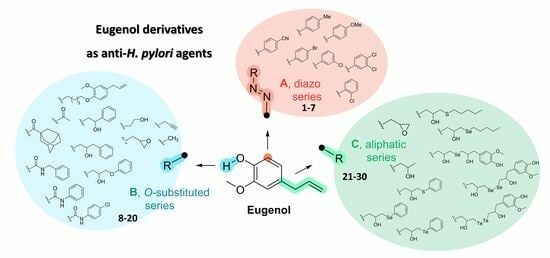
Nature-Inspired Compounds: Synthesis and Antibacterial Susceptibility Testing of Eugenol Derivatives against H. pylori Strains
Abstract
:1. Introduction
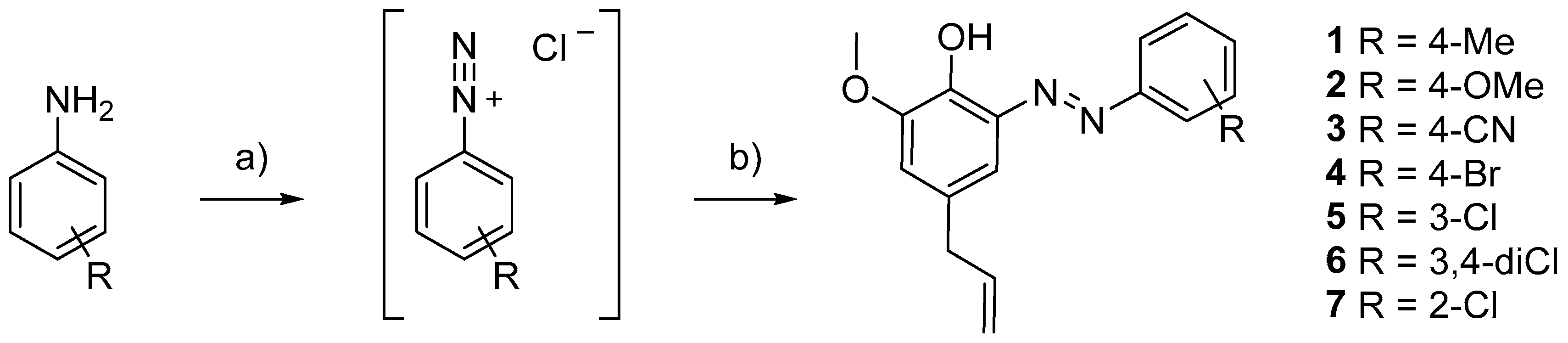
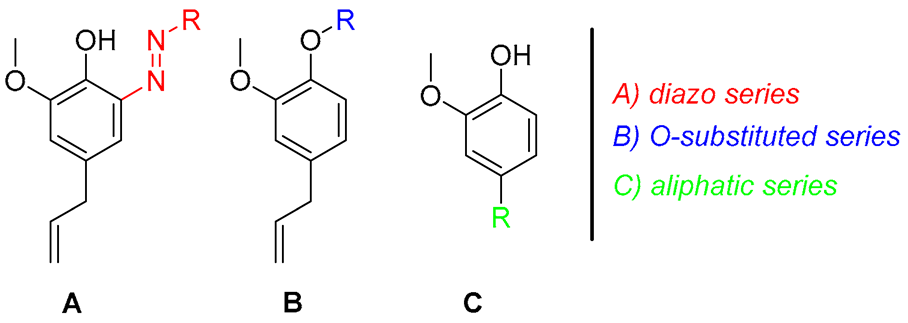
- A, a diazo function was added in the ortho position, to explore the chemical space near the phenolic group and keep the OH function unsubstituted (1–7, Table 1);
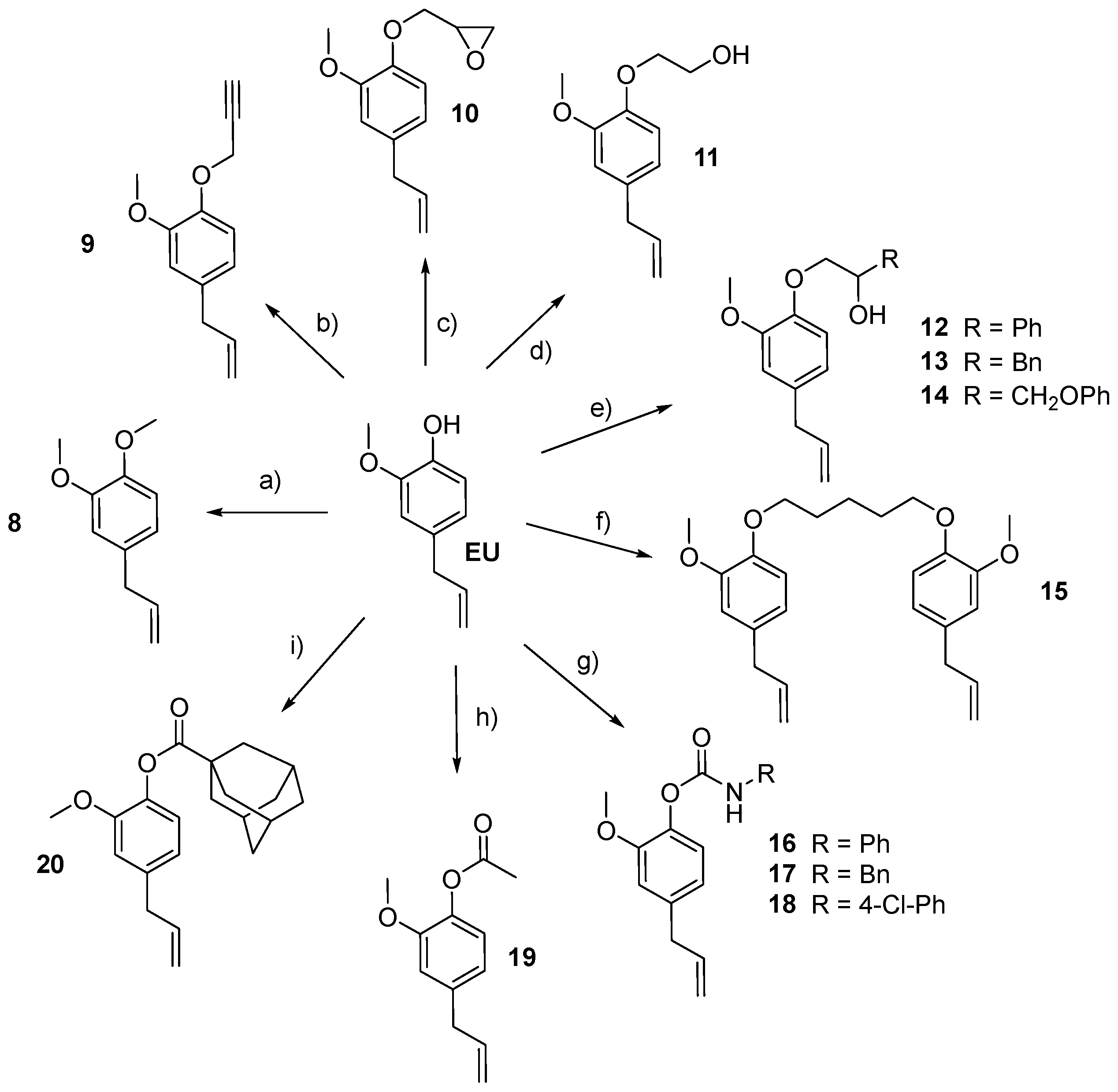
- B, the phenolic group of the EU was alkylated (8–14) or incorporated into a carbamate (16–18) or ester (19 and 20, Table 1) moiety;
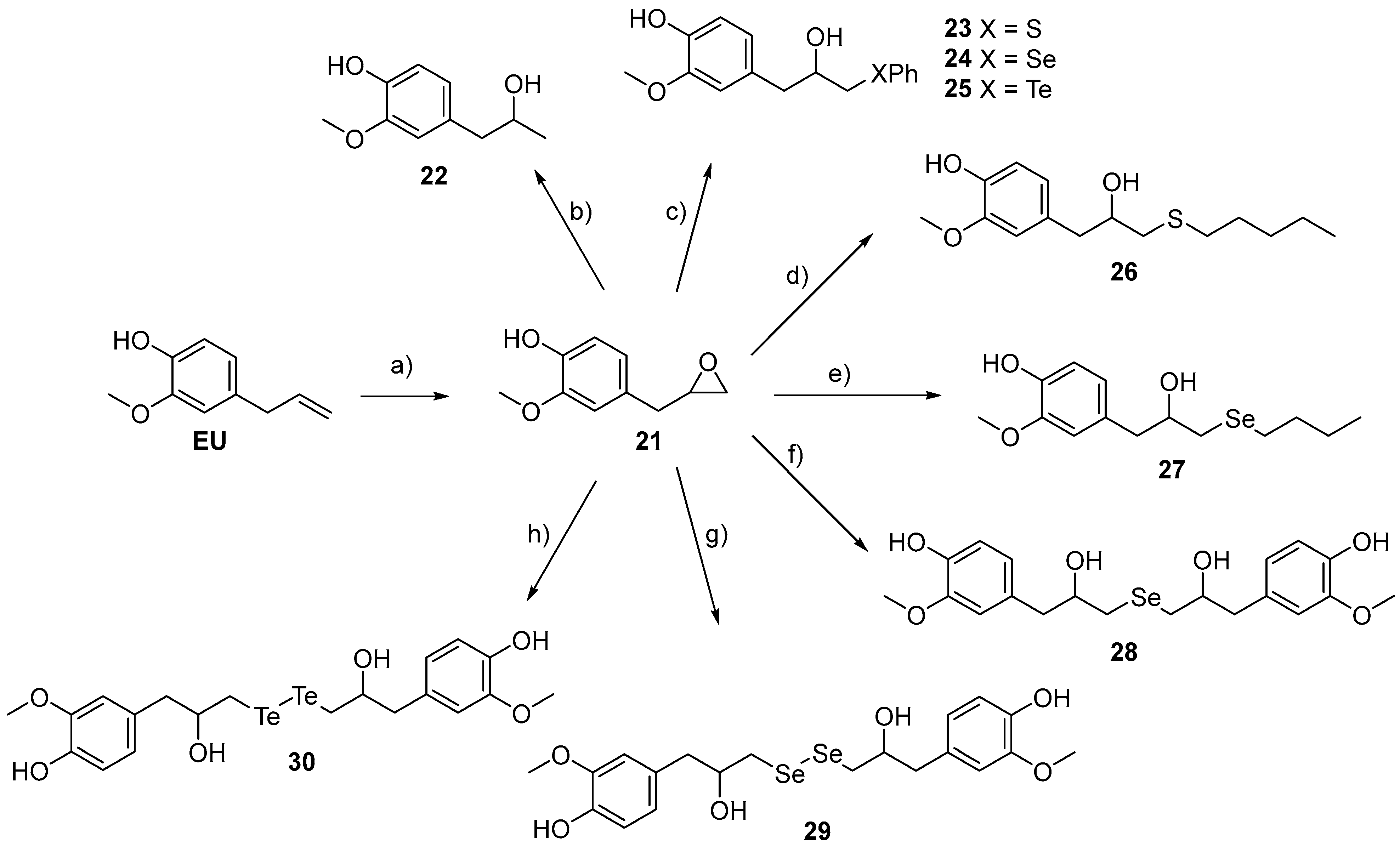
- C, the allylic portion of the EU was replaced by a differently substituted tail, including an epoxide ring (21, Table 1), or alcohol chains (22) containing a chalcogen (23–30).
2. Results and Discussion
2.1. Chemistry
2.2. In Vitro Antibacterial Activity Studies against H. pylori
3. Materials and Methods
3.1. Chemistry
Procedure and Characterization Data for Derivatives 1–30
- General Procedure for the Synthesis of Compounds 1–7
- (E)-4-allyl-2-methoxy-6-(p-tolyldiazenyl)phenol (1)
- (E)-4-allyl-2-methoxy-6-[(4-methoxyphenyl)diazenyl]phenol (2)
- (E)-4-[(5-allyl-2-hydroxy-3-methoxyphenyl)diazenyl]benzonitrile (3)
- (E)-4-allyl-2-((4-bromophenyl)diazenyl)-6-methoxyphenol (4)
- (E)-4-allyl-2-[(3-chlorophenyl)diazenyl]-6-methoxyphenol (5)
- (E)-4-allyl-2-[(3,4-dichlorophenyl)diazenyl]-6-methoxyphenol (6)
- (E)-4-allyl-2-[(2-chlorophenyl)diazenyl]-6-methoxyphenol (7)
- General procedure for the synthesis of 8 and 9
- 4-Allyl-1,2-dimethoxybenzene (8)
- 4-Allyl-2-methoxy-1-(prop-2-yn-1-yloxy)benzene (9)
- Synthesis of 2-[(4-allyl-2-methoxyphenoxy)methyl]oxirane (10)
- Synthesis of 2-(4-allyl-2-methoxyphenoxy)ethanol (11)
- General procedure for the synthesis of alcohols 12–14
- 2-(4-Allyl-2-methoxyphenoxy)-1-phenylethanol (12)
- 1-(4-Allyl-2-methoxyphenoxy)-3-phenylpropan-2-ol (13)
- 1-(4-Allyl-2-methoxyphenoxy)-3-phenoxypropan-2-ol (14)
- Synthesis of 1,5-bis(4-allyl-2-methoxyphenoxy)pentane (15)
- General procedure for the synthesis of carbamates 16–18
- 4-Allyl-2-methoxyphenyl phenylcarbamate (16)
- 4-Allyl-2-methoxyphenyl benzylcarbamate (17)
- 4-Allyl-2-methoxyphenyl (4-chlorophenyl)carbamate (18)
- Synthesis of 4-allyl-2-methoxyphenyl acetate (19)
- Synthesis of 4-allyl-2-methoxyphenyl adamantane-1-carboxylate (20)
- General Procedure for the synthesis of β-phenylcalcogeno alcohols 23–25
- Synthesis of 4-[2-hydroxy-3-(phenylthio)propyl]-2-methoxyphenol (23)
- Synthesis of 4-[2-hydroxy-3-(phenylselanyl)propyl]-2-methoxyphenol (24)
- Synthesis of 4-[2-hydroxy-3-(phenyltellanyl)propyl]-2-methoxyphenol (25)
- Synthesis of 4-[2-hydroxy-3-(pentylthio)propyl]-2-methoxyphenol (26)
- Synthesis of 4-[3-(butylselanyl)-2-hydroxypropyl]-2-methoxyphenol (27)
- Synthesis of 4,4′-[selenobis(2-hydroxypropane-3,1-diyl)]bis(2-methoxyphenol) (28)
3.2. Anti-H. pylori Activity Testing
3.3. In Silico Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Friedrich, L.J.; Efferth, T. Natural Products Targeting Tumor Angiogenesis. Br. J. Pharmacol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Palhares, R.M.; Gonçalves Drummond, M.; Dos Santos Alves Figueiredo Brasil, B.; Pereira Cosenza, G.; das Graças Lins Brandão, M.; Oliveira, G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS ONE 2015, 10, e0127866. [Google Scholar] [CrossRef]
- Ahmad, A.; Elisha, I.L.; van Vuuren, S.; Viljoen, A. Volatile phenolics: A comprehensive review of the anti-infective properties of an important class of essential oil constituents. Phytochemistry 2021, 190, 112864. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of eugenol-A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- FDA, U.S. Food & Drug Administration. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=SCOGS (accessed on 7 September 2023).
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol from the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Rifkind, J.M.; Boindala, S.; Nakka, L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Methods Mol. Biol. 2010, 610, 165–180. [Google Scholar]
- Cheng, S.S.; Liu, J.Y.; Chang, E.H.; Chang, S.T. Antifungal activity of cinnamaldehyde and eugenol congeners against wood-rot fungi. Bioresour. Technol. 2008, 99, 5145–5149. [Google Scholar] [CrossRef]
- Alam, H.; Srivastava, V.; Sekgele, W.; Wani, M.Y.; Al-Bogami, A.S.; Molepo, J.; Ahmad, A. Cellular apoptosis and cell cycle arrest as potential therapeutic targets for eugenol derivatives in Candida auris. PLoS ONE 2023, 18, e0285473. [Google Scholar] [CrossRef] [PubMed]
- Ben Miri, Y.; Nouasri, A.; Herrera, M.; Djenane, D.; Ariño, A. Antifungal Activity of Menthol, Eugenol and Their Combination against Aspergillus ochraceus and Aspergillus niger In Vitro and in Stored Cereals. Foods 2023, 12, 2108. [Google Scholar] [CrossRef] [PubMed]
- Benencia, F.; Courrèges, M.C. In vitro and in vivo activity of eugenol on human herpesvirus. Phytother. Res. 2000, 14, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, B.; Ding, Y.; Meng, J.; Hu, J.; Zhou, X.; Liu, L.; Wu, Z.; Yang, S. Insights into a class of natural eugenol and its optimized derivatives as potential tobacco mosaic virus helicase inhibitors by structure-based virtual screening. Int. J. Biol. Macromol. 2023, 248, 125892. [Google Scholar] [CrossRef]
- Oriola, A.O.; Oyedeji, A.O. Essential Oils and Their Compounds as Potential Anti-Influenza Agents. Molecules 2022, 27, 7797. [Google Scholar] [CrossRef]
- Reis, R.C.F.M.; Dos Santos, E.G.; Benedetti, M.D.; Reis, A.C.C.; Brandão, G.C.; Silva, G.N.D.; Diniz, L.A.; Ferreira, R.S.; Caldas, I.S.; Braga, S.F.P.; et al. Design and synthesis of new 1,2,3-triazoles derived from eugenol and analogues with in vitro and in vivo activity against Trypanosoma cruzi. Eur. J. Med. Chem. 2023, 258, 115622. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; Rahman, U.; Khan, M.R.; Sahar, A.; Mehmoodac, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Matykiewicz, D.; Skórczewska, K. Characteristics and Application of Eugenol in the Production of Epoxy and Thermosetting Resin Composites: A Review. Materials 2022, 15, 4824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhu, X.; Cao, P.; Wei, S.; Lu, Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017, 113, 396–402. [Google Scholar]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]
- Sharma, H.K.; Gupta, P.; Nagpal, D.; Mukherjee, M.; Parmar, V.S.; Lather, V. Virtual screening and antimicrobial evaluation for identification of natural compounds as the prospective inhibitors of antibacterial drug resistance targets in Staphylococcus aureus. Fitoterapia 2023, 168, 105554. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Z.; Hu, S.; Wang, J.; Deng, Y.; Li, X.; Chen, X.; Li, X.; Tang, Y.; Li, X.; et al. A Survey of Helicobacter pylori Antibiotic-Resistant Genotypes and Strain Lineages by Whole-Genome Sequencing in China. Antimicrob. Agents Chemother. 2022, 66, 0218821. [Google Scholar] [CrossRef] [PubMed]
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting Therapeutic Strategies for H. pylori Treatment in the Context of Antibiotic Resistance: Focus on Alternative and Complementary Therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef]
- Sathianarayanan, S.; Ammanath, A.V.; Biswas, R.B.A.; Sukumaran, S.; Venkidasamy, B. A new approach against Helicobacter pylori using plants and its constituents: A review study. Microb. Pathog. 2022, 168, 105594. [Google Scholar] [CrossRef]
- Grande, R.; Carradori, S.; Puca, V.; Vitale, I.; Angeli, A.; Nocentini, A.; Bonardi, A.; Gratteri, P.; Lanuti, P.; Bologna, G.; et al. Selective Inhibition of Helicobacter pylori Carbonic Anhydrases by Carvacrol and Thymol Could Impair Biofilm Production and the Release of Outer Membrane Vesicles. Int. J. Mol. Sci. 2021, 22, 11583. [Google Scholar] [CrossRef]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Spano, M.; Secci, D.; Granese, A.; Sobolev, A.P.; Grande, R.; Campestre, C.; Di Marcantonio, M.C.; et al. Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules 2021, 26, 1829. [Google Scholar] [CrossRef]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Traversi, C.B.; Spano, M.; Sobolev, A.P.; Secci, D.; Di Marcantonio, M.C.; Haloci, E.; Grande, R.; et al. Synthesis and Biological Evaluation of Carvacrol-Based Derivatives as Dual Inhibitors of H. pylori Strains and AGS Cell Proliferation. Pharmaceuticals 2020, 13, 405. [Google Scholar] [CrossRef]
- Ali, S.M.; Khan, A.A.; Ahmed, I.; Musaddiq, M.; Ahmed, K.S.; Polasa, H.; Rao, L.V.; Habibullah, C.M.; Sechi, L.A.; Ahmed, N. Antimicrobial activities of eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. [Google Scholar] [CrossRef]
- Sisto, F.; Carradori, S.; D’Alessandro, S.; Santo, N.; Lattuada, N.; Haynes, R.K.; Taramelli, D.; Grande, R. In Vitro Activity of the Arylaminoartemisinin GC012 against Helicobacter pylori and Its Effects on Biofilm. Pathogens 2022, 11, 740. [Google Scholar] [CrossRef]
- Sengoden, M.; Bhat, G.A.; Darensbourg, D.J. Explorations into the sustainable synthesis of cyclic and polymeric carbonates and thiocarbonates from eugenol-derived monomers and their reactions with CO2, COS, or CS2. Green Chem. 2022, 24, 2535–2541. [Google Scholar] [CrossRef]
- Takeshita, M.; Yaguchi, R.; Akutsu, N. Enzymatic preparation of chiral 1-phenylglycidols and 1-phenyl-1,2-propanediols. Tetrahedron Asymmetry 1992, 3, 1369–1372. [Google Scholar] [CrossRef]
- Talluri, S.K.; Sudalai, A. An organo-catalytic approach to the enantioselective synthesis of (R)-selegiline. Tetrahedron 2007, 63, 9758–9763. [Google Scholar] [CrossRef]
- Tanini, D.; Borgogni, C.; Capperucci, A. Mild and selective silicon-mediated access to enantioenriched 1,2-mercaptoamines and β-amino arylchalcogenides. New J. Chem. 2019, 43, 6388–6393. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Smith, R.A.; Nischwitz, A.K.; Gavin, T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3–TBAI. Tetrahedron Lett. 2005, 46, 8931–8935. [Google Scholar] [CrossRef]
- Tanini, D.; Carradori, S.; Capperucci, A.; Lupori, L.; Zara, S.; Ferraroni, M.; Ghelardini, C.; Di Cesare Mannelli, L.; Micheli, L.; Lucarini, E.; et al. Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly reverted oxaliplatin-induced neuropathy and enhanced antiproliferative action. Eur. J. Med. Chem. 2021, 225, 113793. [Google Scholar] [CrossRef]
- Tanini, D.; Degl’Innocenti, A.; Capperucci, A. Bis(trimethylsilyl)selenide in the selective synthesis of β-hydroxy, β-mercapto and β-amino diorganyl diselenides and selenides through ring opening of strained heterocycles. Eur. J. Org. Chem. 2015, 2, 357–369. [Google Scholar] [CrossRef]
- Tanini, D.; Ricci, L.; Capperucci, A. Rongalite-Promoted on Water Synthesis of Functionalised Tellurides and Ditellurides. Adv. Synth. Catal. 2020, 362, 1323–1332. [Google Scholar] [CrossRef]
- Henriquez-Figuereo, A.; Morán-Serradilla, C.; Angulo-Elizari, E.; Sanmartín, C.; Plano, D. Small molecules containing chalcogen elements (S, Se, Te) as new warhead to fight neglected tropical diseases. Eur. J. Med. Chem. 2023, 246, 115002. [Google Scholar] [CrossRef]
- Nogueira, C.W.; Barbosa, N.V.; Rocha, J.B.T. Toxicology and pharmacology of synthetic organoselenium compounds: An update. Arch. Toxicol. 2021, 95, 1179–1226. [Google Scholar] [CrossRef] [PubMed]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Bergander, K.; Daniliuc, C.G.; Studer, A. Fe-Catalyzed Anaerobic Mukaiyama-Type Hydration of Alkenes using Nitroarenes. Angew. Chem. Int. Ed. 2021, 60, 8313–8320. [Google Scholar] [CrossRef]
- Amaravathi, M.; Kanakaraju, S.; Chandramouli, G.V.P. Synthesis and Characterization of Azobenzene–Porphyrins. J. Heterocyclic Chem. 2013, 50, 268–271. [Google Scholar] [CrossRef]
- Heikes, D.L. SFE with GC and MS determination of safrole and related allylbenzenes in sassafras teas. J. Chromatogr. Sci. 1994, 32, 253–258. [Google Scholar] [CrossRef]
- Dutra, J.A.P.; Maximino, S.C.; Gonçalves, R.D.C.; Morais, P.A.B.; de Lima Silva, W.C.; Rodrigues, R.P.; Neto, Á.C.; Júnior, V.L.; de Souza Borges, W.; Kitagawa, R.R. Anti-Candida, docking studies, and in vitro metabolism-mediated cytotoxicity evaluation of eugenol derivatives. Chem. Biol. Drug Des. 2023, 101, 350–363. [Google Scholar] [CrossRef]
- Genç Bilgiçli, H.; Kestane, A.; Taslimi, P.; Karabay, O.; Bytyqi-Damoni, A.; Zengin, M.; Gulçin, İ. Novel eugenol bearing oxypropanolamines: Synthesis, characterization, antibacterial, antidiabetic, and anticholinergic potentials. Bioorg. Chem. 2019, 88, 102931. [Google Scholar] [CrossRef]
- de Almeida, A.L.; Caleffi-Ferracioli, K.R.; de L Scodro, R.B.; Baldin, V.P.; Montaholi, D.C.; Spricigo, L.F.; Nakamura-Vasconcelos, S.S.; Hegeto, L.A.; Sampiron, E.G.; Costacurta, G.F.; et al. Eugenol and derivatives activity against Mycobacterium tuberculosis, nontuberculous mycobacteria and other bacteria. Future Microbiol. 2019, 14, 331–344. [Google Scholar] [CrossRef]
- Sadeghian, H.; Seyedi, S.M.; Saberi, M.R.; Arghiani, Z.; Riazi, M. Design and synthesis of eugenol derivatives, as potent 15-lipoxygenase inhibitors. Bioorg. Med. Chem. 2008, 16, 890–901. [Google Scholar] [CrossRef]
- SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [CrossRef] [PubMed]
- Sisto, F.; Scaltrito, M.M.; Russello, G.; Bonomi, A.; Dubini, F. Antimicrobial susceptibility testing of Helicobacter pylori determined by microdilution method using a new medium. Curr. Microbiol. 2009, 58, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Eucast: The 13.0 Versions of Breakpoints, Dosing and QC (2023) Published. Available online: https://www.eucast.org/eucast_news/news_singleview?tx_ttnews%5Btt_news%5D=518&cHash=2509b0db92646dffba041406dcc9f20c (accessed on 1 September 2023).
- Josephy, P.D.; Allen-Vercoe, E. Reductive metabolism of azo dyes and drugs: Toxicological implications. Food Chem. Toxicol. 2023, 178, 113932. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Di Stefano, M.; Macchia, M.; Poli, G.; Tuccinardi, T. MolBook UNIPI─Create, Manage, Analyze, and Share Your Chemical Data for Free. J. Chem. Inf. Model. 2023, 63, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Di Stefano, M.; Martinelli, E.; Macchia, M.; Martinelli, A.; Poli, G.; Tuccinardi, T. VenomPred: A Machine Learning Based Platform for Molecular Toxicity Predictions. Int. J. Mol. Sci. 2022, 23, 2105. [Google Scholar] [CrossRef]
 | MIC/MBC (µg/mL) on H. pylori Strains * | MIC50/MIC90 µg/mL | MBC90 µg/mL | |||||
|---|---|---|---|---|---|---|---|---|
| Series | CPD | R | NCTC 11637 ** | F1 *** | 23 *** | F40/499 *** | ||
| A | 1 | 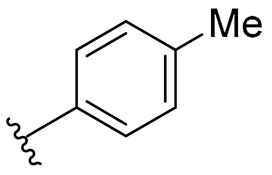 | 64/128 | 64/128 | 64/128 | 128/128 | 64/128 | 128 |
| 2 | 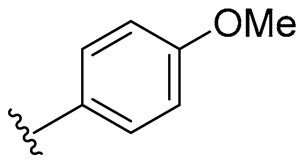 | 32/64 | 32/64 | 32/32 | 32/64 | 32/32 | 64 | |
| 3 | 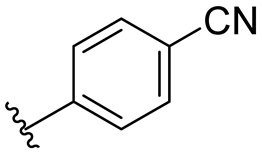 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128/>128 | >128 | |
| 4 | 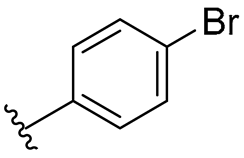 | 128/128 | 128/128 | 128/128 | 128/128 | 128/128 | 128 | |
| 5 |  | 8/8 | 8/8 | 16/16 | 8/8 | 8/16 | 16 | |
| 6 | 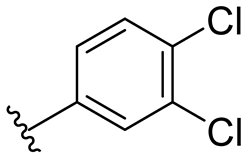 | 128/>128 | 128/128 | 128/>128 | 128/128 | 128/>128 | >128 | |
| 7 | 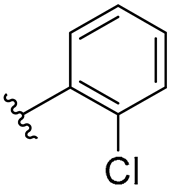 | 8/8 | 8/8 | 16/16 | 8/8 | 8/16 | 16 | |
| B | 8 | 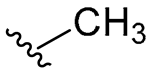 | 64/64 | 64/128 | 128/128 | 128/128 | 64/128 | 128 |
| 9 | 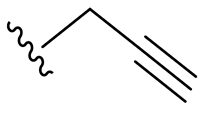 | 32/64 | 32/32 | 32/32 | 64/64 | 32/64 | 64 | |
| 10 | 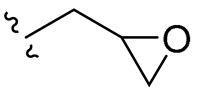 | 32/32 | 32/32 | 32/64 | 32/64 | 32/32 | 64 | |
| 11 | 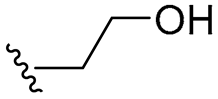 | 64/64 | 32/32 | 64/64 | 32/32 | 32/64 | 64 | |
| 12 |  | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8 | |
| 13 | 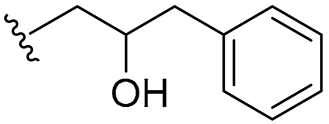 | 16/32 | 8/8 | 16/16 | 8/8 | 8/16 | 32 | |
| 14 | 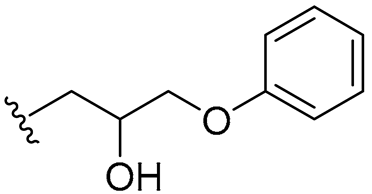 | 16/32 | 8/8 | 16/16 | 8/8 | 8/16 | 32 | |
| 15 | 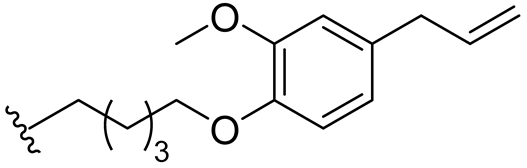 | 128/128 | 32/64 | 128/128 | 32/64 | 32/128 | 128 | |
| 16 | 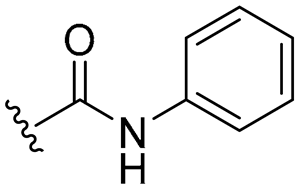 | 16/16 | 16/32 | 32/32 | 16/16 | 16/32 | 32 | |
| 17 | 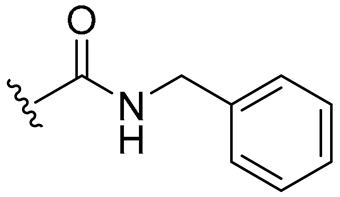 | 8/16 | 8/8 | 8/8 | 8/8 | 8/8 | 16 | |
| 18 |  | 16/32 | 16/16 | 16/16 | 8/8 | 16/16 | 32 | |
| 19 | 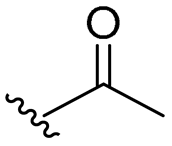 | 64/64 | 64/64 | 128/128 | 64/128 | 64/128 | 128 | |
| 20 | 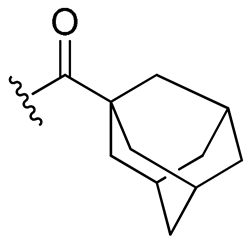 | 32/64 | 32/64 | 64/64 | 32/32 | 32/64 | 64 | |
| C | 21 | 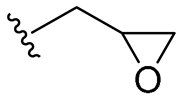 | 64/128 | 64/64 | 128/>128 | 32/32 | 64/128 | >128 |
| 22 | 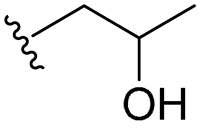 | 128/128 | 64/64 | 128/128 | 64/64 | 64/128 | 128 | |
| 23 | 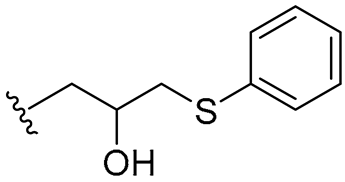 | 8/8 | 8/8 | 8/8 | 8/8 | 8/8 | 8 | |
| 24 | 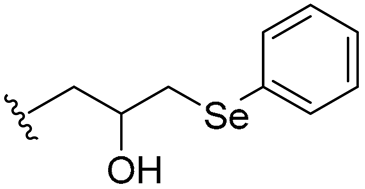 | 8/16 | 8/8 | 4/8 | 2/2 | 4/8 | 16 | |
| 25 | 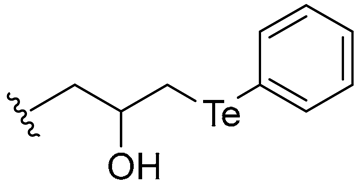 | 16/16 | 4/8 | 4/8 | 4/4 | 4/16 | 16 | |
| 26 | 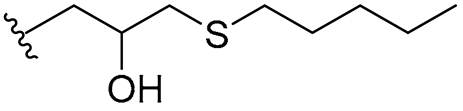 | 16/16 | 8/8 | 8/16 | 8/8 | 8/16 | 16 | |
| 27 | 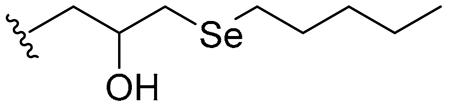 | 16/32 | 16/16 | 16/16 | 16/16 | 16/16 | 32 | |
| 28 | 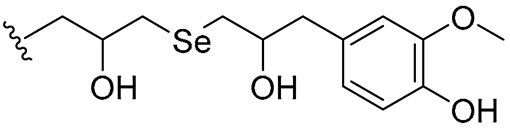 | 64/64 | 32/32 | 32/64 | 16/16 | 32/64 | 64 | |
| 29 | 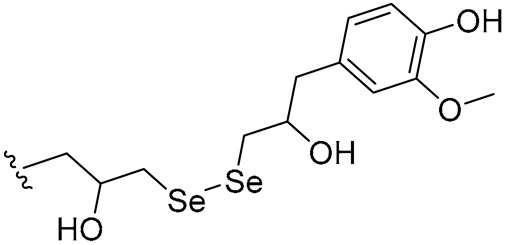 | 4/4 | 4/4 | 2/4 | 2/2 | 2/4 | 4 | |
| 30 | 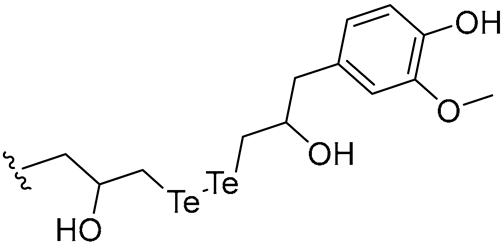 | 16/16 | 4/8 | 4/8 | 4/4 | 4/16 | 16 | |
| EU Parent compound | 32/64 | 64/128 | 64/64 | 64/128 | 64/128 | 128 | ||
| Metronidazole (MTZ) | 256/256 | 2/2 | 1/1 | 32/32 | 2/256 | 256 | ||
| Clarithromycin (CLR) | 0.064/0.064 | 4/8 | 0.064/0.064 | 8/8 | 0.064/8 | 8 | ||
| Amoxicillin (AMX) | 0.016/0.016 | 0.064/0.064 | 0.016/0.016 | 0.016/0.032 | 0.016/0.064 | 0.064 | ||
| Antibiotic susceptibility | MTZ+ CLR− AMX− | MTZ− CLR+ AMX− | MTZ− CLR− AMX− | MTZ+ CLR+ AMX− | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carradori, S.; Ammazzalorso, A.; Niccolai, S.; Tanini, D.; D’Agostino, I.; Melfi, F.; Capperucci, A.; Grande, R.; Sisto, F. Nature-Inspired Compounds: Synthesis and Antibacterial Susceptibility Testing of Eugenol Derivatives against H. pylori Strains. Pharmaceuticals 2023, 16, 1317. https://doi.org/10.3390/ph16091317
Carradori S, Ammazzalorso A, Niccolai S, Tanini D, D’Agostino I, Melfi F, Capperucci A, Grande R, Sisto F. Nature-Inspired Compounds: Synthesis and Antibacterial Susceptibility Testing of Eugenol Derivatives against H. pylori Strains. Pharmaceuticals. 2023; 16(9):1317. https://doi.org/10.3390/ph16091317
Chicago/Turabian StyleCarradori, Simone, Alessandra Ammazzalorso, Sofia Niccolai, Damiano Tanini, Ilaria D’Agostino, Francesco Melfi, Antonella Capperucci, Rossella Grande, and Francesca Sisto. 2023. "Nature-Inspired Compounds: Synthesis and Antibacterial Susceptibility Testing of Eugenol Derivatives against H. pylori Strains" Pharmaceuticals 16, no. 9: 1317. https://doi.org/10.3390/ph16091317
APA StyleCarradori, S., Ammazzalorso, A., Niccolai, S., Tanini, D., D’Agostino, I., Melfi, F., Capperucci, A., Grande, R., & Sisto, F. (2023). Nature-Inspired Compounds: Synthesis and Antibacterial Susceptibility Testing of Eugenol Derivatives against H. pylori Strains. Pharmaceuticals, 16(9), 1317. https://doi.org/10.3390/ph16091317