Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol
Abstract
1. Introduction
2. Results
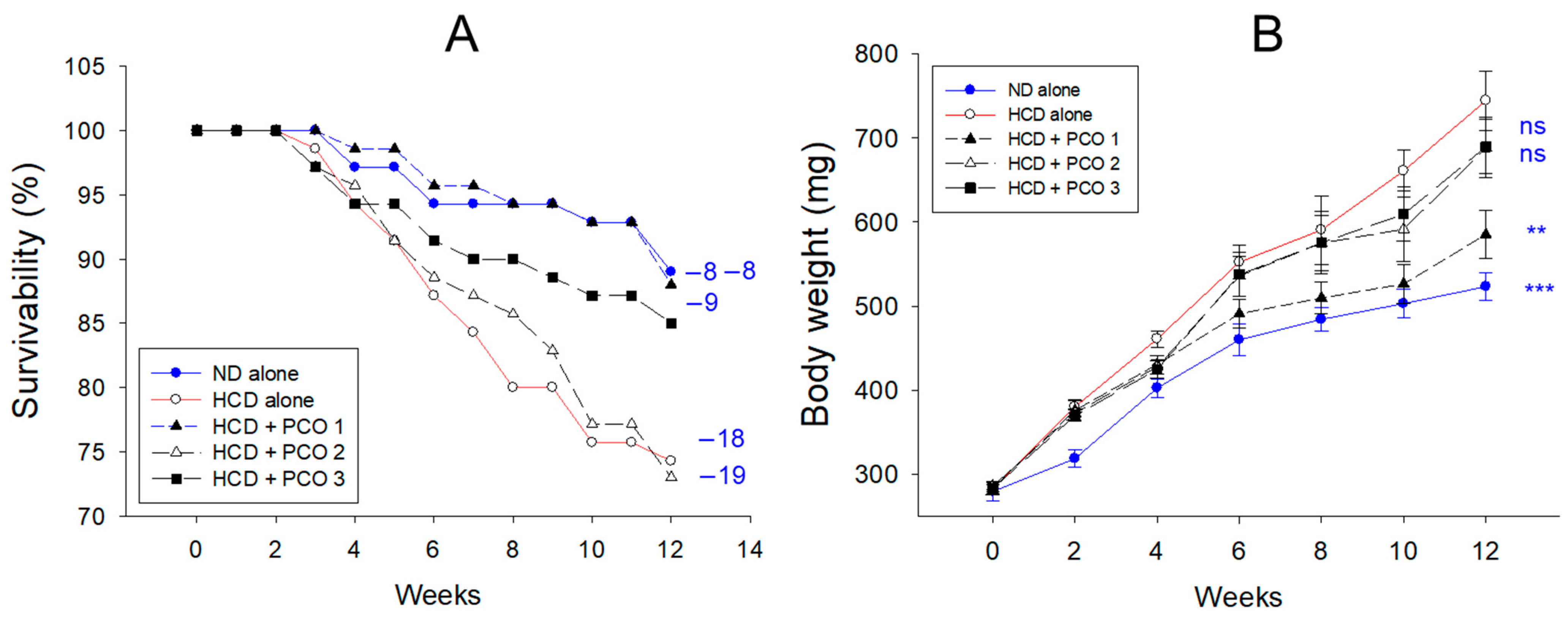
2.1. Change in the Mortality and Body Weight
2.2. Changes in Blood Lipid Profiles
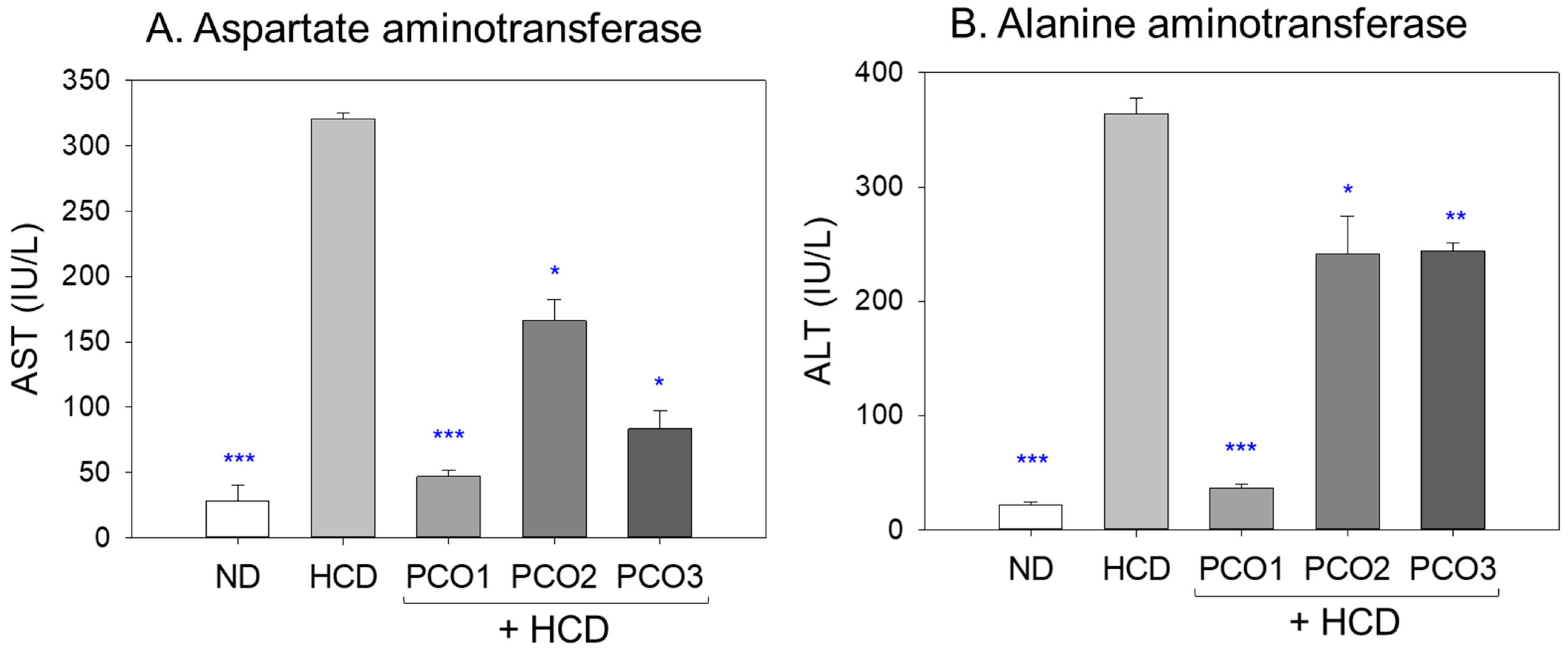
2.3. Liver Function Parameters
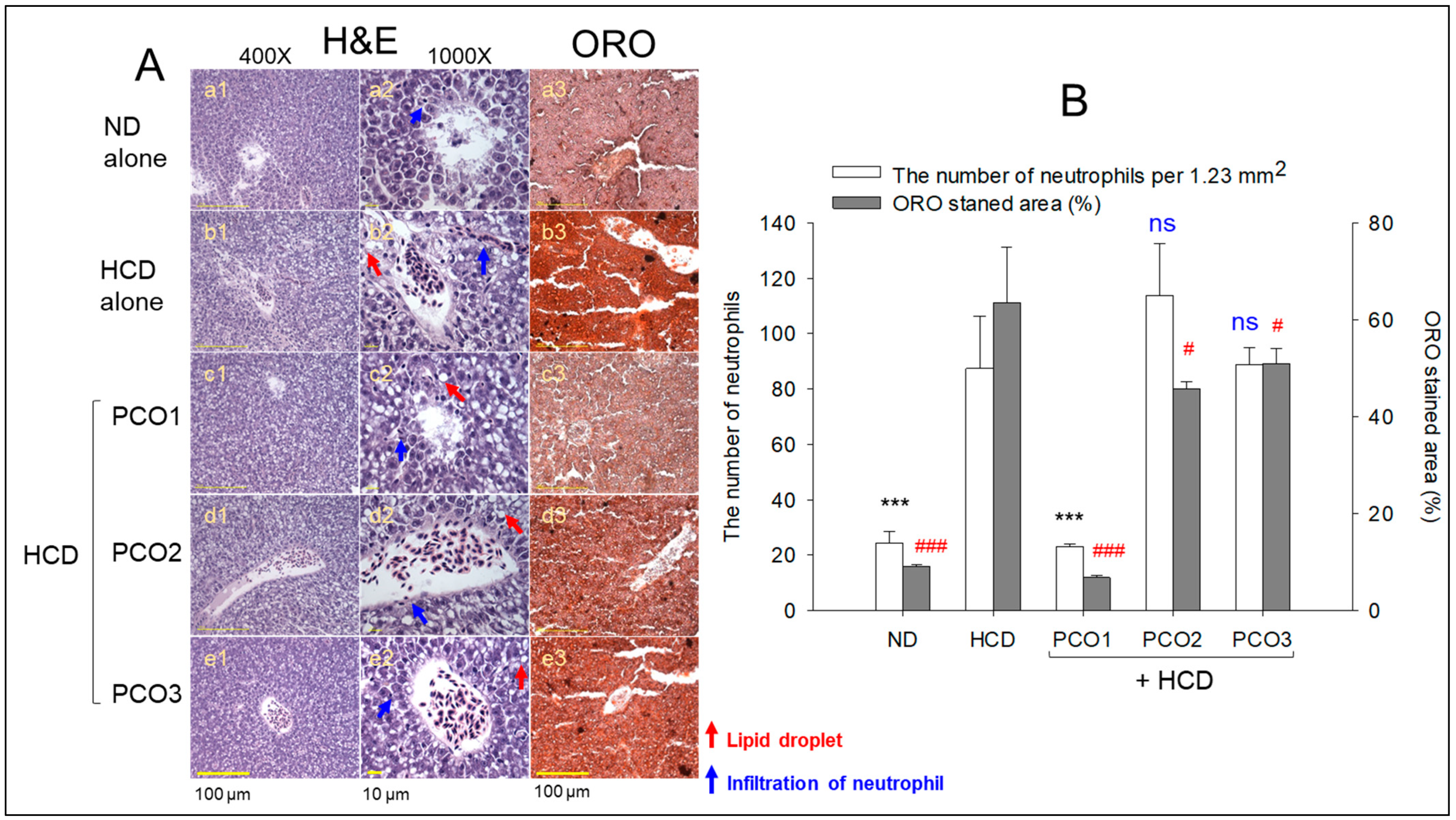
2.4. Analysis of the Liver Tissue (H&E Staining and Oil-Red O Staining)
2.5. Interleukin-6 and ROS Production
2.6. Kidney Tissue Analysis
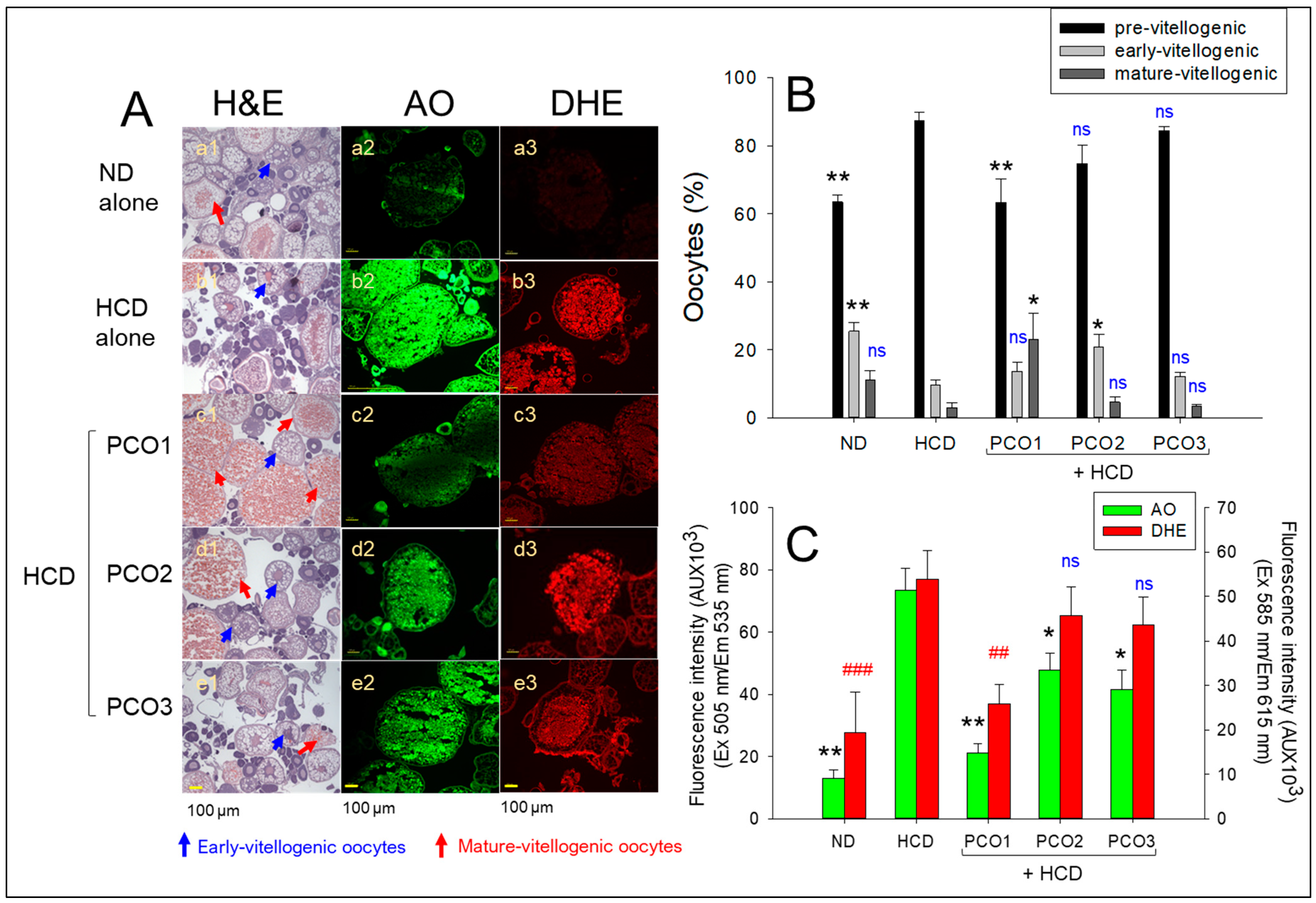
2.7. Ovarian Tissue Analysis
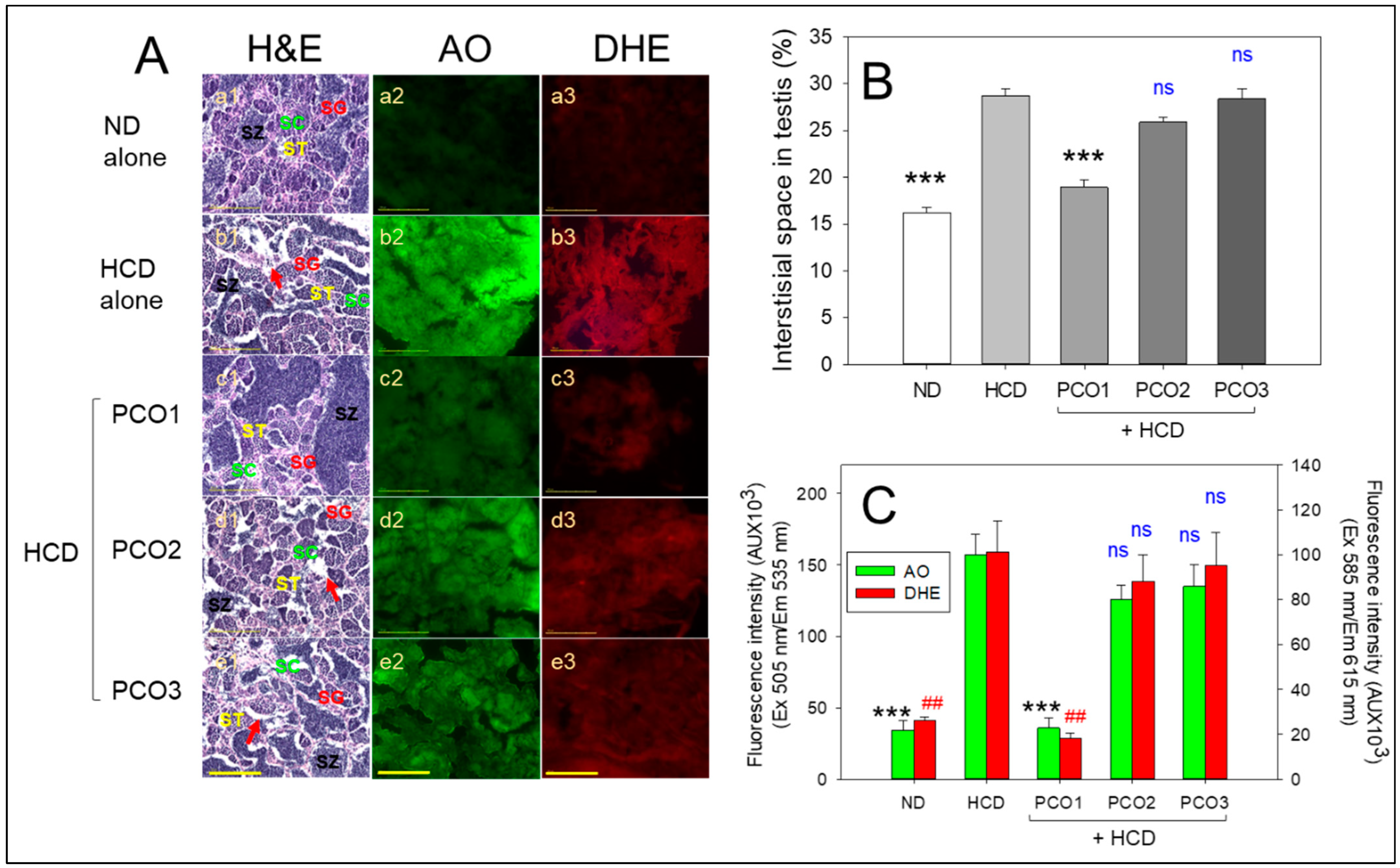
2.8. Analysis of Testicular Cell
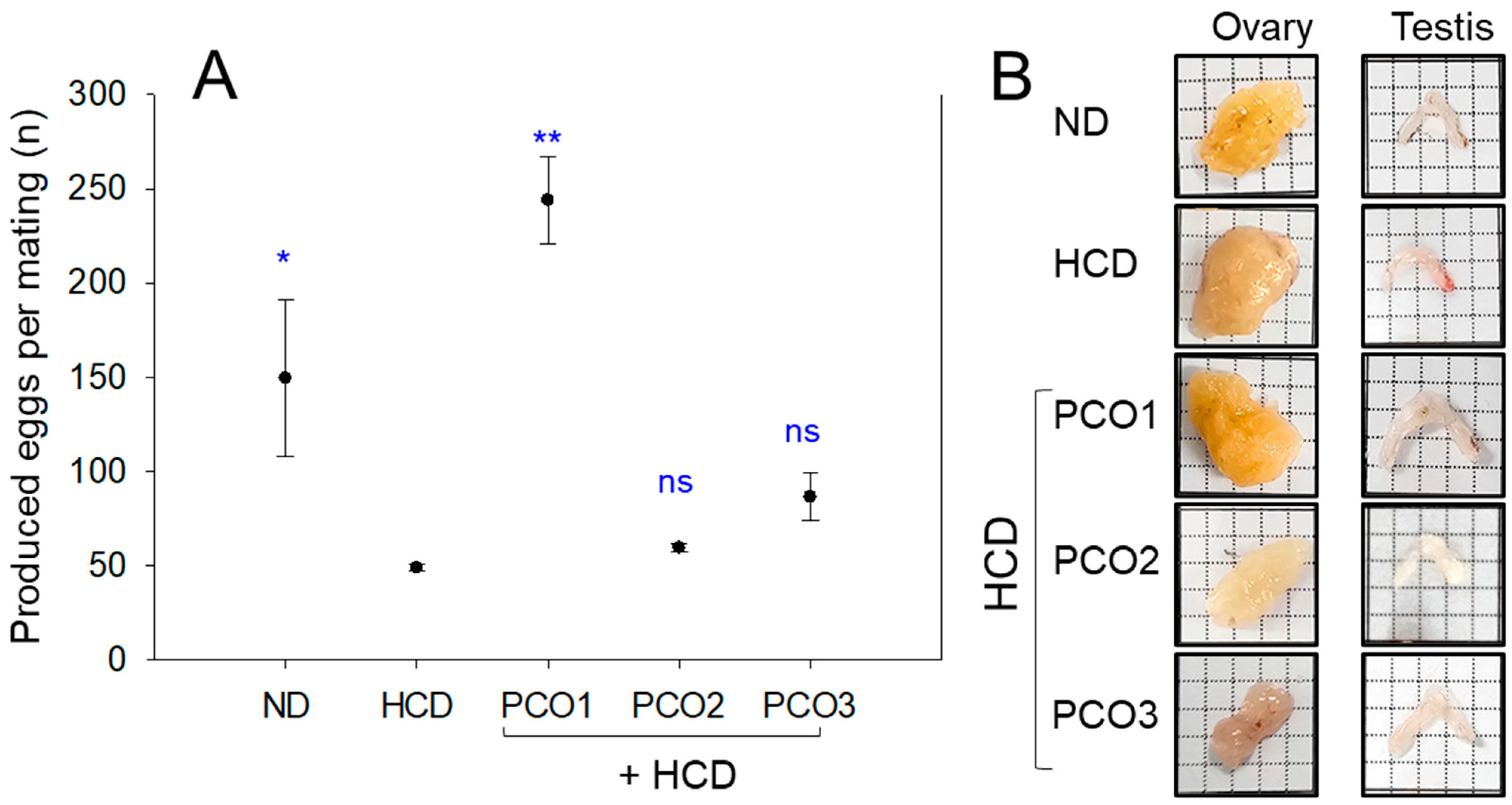
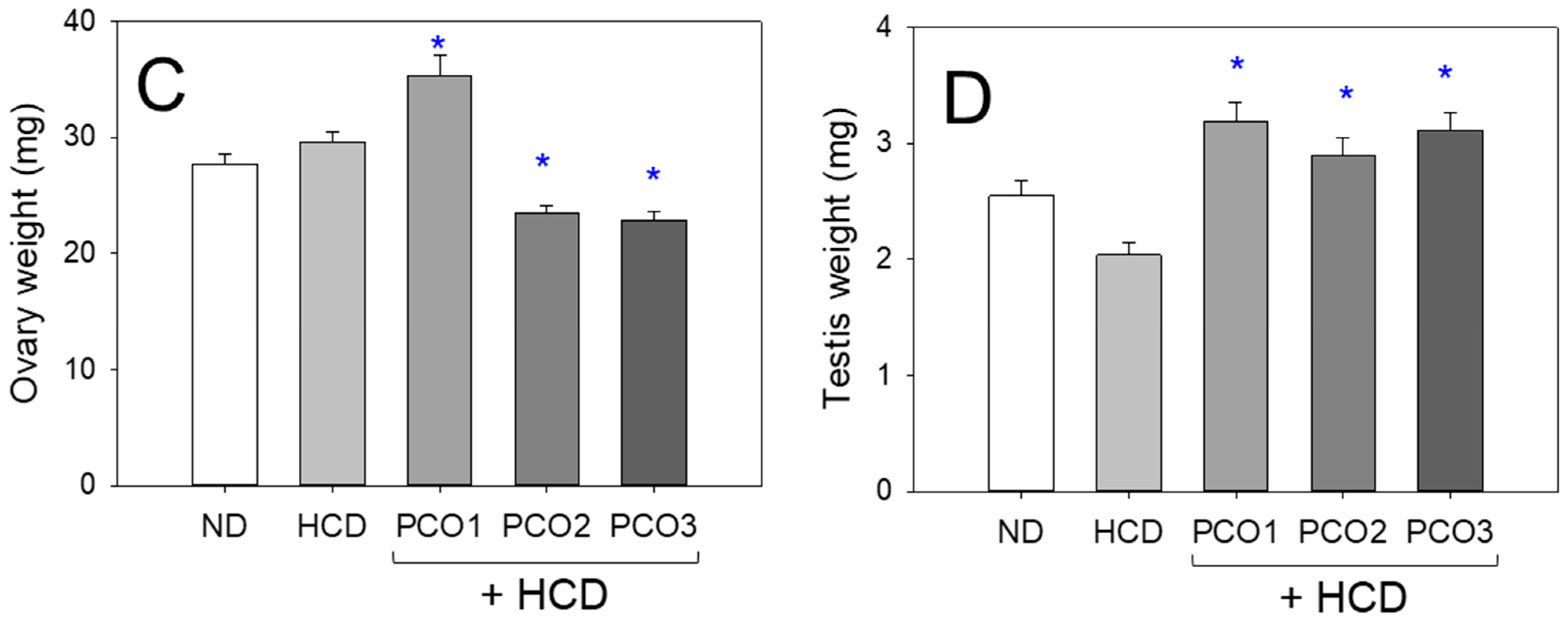
2.9. Embryo Production Ability and Size of the Ovary and Testis
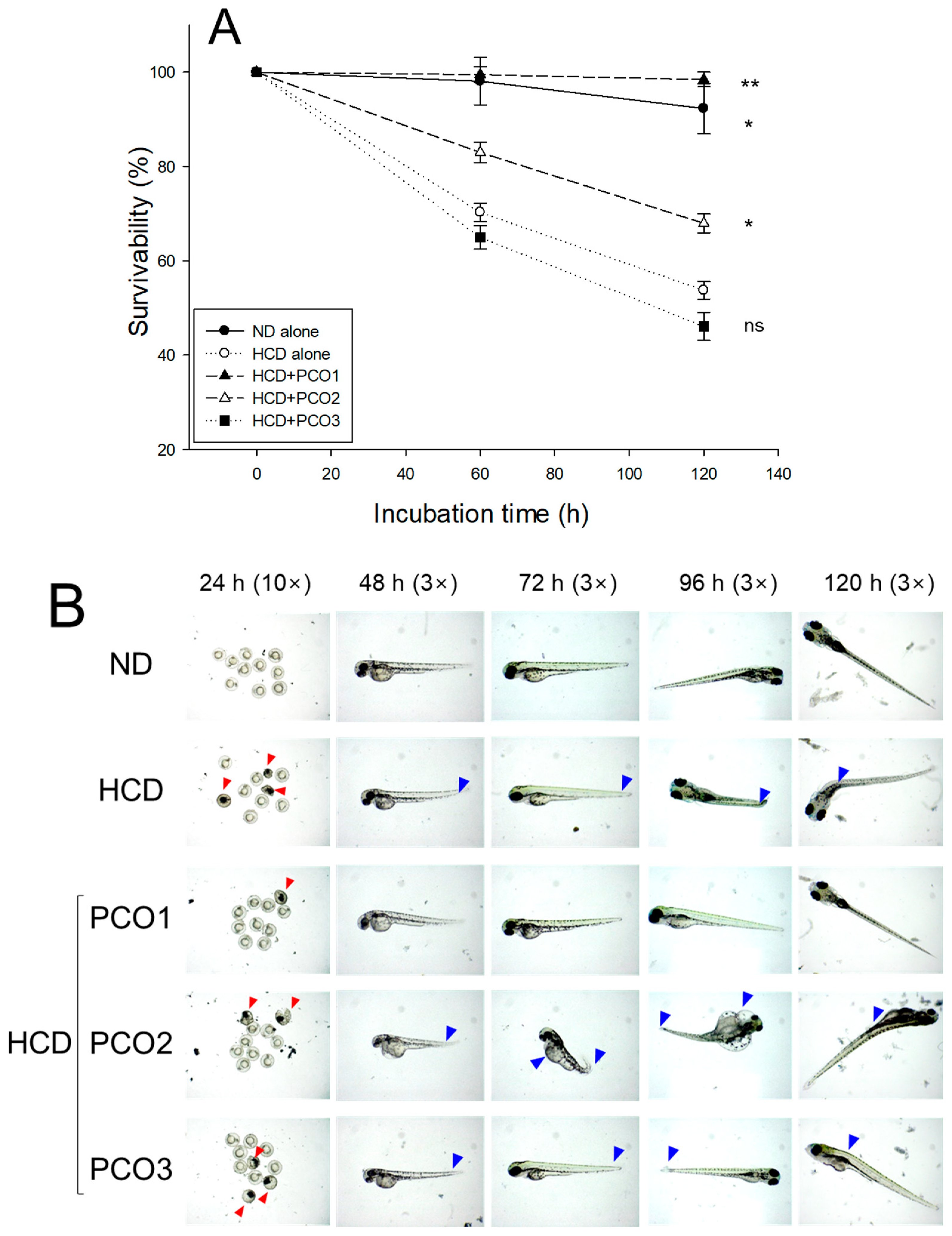
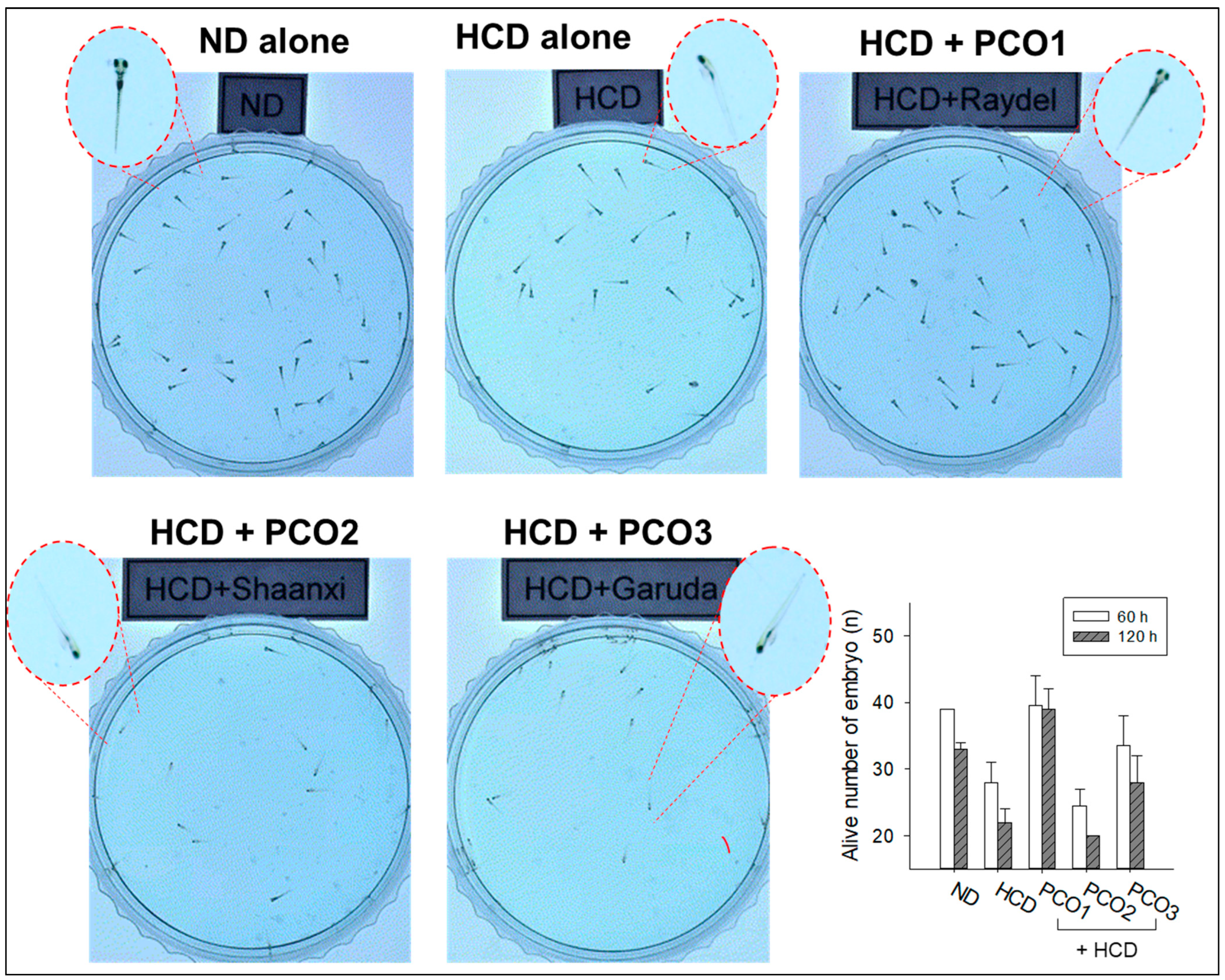
2.10. Embryo Survivability and Developmental Morphology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Maintenace of Zebrafish and Policosanol Supplementation
4.3. Blood Collection and Analysis
4.4. Examination of Liver Tissue
4.5. Histological Examination of Kidney
4.6. Analysis of Ovarian Tissue
4.7. Analysis of Testis Tissue
4.8. Mating and Embryo Production
4.9. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milman, S.; Atzmon, G.; Crandall, J.; Barzilai, N. Phenotypes and genotypes of high density lipoprotein cholesterol in exceptional longevity. Curr. Vasc. Pharmacol. 2013, 12, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Zou, Y.; Tang, J.; Cai, J.; Wei, Y.; Qin, J.; Zhang, Z. Positive association of familial longevity with the moderate-high HDL-C concentration in Bama Aging Study. Aging 2018, 10, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Tamayo, A.R.; Peña-Veites, O.M.; Hernández-González, M.A.; Barrientos-Alvarado, C. Decreased HDL-C Levels as a Predictor of Organ Failure in Acute Pancreatitis in the Emergency Department. Life 2023, 13, 1602. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Impact of High-Density Lipoproteins on Sepsis. Int. J. Mol. Sci. 2022, 23, 12965. [Google Scholar] [CrossRef]
- Arias, A.; Quiroz, A.; Santander, N.; Morselli, E.; Busso, D. Implications of High-Density Cholesterol Metabolism for Oocyte Biology and Female Fertility. Front. Cell Dev. Biol. 2022, 10, 941539. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Sikka, S.C.; Gur, S. A Comprehensive Review of Metabolic Syndrome Affecting Erectile Dysfunction. J. Sex. Med. 2015, 12, 856–875. [Google Scholar] [CrossRef]
- Crudele, L.; De Matteis, C.; Piccinin, E.; Gadaleta, R.M.; Cariello, M.; Di Buduo, E.; Piazzolla, G.; Suppressa, P.; Berardi, E.; Sabbà, C.; et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. 2022, 5, 100627. [Google Scholar] [CrossRef]
- Idzior-Waluś, B.; Trojak, A.; Waluś-Miarka, M.; Woźniakiewicz, E.; Małecki, M.T. Nonalcoholic fatty liver disease is associated with low HDL cholesterol and coronary angioplasty in patients with type 2 diabetes. Med. Sci. Monit. 2013, 19, 1167–1172. [Google Scholar] [CrossRef][Green Version]
- You, A.; Li, Y.; Tomlinson, B.; Yue, L.; Zhao, K.; Fan, H.; Liu, Z.; Zhang, Y.; Zheng, L. Association Between Renal Dysfunction and Low HDL Cholesterol Among the Elderly in China. Front. Cardiovasc. Med. 2021, 8, 644208. [Google Scholar] [CrossRef]
- Osadnik, T.; Goławski, M.; Lewandowski, P.; Morze, J.; Osadnik, K.; Pawlas, N.; Lejawa, M.; Jakubiak, G.K.; Mazur, A.; Schwingschackl, L.; et al. A network meta-analysis on the comparative effect of nutraceuticals on lipid profile in adults. Pharmacol. Res. 2022, 183, 106402. [Google Scholar] [CrossRef]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; Symonds, M.E.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef]
- Arruzazabala, M.; Carbajal, D.; Mas, R.; Garcia, M.; Fraga, V. Effects of policosanol on platelet aggregation in rats. Thromb. Res. 1993, 69, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ishaka, A.; Imam, M.U.; Ismail, M. Nanoemulsification of Rice Bran Wax Policosanol Enhances Its Cardio-protective Effects via Modulation of Hepatic Peroxisome Proliferator-activated Receptor gamma in Hyperlipidemic Rats. J. Oleo Sci. 2020, 69, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, C.; Sun, L.; He, Z.; Feng, Y.; Li, X.; Gan, J.; Chen, X. Effect of policosanol from insect wax on amyloid β-peptide-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. BMC Complement. Med. Ther. 2021, 21, 103. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Kang, D.-J.; Baek, S.-H. Comparison of Policosanols via Incorporation into Reconstituted High-Density Lipoproteins: Cuban Policosanol (Raydel®) Exerts the Highest Antioxidant, Anti-Glycation, and Anti-Inflammatory Activity. Molecules 2023, 28, 6715. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Policosanol: Clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am. Heart J. 2002, 143, 356–365. [Google Scholar] [CrossRef]
- Varady, K.A.; Wang, Y.; Jones, P.J. Role of Policosanols in the Prevention and Treatment of Cardiovascular Disease. Nutr. Rev. 2003, 61, 376–383. [Google Scholar] [CrossRef]
- Dullens, S.P.J.; Mensink, R.P.; Bragt, M.C.E.; Kies, A.K.; Plat, J. Effects of emulsified policosanols with different chain lengths on cholesterol metabolism in heterozygous LDL receptor-deficient mice. J. Lipid Res. 2008, 49, 790–796. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-E.; Baek, S.H. Cuban Policosanol (Raydel®) Potently Protects the Liver, Ovary, and Testis with an Improvement in Dyslipidemia in Hyperlipidemic Zebrafish: A Comparative Study with Three Chinese Policosanols. Molecules 2023, 28, 6609. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-Term Consumption of Cuban Policosanol Lowers Aortic and Peripheral Blood Pressure and Ameliorates Serum Lipid Parameters in Healthy Korean Participants: Randomized, Double-Blinded, and Placebo-Controlled Study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.; Lee, J.; Kim, Y. Comparison of Phytochemical Contents and Cytoprotective Effects of Different Rice Bran Extracts from Indica and Japonica Rice Cultivars. Prev. Nutr. Food Sci. 2020, 25, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kaup, R.M.; Khayyal, M.T.; Verspohl, E.J. Antidiabetic Effects of a Standardized Egyptian Rice Bran Extract. Phytother. Res. 2012, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-T.; Ismail, M.; Tohit, E.R.M.; Abdullah, R.; Zhang, Y.-D. Attenuation of Thrombosis by Crude Rice (Oryza sativa) Bran Policosanol Extract: Ex Vivo Platelet Aggregation and Serum Levels of Arachidonic Acid Metabolites. Evid. Based Complement. Altern. Med. 2016, 2016, 7343942. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A. Zebrafish Models for Dyslipidemia and Atherosclerosis Research. Front. Endocrinol. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Ka, J.; Jin, S.-W. Zebrafish as an Emerging Model for Dyslipidemia and Associated Diseases. J. Lipid Atheroscler. 2021, 10, 42–56. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Juan-García, A.; Bind, M.-A.; Engert, F. Larval zebrafish as an in vitro model for evaluating toxicological effects of mycotoxins. Ecotoxicol. Environ. Saf. 2020, 202, 110909. [Google Scholar] [CrossRef]
- Gupta, H.R.; Patil, Y.; Singh, D.; Thakur, M. Embryonic Zebrafish Model—A Well-Established Method for Rapidly Assessing the Toxicity of Homeopathic Drugs–Toxicity Evaluation of Homeopathic Drugs Using Zebrafish Embryo Model. J. Pharmacopunct. 2016, 19, 319–328. [Google Scholar] [CrossRef]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar] [CrossRef]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y.; et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2017, 62, 1700280. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, L.K.; Jimoh, A.O.; Tukur, U.M.; Imam, M.U. A Review of the Effects of Policosanol on Metabolic Syndrome. Clin. Complement. Med. Pharmacol. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Silvestris, E.; Lovero, D.; Palmirotta, R. Nutrition and Female Fertility: An Interdependent Correlation. Front. Endocrinol. 2019, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.; Whitfield, M.; Pollet-Villard, X.; Levy, R.; Saez, F. Posttesticular sperm maturation, infertility, and hypercholesterolemia. Asian J. Androl. 2015, 17, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Lainez, N.M.; Coss, D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019, 160, 2719–2736. [Google Scholar] [CrossRef]
- Gao, Y.; Zou, Y.; Wu, G.; Zheng, L. Oxidative stress and mitochondrial dysfunction of granulosa cells in polycystic ovarian syndrome. Front. Med. 2023, 10, 1193749. [Google Scholar] [CrossRef]
- Shi, J.F.; Li, Y.K.; Ren, K.; Xie, Y.J.; Yin, W.D.; Mo, Z.C. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Mol. Med. Rep. 2018, 17, 705–713. [Google Scholar] [CrossRef]
- Li, M.; Ma, Z.; Zhang, X.; Guo, L.; Yuan, M. Significance of blood lipid parameters as effective markers for arteriogenic erectile dysfunction. Andrology 2020, 8, 1086–1094. [Google Scholar] [CrossRef]
- Fofana, M.; Maboundou, J.-C.; Bocquet, J.; Le Goff, D. Transfer of cholesterol between high density lipoproteins and cultured rat Sertoli cells. Biochem. Cell Biol. 1996, 74, 681–686. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R. Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women. Med. Sci. 2021, 9, 35. [Google Scholar] [CrossRef]
- Yannas, D.; Frizza, F.; Vignozzi, L.; Corona, G.; Maggi, M.; Rastrelli, G. Erectile Dysfunction Is a Hallmark of Cardiovascular Disease: Unavoidable Matter of Fact or Opportunity to Improve Men’s Health? J. Clin. Med. 2021, 10, 2221. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, W. HDL and Sepsis. Adv. Exp. Med. Biol. 2022, 1377, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-Aging and Tissue Regeneration Ability of Policosanol Along with Lipid-Lowering Effect in Hyperlipidemic Zebrafish via Enhancement of High-Density Lipoprotein Functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Yadav, D.; Kim, S.-J.; Kim, J.-R. Blood Pressure Lowering Effect of Cuban Policosanol is Accompanied by Improvement of Hepatic Inflammation, Lipoprotein Profile, and HDL Quality in Spontaneously Hypertensive Rats. Molecules 2018, 23, 1080. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Komatsu, T.; Uehara, Y. Protection of Liver Functions and Improvement of Kidney Functions by Twelve Weeks Consumption of Cuban Policosanol (Raydel®) with a Decrease of Glycated Hemoglobin and Blood Pressure from a Randomized, Placebo-Controlled, and Double-Blinded Study with Healthy and Middle-Aged Japanese Participants. Life 2023, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Nam, H.-S.; Baek, S.-H.; Kang, D.-J.; Na, H.; Komatsu, T.; Uehara, Y. Beneficial Effect of Cuban Policosanol on Blood Pressure and Serum Lipoproteins Accompanied with Lowered Glycated Hemoglobin and Enhanced High-Density Lipoprotein Functionalities in a Randomized, Placebo-Controlled, and Double-Blinded Trial with Healthy Japanese. Int. J. Mol. Sci. 2023, 24, 5185. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Dahm, R. Zebrafish; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Veter. Res. 2020, 16, 242. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Gewiese-Rabsch, J.; Drucker, C.; Malchow, S.; Scheller, J.; Rose-John, S. Role of IL-6 trans-signaling in CCl4 induced liver damage. Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 1054–1061. [Google Scholar] [CrossRef]
- Hayashi, M.; Sofuni, T.; Ishidate, M. An application of Acridine Orange fluorescent staining to the micronucleus test. Mutat. Res. Lett. 1983, 120, 241–247. [Google Scholar] [CrossRef]
- Patel, U.N.; Patel, U.D.; Khadayata, A.V.; Vaja, R.K.; Modi, C.M.; Patel, H.B. Long-term exposure of the binary mixture of cadmium and mercury damages the developed ovary of adult zebrafish. Environ. Sci. Pollut. Res. 2022, 29, 44928–44938. [Google Scholar] [CrossRef] [PubMed]
- Sutha, J.; Anila, P.A.; Gayathri, M.; Ramesh, M. Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): In vivo and computational analysis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109263. [Google Scholar] [CrossRef] [PubMed]
- Sabaliauskas, N.A.; Foutz, C.A.; Mest, J.R.; Budgeon, L.R.; Sidor, A.T.; Gershenson, J.A.; Joshi, S.B.; Cheng, K.C. High-throughput zebrafish histology. Methods 2006, 39, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Taib, I.S.; Budin, S.B.; Ghazali, A.R.; Jayusman, P.A.; Louis, S.R.; Mohamed, J. Fenitrothion induced oxidative stress and morphological alterations of sperm and testes in male sprague-dawley rats. Clinics 2013, 68, 93–100. [Google Scholar] [CrossRef]
- Koc, N.D.; Teksöz, N.; Ural, M.; Akbulut, C. Histological structure of zebrafish (Danio rerio, Hamilton, 1822) testicles. Elixir Aquac. 2012, 46, 8117–8120. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; Section 2; Organization for Economic Co-Operation and Development (OECD): Paris, France, 2013; ISBN 9789264016194. [Google Scholar]



| ND | HCD | |||||
|---|---|---|---|---|---|---|
| Cohorts (n = 70 in each group) | Control (n = 70) | Control (n = 70) | PCO1 Cuban CNIC Sugarcane Raydel® (n = 70) | PCO2 Chinese Shaanxi Rice bran 400 (n = 70) | PCO3 American Garuda Sugarcane Lesstanol® (n = 70) | |
| Diet composition (%) | Tetrabits 1 | 100 | 96 | 95.9 | 95.9 | 95.9 |
| Cholesterol (%, w/w) | - | 4 | 4 | 4 | 4 | |
| PCO (%, w/w) | - | - | 0.1 | 0.1 | 0.1 | |
| Octacosanol content within in PCO (mg/g) 2 | - | - | 692 (70.5%) 3 | 56 (7.6%) | 546 (60.7%) | |
| Final total PCO amount (mg) 2 | 982 | 739 | 900 | |||
| Total PCO amount on label 4 | 982 | 400 | 900 | |||
| Body weight at week 0 | 279 ± 11 | 282 ± 5 | 284 ± 7 | 285 ± 6 | 283 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Baek, S.-H.; Bahuguna, A. Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol. Pharmaceuticals 2024, 17, 66. https://doi.org/10.3390/ph17010066
Cho K-H, Kim J-E, Nam H-S, Baek S-H, Bahuguna A. Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol. Pharmaceuticals. 2024; 17(1):66. https://doi.org/10.3390/ph17010066
Chicago/Turabian StyleCho, Kyung-Hyun, Ji-Eun Kim, Hyo-Seon Nam, Seung-Hee Baek, and Ashutosh Bahuguna. 2024. "Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol" Pharmaceuticals 17, no. 1: 66. https://doi.org/10.3390/ph17010066
APA StyleCho, K.-H., Kim, J.-E., Nam, H.-S., Baek, S.-H., & Bahuguna, A. (2024). Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol. Pharmaceuticals, 17(1), 66. https://doi.org/10.3390/ph17010066








