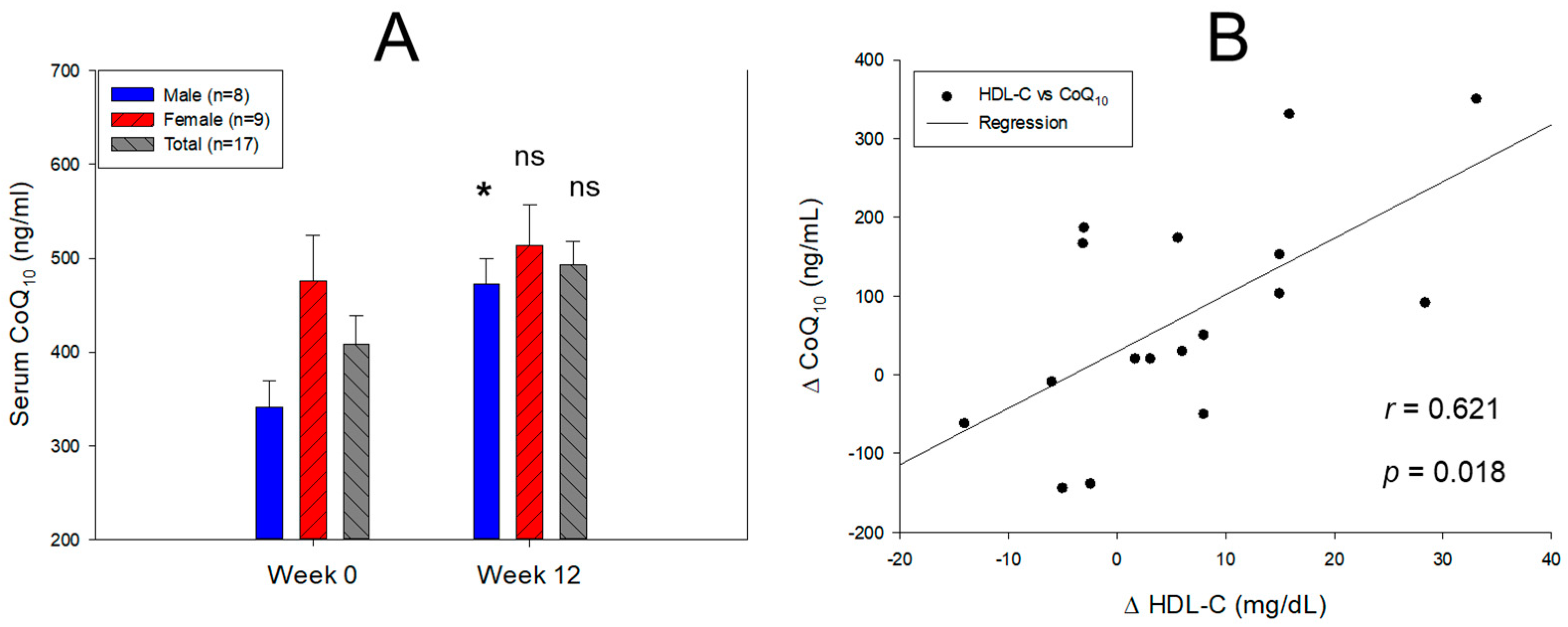
2.2. Change in Serum Coenzyme Q10 and Lipid Profiles
2.3. Change in the Serum Protein Parameters
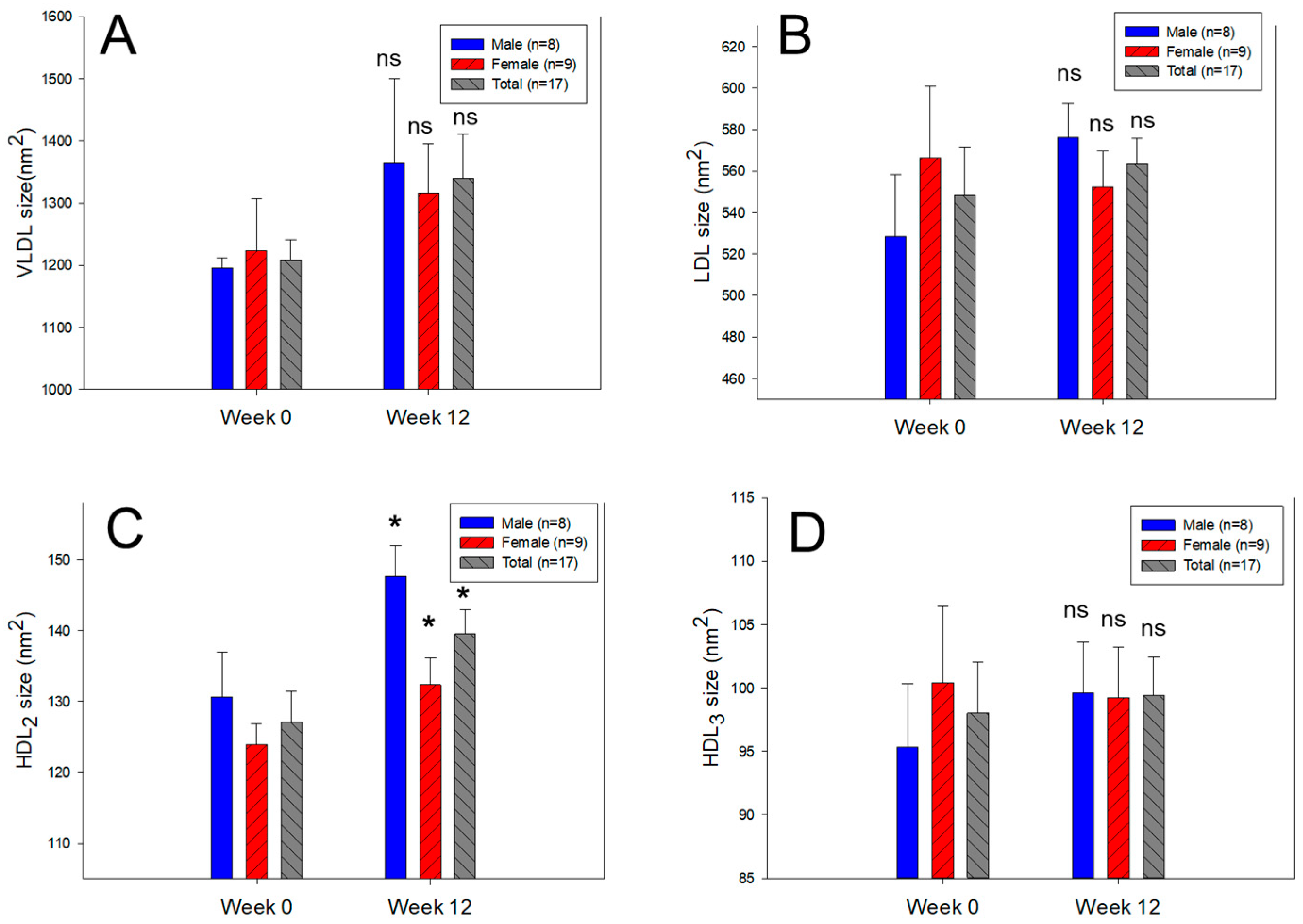
2.4. Change in VLDL and LDL Properties and Compositions
2.5. Electromobility of LDL and Extent of Oxidation
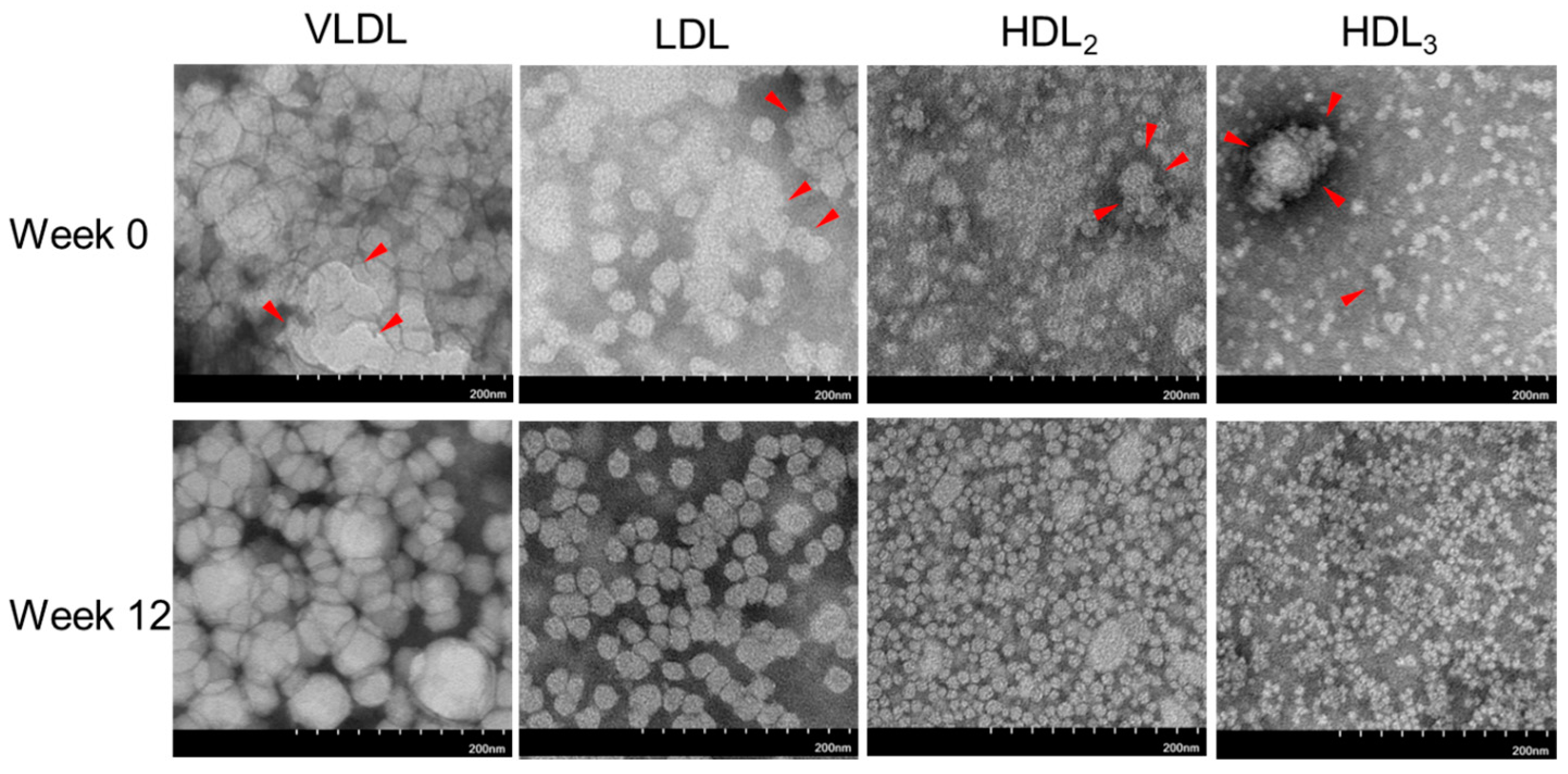
2.6. Electron Microscopic Observation of Lipoprotein Image
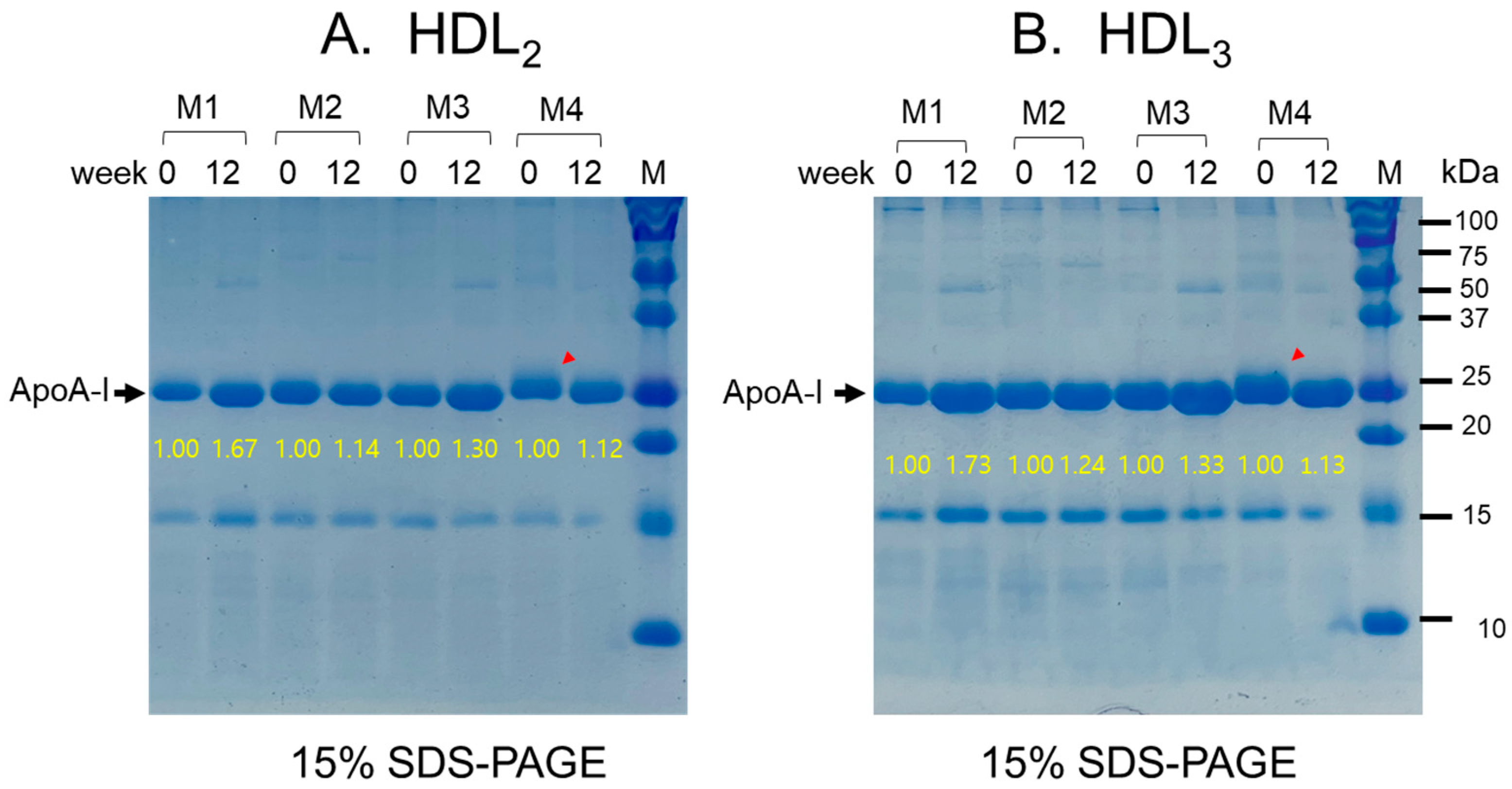
2.7. Change in HDL Quality during 12 Weeks
2.8. Change in apoA-I Expression in HDL
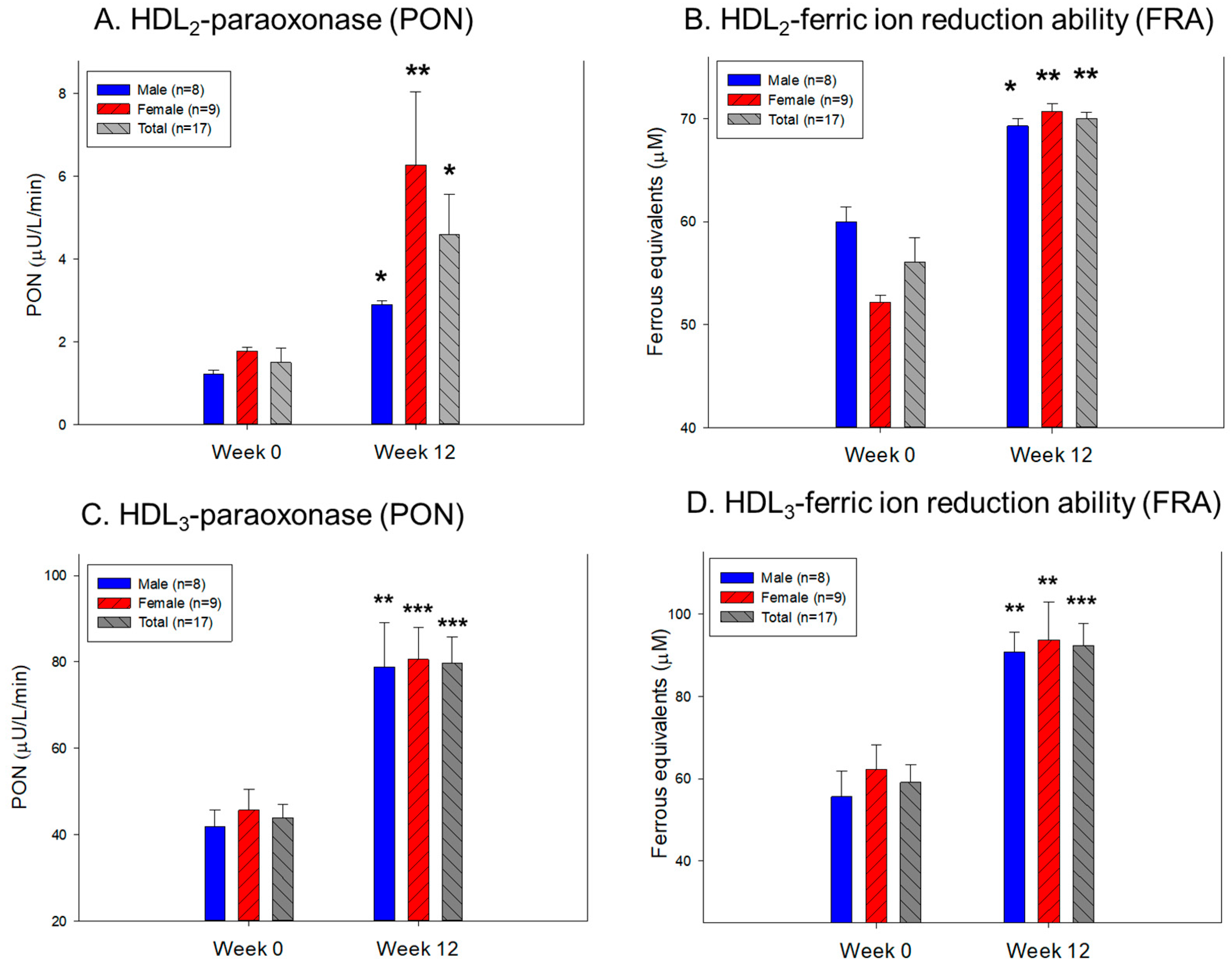
2.9. Change in the Antioxidant Ability in HDL2 and HDL3
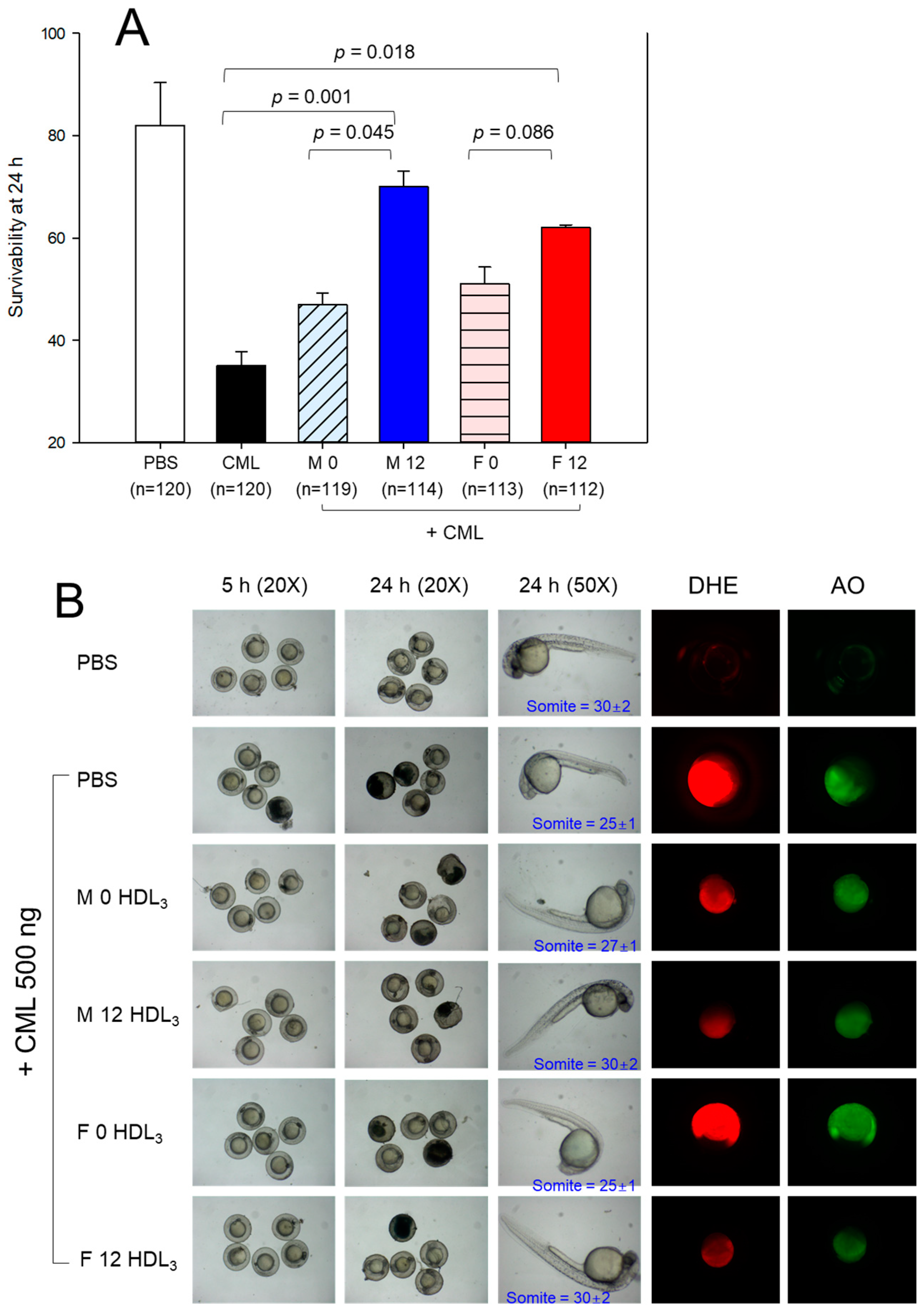
2.10. Embryo Survivability under the Presence of Carboxymethyllysine
3. Discussion
4. Materials and Methods
4.1. Policosanol
4.2. Participants
4.3. Criteria of Inclusion and Exclusion
4.4. Exercise Program
4.5. Anthropometric Analysis
4.6. Blood Analysis
4.7. Quantification of Serum Coenzyme Q10
4.8. Isolation of Lipoproteins
4.9. Characterization of Lipoproteins
4.10. Transmitted Electron Microscopy
4.11. Agarose Gel Electrophoresis
4.12. Antioxidant Activities in the HDL
4.13. Zebrafish Maintenance
4.14. Microinjection of CML and HDL into Zebrafish Embryos
4.15. Imaging of Oxidative Stress, Apoptosis in Embryo
4.16. Statistical Analysis
5. Limitations and Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult population: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef] [PubMed]
- Mainous, A.G.; Tanner, R.J.; Rahmanian, K.P.; Jo, A.; Carek, P.J. Effect of sedentary lifestyle on cardiovascular disease risk among healthy adults with body mass indexes 18.5 to 29.9 kg/m2. Am. J. Cardiol. 2019, 123, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S.; Hyde, R.T.; Wing, A.L.; Lee, I.-M.; Jung, D.L.; Kampert, J.B. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N. Engl. J. Med. 1993, 328, 538–545. [Google Scholar] [CrossRef]
- Kinoshita, K.; Ozato, N.; Yamaguchi, T.; Sudo, M.; Yamashiro, Y.; Mori, K.; Ishida, M.; Katsuragi, Y.; Sasai, H.; Yasukawa, T.; et al. Association of sedentary behaviour and physical activity with cardiometabolic health in Japanese adults. Sci. Rep. 2022, 12, 2262. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Hein, H.O.; Suadicani, P.; Gyntelberg, F. Relation of high TG–Low HDL cholesterol and LDL cholesterol to the incidence of ischemic heart disease: An 8-year follow-up in the Copenhagen male study. Arter. Thromb. Vasc. Biol. 1997, 17, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Paffenbarger, R.S., Jr.; Hyde, R.; Wing, A.L.; Hsieh, C.C. Physical activity, all-cause mortality, and longevity of college alumni. N. Engl. J. Med. 1986, 314, 605–613. [Google Scholar] [CrossRef]
- Blair, S.N.; Kohl, H.W., 3rd; Paffenbarger, R.S., Jr.; Clark, D.G.; Cooper, K.H.; Gibbons, L.W. Physical fitness and all-cause mortality. A prospective study of healthy men and women. J. Am. Med. Assoc. 1989, 262, 2395–2401. [Google Scholar] [CrossRef]
- Couillard, C.; Després, J.-P.; Lamarche, B.; Bergeron, J.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: Evidence from men of the Health, Risk Factors, Exercise Training and Genetics (HERITAGE) Family Study. Arter. Thromb. Vasc. Biol. 2001, 21, 1226–1232. [Google Scholar] [CrossRef]
- Palazón-Bru, A.; Hernández-Lozano, D.; Gil-Guillén, V.F. which physical exercise interventions increase HDL-cholesterol levels? A systematic review of meta-analyses of randomized controlled trials. Sports Med. 2021, 51, 243–253. [Google Scholar] [CrossRef]
- Gao, W.; Lv, M.; Huang, T. Effects of different types of exercise on hypertension in middle-aged and older adults: A network meta-analysis. Front. Public Health 2023, 11, 1194124. [Google Scholar] [CrossRef]
- Lin, M.; Lin, Y.; Li, Y.; Lin, X. Effect of exercise training on blood pressure variability in adults: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292020. [Google Scholar] [CrossRef] [PubMed]
- Toyama, K.; Sugiyama, S.; Oka, H.; Iwasaki, Y.; Sumida, H.; Tanaka, T.; Tayama, S.; Jinnouchi, H.; Ogawa, H. Combination treatment of rosuvastatin or atorvastatin, with regular exercise improves arterial wall stiffness in patients with coronary artery disease. PLoS ONE 2012, 7, e41369. [Google Scholar] [CrossRef] [PubMed]
- Joy, T.R.; Hegele, R.A. Narrative review: Statin-related myopathy. Ann. Intern. Med. 2009, 150, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, M.R.; Oliveira, A.S.B.; Amaral, S.L.D.; Monteiro, H.L. Treatment of dyslipidemia with statins and physical exercises: Recent findings of skeletal muscle responses. Arq. Bras. Cardiol. 2015, 104, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Meador, B.M.; Huey, K.A. Statin-associated myopathy and its exacerbation with exercise. Muscle Nerve 2010, 42, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Mearelli, F.; Biasinutto, C.; Fiotti, N.; Di Girolamo, F.G.; Biolo, G. Statin-Associated Myopathy: Emphasis on Mechanisms and Targeted Therapy. Int. J. Mol. Sci. 2021, 22, 11687. [Google Scholar] [CrossRef]
- Opie, L.H. Exercise-induced myalgia may limit the cardiovascular benefits of statins. Cardiovasc. Drugs Ther. 2013, 27, 569–572. [Google Scholar] [CrossRef]
- Singh, R.B.; Neki, N.S.; Kartikey, K.; Pella, D.; Kumar, A.; Niaz, M.A.; Thakur, A.S. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Vascul. Pharmacol. 2003, 246, 75–82. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-R. Consumption of Cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Yadav, D.; Park, H.-J.; Kim, J.-R.; Cho, K.-H. Long-term consumption of Cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy Korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- Kim, H.R.; Han, M.A. Association between serum liver enzymes and metabolic syndrome in Korean adults. Int. J. Environ. Res. Public Health 2018, 15, 1658. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Hindorf, U.; Persson, P.; Bengtsson, T.; Malmqvist, U.; Werkström, V.; Ekelund, M. Muscular exercise can cause highly pathological liver function tests in healthy men. Br. J. Clin. Pharmacol. 2008, 65, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Zapata, A.; I Zon, L. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Novoa, B.; Bowman, T.; Zon, L.; Figueras, A. LPS response and tolerance in the zebrafish (Danio rerio). Fish Shellfish Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Kim, C.-G. What types of exercise are more effective in reducing obesity and blood pressure for middle-aged women? A systematic review with meta-analysis. Biol. Res. Nurs. 2021, 23, 658–675. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [CrossRef]
- Janikula, M. Policosanol: A new treatment for cardiovascular disease? Altern. Med. Rev. 2002, 7, 203–217. [Google Scholar]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; E Symonds, M.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The effects of exercise and physical activity on weight loss and maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zmuda, J.M.; Yurgalevitch, S.M.; Flynn, M.M.; Bausserman, L.L.; Saratelli, A.; Spannaus-Martin, D.J.; Herbert, P.N.; Thompson, P.D. Exercise training has little effect on HDL levels and metabolism in men with initially low HDL cholesterol. Atherosclerosis 1998, 137, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Tanaka, S.; Saito, K.; Shu, M.; Sone, Y.; Onitake, F.; Suzuki, E.; Shimano, H.; Yamamoto, S.; Kondo, K.; et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch. Intern. Med. 2007, 167, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Ghirlanda, G.; Oradei, A.; Manto, A.; Lippa, S.; Uccioli, L.; Caputo, S.; Greco, A.V.; Littarru, G.P. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: A double-blind, placebo-controlled study. J. Clin. Pharmacol. 1993, 33, 226–229. [Google Scholar] [CrossRef]
- Deichmann, R.; Lavie, C.; Andrews, S. Coenzyme q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010, 10, 16–21. [Google Scholar] [PubMed]
- Pacanowski, M.A.; Frye, R.F.; Enogieru, O.; Schofield, R.S.; Zineh, I. Plasma Coenzyme Q10 Predicts Lipid-lowering Response to High-Dose Atorvastatin. J. Clin. Lipidol. 2008, 2, 289–297. [Google Scholar] [CrossRef]
- Tomasetti, M.; Alleva, R.; Solenghi, M.D.; Littarru, G.P. Distribution of antioxidants among blood components and lipoproteins: Significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. BioFactors 1999, 9, 231–240. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2015, 5, 324–333. [Google Scholar] [CrossRef]
- Da Silva Pereira, E.N.G.; Paula, D.P.; de Araujo, B.P.; Da Fonseca, M.d.J.M.; Diniz, M.d.F.H.S.; Daliry, A.; Griep, R.H. Advanced glycation end product: A potential biomarker for risk stratification of non-alcoholic fatty liver disease in ELSA-Brasil study. World J. Gastroenterol. 2021, 27, 4913–4928. [Google Scholar] [CrossRef]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Quím. 2007, 38, 207–213. [Google Scholar]
- Haskell, W.L.; Lee, I.M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulations 2007, 116, 1081. [Google Scholar] [CrossRef]
- Strath, S.J.; Kaminsky, L.A.; Ainsworth, B.E.; Ekelund, U.; Freedson, P.S.; Gary, R.A.; Richardson, C.R.; Smith, D.T.; Swartz, A.M.; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health and Cardiovascular, Exercise, Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology, and Council. Guide to the assessment of physical activity: Clinical and research applications: A scientific statement from the American Heart Association. Circulations 2013, 128, 2259–2279. [Google Scholar] [CrossRef]
- Havel, R.J.; Eder, H.A.; Bragdon, J.H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Investig. 1955, 34, 1345–1353. [Google Scholar] [CrossRef]
- Markwell, M.A.K.; Haas, S.M.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 87, 206–210. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- McPherson, J.D.; Shilton, B.H.; Walton, D.J. Role of fructose in glycation and cross-linking of proteins. Biochemistry 1988, 27, 1901–1907. [Google Scholar] [CrossRef]
- Noble, R.P. Electrophoretic separation of plasma lipoproteins in agarose gel. J. Lipid Res. 1968, 9, 693–700. [Google Scholar] [CrossRef]
- Mackness, M.; Mackness, B. Effect of dilution on high-density lipoprotein associated paraoxonase-1 activity. Clin. Biochem. 2011, 44, 1270–1271. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Nusslein, V.; Dahm, R. Zebrafish: A Practical Approach; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Na, H.-J. Anti-Inflammatory Activity of CIGB-258 against acute toxicity of carboxymethyllysine in paralyzed zebrafish via enhancement of high-density lipoproteins stability and functionality. Int. J. Mol. Sci. 2022, 23, 10130. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kim, J.-E.; Na, H.-J.; del Carmen Dominguez-Horta, M.; Martinez-Donato, G. CIGB-258 exerts potent anti-inflammatory activity against carboxymethyllysine-induced acute inflammation in hyperlipidemic zebrafish via the protection of apolipoprotein A-I. Int. J. Mol. Sci. 2023, 24, 7044. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Hayashi, M.; Sofuni, T.; Ishidate, M., Jr. An application of acridine orange fluorescent staining to the micronucleus test. Mutat. Res. Lett. 1983, 120, 241–247. [Google Scholar] [CrossRef]


| Groups | Week 0 | Week 12 | Δ Change (%) | p† | |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||||
| SBP (mmHg) | Male (n = 8) | 133.8 ± 6.8 | 119.1 ± 5.2 | −10.9 | 0.109 |
| Female (n = 9) | 122.8 ± 2.8 | 117.2 ± 3.5 | −4.5 | 0.233 | |
| Total (n = 17) | 127.9 ± 3.7 | 118.1 ± 3.0 | −7.7 | 0.046 | |
| DBP (mmHg) | Male (n = 8) | 85.8 ± 5.2 | 69.1 ± 3.9 | −19.4 | 0.022 |
| Female (n = 9) | 79.3 ± 2.1 | 74.2 ± 3.0 | −6.4 | 0.180 | |
| Total (n = 17) | 82.4 ± 2.7 | 71.8 ± 2.4 | −12.8 | 0.007 | |
| BMI (kg/m2) | Male (n = 8) | 31.0 ± 1.9 | 25.0 ± 2.7 | −19.3 | 0.092 |
| Female (n = 9) | 29.0 ± 1.4 | 26.0 ± 1.4 | −10.5 | 0.146 | |
| Total (n = 17) | 30.0 ± 1.1 | 25.5 ± 1.4 | −14.8 | 0.022 | |
| Weight (kg) | Male (n = 8) | 99.9 ± 6.4 | 85.2 ± 5.5 | −14.7 | 0.104 |
| Female (n = 9) | 79.0 ± 4.1 | 71.0 ± 4.2 | −10.2 | 0.190 | |
| Total (n = 17) | 88.8 ± 4.4 | 77.7 ± 3.7 | −12.5 | 0.064 | |
| Waist circumference (cm) | Male (n = 7) | 108.3 ± 3.8 | 89.7 ± 4.6 | −17.2 | 0.009 |
| Female (n = 9) | 95.8 ± 3.7 | 87.4 ± 4.9 | −8.8 | 0.193 | |
| Total (n = 16) | 101.2 ± 3.1 | 88.4 ± 3.3 | −12.7 | 0.008 | |
| Muscle mass (kg) | Male (n = 8) | 65.3 ± 3.2 | 64.1 ± 2.8 | −1.8 | 0.786 |
| Female (n = 9) | 45.2 ± 1.4 | 44.2 ± 1.4 | −2.2 | 0.625 | |
| Total (n = 17) | 54.6 ± 3.0 | 53.6 ± 2.9 | −2.0 | 0.797 | |
| Total fat mass (kg) | Male (n = 8) | 30.6 ± 3.4 | 17.1 ± 3.4 | −44.0 | 0.014 |
| Female (n = 9) | 31.0 ± 3.4 | 24.0 ± 3.4 | −22.6 | 0.163 | |
| Total (n = 17) | 30.8 ± 2.3 | 20.8 ± 2.5 | −32.6 | 0.006 | |
| Subcutaneous fat mass (kg) | Male (n = 8) | 28.9 ± 3.4 | 15.8 ± 3.4 | −45.4 | 0.017 |
| Female (n = 9) | 29.7 ± 3.3 | 22.9 ± 3.3 | −23.1 | 0.162 | |
| Total (n = 17) | 29.3 ± 2.3 | 19.5 ± 2.5 | −33.4 | 0.007 | |
| Visceral fat mass (kg) | Male (n = 8) | 1.7 ± 0.2 | 1.4 ± 0.2 | −20.4 | 0.229 |
| Female (n = 9) | 1.3 ± 0.1 | 1.2 ± 0.1 | −11.8 | 0.200 | |
| Total (n = 17) | 1.5 ± 0.1 | 1.3 ± 0.1 | −16.4 | 0.109 | |
| Body fat percentage (%) | Male (n = 8) | 30.1 ± 1.6 | 19.2 ± 2.8 | −36.3 | 0.005 |
| Female (n = 9) | 38.4 ± 2.8 | 32.7 ± 3.1 | −14.9 | 0.189 | |
| Total (n = 17) | 34.5 ± 1.9 | 26.3 ± 2.7 | −23.7 | 0.018 | |
| Body water content (kg) | Male (n = 8) | 50.8 ± 2.5 | 50.0 ± 2.2 | −1.6 | 0.803 |
| Female (n = 9) | 35.2 ± 1.1 | 34.4 ± 1.1 | −2.2 | 0.626 | |
| Total (n = 17) | 42.5 ± 2.3 | 41.7 ± 2.2 | −1.9 | 0.806 |
| Groups | Week 0 | Week 12 | Δ Change (%) | p† | |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||||
| TC (mg/dL) | Male (n = 8) | 267.7 ± 24.2 | 211.2 ± 12.5 | −21.1 | 0.056 |
| Female (n = 9) | 221.4 ± 8.2 | 195.3 ± 15.3 | −11.8 | 0.152 | |
| Total (n = 17) | 243.2 ± 13.1 | 202.8 ± 9.9 | −16.6 | 0.019 | |
| TG (mg/dL) | Male (n = 8) | 191.4 ± 34.8 | 84.1 ± 17.4 | −56.1 | 0.015 |
| Female (n = 9) | 102.2 ± 9.5 | 52.4 ± 6.5 | −48.7 | 0.001 | |
| Total (n = 17) | 144.2 ± 19.9 | 67.3 ± 9.4 | −53.3 | 0.002 | |
| RC (mg/dL) | Male (n = 8) | 36.2 ± 7.7 | 16.2 ± 3.4 | −55.4 | 0.032 |
| Female (n = 9) | 20.5 ± 1.9 | 12.7 ± 2.2 | −38.0 | 0.017 | |
| Total (n = 17) | 27.9 ± 4.1 | 14.3. ± 2.0 | −48.6 | 0.007 | |
| HDL-C (mg/dL) | Male (n = 8) | 43.8 ± 3.1 | 54.3 ± 4.7 | 23.9 | 0.084 |
| Female (n = 9) | 56.3 ± 3.5 | 58.8 ± 5.9 | 4.4 | 0.721 | |
| Total (n = 17) | 50.4 ± 2.8 | 56.6 ± 3.7 | 12.4 | 0.189 | |
| HDL-C/TC (%) | Male (n = 8) | 17.1 ± 1.7 | 26.2 ± 2.4 | 53.2 | 0.008 |
| Female (n = 9) | 25.7 ± 1.9 | 30.5 ± 2.3 | 18.6 | 0.126 | |
| Total (n = 17) | 21.6 ± 1.6 | 28.5 ± 1.7 | 31.5 | 0.007 | |
| TG/HDL-C (ratio) | Male (n = 8) | 4.7 ± 0.9 | 1.7 ± 0.4 | −63.2 | 0.014 |
| Female (n = 9) | 1.9 ± 0.2 | 1.0 ± 0.1 | −48.6 | 0.005 | |
| Total (n = 17) | 3.2 ± 0.5 | 1.3 ± 0.2 | −58.6 | 0.005 | |
| LDL-C (mg/dL) | Male (n = 8) | 188.0 ± 18.7 | 140.8 ± 10.3 | −25.1 | 0.044 |
| Female (n = 9) | 144.6 ± 8.3 | 123.7 ± 11.7 | −14.5 | 0.164 | |
| Total (n = 17) | 165.0 ± 10.9 | 131.7 ± 7.9 | −20.2 | 0.019 | |
| LDL-C/HDL-C (ratio) | Male (n = 8) | 4.5 ± 0.6 | 2.8 ± 0.4 | −38.2 | 0.029 |
| Female (n = 9) | 2.7 ± 0.3 | 2.2 ± 0.2 | −17.9 | 0.198 | |
| Total (n = 17) | 3.5 ± 0.4 | 2.5 ± 0.2 | −30.0 | 0.022 | |
| Serum CoQ10 (mol/L) | Male (n = 8) | 0.395 ± 0.032 | 0.547 ± 0.031 | 38.2 | 0.027 |
| Female (n = 9) | 0.551 ± 0.056 | 0.595 ± 0.05 | 7.9 | 0.566 | |
| Total (n = 17) | 0.473 ± 0.035 | 0.571 ± 0.029 | 20.6 | 0.090 | |
| CoQ10/TC (mol/mol) | Male (n = 8) | 59.4 ± 7.3 | 102.5 ± 11.1 | 72.6 | 0.007 |
| Female (n = 9) | 99.5 ± 17.0 | 127.3 ± 20.9 | 27.9 | 0.323 | |
| Total (n = 17) | 79.5 ± 10.5 | 114.9 ± 11.9 | 44.5 | 0.034 | |
| CoQ10/HDL-C (mol/mol) | Male (n = 8) | 369.7 ± 75.0 | 395.8 ± 39.7 | 7.1 | 0.764 |
| Female (n = 9) | 400.5 ± 92.2 | 415.5 ± 75.3 | 3.7 | 0.902 | |
| Total (n = 17) | 385.1 ± 57.3 | 405.6 ± 41.0 | 5.3 | 0.773 | |
| CoQ10/LDL-C (mol/mol) | Male (n = 8) | 85.9 ± 12.5 | 156.3 ± 19.4 | 82.0 | 0.010 |
| Female (n = 9) | 155.1 ± 25.1 | 207.5 ± 34.2 | 33.8 | 0.240 | |
| Total (n = 17) | 120.5 ± 16.6 | 181.9 ± 20.2 | 51.0 | 0.026 |
| Groups | Week 0 | Week 12 | Δ Change (%) | p† | |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||||
| AST (IU/L) | Male (n = 8) | 33.5 ± 5.1 | 27.5 ± 2.2 | −17.9 | 0.306 |
| Female (n = 9) | 23.7 ± 2.8 | 27.4 ± 2.8 | 16.0 | 0.352 | |
| Total (n = 17) | 28.3 ± 3.0 | 27.5 ± 1.7 | −2.9 | 0.813 | |
| ALT (IU/L) | Male (n = 8) | 37.8 ± 6.4 | 25.3 ± 2.8 | −33.1 | 0.103 |
| Female (n = 9) | 29.2 ± 8.3 | 28.6 ± 7.0 | −2.3 | 0.952 | |
| Total (n = 17) | 33.2 ± 5.3 | 27.0 ± 3.8 | −18.8 | 0.346 | |
| γ-GTP (IU/L) | Male (n = 8) | 48.0 ± 11.6 | 19.9 ± 2.4 | −58.6 | 0.046 |
| Female (n = 9) | 30.4 ± 6.9 | 15.6 ± 1.7 | −48.9 | 0.065 | |
| Total (n = 17) | 38.7 ± 6.7 | 17.6 ± 1.5 | −54.6 | 0.007 | |
| hsCRP (mg/L) | Male (n = 8) | 2.7 ± 1.1 | 3.4 ± 2.3 | 28.5 | 0.772 |
| Female (n = 9) | 2.0 ± 0.5 | 2.0 ± 0.7 | −0.6 | 0.989 | |
| Total (n = 17) | 2.3 ± 0.6 | 2.7 ± 1.1 | 15.2 | 0.781 | |
| apoA-I (mg/dL) | Male (n = 8) | 144.5 ± 13.6 | 132.6 ± 8.7 | −8.2 | 0.473 |
| Female (n = 9) | 140.9 ± 7.8 | 154.8 ± 10.4 | 9.9 | 0.303 | |
| Total (n = 17) | 142.6 ± 7.4 | 144.4 ± 7.2 | 1.2 | 0.865 | |
| apo-B (mg/dL) | Male (n = 8) | 140.8 ± 16.9 | 105.0 ± 7.2 | −25.4 | 0.072 |
| Female (n = 9) | 89.9 ± 6.3 | 95.1 ± 7.3 | 5.8 | 0.596 | |
| Total (n = 17) | 113.8 ± 10.5 | 99.8 ± 5.1 | −12.4 | 0.237 | |
| apo-B/apoA-I | Male (n = 8) | 1.0 ± 0.1 | 0.8 ± 0.1 | −19.9 | 0.195 |
| Female (n = 9) | 0.7 ± 0.1 | 0.6 ± 0.1 | −4.6 | 0.793 | |
| Total (n = 17) | 0.8 ± 0.1 | 0.7 ± 0.0 | −13.4 | 0.277 | |
| Glucose (mg/dL) | Male (n = 8) | 105.1 ± 5.7 | 97.3 ± 5.0 | −7.5 | 0.320 |
| Female (n = 9) | 90.3 ± 3.7 | 89.2 ± 4.4 | −1.2 | 0.849 | |
| Total (n = 17) | 97.3 ± 3.7 | 93.0 ± 3.4 | −4.4 | 0.398 | |
| Creatinine (mg/dL) | Male (n = 8) | 1.1 ± 0.0 | 1.1 ± 0.1 | −3.0 | 0.619 |
| Female (n = 9) | 1.0 ± 0.1 | 1.0 ± 0.0 | −2.2 | 0.804 | |
| Total (n = 17) | 1.1 ± 0.0 | 1.0 ± 0.0 | −2.6 | 0.627 | |
| e-GRF (mL/min/1.73 m2) | Male (n = 8) | 76.7 ± 2.9 | 80.0 ± 3.8 | 4.3 | 0.505 |
| Female (n = 9) | 72.6 ± 4.2 | 70.1 ± 4.3 | −3.4 | 0.689 | |
| Total (n = 17) | 74.4 ± 2.7 | 74.4 ± 3.1 | 0.1 | 0.988 |
| Groups | Week 0 | Week 12 | Δ Change (%) | p† | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||||
| VLDL | FI (Glycated) | Male (n = 8) | 7131 ± 910 | 4431 ± 374 | −37.9 | 0.052 |
| Female (n = 9) | 9311 ± 2943 | 5085 ± 782 | −45.4 | 0.214 | ||
| All (n = 17) | 8221 ± 1484 | 4758 ± 420 | −42.1 | 0.041 | ||
| MDA (μM) | Male (n = 8) | 27.1 ± 4.8 | 15.0 ± 1.9 | −44.6 | 0.080 | |
| Female (n = 9) | 18.0 ± 6.5 | 10.4 ± 3.4 | −42.4 | 0.339 | ||
| All (n = 17) | 22.6 ± 4.1 | 12.7 ± 2.0 | −43.7 | 0.050 | ||
| Diameter (nm) | Male (n = 8) | 37.6 ± 0.4 | 39.4 ± 2.8 | 4.8 | 0.588 | |
| Female (n = 9) | 38.8 ± 1.0 | 38.5 ± 2.6 | −0.8 | 0.918 | ||
| Total (n = 17) | 38.1 ± 0.5 | 38.9 ± 1.7 | 2.2 | 0.659 | ||
| TC (μg/mg of protein) | Male (n = 8) | 59.3 ± 10.1 | 53.1 ± 4.5 | −10.5 | 0.582 | |
| Female (n = 9) | 66.6 ± 7.3 | 41.2 ± 3.4 | −38.2 | 0.009 | ||
| Total (n = 17) | 63.2 ± 6.0 | 46.8 ± 3.1 | −26.0 | 0.023 | ||
| TG (μg/mg of protein) | Male (n = 8) | 120.6 ± 19.4 | 74.0 ± 15.3 | −38.6 | 0.081 | |
| Female (n = 9) | 131.4 ± 16.4 | 68.4 ± 10.8 | −47.9 | 0.006 | ||
| Total (n = 17) | 126.3 ± 12.3 | 71.0 ± 8.9 | −43.7 | 0.001 | ||
| LDL | FI (Glycated) | Male (n = 8) | 5009 ± 241 | 4358 ± 143 | −13.0 | 0.040 |
| Female (n = 9) | 4907 ± 248 | 4138 ± 165 | −15.7 | 0.020 | ||
| Total (n = 17) | 4955 ± 168 | 4242 ± 110 | −14.4 | 0.001 | ||
| MDA (μM) | Male (n = 8) | 4.5 ± 0.1 | 3.0 ± 0.3 | −32.7 | 0.019 | |
| Female (n = 9) | 3.7 ± 0.4 | 3.4 ± 0.3 | −9.1 | 0.494 | ||
| All (n = 17) | 4.1 ± 0.2 | 3.2 ± 0.2 | −22.0 | 0.012 | ||
| Diameter (nm) | Male (n = 8) | 25.8 ± 0.7 | 27.4 ± 0.4 | 5.9 | 0.091 | |
| Female (n = 9) | 26.7 ± 0.8 | 25.8 ± 0.6 | −3.5 | 0.367 | ||
| Total (n = 17) | 26.3 ± 0.5 | 26.5 ± 0.4 | 0.9 | 0.740 | ||
| TC (μg/mg of protein) | Male (n = 8) | 139.8 ± 14.6 | 103.2 ± 5.0 | −26.2 | 0.043 | |
| Female (n = 9) | 150.1 ± 19.4 | 95.3 ± 4.7 | −36.5 | 0.023 | ||
| Total (n = 17) | 145.3 ± 12.1 | 99.0 ± 3.5 | −31.8 | 0.002 | ||
| TG (μg/mg of protein) | Male (n = 8) | 20.6 ± 2.1 | 12.2 ± 1.3 | −40.8 | 0.005 | |
| Female (n = 9) | 19.7 ± 2.6 | 10.8 ± 1.7 | −45.2 | 0.010 | ||
| Total (n = 17) | 20.1 ± 1.6 | 11.4 ± 1.1 | −43.1 | <0.001 |
| Groups | Week 0 | Week 12 | Δ Change % | p† | ||
|---|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | |||||
| HDL2 | FI (Glycated) | Male (n = 8) | 2234 ± 213 | 1837 ± 180 | −17.8 | 0.175 |
| Female (n = 9) | 1969 ± 166 | 1616 ± 153 | −17.9 | 0.138 | ||
| Total (n = 17) | 2094 ± 133 | 1720 ± 117 | −17.9 | 0.043 | ||
| Diameter (nm) | Male (n = 8) | 12.8 ± 0.4 | 13.5 ± 0.2 | 5.6 | 0.112 | |
| Female (n = 9) | 12.5 ± 0.3 | 13.2 ± 0.4 | 6.0 | 0.180 | ||
| Total (n = 17) | 12.6 ± 0.2 | 13.4 ± 0.2 | 5.8 | 0.038 | ||
| TC (μg/mg of protein) | Male (n = 8) | 84.7 ± 12.5 | 67.2 ± 4.8 | −20.6 | 0.225 | |
| Female (n = 9) | 68.7 ± 7.7 | 69.7 ± 3.6 | 1.4 | 0.910 | ||
| Total (n = 17) | 76.2 ± 7.2 | 68.5 ± 2.9 | −10.1 | 0.332 | ||
| TG (μg/mg of protein) | Male (n = 8) | 14.9 ± 2.8 | 7.5 ± 1.2 | −49.4 | 0.029 | |
| Female (n = 9) | 9.8 ± 0.9 | 6.7 ± 1.0 | −32.2 | 0.033 | ||
| Total (n = 17) | 12.2 ± 1.5 | 7.1 ± 0.8 | −42.1 | 0.004 | ||
| HDL3 | FI (Glycated) | Male (n = 8) | 1885 ± 208 | 1616 ± 159 | −14.3 | 0.322 |
| Female (n = 9) | 1891 ± 241 | 1578 ± 136 | −16.6 | 0.278 | ||
| Total (n = 17) | 1888 ± 156 | 1596 ± 101 | −15.5 | 0.126 | ||
| Diameter (nm) | Male (n = 8) | 9.8 ± 0.3 | 11.2 ± 0.4 | 14.7 | 0.012 | |
| Female (n = 9) | 9.5 ± 0.3 | 10.4 ± 0.4 | 9.5 | 0.070 | ||
| Total (n = 17) | 9.6 ± 0.2 | 10.8 ± 0.3 | 12.0 | 0.002 | ||
| TC (μg/mg of protein) | Male (n = 8) | 37.5 ± 1.3 | 50.0 ± 5.0 | 33.5 | 0.030 | |
| Female (n = 9) | 40.5 ± 1.2 | 52.8 ± 6.7 | 30.5 | 0.107 | ||
| Total (n = 17) | 39.1 ± 0.9 | 51.5 ± 4.2 | 31.8 | 0.009 | ||
| TG (μg/mg of protein) | Male (n = 8) | 6.6 ± 1.2 | 5.6 ± 0.7 | −15.6 | 0.469 | |
| Female (n = 9) | 4.8 ± 1.4 | 4.8 ± 0.7 | −0.2 | 1.000 | ||
| Total (n = 17) | 5.7 ± 0.9 | 5.2 ± 0.5 | −8.7 | 0.643 |
| Groups | At Week 0 | During 12 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | BMI | MET | Total Exercise | Strength Exercise | Aerobic Exercise | MET 2 | Physical Activity 3 | |
| Mean ± SEM | Mean ± SEM | Score/Day | Min/wk | Min/wk | Min/wk | Score/Day | MET × 6 Day × 12 Weeks | |
| Male (n = 8) | 36.9 ± 1.9 | 31.0 ± 1.9 | 1.3 ± 0.1 | 655 ± 97 | 286 ± 9 | 390 ± 103 | 7.0 ± 0.3 | 504 ± 21 |
| Female (n = 9) | 38.4 ± 2.7 | 29.0 ± 1.4 | 1.1 ± 0.0 | 714 ± 95 | 194 ± 31 | 520 ± 84 | 7.4 ± 0.1 | 533 ± 11 |
| Total (n = 17) | 37.7 ± 1.6 | 30.1 ± 1.1 | 1.2 ± 0.1 | 687 ± 66 | 234 ± 21 | 459 ± 66 | 7.2 ± 0.1 | 518 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Nam, H.-S.; Kim, N.-Y.; Lee, M.-S.; Kang, D.-J. Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants. Pharmaceuticals 2024, 17, 132. https://doi.org/10.3390/ph17010132
Cho K-H, Nam H-S, Kim N-Y, Lee M-S, Kang D-J. Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants. Pharmaceuticals. 2024; 17(1):132. https://doi.org/10.3390/ph17010132
Chicago/Turabian StyleCho, Kyung-Hyun, Hyo-Seon Nam, Na-Young Kim, Myeong-Sung Lee, and Dae-Jin Kang. 2024. "Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants" Pharmaceuticals 17, no. 1: 132. https://doi.org/10.3390/ph17010132
APA StyleCho, K.-H., Nam, H.-S., Kim, N.-Y., Lee, M.-S., & Kang, D.-J. (2024). Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants. Pharmaceuticals, 17(1), 132. https://doi.org/10.3390/ph17010132








