Abstract
Stilbenes are phytoalexins, and their biosynthesis can occur through a natural route (shikimate precursor) or an alternative route (in microorganism cultures). The latter is a metabolic engineering strategy to enhance production due to stilbenes recognized pharmacological and medicinal potential. It is believed that in the human body, these potential activities can be modulated by the regulation of the nuclear factor erythroid derived 2 (Nrf2), which increases the expression of antioxidant enzymes. Given this, our review aims to critically analyze evidence regarding E-stilbenes in human metabolism and the Nrf2 activation pathway, with an emphasis on inflammatory and oxidative stress aspects related to the pathophysiology of chronic and metabolic diseases. In this comprehensive literature review, it can be observed that despite the broad number of stilbenes, those most frequently explored in clinical trials and preclinical studies (in vitro and in vivo) were resveratrol, piceatannol, pterostilbene, polydatin, stilbestrol, and pinosylvin. In some cases, depending on the dose/concentration and chemical nature of the stilbene, it was possible to identify activation of the Nrf2 pathway. Furthermore, the use of some experimental models presented a challenge in comparing results. In view of the above, it can be suggested that E-stilbenes have a relationship with the Nrf2 pathway, whether directly or indirectly, through different biological pathways, and in different diseases or conditions that are mainly related to inflammation and oxidative stress.
1. Introduction
Stilbenes account for a vast group of polyphenols characterized by a 1,2-diphenylethylene (C6–C2–C6) skeleton [1]. The medicinal properties of stilbenes, in the human body, can be attributed to their peculiar chemical structures. These include the ability to oligomerize, which increases the interaction with immune components; the presence of the oxidizable catechol group (piceatannol, PIC), which can enhance anti-inflammatory properties; and the presence of two methoxy groups (pterostilbene, PTS), which allow potent antioxidant activity [2,3].
Studies available in the literature have focused on investigating whether these medicinal or nutraceutical properties can be modulated by stilbenes’ molecular action in the nuclear factor pathways, which account for regulating the expression of proinflammatory cytokines and reactive oxygen species (ROS) [4,5].
Two main pathways have been explored to date, namely, the nuclear factor kappa B (NF-κB) pathway and the nuclear factor erythroid-derived 2 (Nrf2) pathways. The NF-κB signaling pathway results from interactions between dimeric transcription factors (NF-κB-inhibitory regulators (IκBs) and the IκB kinase complex (IKK)) that regulate genes involved in human immunological and inflammatory responses. The activation of this pathway is associated with an increase in proinflammatory cytokines, interleukin 1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and adipokines, among others, as well as ROS levels; consequently, it is associated with some acute or chronic diseases [6,7].
Nrf2 is a basic leucine zipper protein (bZIP) that plays an essential role in various processes, such as xenobiotics’ detoxification, heme group metabolism, antioxidant enzymes’ coding catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and inflammatory genes’ suppression. It plays a fundamental role in maintaining cell homeostasis and mitigating different diseases [8,9]. However, the activation of these pathways requires precursors capable of triggering the signaling cascade, such as oxidative stress enhancers (NF-κB) or antioxidants like stilbenes (Nrf2) [6,7,8,9].
Current studies focus, mainly, on investigating the Nrf2 signaling pathway modulated by stilbenes because, although substantial findings about this nuclear factor are available in the literature, their molecular mechanisms and therapeutic potential to be applied in individuals with chronic and metabolic diseases need further study. Considering this, the aim of the current review was to critically analyze evidence regarding stilbenes’ action in both the human metabolism and the Nrf2 activation pathway, with an emphasis on inflammatory and oxidative stress aspects associated with the pathophysiology of chronic and metabolic diseases.
2. Stilbenes
In chemical terms, stilbenes (non-flavonoid polyphenolic class) share a common structure characterized by a 14-carbon skeleton (C6–C2–C6) with two benzene rings linked by an ethylene bridge (Figure 1) [10,11]. Overall, one of the aromatic rings in their structures carries two hydroxyl groups, whereas the other aromatic ring can carry both hydroxy and methoxy groups in different positions [12]. The central ethylenic portion enables two stereoisomers, namely, trans-stilbene (E-stilbene–the natural, stable, and most common of the two) and cis-stilbene (Z-stilbene, less stable) [13].
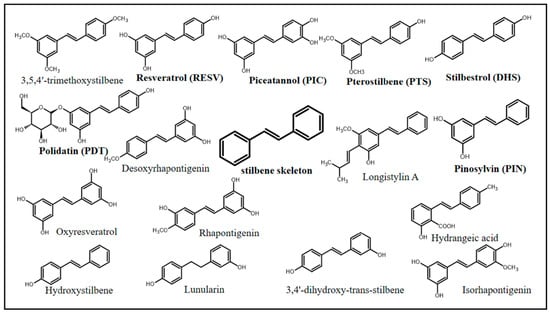
Figure 1.
Chemical structures of stilbenes with documented biological activity.
Stilbenes can be represented by a variety of chemical compounds, some of which have been shown to have biological effects, including resveratrol (RESV), PIC, PTS, polydatin (PDT), stilbestrol (DHS), and pinosylvin (PIN). Natural stilbenes can be found in their free (mostly), glycosylated, prenylated, and methoxylated forms. In addition, they can be monomeric, dimeric, trimeric, and polymeric, which are features capable of affecting their biological activity [12].
Glycosylation is identified as the most frequent change observed in secondary metabolites that can also modify stilbenes’ physical, chemical, and biological properties [14]. This process enables higher stilbene stability and protection against enzymatic reactions by increasing their mean lifetime and their solubility in aqueous media, which preserves their biological properties and enables their transport to different organs [15]. It is worth mentioning that stilbenes are usually stored in their glycosylated form, such as PDT (RESV’s glycosylated form), which can reach a concentration approximately six times higher than that of its free form (RESV) [16]. Glycosylation takes place in plants via glycosyltransferases (GTs) by acting in activating sugars’ (single or multiple) transfer between nucleotide donors (uridine diphosphate (UDP)-glucose, for example) and plants’ molecular receptors [14].
Methylation reactions of phenolic hydroxy, catalyzed by S-adenosyl-L-methionine (SAM)-dependent O-methyltransferases (OMTs) [11], lead to methoxy-stilbenes’ formation, notably, PIN monomethyl ether (3-hydroxy-5-methoxy-stilbene) and PTS (3,5-dimethoxy-4′-hydroxy-stilbene) [17,18]. This reaction can influence stilbenes’ solubility and reactivity, and it can negatively (PIN monomethyl ether’s antifungal and antibacterial activity are lower than those of its free form) and/or positively (higher anticancer activity is associated with RESV methylation) affect their biological activity [19,20,21]. It is known that hydroxy group methylation increases PTS lipophilicity in comparison to RESV; this process leads to greater bioavailability, a fact that justifies the increase in pharmacological interest [22].
Stilbenes’ prenylation takes place in some plant species, such as Macaranga spp., Glycyrrhiza spp., Morus alba, and Arachis hypogaea, via prenyltransferase [23,24,25,26]. Despite their biological relevance, their biosynthetic pathways are yet to be fully understood; however, it is known that, in comparison to their nonprenylated counterparts, stilbenes present higher bioavailability due to increased lipophilicity linked to the prenyl groups [11]. Prenyltransferases are in chloroplasts and are specific to the prenylation of stilbenes [26,27,28].
Multifactorial conditions (isomeric geometric forms Z- and E-; reaction processes; and the stilbene’s type) can interfere with stilbenes’ activity, storage, concentration, biological activity, and bioavailability and can also affect the secondary metabolites’ medicinal and pharmacological beneficial relevance to modulate health and disease processes. Despite some gaps observed in the scientific knowledge, benefits provided by stilbenes, mainly by RESV, PIC, PTS, PDT, DHS, and PIN, are indisputable. Thus, to improve the comprehension of the fundamental mechanism and make rational assumptions about stilbenes’ potential effects on human health, the present investigations have concentrated on examining their biosynthesis and metabolic processes.
2.1. Stilbenes’ Biosynthesis
Stilbenes are secondary metabolites produced by plants to help protect them in response to certain external aggressors (ultraviolet radiation; cracks; fungal, viral, or bacterial attacks; and pesticides, among others). They were initially identified as phytoalexins (defensive substances produced in response to infections), belonging to the class of polyketides [29]. Their Z- and E-isomers accumulate in vegetables’ peel during plants’ developmental stages. This occurs because, in anatomical terms, the peel is a plant’s outermost portion; therefore, it is more susceptible to both biotic and abiotic stressors/aggressors [10,13].
Stilbene biosynthesis can take place either in plants or in microorganisms [10,11,12]. The route identified in plants is the route most often documented for stilbenes and DHS’s biosynthesis, which has shikimate as a precursor. Shikimate, in turn, generates two aromatic amino acids, phenylalanine and tyrosine, which play essential roles in trans-cinnamic acid, or its p-coumaric derivative formation, to initialize the phenylpropanoid pathway accountable for the biosynthesis of several primary and secondary metabolites, such as stilbenes, flavonoids, coumarins, hydrolysable tannins, monolignols, and lignans (Figure 2) [30].
Given this complex cascade for stilbenes’ biosynthesis and low content production, researchers developed a strategy to increase their synthesis by using plant cell cultures, based on the assumption that biosynthesis is triggered by external stressors or aggressors such as ROS, methyl jasmonate (MeJA), and salicylic acid (SA) [31]. Based on the same line of investigation, microbiologists have tried to mimic stilbenes’ biosynthetic pathways in heterologous organisms [32,33].
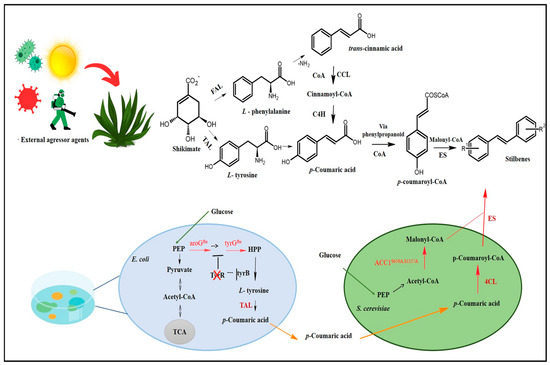
Figure 2.
Stilbenes’ biosynthesis route. Adapted from Yuan [34]. CoA: coenzyme A; COSCoA: acyl CoA thioester; FAL: phenylalanine ammonia-lyase; TAL: tyrosine ammonia-lyase; CCL: cinnamate CoA ligase; C4H: cinnamate-4-hydroxylase (cytochrome P450); ES: stilbene synthetase; HCO3−: bicarbonate ion; ATP: adenosine triphosphate; E. coli: Escherichia coli; S. cerevisiae: Saccharomyces cerevisiae; aroGfbr: feedback-inhibition-resistant 3-deoxy-d-arabinoheptulosonate-7-phosphate synthase; tyrAfbr: feedback-inhibition-resistant chorismate mutase/prephenate dehydrogenase; ACC1S659A,S1157A: inhibition-resistant acetyl-CoA carboxylase; PEP: Phosphoenolpyruvate; HPP: 4-hydroxyphenylpyruvate; tyrB and tyrR: transcriptional regulator; TCA: tricarboxylic acid cycle; 4CL: 4-coumarate-CoA ligase; R1: Substituent 1; R2: Substituent 2.
This revolutionary and innovative field has awakened a new aspect of multidisciplinary research according to which several scientific fields interact with each other to optimally meet the demand to produce secondary plant metabolites through microorganisms and cell cultures. This encouraged the development of new techniques based on metabolic engineering in cellular factories. Yuan et al. [34], for instance, used a co-culture system comprising Escherichia coli and Saccharomyces cerevisiae for RESV biosynthesis, which reached 36 mg/L, whereas Yan et al. [35] managed to biosynthesize 80 mg/L of PTS based on the introduction of genes capable of encoding transcription activator-like (TAL) protein as well as other enzymes and substrates accountable for PTS biosynthesis in Escherichia coli. However, it is necessary to conduct biological studies in vitro and/or in vivo to assess toxicity and safety concerning stilbenes synthesized through microbiological organisms.
2.2. Stilbenes’ Biological Metabolism
Although stilbenes have metabolites that share a common skeleton and demonstrate similar biological activity, the in vivo metabolism of the majority of these compounds remains unknown [36]. Each stilbene has its own functional and structural specificity based on reactions involved in its biosynthesis. This factor reflects its unique behavior, which can be observed in pharmacokinetic studies involving ADME (absorption, distribution, metabolism, and excretion) mechanisms [36,37]. However, studies focused on this research perspective do not yet have enough support, since the dose and administration route set for each stilbene are not yet standardized [3,12].
Phases I and II of biotransformation can be accessed by drugs, medical goods, and/or bioactive substances; of these, stilbenes preferentially travel through phase II due to their bioactivity [36,38,39]. Similar natural processes, including glucuronidation, sulfation, and intestine biotransformation, are shared by stilbenes (Figure 3) [36,37,40,41,42,43]. Sulfation is another important stilbene metabolism pathway, since it is mostly accountable for metabolites’ excretion. This pathway has been associated with stilbenes’ anticancer and cardioprotective activity [44].
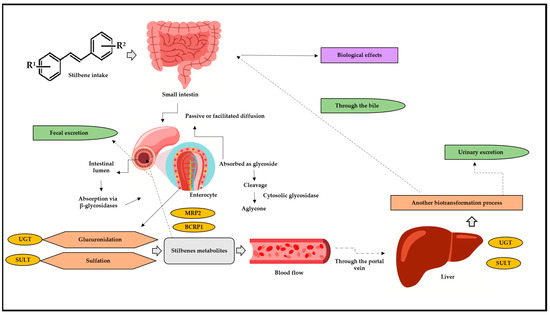
Figure 3.
Stilbenes’ biotransformation in human body. BCRP1: breast cancer resistance protein 1; MRP2: multidrug resistance protein 2; UGT: glucuronosyl-transferase; SULT: sulfotransferase; R1: substituent 1; R2: substituent 2. After oral or intragastric intake, stilbenes reach the small intestine, where they can be absorbed via passive or facilitated diffusion. In passive diffusion, stilbenes must be absorbed as a glycoside and then cleaved, generating aglycone through the action of the cytosolic glycosidase enzyme. In turn, this process can occur through the action of β-glucosidases in the intestinal lumen. Upon reaching the enterocytes, stilbene is metabolized by glucuronidation reactions, through the action of UGT, and via SULT, through sulfotransferase. After these biotransformation steps, the metabolites return to the intestinal lumen through efflux and action of the enzymes BCRP1 and MRP2. Arriving in the bloodstream, these metabolites go through the portal vein and straight to the liver, where they undergo a new biotransformation process. Then, they can return to the small intestine through the bile, be absorbed, and, finally, exert their biological effects and/or can be excreted through urine.
However, in addition to the hepatic pathways listed above, there has been increasing evidence of intestinal biotransformation pathways, mainly associated with RESV. RESV produces dihydroresveratrol, which, in turn, is a metabolite deriving from the intestinal microbiota [41,42,45,46]. Stilbenes delivered through oral or intravenous routes show significantly low concentrations which can range from nano to micromolar; however, they still demonstrate significant biological effects. Experimental results have shown intense intestinal and hepatic biotransformation metabolism 1 h after RESV administration, as observed in a serum RESV concentration ranging from 0.3 to 2.4 µmol/L, whereas its glucuronidated and sulfated metabolites recorded concentrations approximately 20 times higher [47].
Bode et al. [48] assessed human fecal samples collected after RESV supplementation and identified two bacterial strains involved in RESV biotransformation, namely, Slackia equolifaciens and Adlercreutzia equolifaciens. In addition to causing dihydroresveratrol formation, these strains generated 3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxybisbenzyl one (lunularin). However, intestinal bacteria capable of producing dehydroxylated metabolites were not identified, although hydroxy groups’ cleavage plays a key role in the microbial transformation of various compounds, such as lignans, as well as phenolic and bile acids. Therefore, interindividual variability influences intestinal microbiota composition. Nevertheless, external factors, such as diet and physical activity, cannot be ruled out.
On the other hand, Sun et al. [49] investigated PTS metabolites in CD-1 mice’s colonic contents and mucosa. Animals subjected to diet supplemented with PTS for 3 weeks presented pinostilbene metabolite formation. In addition, the concentration of this metabolite in the analyzed colonic content was approximately 10 times higher than that observed for the colonic mucosa. However, it is necessary to conduct further studies to help fill this knowledge gap, since it has been suggested that the intestinal microbiota accounts for PTS demethylation. This result was corroborated by previous investigations associated with microbial demethylases involved in flavonoid, anthocyanin, and lignan demethylation processes, among others [50]. It is worth mentioning that this on-site activity can optimize treatments for dysbiosis and inflammatory bowel diseases (IBDs) such as ulcerative colitis (UC) and Crohn’s disease (CD) [41].
Pharmacokinetics have a significant impact on the experimental data of stilbenes in vivo. In fact, RESV has a low oral bioavailability: less than 30% in a rat model [51] and less than 0.5% in humans [52]. When RESV reaches the colon, it travels to the enterocytes, where it is sulfated (by the SULT1A1) and glucuronidated (through UGT1A1 and UG-TA9). The enterocytes release intact RESV and its metabolites into the portal circulation, where they are transported to the liver, being further conjugated by the same enzyme that was present in the enterocytes [53].
In conclusion, a small fraction of intact RESV and its metabolites enter systemic circulation and are absorbed by peripheral tissues. Conjugated RESV is involved in enterohepatic circulation. Additionally, some of the conjugated metabolites and RESV pass from the small to the large intestine, where the gut microbiota can process them to produce dihydro-resveratrol (DHR), lunularin (L), and 3,4′-dihydroxy-trans-stilbene [48,53].
Despite RESV’s relatively limited bioavailability, several investigations have shown that it has biological activity in vivo in a wide variety of animal trials. Given the presumably non-physiological doses and the exclusion of the role played by RESV metabolites, studies conducted in vitro have shown a wide variety of controversial biological effects [54]. Some scholars believe that metabolites can store stilbenoids [52,55].
PTS is more lipophilic and metabolically stable due to the presence of two methoxy groups, although only one of them is accessible for glucuronidation or sulphation purposes [56]. In fact, RESV was more frequently metabolized by glucuronidation rather than by PTS in human liver microsomes [57]. That study demonstrated gender-based differences in stilbene metabolism. The highest bioavailability rate was recorded for PTS [56], followed by PIC [58]. The lowest oral bioavailability rate was recorded for gnetol (2,3′,5′,6-tetrahydroxy-trans-stilbene) [59], but it has shown a half-life longer than values reported for RESV [51] and PTS [60] after oral administration in rats.
However, despite the encouraging results, further exploration of pharmacokinetic aspects is required, since there are a lack of data on the metabolism and biotransformation of most stilbenes. Future research should investigate these routes, i.e., routes enabling biological activity such as that of nuclear factors (Nrf2 and NF-κB).
3. Stilbenes: Diseases-Based Biological and Pharmacological Activities
Nowadays, we are aware of a good number (almost 100) of stilbene derivatives that have a wide variety of biological effects on several experimental models [12].
Anticancer, antimicrobial, antidiabetic, cardioprotective, anti-inflammatory, antioxidant, and neuroprotective actions are biological effects of these compounds that have been described in the literature. This broad range of biological consequences undoubtedly involves a wide variety of action mechanisms.
3.1. Anticancer
Stilbenes’ anticancer effect appears to depend on blocking a wide variety of signaling pathways involved in tumor growth, as well as in certain cytochrome P450 isoforms, to prevent the metabolic activation of procarcinogens [61,62]. RESV and PTS have shown significant anticancer properties [63]. These compounds inhibit topoisomerase 1 activity, as well as the DNA damage-repair pathway mediated by tyrosyl-DNA phosphodiesterase 1, which accounts for tumors’ resistance to drugs [64]. The RESV methylated derivative 3,5,4′-trimethoxystilbene was capable of inhibiting Caco-2 cells’ growth in human colon cancer, as well as tubulin polymerization, in a dose-dependent manner [65]. Another group of researchers reported that this compound has shown potential antitumor activity in a breast cancer cell model by downregulating phosphatidylinositol 3-kinase/AKT signaling (PI3K-AKT) [66].
A synthetic analog of RESV, named trans-4,4′-dihydroxystilbene (DHS), acted as strong DNA replication inhibitor in mouse models subjected to tumor xenografts and showed effects against pancreatic, ovarian, and colorectal cancer cells [67]. DHS induced cyclin F-mediated downregulation of ribonucleotide reductase regulatory subunit M2 of ribonucleotide reductase (RRM2) by proteasome. Moreover, it was observed to reduce ribonucleotide reductase activity and decrease deoxyribonucleoside triphosphates synthesis through concomitant DNA replication inhibition, cell cycle arrest at S-phase, DNA damage, and, finally, apoptosis [67]. Cyclin is a family of proteins accountable for controlling the progression of a given cell throughout the cell cycle by activating cyclin-dependent kinase (CDK) enzymes. These proteins are also the target of RESV, hemsleyanol D, and (+)-α-viniferin (isolated from the plant species Shorea roxburghii), which have significantly decreased cyclin B1 expression; cyclin B1, in its turn, also suppressed cell cycle progression [68].
A methoxylated stilbene, named isorhapontigenin, induced cell death and cell growth arrest in breast cancer models by activating the caspase pathway [69]. PIC has effects similar to that of RESV on a wide variety of target sites [70,71]. PIC appears to have stronger anticancer activity than RESV, likely because its hydroxyl group is in the ortho-position instead of the meta-position [72]. Hepatocellular carcinomas are among several systemic malignancies whose tumor growth can be slowed by PIC [73]. Cell cycle arrest, modulation of proteins involved in apoptosis regulation, caspase (-3, -7, -8, and -9) activation, mitochondrial potential loss, and cytochrome c release are the mechanisms accounting for mediating anticancer actions [71]. Moreover, PIC inhibits the activation of several transcription factors such as NF-κB, which is a crucial transcriptional regulator activated in response to cell stress [71].
PIN modulates cancer cell growth inhibition and death by controlling the overexpression of TGF-β superfamily member NAG-1 (nonsteroidal anti-inflammatory drug-activated gene), which is linked to tumor progression and development processes [74,75]. In addition, PIN inhibits metastatic oral cancer cells by controlling both metalloproteinase (MMP-2) expression and activity via the ERK pathway (proteins linked to mitogen-activated protein kinase (MAPK) pathway activation, a common incidence in carcinogenesis cases) [76], whereas AMP-activated protein kinase α1 (AMPKalpha1) downregulation is the mechanism leading to leukemia cell death [77].
3.2. Antimicrobial
Stilbenes’ antimicrobial activity is not surprising, since they are substances produced by plants to function as toxins against attacking organisms [78]. These antimicrobial effects were attributed to damage in both the microbial cell wall and cell membrane, to cytoplasm condensation, and to membrane potential disruption.
RESV has shown activity on Gram-negative bacteria, although this activity was lower than that of PIN, which, similarly to PTS, showed higher activity against Gram-positive bacteria [79,80]. Both PIN and PIC presented clear antimicrobial activity through outer membrane destabilization in Gram-negative microorganisms as well as through interactions with cell membrane [80]. RESV, PIN, PIC, and PTS were also active against fungi [81]. Their activity against fungal pathogens was attributed to downregulation of both the ergosterol biosynthesis and the Ras/cAMP pathway, which plays an essential role in controlling and integrating growth, cell cycle progression, and metabolic activity [82].
Recently, kobophenol A, which is a stilbenoid isolated from the Caragana genus, was shown to be capable of blocking the interaction between the ACE2 receptor and the spike receptor binding domain (S1-RBD) of SARS-CoV-2.83 Kobophenol A, and Caragana sinica extracts were previously tested to prevent and treat West Nile virus infection; they demonstrated antiviral activity by inhibiting neuraminidase (patent application no.: KR20200026550A) [83].
A stilbene glycoside (piceid-(1→6)-β-d-glucopyranoside) derived from Parthenocissus tricuspidata demonstrated significant blood schizontocidal activity against Plasmodium berghei in outbred male ICR mice, although its action mechanism remains unknown [84].
Longistylin A, which is an abundant stilbene isolated from Cajanus cajan leaves, presented strong antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA) in vitro in association with bacterial membrane potential disruption and increased membrane permeability. Topical treatment with longistylin A applied to skin injury in vivo improved wound healing and closure in an MRSA-infected wound-healing mouse model [85].
3.3. Antidiabetic
Hydrangeic acid, which is a stilbene derived from processed Hydrangea macrophylla leaves, promoted the adipogenesis of 3T3-L1 cells (fibroblast isolated from mouse embryo and often used to investigate basic cell mechanisms associated with diabetes) [86]. Hydrangeic acid has significantly increased the adiponectin amount released into the medium, 2-deoxyglucose uptake into cells, and glucose transporter 4 translocation (GLUT4). It has also increased the mRNA levels of adiponectin, peroxisome proliferator-activated receptor γ2 (PPARγ2), GLUT4, and fatty acid-binding protein (aP2), although it decreased the expression of TNF-α mRNA. Furthermore, this acid has significantly decreased blood glucose, triglyceride, and free fatty acid levels after it was orally administered to KK-Ay mice (type 2 diabetes model) for 2 weeks [87,88].
Stilbenes, such as 3,5-dimethoxy-4′-O-prenyl-trans-stilbene isolated from the Amazonian plant species Deguelia rufescens, as well as trans-RESV and rumexoid isolated from Rumex bucephalophorus, were capable of inhibiting α-glucosidase in vitro; this finding indicates their potential for use as antidiabetic drugs [89,90].
3.4. Cardiovascular
RESV prevents atherogenesis and promotes thrombus resistance in human vascular endothelial cells by maintaining the balance between vasodilators and vasoconstrictors (nitric oxide and endothelin). Moreover, RESV has antioxidant effects on cholesterol metabolism and prevents platelet aggregation [91]. It also lowers blood pressure in animal models in a dose-dependent manner [92]. Studies conducted with humans have shown systolic blood pressure reduction at high RESV doses [93]. A randomized double-blinded placebo-controlled trial indicated that a high PTS dose reduced both systolic and diastolic blood pressure in humans [94].
3.5. Anti-Inflammatory
Stilbenes can act on various inflammatory process stages and inhibition. Studies have shown that cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) were successfully inhibited by RESV, PIC, PTS, PIN, desoxyrhapontigenin, and rhapontigenin. The NF-κB pathway was suppressed by RESV, oxyresveratrol, PTS, and PIC, which reduced the release of inflammatory cytokines [95,96].
Dietary RESV decreased the death rate in an animal model of chronic dextran sodium sulphate (DSS)-induced colitis by mitigating the severity of clinical symptoms such as body weight loss, diarrhea, and rectal bleeding. RESV decreased prostaglandin E synthase-1 (PGES-1), COX-2, and inducible nitric oxide synthase (NOS) proteins’ expression by downregulating p38, which is a mitogen-activated protein kinase (MAPK) signal pathway. Furthermore, it increased anti-inflammatory cytokine IL-10 expression and decreased the expression of proinflammatory cytokines such as TNF-α and IL-1β [97].
According to a randomized clinical study, RESV has anti-TNF properties useful in the treatment of Takayasu arteritis, a chronic granulomatous inflammatory disease that affects the aorta and its major branches [98].
3.6. Neuroprotection
Antioxidant and anti-inflammatory activities are key components of the neuroprotective features of stilbenes. RESV protected neurons from ROS and enhanced motor coordination in a mouse model subjected to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinson’s [99] by scavenging hydroxyl radicals. It also protected the assessed model from dopaminergic neurodegeneration caused by lipopolysaccharide (LPS) by preventing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibition and microglial activation [100].
Based on a process involving proteasome, RESV promoted intracellular degradation in amyloid-beta peptides produced from several cell lines accountable for expressing wild-type or Swedish mutant amyloid-beta precursor protein 695 [101]. It also reduced learning impairment and mitigated neurodegeneration in the hippocampus of a transgenic Alzheimer’s disease mouse model by decreasing the acetylation of sirtuin 1 (SIRT1) substrates [102].
Patients with Alzheimer’s disease demonstrated a good response to RESV at doses up to 1000 mg, administered twice a day for 52 weeks, in a randomized, placebo-controlled clinical trial [103]. RESV used in this trial prevented a decrease in amyloid-beta 40 levels in the patients’ blood and cerebrospinal fluid in comparison to the placebo group, but it did not consistently affect clinical outcomes or other biomarker trajectories (including plasma Ab42, CSF Ab42, CSF tau, and CSF phospho-tau 181) [103].
Studies currently available in the literature reported that RESV improved the memory and cognition of both healthy individuals and diabetic patients with subclinical cognitive impairment, although it did not show the same effect on individuals with Alzheimer’s disease [104,105,106,107].
RESV prevented neuronal death in a rat model of global cerebral ischemia by activating PI3K-AKT signaling, as well as by decreasing glycogen synthase kinase-3 (GSK-3) and cAMP response element-binding protein (CREB) levels [108]. RESV improved cognition in an animal model of vascular dementia and increased antioxidant enzyme levels in its cerebral cortex and hippocampus, whereas malondialdehyde (lipid peroxidation product) levels decreased [109].
PTS-based treatment improved memory loss caused by streptozotocin in Sprague Dawley rats and enhanced cholinergic transmission by inhibiting cholinesterases [110]. Hydroxystilbene protected rat cortical neurons against damage caused by amyloid beta (25–35) by limiting ROS production, suppressing glutamate release, and by preventing an increase in cytosolic calcium levels [111]. Moreover, oxyresveratrol has significantly decreased brain infarct volume in a murine model of transient middle cerebral artery occlusion by inhibiting both cytochrome c release and caspase-3 activation [112].
4. Stilbenes’ Role in Activating the Nrf2 Pathway
The human body has pre-established regulatory mechanisms, such as nuclear factors NF-κB and Nrf2, that enable it to respond and adapt to both exogenous (such as pollution, UV radiation, pollution, physical inactivity, smoking, and alcohol consumption) and endogenous (such as cortisol, ROS, proinflammatory cytokines, hydroperoxides, and quinones) stressors [8,9,113]. Because nuclear pathways are strictly regulated, stress can cause NF-κB dysregulation, which then activates its pathway. This worsens endogenous inflammation by increasing the expression of proinflammatory agents such as chemokines, adhesion molecules, and cytokines [7,114]. Unlike NF-κB, the Nrf2 pathway can be mainly activated by key components of antioxidant systems, i.e., by direct antioxidants, which are molecules with redox-active properties capable of ruling out ROS (reduced glutathione, GSH; ascorbate; tocopherols) and enzyme systems (GPx and thioredoxin system (TXN), among others) as well as by indirect antioxidants, which are susceptible to the induction of cytoprotective genes capable of recycling and/or regenerating direct antioxidants (natural polyphenols, such as stilbenes and isothiocyanates) [115].
Despite the undeniable importance of both nuclear factors listed above, the current review focuses only on investigating the modulation of the Nrf2 pathway. After understanding its essential role in redox homeostasis, drug/xenobiotic metabolism, mitochondrial function, and deoxyribonucleic acid (DNA) repair, researchers have expanded their interest in modulating the Nrf2 pathway, mainly in the human health field. Once stimulating this pathway, the likelihood of treating or mitigating unfavorable outcomes in chronic and/or metabolic diseases increases [113]. The Nrf2 controls basal gene expression both under homeostasis and oxidative stress conditions. Furthermore, it accounts for regulating approximately 250 genes involved in a wide mechanistic range of cell functions [115,116].
Nrf2 is a modular protein comprising 605 amino acids in humans; in addition, it possesses seven conserved domains in different functions to control Nrf2 transcriptional activity (Figure 4) [117,118]. These domains are homologous to a protein deriving from erythroid cells with CNC (cap‘n’collar) homology (ECH) named Nrf2-ECH (Neh) [117]. The Neh1 domain comprises conserved region CNC-bZIP, which plays an essential role as a transcription factor and in heterodimerization processes associated with other bZIP proteins such as musculoaponeurotic fibrosarcoma (sMAF) proteins; these proteins can be found in their MafF, MafG, and MafK forms, which can recognize antioxidant response elements (AREs) capable of activating gene transcription [119]. The Neh2 N-terminal domain has negative control over the Nrf2 activity, i.e., it mediates Nrf2 ubiquitination and degradation processes. Neh2 has two highly conserved peptide sequences, namely, ETGE (high affinity) and DLG (low affinity) degrons, which interact specifically with the transcription factor mediated by ECH-associated protein 1 (Keap1); consequently, they play a key role in proteasomal degradation processes [119,120].
The Neh3, Neh4, and Neh5 domains are involved in transcriptional activation processes, since they can bind to different transcriptional machinery components. The Neh3 domain is located in the C-terminal region. However, it is worth highlighting that removing 16 amino acids from the C-terminus of this protein inactivates the CNC-bZIP factor; this indicates the role played by it in target genes’ transactivation processes. Furthermore, the Neh3 domain can interact with Chromodomain Helicase DNA Binding Protein 6 (CHD6), a fact that corroborates its role in transcriptional activation processes [121,122].
The Neh4 and Neh5 domains act in a cooperative manner by interacting with cyclic adenosine 3′,5′-monophosphate (cAMP)-responsive binding protein (CREB) and by synergistically increasing gene transcription rates. In addition, these domains can bind to HMG-CoA reductase degradation protein 1 (HRD1) to mediate Nrf2 degradation [122,123].
Neh6 is another domain accounting for Nrf2-negative regulation as well as for its Keap1-independent regulation [123]. Like Neh2, the Neh6 domain presents two peptide degrons, namely, DSGIS and DSAPGS. These degrons are recognized by the β-transducin-repeat-containing protein (β-TrCP), which accounts for mediating Nrf2 degradation in cells under distress conditions. It is important to emphasize that degron DSGIS has a phosphorylation site for the glycogen synthase kinase-3 (GSK-3) enzyme, which increases β-TrCP’s ability to suppress Nrf2 when it is modified by GSK-3 [115,124].
Finally, Neh7 is the most recently described domain and has a region that is yet to be fully explained. This region interacts with retinoic receptor X α (RXRα) to suppress Nrf2 activity and to prevent co-activators’ recruitment to the Neh4 and Neh5 domains [125]. Overall, these domains act in Nrf2 stability modulation as well as in the transcriptional activation of its target genes, such as transcriptional, post-transcriptional and post-translational regulation [115,118].
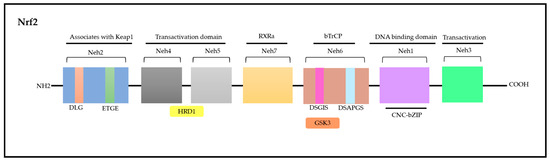
Figure 4.
Nrf2 molecule—seven domains with various functions that control transcriptional activity. Adapted from Itoh [117] and Canning [122]. Nrf2: nuclear factor erythroid-derived 2; CNC: cap‘n’collar; ECH: erythroid cells with CNC homology; Neh: Nrf2-ECH; Keap1: ECH-associated protein 1; DLG: polypeptide sequence containing the amino acids aspartic acid, leucine and glycine; ETGE: polypeptide sequence containing the amino acids glutamate, threonine, glycine and glutamate; HRD1: HMG-CoA reductase degradation protein 1; RXRα: retinoic receptor X α; DSGIS and DSAPGS: peptidic degrons; GSK3: glycogen synthase kinase-3 β enzyme; bzip: Basic Leucine Zipper Domain; bTrCP: β-transducin-repeat-containing protein; DNA: desoxyribonucleic acid.
Overall, Nrf2 has a short half-life (approximately 15 min) under homeostatic conditions; further, it is linked to domains (Keap1 or β-TrCP or HRD1) capable of keeping it inactivated [9,126]. Its structure plays an essential role in helping us achieve a good understanding of its metabolic routes, since Nrf2 regulation mainly takes place through E3 ubiquitin ligase substrates involved in its ubiquitination and activation processes. These substrates comprise the Keap1-CUL3-RBX1 complex, SCF/β-TrCP, and HRD1 [113].
Each one of these complexes mediates Nrf2 degradation. In other words, they interrupt the connection with the above-listed domains to activate Nrf2, based on different stimuli; namely, Keap1-CUL3-RBX1 complex responds to electrophilic/oxidative modification of key cysteines, mTOR, and CUL3-Ring E3 ligase (CRL) inhibitors as well as to the competitive binding of ETGE-containing proteins and to increased p62/SQSTM1 levels, whereas SCF/β-TrCP can be modulated through metabolic changes taking place both in the cytosol and in cell nucleus (Figure 5). These changes are regulated by the glycogen synthase kinase-3 β enzyme, (GSK3β), by insulin or growth factors, and by CRL inhibitors. HRD1 ubiquitylates Nrf2 under endoplasmic reticulum stress [113].
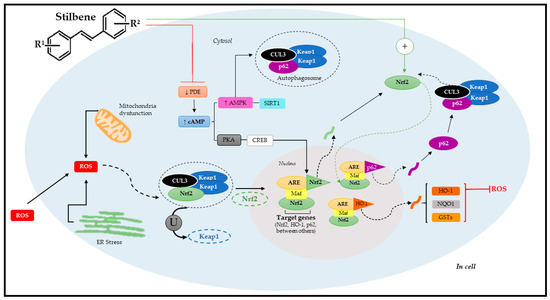
Figure 5.
Molecular mechanism of Nrf2 pathway activation through stilbenes. Adapted from Reinisalo [4] and Niture [8]. R1: substituent 1; R2: substituent 2; ROS: reactive oxygen species; CUL3: cullin-3; Nrf2: nuclear factor erythroid-derived 2; ER: endoplasmic reticulum; ARE: antioxidant response elements; Keap1: ECH-associated protein 1; U: ubiquitination; PDE: phosphodiesterase; cAMP: cyclic adenosine 3′,5′-monophosphate; PKA: protein kinase A; CREB: cAMP-responsive binding protein; AMPK; SIRT1: sirtuin 1; p62: autophagy substrate; HO-1: Heme oxygenase-1; NQO1: NAD(P)H:quinone acceptor oxidoreductase 1; GSTs: glutathione S-transferases; Maf: musculoaponeurotic fibrosarcoma proteins.
However, it is important to emphasize that Nrf2 can also be regulated by other signaling pathways such as epigenetic (methylation, acetylation, and/or microRNAs) and post-translational factors (phosphorylation, ubiquitination, acetylation, and/or methylation) [8,113]. As previously mentioned, polyphenols, such as stilbenes, can activate Nrf2. Studies have shown that these phytochemicals often use signal transduction mechanisms involving a complex cascade of events that comprise the following phases: basal, pre-induction, induction, and post-induction [8]. After cell exposure to stilbenes, there is a pre-induction response via negative Nrf2 regulators, which are translocated from the cell nucleus to its cytoplasm. The induction phase takes place simultaneously to Nrf2 trans-location to the nucleus. This process is followed by stabilization and heterodimerization, which activate ARE-mediated cytoprotective gene expression and trigger the post-induction phase to interrupt Nrf2 activation [127,128].
When it comes to stilbenes, the most investigated pathway is the pathway acting through ARE; Nrf2 plays a key role in regulating antioxidant genes and phase-II metabolites. Heme oxygenase-1 (HO-1) and NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1) were mostly identified among Nrf2 target genes [129,130]. The Nfr2/ARE pathway is suppressed under optimal conditions; Nrf2 remains trapped in the cytosol linked to the Keap1 domain that, together with cullin-3 (CUL3), forms the Keap1-CUL3-RBX1 complex, which is constantly exposed to ubiquitination and proteasomal degradation [131].
However, Nrf2 under stress conditions dissociates from the Keap1-CUL3-RBX1 complex through two pathways. The first relies on specific Keap1 cysteine residues’ modification by oxidants, phytochemicals (such as stilbenes), and/or electrophiles; whereas the second refers to specific Keap1 cysteine residues’ modification by p62 involved in autophagy process [131]. Both pathways enable Nrf2 translocation to the cell nucleus. Upon arriving in the cell nucleus, Nrf2 undergoes heterodimerization with small Maf proteins (sMaf) that play an essential role in its binding to ARE and, consequently, in the activation of target genes [4].
Alternatively, data have demonstrated that stilbenes can also act via new molecular targets (auxiliary mechanisms), such as via cAMP signaling, via AMP-activated protein kinase (AMPK), which accounts for regulating energy homeostasis, the estrogen-related receptor α (ERRα), and estrogen receptors (ER) as well as the enzymatic cofactor tetrahydrobiopterin (BH4)—an essential cofactor of the nitric oxide synthetase (NOS) enzyme, which accounts for nitric oxide (•NO) synthesis. Stilbenes can also act via phosphodiesterase (PDE, enzymes accountable for cAMP and cGMP degradation) mediated by increased cellular cAMP levels [132,133]. Therefore, the dimension surrounding stilbenes in the Nrf2 pathway activation process is quite complex, since it involves several factors accounting for activating specific target genes. This specificity means that stilbenes have therapeutic functions in some chronic and metabolic diseases. The latest evidence of stilbenes’ therapeutic effects via Nrf2 pathway mediation will be discussed below.
5. Stilbenes: Compounds-Based Approach
Although the literature reports several studies focused on investigating stilbenes, most of them remain at the preclinical testing stage, comprising models in vivo and in vitro, and show preference for RESV, a prototype of the class, over other compounds. The therapeutic action of other stilbenes, such as PIC, PIN, DHS, PTS, and PDT, has been recently explored. This section focuses on investigating each of the aforementioned stilbenes and their association with the mitigation of different diseases via Nrf2 nuclear pathway activation.
5.1. RESV
RESV (3,5,4′-trihydroxystilbene) is a natural polyphenol first identified in 1940 by Japanese scientists. RESV was found in the roots of Veratrum grandiflorum and, later, in the roots of Polygonum cupsidatum, the latter being an important traditional medicine in China [36,134,135,136,137]. RESV can be found in several plants, including peanuts (Arachis hypogea), blueberries and cranberries (Vaccinium spp.), Japanese knotweed (Polygonum cuspidatum)—a traditional local herbal medicine—and, even more widely and abundantly, in grapevine (Vitis vinifera) and its derivatives, such as red wines and whole grape juices [135,138,139].
Regarding RESV’s ability to promote health benefits through its role in activating Nrf2, several experimental studies have been conducted in this context; the vast majority of these studies were carried out in vivo (Table 1) and in vitro (Table 2), but some randomized clinical trials were also reported (Table 3) [140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232].

Table 1.
List of resveratrol (RESV) uses and Nrf2 pathway-based activity in animal models.

Table 2.
List of resveratrol (RESV) uses and Nrf2 pathway-based activity in vitro studies.

Table 3.
List of resveratrol (RESV) uses and Nrf2 pathway-based activity in randomized clinical trials.
In view of the above, it is possible to note that the literature contains several intervention studies carried out with the administration of RESV in numerous contexts. Among them, most have been carried out on animal models, followed by combinations with or without cellular models; little research has been carried out on humans. When it comes to animal studies, most research has included rats and mice. However, experiments on birds and fish were also considered. RESV was administered orally, by gavage or intraperitoneally. The doses used varied in increasing concentrations and the administration periods ranged from a single dose to administration over a few days or months. The conditions tested were wide and varied, including bone, joint, kidney, cardiovascular, and neurodegenerative diseases, cancer, ageing, and wound healing. Although the investigations included several conditions, the main findings were uniform in observing the role of RESV in increasing the expression and/or signaling pathways involving Nrf2 as well as the antioxidant enzymes regulated by it. Furthermore, the action of this compound is also seen in negatively regulating NF-κB, attenuating inflammatory processes. It is also worth highlighting that the findings were independent of the dose and time of administration, emphasizing the importance of this antioxidant in situations involving oxidative stress and inflammation in animal models. Furthermore, in most cases among the studies analyzed, the administration of RESV was shown to alleviate clinical symptoms of the diseases, including improvement in insulin resistance, healing, as well as improvement in the lipid profile.
Like animal research, cellular model studies cover a wide range of varied conditions or diseases. The intervention time ranged from 4 to 72 h, with increasing concentrations, and the findings are unanimous in highlighting the important role of RESV in attenuating oxidative stress, increasing the expression of antioxidants, and/or promoting Nrf2 activation directly or indirectly through lower expression of inflammatory components, via NF-κB, as observed in animal models. In turn, very few clinical studies in humans have been carried out regarding the action of RESV on the activation of Nrf2, and only two are presented in the present study. Although both are of high methodological quality, randomized, double blind, and crossover, the findings are conflicting and underline the need for more research to be conducted from this perspective, aiming to analyze whether the promising results observed in animal models and in vitro can be confirmed in human populations. This strategy is attractive and capable of helping in the prevention and/or treatment of various adverse health situations. Furthermore, it is necessary to define better doses and administration times for the antioxidant for each condition evaluated to obtain more effective results and better determine RESV’s clinical applicability.
5.2. PTS
The first reports about PTS (trans-3,5-dimethoxy-4-hydroxystilbene) occurred in the 1940s when it was identified as a polyphenol in the bark of Pterocarpus marsupium (a deciduous tree); after a year, it was recognized and validated via synthesis. Subsequently, it was found in grapes, blueberries, and peanuts [232,233,234,235,236]. Its discovery redirected scientific interest, since previous attention regarding the use of stilbenes in health was centered on RESV [237].
PTS is a dimethylated analogue of RESV, which strongly affects its lipophilicity, increasing its availability in biological media, making it a more potent therapeutic agent than RESV [36,238,239]. Among its pharmacological properties, its antidiabetic property was the first to be ratified, through Ayurvedic medicine; however, amid scientific and technological advances in the area of phytotherapy applied to human health, other therapeutic activities were also found against numerous health problems and diseases [240].
In parallel with these findings, and sensitized by the discovery that pathological processes, in most cases, are initiated or intensified through the exacerbation of oxidative stress and inflammation, new scientific insights have emerged focusing on products that activate or suppress nuclear factors (Nrf2 and NF-κB), which modulate serum levels of ROS and proinflammatory cytokines. Given this, mechanistic studies, which analyze the pharmacokinetics of phytoalexins, identified that the biological capacity to attenuate the pathological processes of PTS mainly came from its ability to activate the Nrf2 pathway. Table 4 and Table 5 bring together the experimental studies in which PTS presented health benefits, via activation of Nrf2, with studies carried out in vitro and in vivo, respectively [241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268].

Table 4.
List of pterostilbene (PTS) uses and Nrf2 pathway-based activity in vitro studies.

Table 5.
List of pterostilbene (PTS) uses and Nrf2 pathway-based activity in animal models.
Given these findings, mostly in vivo studies could be identified (72.5%; 21/29 articles). The cellular viability of PTS was investigated in the concentration (dose) range of 2–50 µM, proving to be safe. However, it is noteworthy that depending on the cell culture, the tolerance threshold dose may be changed. For the animal model, doses ranging from 5 to 100 mg/kg were used, depending on the animal model used (rodents or zebrafish). It is notable that the activation of the Nrf2 pathway in the animal model was dependent on the PTS dose.
As tolerance data might vary as a result of the animal model, the use of several animal models may present a challenge when comparing results. Since the zebrafish model is a more modern animal model than the regularly used rats and/or mice, it has received less attention in the scientific literature while being regarded as a safe alternative model because of its genetic resemblance to humans.
5.3. PIC
PIC (3,3′,4,5′-trans-tetrahydroxystilbene) belongs to the stilbenes class and is an hydroxilated analogue of RESV; it was reported for the first time in 1956, isolated from the Vouacapoua americana species, also known as acapu, and is widely found in natural sources such as fruits, vegetables, and medicinal plants [269,270]. Studies report the presence of PIC in grapes, passion fruit, blueberries, white tea, and rhubarb [271].
Regarding the potential of this stilbene, it has demonstrated several biological activities such as antioxidant, antiviral, anticancer, antiglycant, antidiabetic, and anti-inflammatory activities [139,272,273,274,275]. Among its biological activities, modulation via Nrf2 was mainly found in animal models (Table 6) and in vitro (Table 7) [276,277,278,279,280,281,282,283,284,285]. It was observed that PIC positively regulated the expression of Nrf2 and its mRNA, increasing the expressions of NQO1, HO-1, γGCS, and GPx and, via Nrf2/Keap1, acting in testicular protection and attenuation of oxidative stress. In addition, a decrease in MDA and an increase in antioxidant enzymes SOD, CAT, and GPx were observed. The pathway observed in this study was Nrf2/Keap1, in which Nrf2 uncouples from Keap1, translocating Nrf2 to the cell nucleus, thus regulating target genes to promote an antioxidant response [279].

Table 6.
List of piceatannol (PIC) uses and Nrf2 pathway-based activity in animal models.

Table 7.
List of piceatannol (PIC) uses and Nrf2 pathway-based activity in vitro studies.
The concentration range of 5–20 mg/kg of PIC was found to be safe for use in animal model studies provided that the maximum dosage of 20 mg/kg is not exceeded. A concentration of 10 μM is utilized in most of the works pertaining to in vitro research. The highest concentration used among the studies reported was 40 μM. Nevertheless, in the current work, cell viability was assessed using concentrations as high as 40 μM, and it was found that PIC did not significantly affect cell viability at the concentrations tested [285].
Six hours to twelve weeks were employed for the evaluation of PIC intervention times, with 24 h being the most common intervention time in experimental methods. Kil et al. [274] found that, despite a shorter intervention period than prior research, there was a similar drop in ROS and rise in HO-1.
We observed that all studies have the same purpose, i.e., the activation of the Nrf2 pathway. The majority of studies using PIC as an activator of the Nrf2 pathway identified a common antioxidant enzyme, HO-1, which is important and plays an important role in protecting against oxidative injuries, modulating inflammation, regulating apoptosis, and contributing to angiogenesis [275]. Some studies evaluated greater activation pathways than others, depending on the analyzed conditions/diseases. Among the studies listed in Table 7, Achy-Brou et al. [283] evaluated the activation of Nrf2 through the reduction in ●NO levels, differing from other researchers who generally evaluated the production of antioxidant enzymes related to the activation of the Nrf2 pathway.
5.4. PIN and DHS
PIN (3,5-dihydroxy-trans-stilbene) is found in the Pinaceae family, mainly in the heartwood of Pinus sylvestris (also known as Scots pine), and can also be found in the leaves of Pinus densiflora [286,287]. Pine tree parts are traditionally used in East Asia to treat a variety of health conditions, including inflammation, liver toxicity, and stomach disorders. This compound is being studied extensively because it is very significant in plants and due to its positive effects on human health, including antioxidant, neuroprotective, and antiallergic properties [288]. Other activities reported for this compound are described in the literature, such as antibacterial [289] and anticancer activity [290]. Erasalo et al. [291] observed the anti-inflammatory effect in in vitro and in vivo models, inhibiting the PI3K/Akt signaling pathway.
DHS (4,4′-dihydroxy-trans-stilbene) can be found in the methanolic extract of Yucca periculosa bark [292]. Despite being less investigated, antioxidant and anticancer activity can be included among its biological properties [293,294]. According to Chen et al. [295], DHS was effective against pancreatic, ovarian, and colorectal cancer cells. This compound also showed an antimetastatic effect in vivo in a model of melanoma-mediated lung metastasis, where it was observed that DHS reduced the formation of large melanoma nodules [296,297].
To prevent information duplication, PIN and DHS were presented together in this section; some articles that studied their actions via Nrf2 also evaluated their pharmacokinetic features in a comparable manner. It is also valuable to note that, in comparison to the others, there are not many publications concerning these compounds in the scientific literature, perhaps because they were discovered more recently. Thus, Table 8 shows the results of in vitro tests, and Table 9 lists the in vivo experimental studies (in animal models) [298,299,300].

Table 8.
List of pinosylvin (PIN), stilbestrol (DHS) uses and Nrf2 pathway-based activity in vitro studies.

Table 9.
List of pinosylvin (PIN) and stilbestrol (DHS) uses and Nrf2 pathway-based activity in animal models.
Wang et al. [300] evaluated the activation of the Nrf2 pathway in oligoasthenospermia for three stilbenes: PIN, DHS, and REVS. The dose tested in this study was 100 mg/kg, a much higher level compared to other studies carried out. Their research also evaluated changes in antioxidant enzymes, and a reduction in oxidative stress in the studied disease was observed.
There were two in vitro studies of PIN and DHS, both treating different conditions or diseases. PIN was used to treat oxidative stress-induced cell death, while DHS was used to treat COPD. Both studies shared a 24 h evaluation period and a concentration range very close to PIN (5 μM) and DHS (0.5–4 μM). PIN at a concentration of 5 μM elevated HO-1 levels, whereas Nrf2 levels remained basal in the study of PIN against oxidative stress induced in human retinal epithelial cells. The authors evaluated the role of p62, investigating the expression level of p62 mRNA in protection by PIN. They suggested that this may have occurred due to accumulation of the p62 protein [298].
5.5. PDT
PDT (3,4′,5-trihydroxystilbene-3-β-D-glucoside) is a glycoside derivative of RESV [301,302]. Like PTS, it has more prominent biological activity than RESV, particularly as an antioxidant [303]. It can be obtained from the Vitaceae, Liliaceae, and Leguminosae families; however, Polygonum cuspidatum and Reynoutria japonica are the main sources for extraction on an industrial scale [304,305].
The therapeutic and protective effects of PDT have been widely investigated, mainly regarding its role in modulating nuclear factors, such as Nrf2, in order to improve antioxidant defense against pathological processes [302,306,307]. In view of this, Table 10 lists studies that focused on the pharmacokinetic performance of polidatin in various diseases or pathological conditions such as liver disease, inflammatory bowel disease, neurodegeneration, endometriosis, diabetes mellitus, kidney disease, ophthalmological conditions, acute myocardial infarction, osteoarthritis, lung disease, auditory disease, asthma, and allergy [307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327].

Table 10.
List of polidatin (PDT) uses and Nrf2 pathway-based activity in animal models.
All the publications examined PDT’s therapeutic potential using animal models (rats, mice, and/or guinea pigs) and doses ranging from 7.5 to 500 mg/kg. Some of the research found a direct correlation between PDT’s medicinal effects and dosage. Due to the fact that the treatment time varied based on the dose administered, a dose discrepancy can also be seen, making a comparative examination of its effects problematic. While PDT can indeed act to increase the expression of Nrf2 and its antioxidant enzymes, it is unclear how this leads to an improvement in the serum levels of endogenous markers. Therefore, studies that standardize the dose and duration of intervention are required to investigate the long-term effects of PDT in order to provide scientific evidence to conduct more rigorous and well-designed studies in humans.
6. Conclusions and Future Perspectives
Based on the present review, it is evident that stilbenes could activate Nrf2 either directly or indirectly. They achieve this by affecting NF-κB and utilizing distinct biological pathways. This activation has implications for treating diseases or health conditions that involve inflammation and redox imbalance. Nevertheless, most documented experimental experiments were carried out using cellular or animal models. Although the positive outcomes are evident, it is crucial to conduct multiple studies that adopt a multidisciplinary approach to thoroughly investigate the role of stilbenes in the Nrf2 pathway and their potential therapeutic uses. This entails gaining a comprehensive understanding of the biology and biochemistry of how these compounds interact with other molecules, developing other laboratorial methods (enzyme interaction investigations), conducting dose–response studies, and performing preclinical and clinical controlled trials, as well as searching for and guaranteeing the quality, purity, and stability of the chemical compounds or extracts used. These investigations are of utmost importance in exploring the therapeutic properties of natural products and their behavior in pathophysiological conditions. Consequently, investigations conducted on cellular and animal models play a vital role in assessing the safety and effectiveness of herbal medicines. These studies pave the way for further research involving human subjects and ultimately lead to the development of new formulations that can enhance the well-being and quality of life for individuals afflicted with health conditions that pose a threat to their overall health. In addition, it is crucial to consider the potential interaction of stilbenes with other metabolic or physiological processes. This contact could result in broader impacts across the body when these chemicals are used. Therefore, it is imperative to further explore this aspect. Furthermore, it is important to emphasize the essential significance of stereochemistry in the profound modification of the reactivity and biological activity of stilbenes. Therefore, this research specifically examines the arrangement of E-stilbene isomers and their hybrid derivatives, since they possess greater stability, lower cytotoxicity, and more significant biological activity as compared to the Z-stilbenes configuration. There is a deficiency in scientific and technological expertise. To ensure optimal activation of Nrf2 and/or negative regulation of NF-κB, it is crucial to establish precise dosages, delivery methods, and timing for each type of stilbene and for each specific situation. This will enable the standardization of supplementation with each chemical. Furthermore, it is imperative to assess the safety of stilbenes in the context of different situations where their potential benefits have been proposed, with the goal of offering more reliable and secure data.
Author Contributions
Conceptualization, E.L.S.S.M., M.O.F.G. and L.S.; methodology, E.L.S.S.M., J.A.X., M.B.T.F., M.O.S. and M.O.F.G.; formal analysis, E.L.S.S.M., J.A.X., M.B.T.F., M.O.S. and P.T.; investigation, E.L.S.S.M., J.A.X., M.B.T.F., M.O.S. and P.T.; writing—original draft preparation, E.L.S.S.M., J.A.X., M.B.T.F., M.O.S. and P.T.; writing—review and editing, M.O.F.G. and L.S.; visualization, A.C.M.O. and P.B.E.; supervision, M.O.F.G. and L.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support of the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) [435704/2018-4], INCT-Bioanalítica (Instituto Nacional de Ciências e Tecnologia em Bioanalítica) [465389/2014-7], CAPES/RENORBIO/PROAP (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) [88881.647234/2021-01], and FAPEAL (Fundação de Amparo à Pesquisa do Estado de Alagoas).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nagumo, M.; Ninomiya, M.; Oshima, N.; Itoh, T.; Tanaka, K.; Nishina, A.; Koketsu, M. Comparative analysis of stilbene and benzofuran neolignan derivatives as acetylcholinesterase inhibitors with neuroprotective and anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2019, 29, 2475–2479. [Google Scholar] [CrossRef]
- Zhan, J.; Hu, T.; Shen, J.; Yang, G.; Ho, C.; Li, S. Pterostilbene is more efficacious than hydroxystilbenes in protecting liver fibrogenesis in a carbon tetracholride-induced rat model. J. Funct. Foods 2021, 84, 104604. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Mascarenhas, R.; Harish, H.M.; Gowda, Y.; Lakshmaiah, V.V.; Nagella, P.; Al-Mssallem, M.Q.; Alessa, F.M.; Almaghasla, M.I.; Rezk, A.A. Stilbenes, a Versatile Class of Natural Metabolites for Inflammation—An Overview. Molecules 2023, 28, 3786. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol stilbenes: Molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxidative Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Bhandari, R.; Khanna, G.; Kaushik, D.; Kuhad, A. Divulging the Intricacies of Crosstalk between NF-Kb and Nrf2-Keap1 Pathway in Neurological Complications of COVID-19. Mol. Neurobiol. 2021, 58, 3347–3361. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, E.L.S.S.; Fragoso, M.B.T.; de Oliveira, J.M.; Xavier, J.A.; Goulart, M.O.F.; de Oliveira, A.C.M. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants 2022, 11, 129. [Google Scholar] [CrossRef]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2—An update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, C., II; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2019, 18, 37–67. [Google Scholar] [CrossRef]
- Wang, J.; Hou, B. Glycosyltransferases: Key players involved in the modification of plant secondary metabolites. Front. Biol. China 2009, 4, 39–46. [Google Scholar] [CrossRef]
- Shimoda, K.; Kubota, N.; Uesugi, D.; Kobayashi, Y.; Hamada, H.; Hamada, H. Glycosylation of stilbene compounds by cultured plant cells. Molecules 2020, 25, 1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, L.; Guo, Y.X.; Dong, Y.S.; Zhang, D.J.; Xiu, Z.L. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2007, 75, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Ray, A.B.; Dc’ah, F.K.; Slatkin, D.J.; Schiff, P.L. Constituents od Pterocarpus marsupium. J. Nat. Prod. 1984, 47, 179–181. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.C.; Corio-Costet, M.F.; Mérillon, J.M. Pinus pinaster Knot: A Source of Polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Jeong, Y.J.; An, C.H.; Woo, S.G.; Jeong, H.J.; Kim, Y.M.; Park, S.J.; Yoon, B.D.; Kim, C.Y. Production of pinostilbene compounds by the expression of resveratrol O-methyltransferase genes in Escherichia coli. Enzym. Microb. Technol. 2014, 54, 8–14. [Google Scholar] [CrossRef]
- Vek, V.; Poljanšek, I.; Humar, M.; Willför, S.; Oven, P. In vitro inhibition of extractives from knotwood of Scots pine (Pinus sylvestris) and black pine (Pinus nigra) on growth of Schizophyllum commune, Trametes versicolor, Gloeophyllum trabeum and Fibroporia vaillantii. Wood Sci. Technol. 2020, 54, 1645–1662. [Google Scholar] [CrossRef]
- Martínez-Márquez, A.; Morante-Carriel, J.A.; Palazon, J.; Bru-Martínez, R. Rosa hybrida orcinol O-methyl transferase-mediated production of pterostilbene in metabolically engineered grapevine cell cultures. New Biotechnol. 2018, 42, 62–70. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Potter, T.L.; Horn, B.W. Prenylated stilbenes from peanut root mucilage. Phytochem. Anal. 2006, 17, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Leláková, V.; Béraud-Dufour, S.; Hošek, J.; Šmejkal, K.; Prachyawarakorn, V.; Pailee, P.; Widmann, C.; Václavík, J.; Coppola, T.; Mazella, J.; et al. Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J. Ethnopharmacol. 2020, 263, 113147. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Zhu, W.; Liu, S.; Guan, Q.; Chen, X.; Huang, W.; Wang, T.; Yang, B.; Tian, J. Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba. Plant Cell Physiol. 2018, 59, 2214–2227. [Google Scholar] [CrossRef]
- Munakata, R.; Olry, A.; Karamat, F.; Courdavault, V.; Sugiyama, A.; Date, Y.; Krieger, C.; Silie, P.; Foureau, E.; Papon, N.; et al. Molecular evolution of parsnip (Pastinaca sativa) membrane-bound prenyltransferases for linear and/or angular furanocoumarin biosynthesis. New Phytol. 2016, 211, 332–344. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Sanders, S.; Jayanthi, S.; Rajan, G.; Podicheti, R.; Thallapuranam, S.K.; Mockaitis, K.; Medina-Bolivar, F. Stilbenoid prenyltransferases define key steps in the diversification of peanut phytoalexins. J. Biol. Chem. 2018, 293, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R. A new class of phytoalexins from grapevines. Experientia 1977, 33, 151–152. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, S.J.; Cui, T.B.; Liu, Z.Y.; Zhao, W. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glycoside biosynthesis by suspension cells cultures of Polygonum multiflorum thunb and production enhancement by methyl jasmonate and salicylic acid. Molecules 2012, 17, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Mehrshahi, P.; Smith, A.G.; Goossens, A. Synthetic biology approaches for the production of plant metabolites in unicellular organisms. J. Exp. Bot. 2017, 68, 4057–4074. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Yuan, S.F.; Yi, X.; Johnston, T.G.; Alper, H.S. De novo resveratrol production through modular engineering of an Escherichia coli-Saccharomyces cerevisiae co-culture. Microb. Cell Fact. 2020, 19, 143. [Google Scholar] [CrossRef]
- Yan, Z.B.; Liang, J.L.; Niu, F.X.; Shen, Y.P.; Liu, J.Z. Enhanced Production of Pterostilbene in Escherichia coli Through Directed Evolution and Host Strain Engineering. Front. Microbiol. 2021, 12, 710405. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Roupe, K.A.; Remsberg, C.M.; Yáñez, J.A.; Davies, N.M. Pharmacometrics of Stilbenes: Seguing Towards the Clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef]
- Nunes, S.; Danesi, F.; Del Rio, D.; Silva, P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Danesi, F.; Ferguson, L.R. Could pomegranate juice help in the control of inflammatory diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef]
- Fu, J.; Wu, S.; Wang, M.; Tian, Y.; Zhang, Z.; Song, R. Intestinal metabolism of Polygonum cuspidatum in vitro and in vivo. Biomed. Chromatogr. 2018, 32, e4190. [Google Scholar] [CrossRef]
- Miksits, M.; Maier-Salamon, A.; Aust, S.; Thalhammer, T.; Reznicek, G.; Kunert, O.; Haslinger, E.; Szekeres, T.; Jaeger, W. Sulfation of resveratrol in human liver: Evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica 2005, 35, 1101–1119. [Google Scholar] [CrossRef]
- Jenner, A.M.; Rafter, J.; Halliwell, B. Human fecal water content of phenolics: The extent of colonic exposure to aromatic compounds. Free Radic. Biol. Med. 2005, 38, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Ferguson, L.R. Dietary interactions with the bacterial sensing machinery in the intestine: The plant polyphenol case. Front. Genet. 2014, 5, 64. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, X.; Cai, X.; Song, M.; Zheng, J.; Pan, C.; Qiu, P.; Zhang, L.; Zhou, S.; Tang, Z.; et al. Identification of pinostilbene as a major colonic metabolite of pterostilbene and its inhibitory effects on colon cancer cells. Mol. Nutr. Food Res. 2016, 60, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Marier, J.F.; Vachon, P.; Gritsas, A.; Zhang, J.; Moreau, J.P.; Ducharme, M.P. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J. Pharmacol. Exp. Ther. 2002, 302, 369–373. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and its human metabolites—Effects on metabolic health and obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Del Rio, D. Gold Standards for Realistic (Poly)phenol Research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef] [PubMed]
- Calamini, B.; Ratia, K.; Malkowski, M.G.; Cuendet, M.; Pezzuto, J.M.; Santarsiero, B.D.; Mesecar, A.D. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem. J. 2010, 429, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Garcia AM, G.; Meyskens, F.L. Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [Google Scholar] [CrossRef]
- Lin, H.S.; Tringali, C.; Spatafora, C.; Wu, C.; Ho, P.C. A simple and sensitive HPLC-UV method for the quantification of piceatannol analog trans-3,5,3′,4′-tetramethoxystilbene in rat plasma and its application for a pre-clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 679–684. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Martinez, S.E.; Akinwumi, B.C.; Anderson, H.D.; Takemoto, J.K.; Sayre, C.L.; Davies, N.M. Preclinical Pharmacokinetics and Pharmacodynamics and Content Analysis of Gnetol in Foodstuffs. Phytother. Res. 2015, 29, 1168–1179. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene 169 Pharmacometrics of Pterostilbene: Preclinical Pharmacokinetics and Metabolism, Anticancer, Antiinflammatory, Antioxidant and Analgesic Activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Chang, T.K.H.; Lee, W.B.K.; Ko, H.H. Trans-resveratrol modulates the catalytic activity and mRNA expression of the procarcinogen-activating human cytochrome P450 1B1. Can. J. Physiol. Pharmacol. 2000, 78, 874–881. [Google Scholar] [CrossRef]
- Chun, Y.J.; Oh, Y.K.; Kim, B.J.; Kim, D.; Kim, S.S.; Choi, H.K.; Kim, M.Y. Potent inhibition of human cytochrome P450 1B1 by tetramethoxystilbene. Toxicol. Lett. 2009, 189, 84–89. [Google Scholar] [CrossRef]
- Lin, W.S.; Leland, J.V.; Ho, C.T.; Pan, M.H. Occurrence, Bioavailability, Anti-inflammatory, and Anticancer Effects of Pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Sun, C.; Chen, X.; Han, L.; Wang, T.; Liu, J.; Chen, X.; Zhao, D. Effect of pterostilbene, a natural derivative of resveratrol, in the treatment of colorectal cancer through top1/tdp1-mediated dna repair pathway. Cancers 2021, 13, 4002. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.; Chabert, P.; Stutzmann, J.; Coelho, D.; Fougerousse, A.; Gossé, F.; Launay, J.F.; Brouillard, R.; Raul, F. Resveratrol analog (Z)-3,5,4′-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int. J. Cancer 2003, 107, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H.; Way, T.D.; Chen, W.J. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Li, Y.; Hu, S.; Zhou, W.; Meng, Y.; Li, Z.; Zhang, Y.; Sun, J.; Bo, Z.; DePamphilis, M.L.; et al. DHS (trans−4,4′-dihydroxystilbene) suppresses DNA replication and tumor growth by inhibiting RRM2 (ribonucleotide reductase regulatory subunit M2). Oncogene 2019, 38, 2364–2379. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Moriyama, M.; Ninomiya, K.; Morikawa, T.; Hayakawa, T. Inhibitory Effects of Oligostilbenoids from the Bark of Shorea roxburghii on Malignant Melanoma Cell Growth: Implications for Novel Topical Anticancer Candidates. Biol. Pharm. Bull. 2016, 39, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Teli, M.K.; Lee, J.H.; Gaire, B.P.; Kim, M.H.; Kim, S.Y. A stilbenoid isorhapontigenin as a potential anti-cancer agent against breast cancer through inhibiting sphingosine kinases/tubulin stabilization. Cancers 2019, 11, 1947. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.A.; Patterson, L.H.; Wanogho, E.; Perry, P.J.; Butler, P.C.; Ijaz, T.; Ruparelia, K.C.; Lamb, J.H.; Farmer, P.B.; Stanley, L.A.; et al. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. Br. J. Cancer 2002, 86, 774–778. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari SN, A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, bioactivity, encapsulation and structural modifications. A review of their current limitations and promising approaches. Crit. Rev. Food Sci. Nutr. 2022, 63, 7269–7287. [Google Scholar] [CrossRef]
- Kita, Y.; Miura, Y.; Yagasaki, K. Antiproliferative and anti-invasive effect of Piceatannol, a polyphenol present in grapes and wine, against hepatoma AH109A cells. J. Biomed. Biotechnol. 2012, 2012, 672416. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Kim, J.; Khan, I.A.; Walker, L.A.; Khan, S.I. Nonsteroidal anti-inflammatory drug activated gene-1 (NAG-1) modulators from natural products as anti-cancer agents. Life Sci. 2014, 100, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Eling, T.E.; Baek, S.J.; Shim, M.; Lee, C.H. NSAID activated gene (NAG-1), a modulator of tumorigenesis—PubMed. J. Biochem. Mol. Biol. 2006, 39, 649–655. [Google Scholar]
- Chen, M.K.; Liu, Y.T.; Lin, J.T.; Lin, C.C.; Chuang, Y.C.; Lo, Y.S.; Hsi, Y.T.; Hsieh, M.J. Pinosylvin reduced migration and invasion of oral cancer carcinoma by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomed. Pharmacother. 2019, 117, 109160. [Google Scholar] [CrossRef]
- Song, J.; Seo, Y.; Park, H. Pinosylvin enhances leukemia cell death via down-regulation of AMPKα expression. Phytother. Res. 2018, 32, 2097–2104. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial activity of resveratrol structural analogues: A mechanistic evaluation of the structure-activity relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Väkeväinen, K.; Komulainen, H.; Rautiainen, M.; Smeds, A.; Raitanen, J.E.; Eklund, P.; Willför, S.; Alakomi, H.L.; Saarela, M.; et al. The antimicrobial effects of wood-associated polyphenols on food pathogens and spoilage organisms. Int. J. Food Microbiol. 2013, 164, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Mattio, L.; Catinella, G.; Iriti, M.; Vallone, L. Inhibitory activity of stilbenes against filamentous fungi. Ital. J. Food Saf. 2021, 10, 8461. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene derivatives as new perspective in antifungal medicinal chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, S.; Badavath, V.N.; Thakur, A.; Yin, N.; De Jonghe, S.; Acevedo, O.; Jochmans, D.; Leyssen, P.; Wang, K.; Neyts, J.; et al. Kobophenol A Inhibits Binding of Host ACE2 Receptor with Spike RBD Domain of SARS-CoV-2, a Lead Compound for Blocking COVID-19. J. Phys. Chem. Lett. 2021, 12, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Park, W.H.; Lee, S.J.; Moon, H.I. Antimalarial activity of a new stilbene glycoside from Parthenocissus tricuspidata in mice. Antimicrob. Agents Chemother. 2008, 52, 3451–3453. [Google Scholar] [CrossRef]
- Wu, J.; Li, B.; Xiao, W.; Hu, J.; Xie, J.; Yuan, J.; Wang, L. Longistylin A, a natural stilbene isolated from the leaves of Cajanus cajan, exhibits significant anti-MRSA activity. Int. J. Antimicrob. Agents 2020, 55, 105821. [Google Scholar] [CrossRef]
- Cave, E.; Crowther, N.J. The Use of 3T3-L1 Murine Preadipocytes as a Model of Adipogenesis. Methods Mol. Biol. 2019, 1916, 263–272. [Google Scholar] [PubMed]
- Zhang, H.; Matsuda, H.; Yamashita, C.; Nakamura, S.; Yoshikawa, M. Hydrangeic acid from the processed leaves of Hydrangea macrophylla var. thunbergii as a new type of anti-diabetic compound. Eur. J. Pharmacol. 2009, 606, 255–261. [Google Scholar]
- Tomino, Y. Lessons from the KK-Ay mouse, a spontaneous animal model for the treatment of human type 2 diabetic nephropathy. Nephrourol. Mon. 2012, 4, 524–529. [Google Scholar] [CrossRef]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant Activity and Inhibition of r-Glucosidase by trans-Resveratrol, Piceid, and a Novel trans-Stilbene from the Roots of Israeli Rumex bucephalophorus L. J. Agric. Food Chem. 2006, 54, 1243–1247. [Google Scholar] [CrossRef]
- Pereira, A.C.; Arruda, M.S.; da Silva, E.A.; da Silva, M.N.; Lemos, V.S.; Cortes, S.F. Inhibition of α-glucosidase and hypoglycemic effect of stilbenes from the Amazonian plant Deguelia rufescens var. urucu (Ducke) A.M.G. Azevedo (Leguminosae). Planta Med. 2012, 78, 36–38. [Google Scholar] [CrossRef]
- Colica, C.; Milanović, M.; Milić, N.; Aiello, V.; De Lorenzo, A.; Abenavoli, L. A Systematic Review on Natural Antioxidant Properties of Resveratrol. Nat. Prod. Commun. 2018, 13, 1195–1203. [Google Scholar] [CrossRef]
- Li, X.; Dai, Y.; Yan, S.; Shi, Y.; Li, J.; Liu, J.; Cha, L.; Mu, J. Resveratrol lowers blood pressure in spontaneously hypertensive rats via calcium-dependent endothelial NO production. Clin. Exp. Hypertens. 2016, 38, 287–293. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef]
- Riche, D.M.; Riche, K.D.; Blackshear, C.T.; McEwen, C.L.; Sherman, J.J.; Wofford, M.R.; Griswold, M.E. Pterostilbene on metabolic parameters: A randomized, double-blind, and placebo-controlled trial. Evid.-Based Complement. Altern. Med. 2014, 2014, 459165. [Google Scholar] [CrossRef]
- Wongwat, T.; Srihaphon, K.; Pitaksutheepong, C.; Boonyo, W.; Pitaksuteepong, T. Suppression of inflammatory mediators and matrix metalloproteinase (MMP)-13 by Morus alba stem extract and oxyresveratrol in RAW 264.7 cells and C28/I2 human chondrocytes. J. Tradit. Complement. Med. 2020, 10, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Sánchez-Fidalgo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef]
- Shi, G.; Hua, M.; Xu, Q.; Ren, T. Resveratrol improves treatment outcome and laboratory parameters in patients with Takayasu arteritis: A randomized double-blind and placebo-controlled trial. Immunobiology 2017, 222, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Ko, M.C.; Chen, B.Y.; Huang, J.C.; Hsieh, C.W.; Lee, M.C.; Chiou, R.Y.; Wung, B.S.; Peng, C.H.; Yang, Y.L. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.S.; Zhou, H.; Wilson, B.; Hong, J.S.; Gao, H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Raederstorff, D.; Howe, P.R.C. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016, 8, 425. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Nealon, R.S.; Scholey, A.; Howe, P.R.C. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef]
- Simão, F.; Matté, A.; Pagnussat, A.S.; Netto, C.A.; Salbego, C.G. Resveratrol prevents CA1 neurons against ischemic injury by parallel modulation of both GSK-3β and CREB through PI3-K/Akt pathways. Eur. J. Neurosci. 2012, 36, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol improves cognition and reduces oxidative stress in rats with vascular dementia. Neural Regen. Res. 2013, 8, 2050–2059. [Google Scholar] [PubMed]
- Naik, B.; Nirwane, A.; Majumdar, A. Pterostilbene ameliorates intracerebroventricular streptozotocin induced memory decline in rats. Cogn. Neurodyn. 2017, 11, 35–49. [Google Scholar] [CrossRef]
- Ban, J.Y.; Jeon, S.Y.; Nguyen, T.T.; Bae, K.; Song, K.S.; Seong, Y.H. Neuroprotective Effect of Oxyresveratrol from Smilacis Chinae Rhizome on Amyloid b Protein (25–35)-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Biol. Pharm. Bull. 2006, 29, 2419–2424. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Spina, M.G.; Lorenz, P.; Ebmeyer, U.; Wolf, G.; Horn, T.F. Oxyresveratrol (trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Res. 2004, 1017, 98–107. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in disease: Timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Igarashi, K.; Hayashi, N.; Nishizawa, M.; Yamamoto, M. Cloning and Characterization of a Novel Erythroid Cell-Derived CNC Family Transcription Factor Heterodimerizing with the Small Maf Family Proteins. Mol. Cell. Biol. 1995, 15, 4184–4193. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Yamamoto, M. The KEAP1NRF2 system in cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004, 279, 31556–31567. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The Carboxy-Terminal Neh3 Domain of Nrf2 Is Required for Transcriptional Activation. Mol. Cell Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.; Killeen, E.; Naquin, R.; Alam, S.; Alam, J. Degradation of transcription factor Nrf2 via the ubiquitin-proteasome pathway and stabilization by cadmium. J. Biol. Chem. 2003, 278, 2396–2402. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Jaiswal, A.K. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J. Biol. Chem. 2010, 285, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shelton, P.; Jaiswal, A.K. The transcription factor NF-E2-related factor 2 (nrf2): A protooncogene? FASEB J. 2013, 27, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme Oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonenn, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Kokot, A.; Metze, D.; Mouchet, N.; Galibert, M.D.; Schiller, M.; Luger, T.A.; Böhm, M. α-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology 2009, 150, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Shin, S.; Slocum, S.L.; Agoston, E.S.; Wakabayashi, J.; Kwak, M.K.; Misra, V.; Biswal, S.; Yamamoto, M.; Kensler, T.W. Regulation of Notch1 signaling by Nrf2: Implications for tissue regeneration. Sci. Signal 2010, 3, ra52. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive compounds in oxidative stress-mediated diseases: Targeting the nrf2/are signaling pathway and epigenetic regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef] [PubMed]
- Niesen, D.B.; Hessler, C.; Seeram, N.P. Beyond resveratrol: A review of natural stilbenoids identified from 2009-2013. J. Berry Res. 2013, 3, 181–196. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef]
- Dai, M.; Yuan, D.; Lei, Y.; Li, J.; Ren, Y.; Zhang, Y.; Cang, H.; Gao, W.; Tang, Y. Expression, purification and structural characterization of resveratrol synthase from Polygonum cuspidatum. Protein Expr Purif. 2022, 191, 106024. [Google Scholar] [CrossRef]
- Dos Santos, F.A.R.; Xavier, J.A.; da Silva, F.C.; Merlin, J.P.J.; Goulart, M.O.F.; Rupasinghe, H.P.V. Antidiabetic, Antiglycation, and Antioxidant Activities of Ethanolic Seed Extract of Passiflora edulis and Piceatannol In Vitro. Molecules 2022, 27, 4064. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Averilla, J.N.; Kim, H.J.; Kim, J.S.; Kim, J.S. Grape Peel Extract and Resveratrol Inhibit Wrinkle Formation in Mice Model Through Activation of Nrf2/HO-1 Signaling Pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xie, X.; Yuan, L.; Qiu, J.; Duan, W.; Xu, B.; Chen, X. Resveratrol ameliorates rheumatoid arthritis via activation of SIRT1-Nrf2 signaling pathway. BioFactors 2020, 46, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yan, Y.; He, X.Y.; Yang, H.; Liang, B.; Wang, J.; He, Y.; Ding, Y.; Yu, H. Effects of Resveratrol on the Mechanisms of Antioxidants and Estrogen in Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 8983752. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxidative Med. Cell Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol accelerates wound healing by attenuating oxidative stress-induced impairment of cell proliferation and migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Xun, W.; Fu, Q.; Shi, L.; Cao, T.; Jiang, H.; Ma, Z. Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. Int. Immunopharmacol. 2021, 99, 107989. [Google Scholar] [CrossRef]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Babaei, K.R.; Namvarjah, F.; Izadi, P.; Meshkani, R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, L.; Wang, R.; Chen, T.; Liu, X.; Jin, F. 3,5,4′-Tri-O-acetylresveratrol attenuates seawater inhalation-induced acute respiratory distress syndrome via thioredoxin 1 pathway. Int. J. Mol. Med. 2018, 41, 3493–3500. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X. Dietary resveratrol alleviated lipopolysaccharide-induced ileitis through Nrf2 and NF-κB signalling pathways in ducks (Anas platyrhynchos). J. Anim. Physiol. Anim. Nutr. 2022, 106, 1306–1320. [Google Scholar] [CrossRef]
- Rasheed, M.S.U.; Tripathi, M.K.; Patel, D.K.; Singh, M.P. Resveratrol Regulates Nrf2-Mediated Expression of Antioxidant and Xenobiotic Metabolizing Enzymes in Pesticides-Induced Parkinsonism. Protein Pept. Lett. 2020, 27, 1038–1045. [Google Scholar] [CrossRef]
- Recalde, M.D.; Miguel, C.A.; Noya-Riobó, M.V.; González, S.L.; Villar, M.J.; Coronel, M.F. Resveratrol exerts anti-oxidant and anti-inflammatory actions and prevents oxaliplatin-induced mechanical and thermal allodynia. Brain Res. 2020, 1748, 147079. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, X.; Zhao, L.; Li, Z.; Liu, B. Resveratrol Prevents Diabetic Cardiomyopathy by Increasing Nrf2 Expression and Transcriptional Activity. BioMed Res. Int. 2018, 2018, 2150218. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Hu, S.; Ge, S.; Jia, M.; Wang, N. Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. Int. Immunopharmacol. 2021, 93, 107426. [Google Scholar] [CrossRef]
- Trusov, N.V.; Semin, M.O.; Shipelin, V.A.; Apryatin, S.A.; Gmoshinski, I.V. Liver gene expression in normal and obese rats received resveratrol and L-carnitine. Vopr. Pitan. 2021, 90, 25–37. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, F.; Liu, M.; Xue, J.; Huang, H. Resveratrol ameliorates sepsis-induced acute kidney injury in a pediatric rat model via Nrf2 signaling pathway. Exp. Ther. Med. 2018, 16, 3233–3240. [Google Scholar] [CrossRef]
- Wu, M.; Ma, L.; Xue, L.; Ye, W.; Lu, Z.; Li, X.; Jin, Y.; Qin, X.; Chen, D.; Tang, W.; et al. Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging 2019, 11, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M.; Guan, P.; Luo, L.F.; Qin, L.Y.; Wang, N.; Zhao, Y.S.; Ji, E.S. Resveratrol protects against CIH-induced myocardial injury by targeting Nrf2 and blocking NLRP3 inflammasome activation. Life Sci. 2020, 245, 117362. [Google Scholar] [CrossRef]
- Kabel, A.M.; Atef, A.; Estfanous, R.S. Ameliorative potential of sitagliptin and/or resveratrol on experimentally-induced clear cell renal cell carcinoma. Biomed. Pharmacother. 2018, 97, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Zhang, S.; Huang, J.; Lin, T.; Lin, Q. Resveratrol Attenuates Intermittent Hypoxia-Induced Lung Injury by Activating the Nrf2/ARE Pathway. Lung 2020, 198, 323–331. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Liu, M.; Liu, X.; Jiao, Y.; Jin, S.; Shan, A.; Feng, X. Effects of dietary resveratrol supplementation on growth performance and anti-inflammatory ability in ducks (Anas platyrhynchos) through the nrf2/ho-1 and tlr4/nf-κb signaling pathways. Animals 2021, 11, 3588. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Wang, J.; Wang, X.; Chen, C.; Yin, D.; Zhao, F.; Yin, J.; Guo, M.; Zhang, L.; et al. Resveratrol represses estrogen-induced mammary carcinogenesis through NRF2-UGT1A8-estrogen metabolic axis activation. Biochem. Pharmacol. 2018, 155, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhao, X.; Fu, J.; Wang, X. Resveratrol increase myocardial Nrf2 expression in type 2 diabetic rats and alleviate myocardial ischemia/reperfusion injury (MIRI). Ann. Palliat. Med. 2019, 8, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, V.; Palomera-Ávalos, V.; López-Ruiz, S.; Canudas, A.M.; Pallàs, M.; Griñán-Ferré, C. Maternal resveratrol supplementation prevents cognitive decline in senescent mice offspring. Int. J. Mol. Sci. 2019, 20, 1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Xiong, Q.; Zhou, M.; Tian, X.; Yue, K.; Li, Y.; Shu, X.; Ru, Q. Resveratrol attenuates methamphetamine-induced memory impairment via inhibition of oxidative stress and apoptosis in mice. J. Food Biochem. 2021, 45, e13622. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.; Wang, T.; Tong, C.; Liu, Y.; Shi, L.; Mao, S.; Shi, X.; Jin, H.; Liu, Y.; Hou, M. Resveratrol ameliorates thoracic blast exposure-induced inflammation, endoplasmic reticulum stress and apoptosis in the brain through the Nrf2/Keap1 and NF-κB signaling pathway. Injury 2021, 52, 2795–2802. [Google Scholar] [CrossRef]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, 840–846. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Z.; Li, C.; Pan, Y.; Tang, X.; Wang, X.J. Synthetic Imine Resveratrol Analog 2-Methoxyl-3,6-Dihydroxyl-IRA Ameliorates Colitis by Activating Protective Nrf2 Pathway and Inhibiting NLRP3 Expression. Oxidative Med. Cell Longev. 2019, 2019, 7180284. [Google Scholar] [CrossRef] [PubMed]
- Pierre, C.J.; Azeez, T.A.; Rossetti, M.L.; Gordon, B.S.; La Favor, J.D. Long-term administration of resveratrol and MitoQ stimulates cavernosum antioxidant gene expression in a mouse castration model of erectile dysfunction. Life Sci. 2022, 310, 121082. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Chen, X.; Geng, Z.; Hu, H.; Zhang, C. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals 2021, 11, 1427. [Google Scholar] [CrossRef]
- Ma, S.; Feng, J.; Zhang, R.; Chen, J.; Han, D.; Li, X.; Yang, B.; Li, X.; Fan, M.; Li, C.; et al. SIRT1 Activation by Resveratrol Alleviates Cardiac Dysfunction via Mitochondrial Regulation in Diabetic Cardiomyopathy Mice. Oxidative Med. Cell Longev. 2017, 2017, 4602715. [Google Scholar] [CrossRef]
- Wang, X.; Fang, H.; Xu, G.; Yang, Y.; Xu, R.; Liu, Q.; Xue, X.; Liu, J.; Wang, H. Resveratrol prevents cognitive impairment in type 2 diabetic mice by upregulating Nrf2 expression and transcriptional level. Diabetes Metab. Syndr. Obes. 2020, 13, 1061–1075. [Google Scholar] [CrossRef]
- Ikeda, E.; Tanaka, D.; Glogauer, M.; Tenenbaum, H.C.; Ikeda, Y. Healing effects of monomer and dimer resveratrol in a mouse periodontitis model. BMC Oral Health 2022, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-induced kidney injury in mice is counteracted by a flavonoid-rich extract of bergamot juice, alone or in association with curcumin and resveratrol, via the enhancement of different defense mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.N.; Lu, H.P.; Peng, Y.L.; Zhang, B.S.; Gong, X.B.; Su, J.; Zhou, Y.; Pan, M.H.; Xu, L. Oxyresveratrol prevents lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Int. Immunopharmacol. 2018, 56, 105–112. [Google Scholar] [CrossRef]
- Seo, Y.; Park, J.; Choi, W.; Ju Son, D.; Sung Kim, Y.; Kim, M.K.; Yoon, B.E.; Pyee, J.; Tae Hong, J.; Go, Y.M.; et al. Antiatherogenic Effect of Resveratrol Attributed to Decreased Expression of ICAM-1 (Intercellular Adhesion Molecule-1). Arterioscler. Thromb. Vasc. Biol. 2019, 39, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, H.; Lin, Q.; Liu, X.; Cheng, Y.; Deng, B. Anti-inflammatory effect of resveratrol attenuates the severity of diabetic neuropathy by activating the Nrf2 pathway. Aging 2021, 13, 10659–10671. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Stefański, T.; Sobiak, S.; Cichocki, M.; Baer-Dubowska, W. The effect of resveratrol and its methylthio-derivatives on the Nrf2-ARE pathway in mouse epidermis and HaCaT keratinocytes. Cell Mol. Biol. Lett. 2014, 19, 500–516. [Google Scholar] [CrossRef]
- Zhou, Y.; Lan, R.; Xu, Y.; Zhou, Y.; Lin, X.; Miao, J. Resveratrol alleviates oxidative stress caused by Streptococcus uberis infection via activating the Nrf2 signaling pathway. Int. Immunopharmacol. 2020, 89, 107076. [Google Scholar] [CrossRef]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates Inflammation and Oxidative Stress Induced by Myocardial Ischemia-Reperfusion Injury: Role of Nrf2/ARE Pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420–10428. [Google Scholar] [PubMed]
- Abd El-Fattah, A.A.; Fahim, A.T.; Sadik, N.A.H.; Ali, B.M. Resveratrol and curcumin ameliorate di-(2-ethylhexyl) phthalate induced testicular injury in rats. Gen. Comp. Endocrinol. 2016, 225, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.S.; Ayo, J.O.; Danjuma, N.M.; AbdulWahab, A.; Isa, A.S.; Umar, A.H. Modulation of Memory and Neurochemical Changes by Resveratrol and Environmental Enrichment in Rodent Model of Alzheimer’s Disease. Niger. J. Physiol. Sci. 2022, 37, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, E.M.; Ahmed, K.A.; Abdelmonem, M. Resveratrol mitigates diclofenac-induced hepatorenal toxicity in rats via modulation of miR-144/Nrf2/GSH axis. J. Biochem. Mol. Toxicol. 2022, 36, e23129. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Li, Y.; Cao, L.; Du, J.; Zheng, T.; Qian, H.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, anti-inflammatory and hepatoprotective effects of resveratrol on oxidative stress-induced liver damage in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 215, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.S.; Cheng, Y.H.; Lee, C.Y.; Chung, C.Y.; Chang, W.C. Resveratrol protects against methylglyoxal-induced hyperglycemia and pancreatic damage in vivo. Nutrients 2015, 7, 2850–2865. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol relieved acute liver damage in ducks (Anas platyrhynchos) induced by afb1 via modulation of apoptosis and nrf2 signaling pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef]
- Zhou, N.; Tian, Y.; Liu, W.; Tu, B.; Xu, W.; Gu, T.; Zou, K.; Lu, L. Protective Effects of Resveratrol and Apigenin Dietary Supplementation on Serum Antioxidative Parameters and mRNAs Expression in the Small Intestines of Diquat-Challenged Pullets. Front. Vet. Sci. 2022, 9, 850769. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, R.; Man, K.; Yang, X.B. Enhancing osteogenic potential of hDPSCs by resveratrol through reducing oxidative stress via the Sirt1/Nrf2 pathway. Pharm. Biol. 2022, 60, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Chang, J.C.; Lin, W.Y.; Li, C.C.; Hsieh, M.; Chen, H.W.; Wang, T.S.; Wu, W.T.; Liu, C.S.; Liu, K.L. Caffeic acid and resveratrol ameliorate cellular damage in cell and Drosophila models of spinocerebellar ataxia type 3 through upregulation of Nrf2 pathway. Free Radic. Biol. Med. 2018, 115, 309–317. [Google Scholar] [CrossRef]
- Hussein, M.M.A.; Mahfouz, M.K. Effect of resveratrol and rosuvastatin on experimental diabetic nephropathy in rats. Biomed. Pharmacother. 2016, 82, 685–692. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Wang, S.; Zhang, C.; Zheng, L.; Jia, Y.; Xu, M.; Zhu, T.; Zhang, Y.; Rong, R. Resveratrol Alleviates Inflammatory Responses and Oxidative Stress in Rat Kidney Ischemia-Reperfusion Injury and H2O2-Induced NRK-52E Cells via the Nrf2/TLR4/NF-κB Pathway. Cell. Physiol. Biochem. 2018, 45, 1677–1689. [Google Scholar] [CrossRef]
- Tamaki, N.; Orihuela-Campos, C.R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ma, Y.; Jin, J.; Wang, X. Activation of AMPK/p38/Nrf2 is involved in resveratrol alleviating myocardial ischemia-reperfusion injury in diabetic rats as an endogenous antioxidant stress feedback. Ann. Transl. Med. 2022, 10, 890. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, R.; Wang, J.; Yang, X.; Wen, L.; Feng, J. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via nrf2/ho-1 pathway. Pharm. Biol. 2018, 56, 440–449. [Google Scholar] [CrossRef]
- Li, X.N.; Ma, L.Y.; Ji, H.; Qin, Y.H.; Jin, S.S.; Xu, L.X. Resveratrol protects against oxidative stress by activating the keap-1/nrf2 antioxidant defense system in obese-asthmatic rats. Exp. Ther. Med. 2018, 16, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, G.; Chen, L.; Ding, Y.; Lian, J.; Hong, G.; Lu, Z. Resveratrol protects mice from paraquat-induced lung injury: The important role of SIRT1 and NRF2 antioxidant pathways. Mol. Med. Rep. 2016, 13, 1833–1838. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways Downloaded from. Eccles. Health Sci. Lib-Serials 2014, 35, 1872–1880. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Q.; Li, J.; Xiao, M.; Gao, T.; Zhang, X.; Lu, G.; Wang, J.; Guo, Y.; Wen, P.; et al. Co-Treatment with Resveratrol and FGF1 Protects against Acute Liver Toxicity After Doxorubicin Treatment via the AMPK/NRF2 Pathway. Front. Pharmacol. 2022, 13, 940406. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Q.; Gao, T.; Li, J.; Zhang, J.; Chen, O.; Cao, C.; Mao, M.; Xiao, M.; Zhang, X.; et al. Resveratrol and FGF1 Synergistically Ameliorates Doxorubicin-Induced Cardiotoxicity via Activation of SIRT1-NRF2 Pathway. Nutrients 2022, 14, 4017. [Google Scholar] [CrossRef]
- Meng, Q.; Guo, T.; Li, G.; Sun, S.; He, S.; Cheng, B.; Shi, B.; Shan, A. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J. Anim. Sci. Biotechnol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Celi, P.; Zhuo, Y.; Ding, X.; Zeng, Q.; Bai, S.; Xu, S.; Yin, H.; Lv, L.; et al. Resveratrol Alleviating the Ovarian Function Under Oxidative Stress by Alternating Microbiota Related Tryptophan-Kynurenine Pathway. Front. Immunol. 2022, 13, 911381. [Google Scholar] [CrossRef]
- Achy-Brou CA, A.; Billack, B. Lipopolysaccharide Attenuates the Cytotoxicity of Resveratrol in Transformed Mouse Macrophages. Plant Foods Human. Nutr. 2016, 71, 272–276. [Google Scholar] [CrossRef]
- Li, H.; Shen, Y.; Sun, W.; Xiao, H. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar]
- Hosoda, R.; Hamada, H.; Uesugi, D.; Iwahara, N.; Nojima, I.; Horio, Y.; Kuno, A. Different antioxidative and antiapoptotic effects of piceatannol and resveratrol. J. Pharmacol. Exp. Ther. 2021, 376, 385–396. [Google Scholar] [CrossRef]
- Chen, S.; Tamaki, N.; Kudo, Y.; Tsunematsu, T.; Miki, K.; Ishimaru, N.; Ito, H.O. Protective effects of resveratrol against 5-fluorouracil-induced oxidative stress and inflammatory responses in human keratinocytes. J. Clin. Biochem. Nutr. 2021, 69, 238–246. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transpl. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef] [PubMed]
- Daverey, A.; Agrawal, S.K. Pre and post treatment with curcumin and resveratrol protects astrocytes after oxidative stress. Brain Res. 2018, 1692, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Caldeira-Dias, M.; Viana-Mattioli, S.; Machado, J.S.R.; Carlström, M.; Cavalli, R.C.; Sandrim, V.C. Resveratrol and grape juice: Effects on redox status and nitric oxide production of endothelial cells in in vitro preeclampsia model. Pregnancy Hypertens. 2021, 23, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Afzal, S.; Zheng, Y.F.; Münch, G.; Li, C.G. Synergistic Protective Effect of Curcumin and Resveratrol against Oxidative Stress in Endothelial EAhy926 Cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 2661025. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2–Keap1 pathway–mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide–treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef]
- Leong, C.W.; Wong, C.H.; Lao, S.C.; Leong, E.C.; Lao, I.F.; Law, P.T.; Fung, K.P.; Tsang, K.S.; Waye, M.M.; Tsui, S.K.; et al. Effect of resveratrol on proliferation and differentiation of embryonic cardiomyoblasts. Biochem Biophys Res Commun. 2007, 360, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sabzevary-Ghahfarokhi, M.; Soltani, A.; Luzza, F.; Larussa, T.; Rahimian, G.; Shirzad, H.; Bagheri, N. The protective effects of resveratrol on ulcerative colitis via changing the profile of Nrf2 and IL-1β protein. Mol. Biol. Rep. 2020, 47, 6941–6947. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.C.; Nicol, C.J.B.; Lo, S.S.; Hung, S.W.; Wang, C.J.; Lin, C.H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 11678. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, L.; Wang, J.; Zhao, B.; Pan, J.; Wang, L.; Liu, X.; Liu, X.; Liu, Z. Resveratrol Prevents Acrylamide-Induced Mitochondrial Dysfunction and Inflammatory Responses via Targeting Circadian Regulator Bmal1 and Cry1 in Hepatocytes. J. Agric. Food Chem. 2019, 67, 8510–8519. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Yu, H.; Shan, A.; Shen, J.; Zhou, C.; Zhao, Y.; Fang, H.; Wang, X.; Wang, J.; et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway. Toxicon 2019, 164, 10–15. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Feng, Z.; Bergan, R.; Li, B.; Qin, Y.; Zhao, L.; Zhang, Z.; Shi, M. Involvement of the PI3K/Akt/Nrf2 Signaling Pathway in Resveratrol-Mediated Reversal of Drug Resistance in HL-60/ADR Cells. Nutr. Cancer 2019, 71, 1007–1018. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yang, J.; He, Y.; Wan, H.; Li, C. Analogs of imine resveratrol alleviate oxidative stress-induced neurotoxicity in PC12 cells via activation of Nrf2. FEBS Open Bio 2021, 11, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, X.; Tao, Z.; Wang, X.J.; Pan, Y. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, E.Y.; Ha, H.K.; Jo, C.M.; Lee, W.J.; Lee, S.S.; Kim, J.W. Resveratrol-loaded nanoparticles induce antioxidant activity against oxidative stress. Asian-Australas. J. Anim. Sci. 2016, 29, 288–298. [Google Scholar] [CrossRef]
- Csiszár, A.; Csiszar, A.P.J.T.; Gautam, T.; Kleusch, C.; Hoffmann, B.; Tucsek, Z.; Toth, P.; Sonntag, W.E.; Ungvari, Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 303–313. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Moghadam, D.; Zarei, R.; Vakili, S.; Ghojoghi, R.; Zarezade, V.; Veisi, A.; Sabaghan, M.; Azadbakht, O.; Behrouj, H. The effect of natural polyphenols Resveratrol, Gallic acid, and Kuromanin chloride on human telomerase reverse transcriptase (hTERT) expression in HepG2 hepatocellular carcinoma: Role of SIRT1/Nrf2 signaling pathway and oxidative stress. Mol. Biol. Rep. 2023, 50, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Wang, J.; Zhang, X.; Wang, J.; Li, J.; Liu, Q.; Lu, G.; Xiao, M.; Gao, T.; Guo, Y.; et al. Resveratrol Attenuates High Glucose-Induced Osteoblast Dysfunction via AKT/GSK3β/FYN-Mediated NRF2 Activation. Front. Pharmacol. 2022, 13, 862618. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, M.; Shen, Y.; Kang, L.; Zhu, X.; Dong, W.; Lei, X. Resveratrol Attenuates Hyperoxia Lung Injury in Neonatal Rats by Activating SIRT1/PGC-1α Signaling Pathway. Am. J. Perinatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, M.N.; Deng, J.; Zhang, C.H.; Liu, M.W. Resveratrol exhibits neuroprotection against paraquat-induced PC12 cells via heme oxygenase 1 upregulation by decreasing MiR-136-5p expression. Bioengineered 2022, 13, 7065–7081. [Google Scholar] [CrossRef] [PubMed]
- Gurusinghe, S.; Cox, A.G.; Rahman, R.; Chan, S.T.; Muljadi, R.; Singh, H.; Leaw, B.; Mockler, J.C.; Marshall, S.A.; Murthi, P.; et al. Resveratrol mitigates trophoblast and endothelial dysfunction partly via activation of nuclear factor erythroid 2-related factor-2. Placenta 2017, 60, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Xue, M.; Weickert, M.O.; Thornalley, P.J. Reversal of insulin resistance in overweight and obese subjects by trans-resveratrol and hesperetin combination—Link to dysglycemia, blood pressure, dyslipidemia, and low-grade inflammation. Nutrients 2021, 13, 2374. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.O.; Rizzetto, F.; Grimmer, G.H.; Ribeiro-Alves, M.; Daleprane, J.B.; Carraro-Eduardo, J.C.; Mafra, D. Effects of Resveratrol Supplementation in Nrf2 and NF-κB Expressions in Nondialyzed Chronic Kidney Disease Patients: A Randomized, Double-Blind, Placebo-Controlled, Crossover Clinical Trial. J. Ren. Nutr. 2016, 26, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Spath, E.; Kromp, K. Components of red sandalwood. III. The synthesis of pterostilbene. Ber. Dtsch. Chem. Ges. B 1941, 74, 189–192. [Google Scholar]
- Spath, E.; Schlager, J. On the constituents of ‘Red Sandalwood’ [Pterocarpus santalinus]. 2: The constitution of pterostilbene. Ber. Dtsch. Chem. Ges. B 1941, 73, 881–884. [Google Scholar]
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of pterostilbene as a phytoalexin from vitis vinifera leaves. Phytochemistry 1979, 18, 1025–1027. [Google Scholar] [CrossRef]
- Rimando, A.M.; Kalt, W.; Magee, J.B.; Dewey, J.; Ballington, J.R. Resveratrol, pterostilbene, and piceatannol in Vaccinium berries. J. Agric. Food Chem. 2004, 52, 4713–4719. [Google Scholar] [CrossRef]
- Medina-Bolivar, F.; Condori, J.; Rimando, A.M.; Hubstenberger, J.; Shelton, K.; O’Keefe, S.F.; Bennett, S.; Dolan, M.C. Production and secretion of resveratrol in hairy root cultures of peanut. Phytochemistry 2007, 68, 1992–2003. [Google Scholar] [CrossRef]
- Cassiano, C.; Eletto, D.; Tosco, A.; Riccio, R.; Monti, M.C.; Casapullo, A. Determining the effect of pterostilbene on insulin secretion using chemoproteomics. Molecules 2020, 25, 2885. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, K.H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef] [PubMed]
- Bhakkiyalakshmi, E.; Sireesh, D.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via Nrf2 signaling in experimental diabetes. Eur. J. Pharmacol. 2016, 777, 9–16. [Google Scholar] [CrossRef]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene activates the Nrf2-dependent antioxidant response to ameliorate arsenic-induced intracellular damage and apoptosis in human keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Bhakkiyalakshmi, E.; Shalini, D.; Sekar, T.V.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. Therapeutic potential of pterostilbene against pancreatic beta-cell apoptosis mediated through Nrf2. Br. J. Pharmacol. 2014, 171, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, H.; Ho, Z.Y.; Dai, X.Y.; Chen, Q.; Li, R.; Liang, B.; Zhu, H. Pterostilbene’s protective effects against photodamage caused by UVA/UVB irradiation. Pharmazie 2018, 73, 651–658. [Google Scholar] [PubMed]
- Li, H.; Jiang, N.; Liang, B.; Liu, Q.; Zhang, E.; Peng, L.; Deng, H.; Li, R.; Li, Z.; Zhu, H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. Report. 2017, 22, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Xiao, W.; Gu, W.T.; Zhang, Z.T.; Xu, S.H.; Chen, Z.Q.; Xu, Y.H.; Zhang, L.Y.; Wang, S.M.; Nie, H. Pterostilbene prevents methylglyoxal-induced cytotoxicity in endothelial cells by regulating glyoxalase, oxidative stress and apoptosis. Food Chem. Toxicol. 2021, 153, 112244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Yeh, C.T.; Kuo, K.T.; Yadav, V.K.; Fong, I.H.; Kounis, N.G.; Hu, P.; Hung, M.Y. Pterostilbene increases LDL metabolism in HL-1 cardiomyocytes by modulating the PCSK9/HNF1α/SREBP2/LDLR signaling cascade, upregulating epigenetic hsa-miR-335 and hsa-miR-6825, and LDL receptor expression. Antioxidants 2021, 10, 1280. [Google Scholar] [CrossRef]
- Xu, C.; Song, Y.; Wang, Z.; Jiang, J.; Piao, Y.; Li, L.; Jin, S.; Li, L.; Zhu, L.; Yan, G. Pterostilbene suppresses oxidative stress and allergic airway inflammation through AMPK/Sirt1 and Nrf2/HO-1 pathways. Immun. Inflamm. Dis. 2021, 9, 1406–1417. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Q.; Mi, Y.; Zhou, D.; Meng, Q.; Chen, G.; Li, N.; Hou, Y. Pterostilbene Alleviates Aβ1-42-Induced Cognitive Dysfunction via Inhibition of Oxidative Stress by Activating Nrf2 Signaling Pathway. Mol. Nutr. Food Res. 2021, 65, 2000711. [Google Scholar] [CrossRef]
- Kosuru, R.; Kandula, V.; Rai, U.; Prakash, S.; Xia, Z.; Singh, S. Pterostilbene Decreases Cardiac Oxidative Stress and Inflammation via Activation of AMPK/Nrf2/HO-1 Pathway in Fructose-Fed Diabetic Rats. Cardiovasc. Drugs Ther. 2018, 32, 147–163. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Gowrisankar, Y.V.; Wang, L.W.; Zhang, Y.Z.; Chen, X.Z.; Huang, P.J.; Yen, H.R.; Yang, H.L. The in vitro and in vivo depigmenting activity of pterostilbene through induction of autophagy in melanocytes and inhibition of UVA-irradiated α-MSH in keratinocytes via Nrf2-mediated antioxidant pathways. Redox Biol. 2021, 44, 102007. [Google Scholar] [CrossRef]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988–42000. [Google Scholar] [CrossRef]
- Zhang, E.; Huang, J.; Wang, K.; Yu, Q.; Zhu, C.; Ren, H. Pterostilbene Protects against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Upregulating the Nrf2 Pathway and Inhibiting NF-κB, MAPK, and NLRP3 Inflammasome Activation. J. Med. Food 2020, 23, 952–960. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Z.; Jiang, A.; Wu, D.; Li, S.; Liu, Z.; Wei, Z.; Yang, Z.; Guo, C. Protective Effects of Pterostilbene on Lipopolysaccharide-Induced Acute Lung Injury in Mice by Inhibiting NF-κB and Activating Nrf2/HO-1 Signaling Pathways. Front. Pharmacol. 2021, 11, 591836. [Google Scholar] [CrossRef]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells in Vivo: A Physiological Glucocorticoids-and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016, 24, 974–990. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L.; Huang, J.; Lv, H.; Deng, X.; Ci, X. Pterostilbene Reduces Acetaminophen-Induced Liver Injury by Activating the Nrf2 Antioxidative Defense System via the AMPK/Akt/GSK3β Pathway. Cell. Physiol. Biochem. 2018, 49, 1943–1958. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, D.; Ortiz, V.; Türck, P.; Campos-Carraro, C.; Zimmer, A.; Teixeira, R.; Bianchi, S.; de Castro, A.L.; Schenkel, P.C.; Belló-Klein, A.; et al. Stilbenoid pterostilbene complexed with cyclodextrin preserves left ventricular function after myocardial infarction in rats: Possible involvement of thiol proteins and modulation of phosphorylated GSK-3β. Free Radic. Res. 2018, 52, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Lian, W.; Wang, G.; Qiu, M.; Lin, L.; Zeng, R. Pterostilbene induces Nrf2/HO-1 and potentially regulates NF-κB and JNK–Akt/mTOR signaling in ischemic brain injury in neonatal rats. 3 Biotech 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Zhang, Y.; Wu, D.; Li, S.; Jiang, A.; Du, C.; Xie, G. Pterostilbene Exerts Hepatoprotective Effects through Ameliorating LPS/D-Gal-Induced Acute Liver Injury in Mice. Inflammation 2021, 44, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Millán, I.; Desco, M.D.C.; Torres-Cuevas, I.; Pérez, S.; Pulido, I.; Mena-Mollá, S.; Mataix, J.; Asensi, M.; Ortega, Á.L. Pterostilbene prevents early diabetic retinopathy alterations in a rabbit experimental model. Nutrients 2020, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador, R.; Marchio, P.; López-Blanch, R.; Jihad-Jebbar, A.; Rivera, P.; Vallés, S.L.; Banacloche, S.; Alcácer, J.; Colomer, N.; et al. Nicotinamide Riboside and Pterostilbene Cooperatively Delay Motor Neuron Failure in ALS SOD1G93A Mice. Mol. Neurobiol. 2021, 58, 1345–1371. [Google Scholar] [CrossRef] [PubMed]
- Dornadula, S.; Thiruppathi, S.; Palanisamy, R.; Umapathy, D.; Suzuki, T.K.; Mohanram, R. Differential proteomic profiling identifies novel molecular targets of pterostilbene against experimental diabetes. J. Cell Physiol. 2019, 234, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Duan, Z.; Xu, J.; Liang, J.; Zhang, S.; Zhang, H.; Zhang, X.; Wang, Y. Pterostilbene reduces endothelial cell injury in vascular arterial walls by regulating the Nrf2 mediated AMPK/STAT3 pathway in an atherosclerosis rat model. Exp. Ther. Med. 2019, 19, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Lu, W.; Zhang, K.; Zhou, W. Pterostilbene reduces endothelial cell apoptosis by regulation of the Nrf2 mediated TLR 4/MyD88/NF κB pathway in a rat model of atherosclerosis. Exp. Ther. Med. 2020, 20, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hua, C.; Yang, X.; Fan, X.; Song, H.; Peng, L.; Ci, X. Pterostilbene prevents LPS-induced early pulmonary fibrosis by suppressing oxidative stress, inflammation and apoptosis: In vivo. Food Funct. 2020, 11, 4471–4484. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Fu, S.; Ren, B.; Ma, L.; Sun, D. Based on Network Pharmacology and Gut Microbiota Analysis to Investigate the Mechanism of the Laxative Effect of Pterostilbene on Loperamide-Induced Slow Transit Constipation in Mice. Front. Pharmacol. 2022, 13, 913420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, F.; Zhang, X.; Liu, S.; Mu, P. SIRT1 Is Involved in the Neuroprotection of Pterostilbene against Amyloid β 25–35-Induced Cognitive Deficits in Mice. Front. Pharmacol. 2022, 13, 877098. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef]
- Zomer AP, L.; Rodrigues, C.A.; Maldaner, L. Piceatannol: Um estilbeno natural com um espectro amplo de atividades biológicas. Res. Soc. Dev. 2022, 11, e49211932221. [Google Scholar] [CrossRef]
- Krambeck, K.; Santos, D.; Sousa Lobo, J.M.; Amaral, M.H. Benefits of skin application of piceatannol—A minireview. Australas. J. Dermatol. 2023, 64, e21–e25. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Hao, Y.; Wang, Z.; Liu, J.; Wang, J. Piceatannol alleviate ROS-mediated PC-12 cells damage and mitochondrial dysfunction through SIRT3/FOXO3a signaling pathway. J. Food Biochem. 2022, 46, e13820. [Google Scholar] [CrossRef]
- Khan, I.; Preeti, K.; Kumar, R.; Khatri, D.K.; Singh, S.B. Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int Immunopharmacol. 2023, 116, 109793. [Google Scholar] [CrossRef] [PubMed]
- Kil, J.S.; Jeong, S.O.; Chung, H.T.; Pae, H.O. Piceatannol attenuates homocysteine-induced endoplasmic reticulum stress and endothelial cell damage via heme oxygenase-1 expression. Amino Acids 2017, 49, 735–745. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Gao, Y.; Niu, X.; Deng, X.; Wang, J.; Feng, H.; Guo, Z.; Qiu, J. Piceatannol Alleviates Clostridium perfringens Virulence by Inhibiting Perfringolysin O. Molecules 2022, 27, 5145. [Google Scholar] [CrossRef] [PubMed]
- Binmahfouz, L.S.; Eid, B.G.; Bagher, A.M.; Shaik, R.A.; Binmahfouz, N.S.; Abdel-Naim, A.B. Piceatannol SNEDDS Attenuates Estradiol-Induced Endometrial Hyperplasia in Rats by Modulation of NF-κB and Nrf2/HO-1 Axes. Nutrients 2022, 14, 1891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.H.; Chen, X.; Zhang, N.; Li, G. Piceatannol attenuates behavioral disorder and neurological deficits in aging mice: Via activating the Nrf2 pathway. Food Funct. 2018, 9, 371–378. [Google Scholar] [CrossRef]
- Shi, X.; Fu, L. Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des. Dev. Ther. 2019, 13, 2811–2824. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem. Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, S.A.; Azab, S.S.; Elsherbiny, D.A.; El-Demerdash, E. Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1331–1345. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Wang, Z.; Yu, L.L.; Wang, J. Piceatannol protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis through modulating PI3K/Akt Signaling Pathway. Nutrients 2019, 11, 1515. [Google Scholar] [CrossRef]
- Adiabouah Achy-Brou, C.A.; Billack, B. A comparative assessment of the cytotoxicity and nitric oxide reducing ability of resveratrol, pterostilbene and piceatannol in transformed and normal mouse macrophages. Drug Chem. Toxicol. 2017, 40, 36–46. [Google Scholar] [CrossRef]
- Zhu, T.; Fang, F.; Sun, D.; Yang, S.; Zhang, X.; Yu, X.; Yang, L. Piceatannol Inhibits P. acnes–Induced Keratinocyte Proliferation and Migration by Downregulating Oxidative Stress and the Inflammatory Response. Inflammation 2020, 43, 347–357. [Google Scholar] [CrossRef]
- Wang, L.; Guo, Y.; Ye, J.; Pan, Z.; Hu, P.; Zhong, X.; Qiu, F.; Zhang, D.; Huang, Z. Protective Effect of Piceatannol against Cerebral Ischaemia–Reperfusion Injury Via Regulating Nrf2/HO-1 Pathway In Vivo and Vitro. Neurochem. Res. 2021, 46, 1869–1880. [Google Scholar] [CrossRef]
- Kivimäki, K.; Leppänen, T.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E. Pinosylvin shifts macrophage polarization to support resolution of inflammation. Molecules 2021, 26, 2772. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, C.; Zou, L.; Zheng, Z.; Ouyang, J. Efficient biosynthesis of pinosylvin from lignin-derived cinnamic acid by metabolic engineering of Escherichia coli. Biotechnol. Biofuels Bioprod. 2022, 15, 136. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Machate, H.; Benali, T.; Sahib, N.; Jaouadi, I.; Omari, N.E.; Aboulaghras, S.; Bangar, S.P.; Lorenzo, J.M.; Zengin, G.; et al. Natural Sources and Pharmacological Properties of Pinosylvin. Plants 2022, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Bien-Aime, S.; Yu, W.; Uhrich, K.E. Pinosylvin-Based Polymers: Biodegradable Poly(Anhydride-Esters) for Extended Release of Antibacterial Pinosylvin. Macromol. Biosci. 2016, 16, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Hsieh, M.C.; Lin, C.C.; Lo, Y.S.; Ho, H.Y.; Hsieh, M.J.; Lin, J.T. Pinosylvin inhibits migration and invasion of nasopharyngeal carcinoma cancer cells via regulation of epithelial-mesenchymal transition and inhibition of MMP-2. Oncol. Rep. 2021, 46, 143. [Google Scholar] [CrossRef] [PubMed]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Avila, J.G.; de Vivar, A.R.; García, A.M.; Marín, J.C.; Aranda, E.; Céspedes, C.L. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry 2003, 64, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.J.; Liu, X.D.; Qian, Y.P.; Shang, Y.J.; Li, X.Z.; Dai, F.; Fang, J.G.; Jin, X.L.; Zhou, B. 4,4′-Dihydroxy-trans-stilbene, a resveratrol analogue, exhibited enhanced antioxidant activity and cytotoxicity. Bioorg. Med. Chem. 2009, 17, 2360–2365. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Baba, K. Antitumor and Antimetastatic Activity of Synthetic Hydroxystilbenes Through Inhibition of Lymphangiogenesis and M2 Macrophage Differentiation of Tumor-associated Macrophages. Anticancer Res. 2016, 36, 137–148. [Google Scholar] [PubMed]
- Chen, L.; Chen, Z.; Xu, Z.; Feng, W.; Yang, X.; Qi, Z. Polydatin protects Schwann cells from methylglyoxal induced cytotoxicity and promotes crushed sciatic nerves regeneration of diabetic rats. Phytother. Res. 2021, 35, 4592–4604. [Google Scholar] [CrossRef] [PubMed]
- Savio, M.; Ferraro, D.; Maccario, C.; Vaccarone, R.; Jensen, L.D.; Corana, F.; Mannucci, B.; Bianchi, L.; Cao, Y.; Stivala, L.A. Resveratrol analogue 4,4′-dihydroxy-trans-stilbene potently inhibits cancer invasion and metastasis. Sci Rep. 2016, 6, 19973. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Pai, G.B.; Subramanian, M.; Gupta, P.; Tyagi, M.; Patro, B.S.; Chattopadhyay, S. Resveratrol analogue, trans-4,4′-dihydroxystilbene (DHS), inhibits melanoma tumor growth and suppresses its metastatic colonization in lungs. Biomed. Pharmacother. 2018, 107, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Koskela, A.; Reinisalo, M.; Hyttinen, J.; Kaarniranta, K.; Karjalainen, R. Pinosylvin-mediated protection against oxidative stress in human retinal pigment epithelial cells. Mol. Vis. 2014, 2, 760–769. [Google Scholar]
- Wang, T.; Dai, F.; Li, G.H.; Chen, X.M.; Li, Y.R.; Wang, S.Q.; Ren, D.M.; Wang, X.N.; Lou, H.X.; Zhou, B.; et al. Trans-4,4′-dihydroxystilbene ameliorates cigarette smoke-induced progression of chronic obstructive pulmonary disease via inhibiting oxidative stress and inflammatory response. Free Radic. Biol. Med. 2020, 152, 525–539. [Google Scholar] [CrossRef]
- Wang, C.N.; Sang, M.M.; Gong, S.N.; Yang, J.F.; Cheng, C.Y.; Sun, F. Two resveratrol analogs, pinosylvin and 4,4′-dihydroxystilbene, improve oligoasthenospermia in a mouse model by attenuating oxidative stress via the Nrf2-ARE pathway. Bioorganic Chem. 2020, 104, 104295. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent advances in chemistry, therapeutic properties and sources of polydatin. Phytochem. Rev. 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Karami, A.; Fakhri, S.; Kooshki, L.; Khan, H. Polydatin: Pharmacological Mechanisms, Therapeutic Targets, Biological Activities, and Health Benefits. Molecules 2022, 27, 6474. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The neuroprotective role of polydatin: Neuropharmacological mechanisms, molecular targets, therapeutic potentials, and clinical perspective. Molecules 2021, 26, 5985. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wang, Y.; Li, J.; Liu, Z.; Lang, S.; Ouyang, W.; Zhang, J. Comprehensive metabolism study of polydatin in rat plasma and urine using ultra-high performance liquid chromatography coupled with high-resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1117, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Negrea, B.-M.; Stoilov-Linu, V.; Pop, C.-E.; Deák, G.; Crăciun, N.; Făgăraș, M.M. Expansion of the Invasive Plant Species Reynoutria japonica Houtt in the Upper Bistrița Mountain River Basin with a Calculus on the Productive Potential of a Mountain Meadow. Sustainability 2022, 14, 5737. [Google Scholar] [CrossRef]
- Huang, B.; Liu, J.; Meng, T.; Li, Y.; He, D.; Ran, X.; Chen, G.; Guo, W.; Kan, X.; Fu, S.; et al. Polydatin prevents lipopolysaccharide (LPS)-induced Parkinson’s disease via regulation of the AKt/GSK3β-Nrf2/NF-κB signaling axis. Front. Immunol. 2018, 9, 2527. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Lei, X.; Liu, H.; Zhao, H.; Guo, J.; Chen, Y.; Xu, Y.; Cheng, Y.; Liu, C.; Cui, J.; et al. Polydatin alleviated radiation-induced lung injury through activation of Sirt3 and inhibition of epithelial–mesenchymal transition. J. Cell Mol. Med. 2017, 21, 3264–3276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Chen, G.; Yang, Z.; Wen, D.; Guo, J.; Xiong, Q.; Li, P.; Zhao, L.; Wang, J.; Wu, C.; Dong, L. Polydatin has anti-inflammatory and antioxidant effects in LPS-induced macrophages and improves DSS-induced mice colitis. Immun. Inflamm. Dis. 2021, 9, 959–970. [Google Scholar] [CrossRef]
- Zhao, X.; Qin, J.; Li, H.; Feng, X.; Lv, Y.; Yang, J. Effect of Polydatin on Neurological Function and the Nrf2 Pathway during Intracerebral Hemorrhage. J. Mol. Neurosci. 2020, 70, 1332–1337. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Zhang, L.; Zhang, Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019, 217, 119–127. [Google Scholar] [CrossRef]
- Li, R.; Maimai, T.; Yao, H.; Liu, X.; He, Z.; Xiao, C.; Wang, Y.; Xie, G. Protective effects of polydatin on LPS-induced endometritis in mice. Microb. Pathog. 2019, 137, 103720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Pei, Z.; Gao, H.; Shi, W.; Sun, M.; Xu, Q.; Zhao, J.; Meng, W.; Xiao, K. Protective effects of polydatin against sulfur mustard-induced hepatic injury. Toxicol. Appl. Pharmacol. 2019, 367, 1–11. [Google Scholar] [CrossRef]
- Bheereddy, P.; Yerra, V.G.; Kalvala, A.K.; Sherkhane, B.; Kumar, A. SIRT1 Activation by Polydatin Alleviates Oxidative Damage and Elevates Mitochondrial Biogenesis in Experimental Diabetic Neuropathy. Cell. Mol. Neurobiol. 2021, 41, 1563–1577. [Google Scholar] [CrossRef]
- Gu, L.; Liu, J.; Xu, D.; Lu, Y. Polydatin prevents LPS-induced acute kidney injury through inhibiting inflammatory and oxidative responses. Microb. Pathog. 2019, 137, 103688. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Min, J.; Chen, Y.; Li, H.; Zhang, Y. Polydatin attenuates orbital oxidative stress in Graves’ orbitopathy through the NRF2 pathway. Chem. Biol. Interact. 2020, 315, 108894. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.T.; Zhang, D.M.; Wan, Z.Y.; Li, T.S.; Jiao, R.Q.; Chen, T.Y.; Zhao, X.J.; Kong, L.D. Polydatin enhances glomerular podocyte autophagy homeostasis by improving Nrf2-dependent antioxidant capacity in fructose-fed rats. Mol. Cell Endocrinol. 2021, 520, 111079. [Google Scholar] [CrossRef]
- Zhan, J.; Li, X.; Luo, D.; Yan, W.; Hou, Y.; Hou, Y.; Chen, S.; Luan, J.; Zhang, Q.; Lin, D. Polydatin Attenuates OGD/R-Induced Neuronal Injury and Spinal Cord Ischemia/Reperfusion Injury by Protecting Mitochondrial Function via Nrf2/ARE Signaling Pathway. Oxidative Med. Cell Longev. 2021, 2021, 6687212. [Google Scholar] [CrossRef]
- Chen, G.; Liu, G.; Cao, D.; Jin, M.; Guo, D.; Yuan, X. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J. Nat. Med. 2019, 73, 85–92. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Chen, Z.; Huang, J.; Chen, Q.; Cai, W.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic. Biol. Med. 2017, 106, 393–405. [Google Scholar] [CrossRef]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin Protects Rat Liver against Ethanol-Induced Injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-B p65 Pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin ameliorates chemotherapy-induced cognitive impairment (chemobrain) by inhibiting oxidative stress, inflammatory response, and apoptosis in rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210. [Google Scholar] [CrossRef]
- Tang, S.; Tang, Q.; Jin, J.; Zheng, G.; Xu, J.; Huang, W.; Li, X.; Shang, P.; Liu, H. Polydatin inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes by activating the Nrf2 signaling pathway and ameliorates murine osteoarthritis. Food Funct. 2018, 9, 1701–1712. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Xu, P.; Lin, Q.; Zhao, T.; Li, C.; Cui, Z.K.; Tian, G. Polydatin activates the Nrf2/HO-1 signaling pathway to protect cisplatin-induced hearing loss in guinea pigs. Front. Pharmacol. 2022, 13, 887833. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, Y.; Gu, Y.; Wang, J.; Zhang, H.; Gao, H.; Jin, Q.; Zhao, L. Polydatin attenuates reactive oxygen species-induced airway remodeling by promoting Nrf2-mediated antioxidant signaling in asthma mouse model. Life Sci. 2019, 218, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating Sirt1 to resist AGEs-induced upregulation of fibronetin and transforming growth factor-β1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef]
- Ye, J.; Piao, H.; Jiang, J.; Jin, G.; Zheng, M.; Yang, J.; Jin, X.; Sun, T.; Choi, Y.H.; Li, L.; et al. Polydatin inhibits mast cell-mediated allergic inflammation by targeting PI3K/Akt, MAPK, NF-κB and Nrf2/HO-1 pathways. Sci. Rep. 2017, 7, 11895. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).