Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents
Abstract
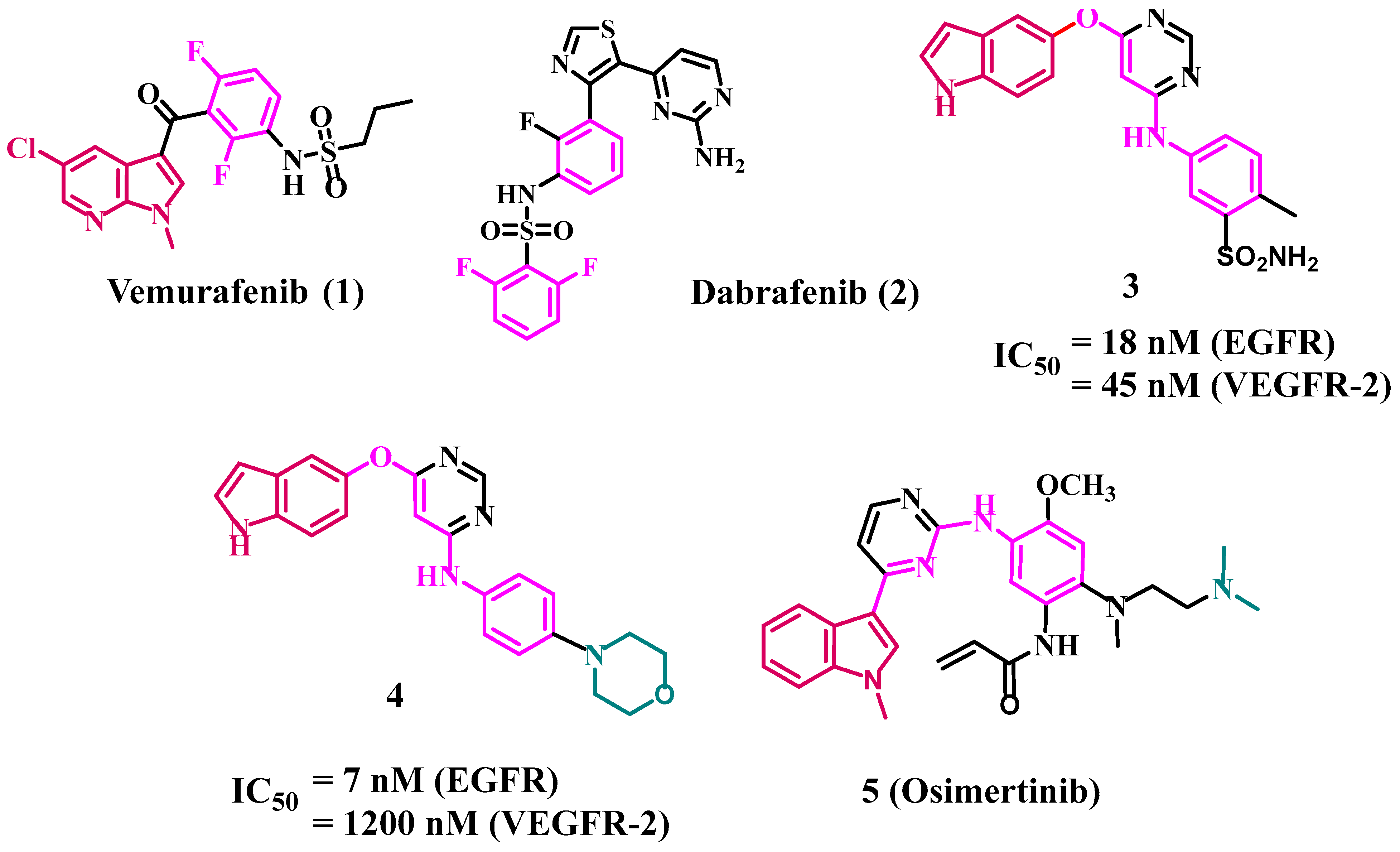
1. Introduction
2. Results and Discussion
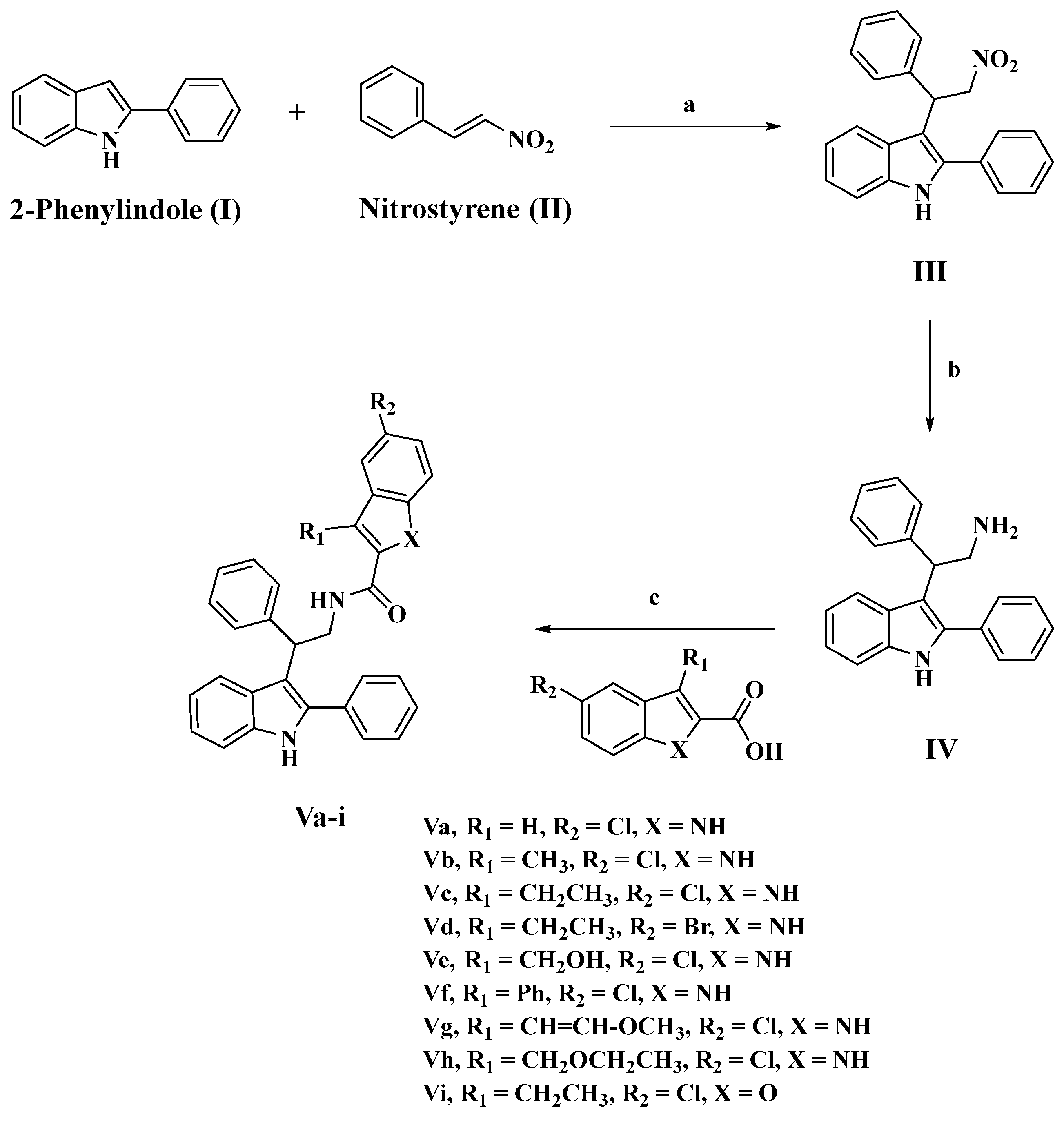
2.1. Chemistry
2.2. Biology
2.2.1. Assay for Cell Viability
2.2.2. Antiproliferative Assay
2.2.3. EGFR Inhibitory Assay
2.2.4. BRAFV600E Inhibitory Assay
2.2.5. VEGFR-2 Inhibitory Assay
2.3. Apoptotic Marker Assays
2.3.1. Caspase 3 Assay
2.3.2. Caspase-8, Bax, and Bcl-2 Level Assays
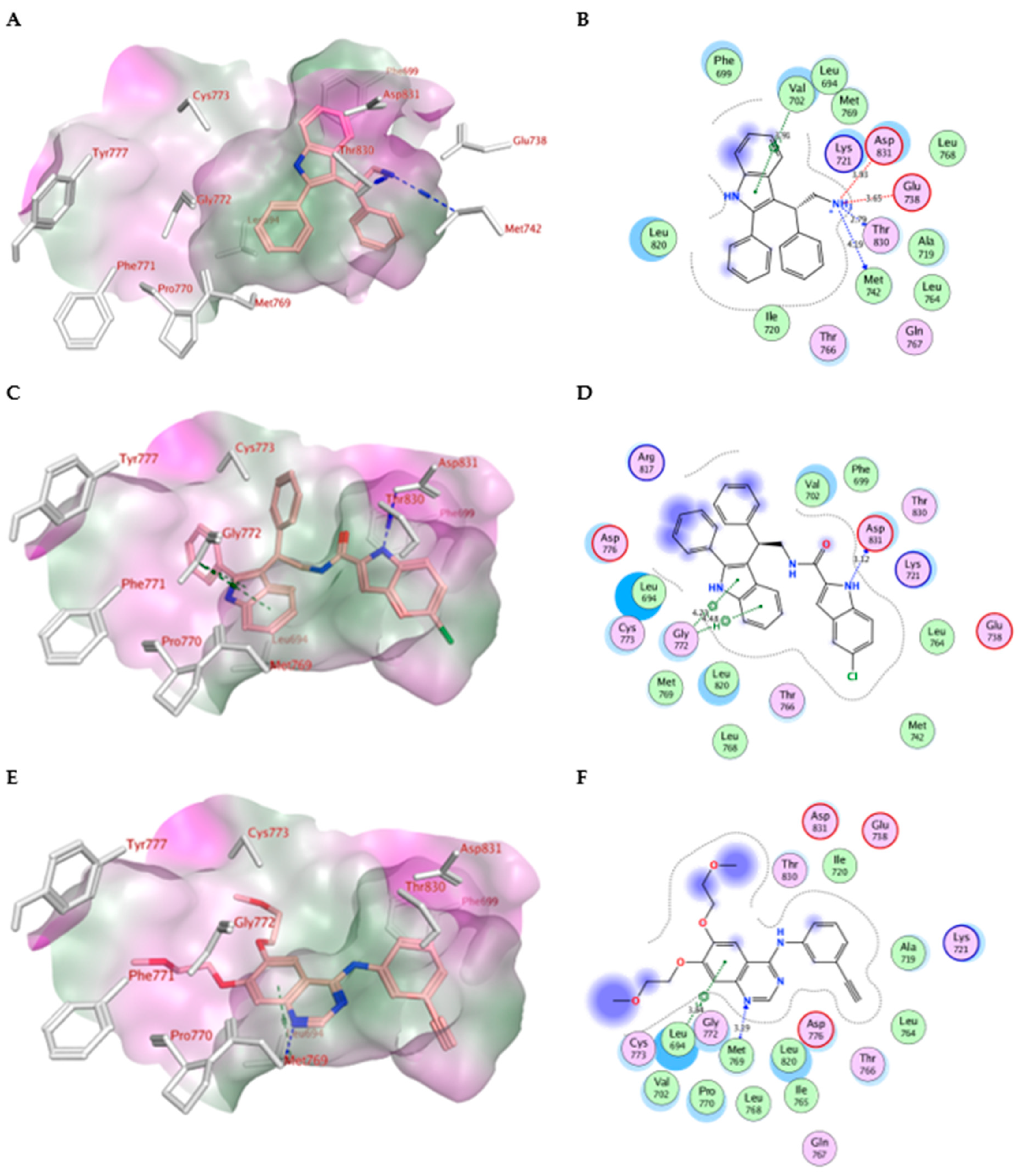
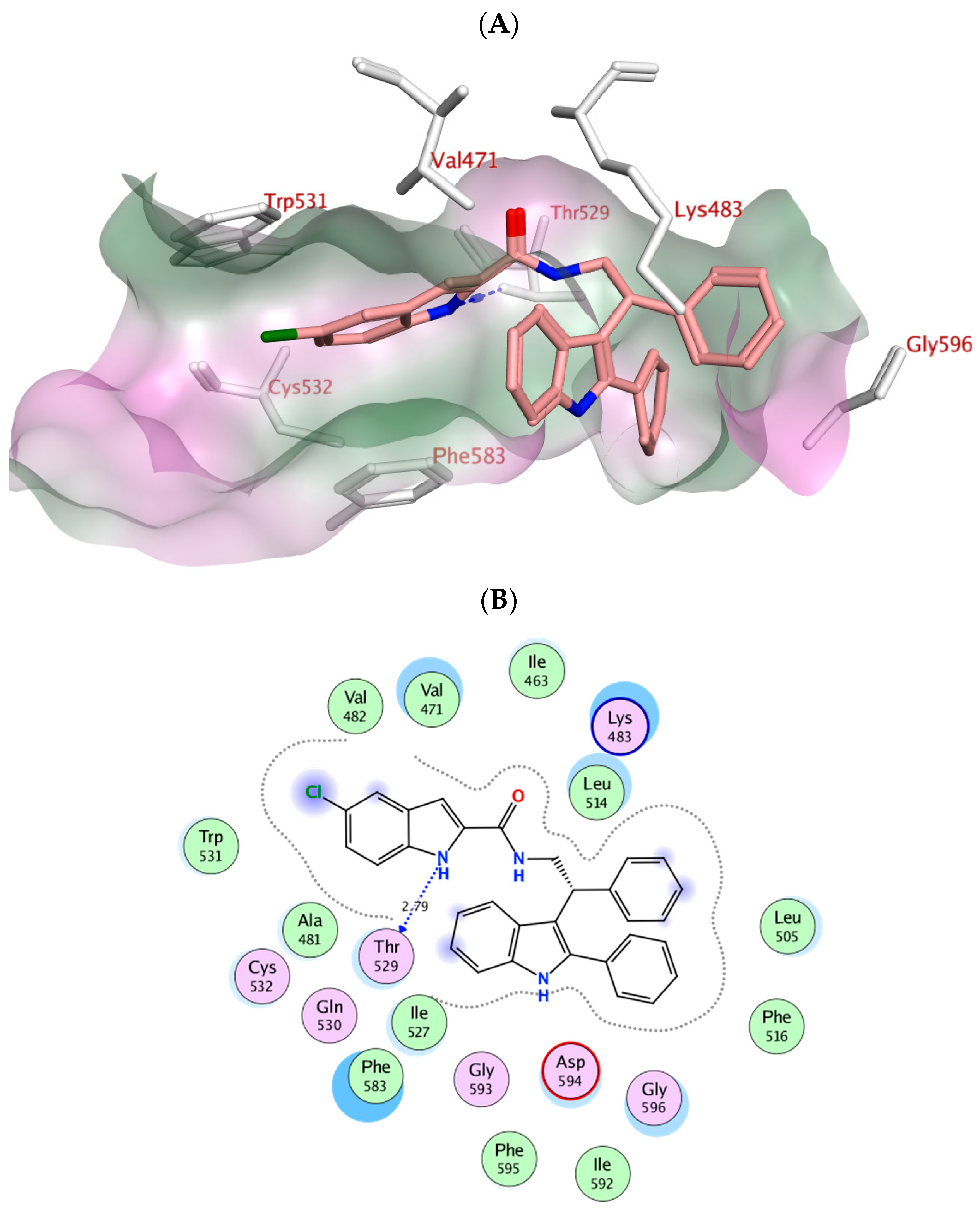
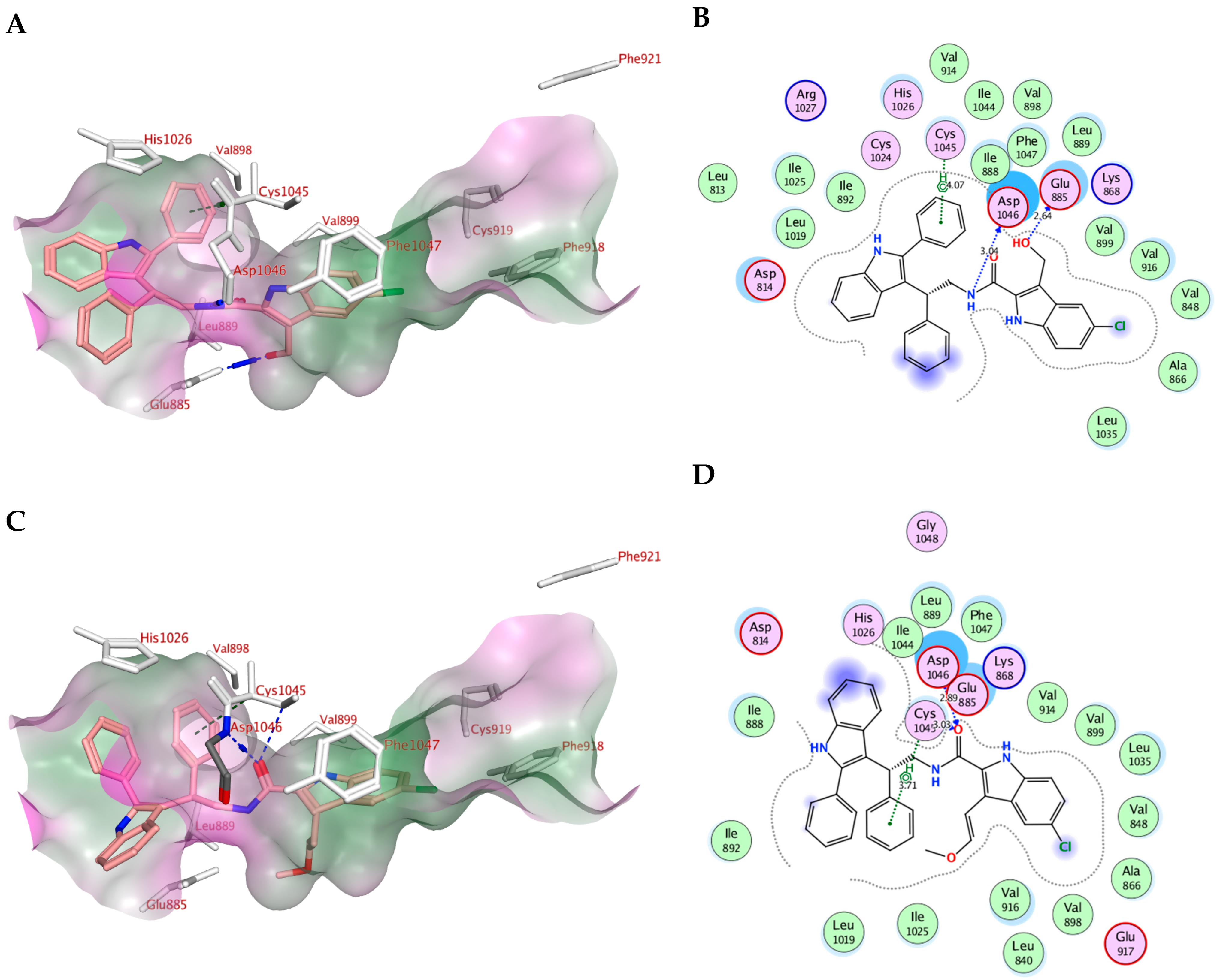
2.4. Molecular Modeling
2.5. In Silico ADME/Pharmacokinetics Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of 3-(2-nitro-1-phenylethyl)-2-phenyl-1H-indole (III)
3.1.2. Synthesis of 2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethan-1-amine (IV)
3.1.3. Synthesis of N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamides (Va-i)
5-Chloro-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Va)
5-Chloro-3-methyl-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vb)
5-Chloro-3-ethyl-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vc)
5-Bromo-3-ethyl-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vd)
5-Chloro-3-(hydroxymethyl)-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Ve)
5-Chloro-3-phenyl-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vf)
(E)-5-Chloro-3-(2-methoxyvinyl)-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vg)
5-Chloro-3-(ethoxymethyl)-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)-1H-indole-2-carboxamide (Vh)
5-Chloro-3-ethyl-N-(2-phenyl-2-(2-phenyl-1H-indol-3-yl)ethyl)benzofuran-2-carboxamide (Vi)
3.2. Biology
3.2.1. Cell Viability Assay
3.2.2. Antiproliferative Assay
3.2.3. EGFR Inhibitory Assay
3.2.4. BRAFV600E Inhibitory Assay
3.2.5. VEGFR-2 Inhibitory Assay
3.3. Apoptotic Markers Assays
3.3.1. Caspase-3 Assay
3.3.2. Caspase-8, Bax, and Bcl-2 Level Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioorg. Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Mahadevan, D. A comprehensive review of protein kinase inhibitors for cancer therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.V. Kinase-targeted cancer therapies: Progress, challenges and future directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Mahapatra, D.K.; Bharti, S.K. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2016, 109, 314–341. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2021 update. Pharmacol. Res. 2021, 165, 105463. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yang, X.; Duan, Y.; Han, J.; Liao, C. Small-molecule kinase inhibitors for the treatment of nononcologic diseases. J. Med. Chem. 2021, 64, 1283–1345. [Google Scholar] [CrossRef]
- Kim, G.; Ko, Y.T. Small molecule tyrosine kinase inhibitors in glioblastoma. Arch. Pharm. Res. 2020, 43, 385–394. [Google Scholar] [CrossRef]
- Du, J.; Yan, H.; Xu, Z.; Yang, B.; He, Q.; Wang, X.; Luo, P. Cutaneous toxicity of FDA-approved small-molecule kinase inhibitors. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1311–1325. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef]
- Ivy, S.P.; Wick, J.Y.; Kaufman, B.M. An overview of small-molecule inhibitors of VEGFR signaling. Nat. Rev. Clin. Oncol. 2009, 6, 569–579. [Google Scholar] [CrossRef]
- Kaufman, N.E.; Dhingra, S.; Jois, S.D.; Vicente, M.d.G.H. Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Hu, W.; Liu, Y.; Chen, J. Concurrent gene alterations with EGFR mutation and treatment efficacy of EGFR-TKIs in Chinese patients with non-small cell lung cancer. Oncotarget 2017, 8, 25046. [Google Scholar] [CrossRef]
- Nagy, J.A.; Dvorak, A.M.; Dvorak, H.F. VEGF-A164/165 and PlGF: Roles in angiogenesis and arteriogenesis. Trends Cardiovasc. Med. 2003, 13, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kitadai, Y.; Bucana, C.D.; Cleary, K.R.; Ellis, L.M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995, 55, 3964–3968. [Google Scholar] [PubMed]
- Poon, R.T.-P.; Fan, S.-T.; Wong, J. Clinical implications of circulating angiogenic factors in cancer patients. J. Clin. Oncol. 2001, 19, 1207–1225. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Kamal, M.A.; Akhtar, S. Tumor angiogenesis and VEGFR-2: Mechanism, pathways and current biological therapeutic interventions. Curr. Drug Metab. 2021, 22, 50–59. [Google Scholar]
- Azzoli, C.G.; Baker, S., Jr.; Temin, S.; Pao, W.; Aliff, T.; Brahmer, J.; Johnson, D.H.; Laskin, J.L.; Masters, G.; Milton, D. American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non–small-cell lung cancer. J. Clin. Oncol. 2009, 27, 6251. [Google Scholar] [CrossRef]
- Qin, Y.; Jian, H.; Tong, X.; Wu, X.; Wang, F.; Shao, Y.W.; Zhao, X. Variability of EGFR exon 20 insertions in 24 468 Chinese lung cancer patients and their divergent responses to EGFR inhibitors. Mol. Oncol. 2020, 14, 1695–1704. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Z.; Bai, Z.; Xie, R.; Sun, L.; Lin, K. Development and strategies of VEGFR-2/KDR inhibitors. Future Med. Chem. 2012, 4, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Enokida, T.; Tahara, M. Management of VEGFR-Targeted TKI for thyroid Cancer. Cancers 2021, 13, 5536. [Google Scholar] [CrossRef] [PubMed]
- Pitoia, F.; Jerkovich, F. Selective use of sorafenib in the treatment of thyroid cancer. Drug Des. Dev. Ther. 2016, 2016, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Wellbrock, C.; Karasarides, M.; Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004, 5, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Ammar, U.M.; Abdel-Maksoud, M.S.; Oh, C.-H. Recent advances of RAF (rapidly accelerated fibrosarcoma) inhibitors as anti-cancer agents. Eur. J. Med. Chem. 2018, 158, 144–166. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK pathway: New concepts of activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. RAF protein-serine/threonine kinases: Structure and regulation. Biochem. Biophys. Res. Commun. 2010, 399, 313–317. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS–ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 2014, 13, 928–942. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Gouda, A.M.; Abou-Ghadir, O.F.; Salem, O.I.; Ali, A.T.; Farghaly, H.S.; Abdelrahman, M.H.; Trembleau, L.; Abdu-Allah, H.H.; Youssif, B.G. Design and synthesis of novel 2, 3-dihydropyrazino [1, 2-a] indole-1, 4-dione derivatives as antiproliferative EGFR and BRAFV600E dual inhibitors. Bioorg. Chem. 2020, 104, 104260. [Google Scholar] [CrossRef]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Gouda, A.M.; Youssif, B.G.; Tateishi, H.; Fujita, M.; Otsuka, M.; Abdel-Aziz, M. Design and synthesis of novel quinoline/chalcone/1, 2, 4-triazole hybrids as potent antiproliferative agent targeting EGFR and BRAFV600E kinases. Bioorg. Chem. 2021, 106, 104510. [Google Scholar] [CrossRef] [PubMed]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The first drug approved for BRAF-mutant cancer. Nat. Rev. Drug Discov. 2012, 11, 873–886. [Google Scholar] [CrossRef]
- Khoja, L.; Hogg, D. Dabrafenib in the treatment of metastatic or unresectable melanoma. Expert Rev. Anticancer Ther. 2015, 15, 265–276. [Google Scholar] [CrossRef]
- Prahallad, A.; Sun, C.; Huang, S.; Di Nicolantonio, F.; Salazar, R.; Zecchin, D.; Beijersbergen, R.L.; Bardelli, A.; Bernards, R. Unresponsiveness of colon cancer to BRAF (V600E) inhibition through feedback activation of EGFR. Nature 2012, 483, 100–103. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, W.-g.; Song, Q.-b.; Wei, J. BRAF V600E mutation as a predictive factor of anti-EGFR monoclonal antibodies therapeutic effects in metastatic colorectal cancer: A meta-analysis. Chin. Med. Sci. J. 2014, 29, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; Amaro, A.; Levati, L.; Alvino, E.; Lacal, P.M.; Mastroeni, S.; Ruffini, F.; Bonmassar, L.; Antonini Cappellini, G.C.; Felli, N. miR-126-3p down-regulation contributes to dabrafenib acquired resistance in melanoma by up-regulating ADAM9 and VEGF-A. J. Exp. Clin. Cancer Res. 2019, 38, 272. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-I.; Liao, C.-B.; Chen, C.-S.; Cheng, F.-Y.; Chung, Y.-H.; Wang, Y.-C.; Ciou, S.-Y.; Hsueh, W.-Y.; Lo, T.-H.; Huang, G.-R. Design and synthesis of 4-anilinoquinazolines as Raf kinase inhibitors. Part 1. Selective B-Raf/B-RafV600E and potent EGFR/VEGFR2 inhibitory 4-(3-hydroxyanilino)-6-(1H-1, 2, 3-triazol-4-yl) quinazolines. Bioorg. Chem. 2021, 109, 104715. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.; Brungs, D.; Szeto, E.; Epstein, R. Anticancer activity of combination targeted therapy using cetuximab plus vemurafenib for refractory BRAFV600E-mutant metastatic colorectal carcinoma. Curr. Oncol. 2014, 21, e151. [Google Scholar] [CrossRef]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef]
- Fondevila, F.; Méndez-Blanco, C.; Fernández-Palanca, P.; González-Gallego, J.; Mauriz, J.L. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Comunanza, V.; Corà, D.; Orso, F.; Consonni, F.M.; Middonti, E.; Di Nicolantonio, F.; Buzdin, A.; Sica, A.; Medico, E.; Sangiolo, D. VEGF blockade enhances the antitumor effect of BRAFV 600E inhibition. EMBO Mol. Med. 2017, 9, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Torres-Collado, A.X.; Knott, J.; Jazirehi, A.R. Reversal of resistance in targeted therapy of metastatic melanoma: Lessons learned from Vemurafenib (BRAFV600E-specific inhibitor). Cancers 2018, 10, 157. [Google Scholar] [CrossRef]
- Cheng, H.; Chang, Y.; Zhang, L.; Luo, J.; Tu, Z.; Lu, X.; Zhang, Q.; Lu, J.; Ren, X.; Ding, K. Identification and optimization of new dual inhibitors of B-Raf and epidermal growth factor receptor kinases for overcoming resistance against vemurafenib. J. Med. Chem. 2014, 57, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Kumar, A.; Kumar, P. Anticancer potential of plants and natural products. Am J Pharmacol Sci 2013, 1, 104–115. [Google Scholar] [CrossRef]
- Sravanthi, T.; Manju, S. Indoles—A promising scaffold for drug development. Eur. J. Pharm. Sci. 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, A.; Sharma, R.; Singh, R.K. Target-based anticancer indole derivatives and insight into structure‒activity relationship: A mechanistic review update (2018–2021). Acta Pharm. Sin. B 2022, 12, 3006–3027. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.; Abdelrahman, M.H.; Abdelazeem, A.H.; Ibrahim, H.M.; Salem, O.I.; Mohamed, M.F.; Treambleau, L.; Bukhari, S.N.A. Design, synthesis, mechanistic and histopathological studies of small-molecules of novel indole-2-carboxamides and pyrazino [1, 2-a] indol-1 (2H)-ones as potential anticancer agents effecting the reactive oxygen species production. Eur. J. Med. Chem. 2018, 146, 260–273. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The importance of indole and azaindole scaffold in the development of antitumor agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Li, W.; Qi, Y.Y.; Wang, Y.Y.; Gan, Y.Y.; Shao, L.H.; Zhang, L.Q.; Tang, Z.H.; Zhu, M.; Tang, S.Y.; Wang, Z.C. Design, synthesis, and biological evaluation of sorafenib derivatives containing indole (ketone) semicarbazide analogs as antitumor agents. J. Heterocycl. Chem. 2020, 57, 2548–2560. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. Molecular dynamics guided development of indole based dual inhibitors of EGFR (T790M) and c-MET. Bioorg. Chem. 2018, 79, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Des. Dev. Ther. 2016, 10, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yoo, J.; Kwon, A.; Kim, D.; Nguyen, H.K.; Lee, B.-Y.; Suh, W.; Min, K.H. Structure-activity relationship of indole-tethered pyrimidine derivatives that concurrently inhibit epidermal growth factor receptor and other angiokinases. PLoS ONE 2015, 10, e0138823. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Choudhary, A. Kinase Inhibitor Drugs. Success. Drug Discov. 2018, 3, 65–93. [Google Scholar]
- Ward, R.A.; Goldberg, F.W. Kinase Drug Discovery: Modern Approaches; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Al-Wahaibi, L.H.; Mohammed, A.F.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, Synthesis, and Antiproliferative Activity of New 5-Chloro-indole-2-carboxylate and Pyrrolo [3, 4-b] indol-3-one Derivatives as Potent Inhibitors of EGFRT790M/BRAFV600E Pathways. Molecules 2023, 28, 1269. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mostafa, Y.A.; Abdelrahman, M.H.; El-Bahrawy, A.H.; Trembleau, L.; Youssif, B.G. Synthesis and Biological Evaluation of Indole-2-Carboxamides with Potent Apoptotic Antiproliferative Activity as EGFR/CDK2 Dual Inhibitors. Pharmaceuticals 2022, 15, 1006. [Google Scholar] [CrossRef]
- Gomaa, H.A.; Shaker, M.E.; Alzarea, S.I.; Hendawy, O.; Mohamed, F.A.; Gouda, A.M.; Ali, A.T.; Morcoss, M.M.; Abdelrahman, M.H.; Trembleau, L. Optimization and SAR investigation of novel 2, 3-dihydropyrazino [1, 2-a] indole-1, 4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg. Chem. 2022, 120, 105616. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Gomaa, H.A.; Hendawy, O.; Ali, A.T.; Farghaly, H.S.; Gouda, A.M.; Abdelazeem, A.H.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G. Design, synthesis, and biological evaluation of novel EGFR inhibitors containing 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity. Bioorg. Chem. 2021, 112, 104960. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Alakilli, S.Y.; El Azab, E.F.; Baawad, F.A.; Shaaban, E.I.A.; Alrub, H.A.; Hendawy, O.; Gomaa, H.A.; Bakr, A.G.; Abdelrahman, M.H. Discovery of new 5-substituted-indole-2-carboxamides as dual epidermal growth factor receptor (EGFR)/cyclin dependent kinase-2 (CDK2) inhibitors with potent antiproliferative action. RSC Med. Chem. 2023, 14, 734–744. [Google Scholar] [CrossRef]
- Gomaa, H.A.; El-Sherief, H.A.; Hussein, S.; Gouda, A.M.; Salem, O.I.; Alharbi, K.S.; Hayallah, A.M.; Youssif, B.G. Novel 1, 2, 4-triazole derivatives as apoptotic inducers targeting p53: Synthesis and antiproliferative activity. Bioorg. Chem. 2020, 105, 104369. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, A.A.; Abdel-Aziz, S.A.; Abdelrahman, K.S.; Wanas, A.S.; Gouda, A.M.; Youssif, B.G.; Abdel-Aziz, M. Design and synthesis of new 1, 6-dihydropyrimidin-2-thio derivatives targeting VEGFR-2: Molecular docking and antiproliferative evaluation. Bioorg. Chem. 2020, 102, 104090. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Mahmoud, M.A.; Mohammed, A.F.; Salem, O.I.; Gomaa, H.A.; Youssif, B.G. New 1, 3, 4-oxadiazoles linked with the 1, 2, 3-triazole moiety as antiproliferative agents targeting the EGFR tyrosine kinase. Arch. Pharm. 2022, 355, 2200009. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, S.A.; Taher, E.S.; Lan, P.; Asaad, G.F.; Gomaa, H.A.; El-Koussi, N.A.; Youssif, B.G. Design, synthesis, and biological evaluation of new pyrimidine-5-carbonitrile derivatives bearing 1, 3-thiazole moiety as novel anti-inflammatory EGFR inhibitors with cardiac safety profile. Bioorg. Chem. 2021, 111, 104890. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zied, H.A.; Beshr, E.A.; Gomaa, H.A.; Mostafa, Y.A.; Youssif, B.G.; Hayallah, A.M.; Abdel-Aziz, M. Discovery of new cyanopyridine/chalcone hybrids as dual inhibitors of EGFR/BRAFV600E with promising antiproliferative properties. Arch. Pharm. 2022, 356, e2200464. [Google Scholar] [CrossRef]
- La, D.S.; Belzile, J.; Bready, J.V.; Coxon, A.; DeMelfi, T.; Doerr, N.; Estrada, J.; Flynn, J.C.; Flynn, S.R.; Graceffa, R.F. Novel 2, 3-dihydro-1, 4-benzoxazines as potent and orally bioavailable inhibitors of tumor-driven angiogenesis. J. Med. Chem. 2008, 51, 1695–1705. [Google Scholar] [CrossRef]
- Qiao, L.; Liang, N.; Zhang, J.; Xie, J.; Liu, F.; Xu, D.; Yu, X.; Tian, Y. Advanced research on vasculogenic mimicry in cancer. J. Cell. Mol. Med. 2015, 19, 315–326. [Google Scholar] [CrossRef]
- Okamoto, K.; Ikemori-Kawada, M.; Jestel, A.; von König, K.; Funahashi, Y.; Matsushima, T.; Tsuruoka, A.; Inoue, A.; Matsui, J. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med. Chem. Lett. 2015, 6, 89–94. [Google Scholar] [CrossRef]
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 108–121. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Mohammed, A.F.; Salem, O.I.; Rabea, S.M.; Youssif, B.G. Design, synthesis, and antiproliferative properties of new 1, 2, 3-triazole-carboximidamide derivatives as dual EGFR/VEGFR-2 inhibitors. J. Mol. Struct. 2023, 1282, 135165. [Google Scholar] [CrossRef]
- Abou-Zied, H.A.; Youssif, B.G.; Mohamed, M.F.; Hayallah, A.M.; Abdel-Aziz, M. EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019, 89, 102997. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Lim, B. Targeting apoptosis in cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Martin, S. Caspases: Executioners of apoptosis. Pathobiol. Hum. Dis. 2014, 145–152. [Google Scholar]
- Choudhary, G.S.; Al-Harbi, S.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. In Apoptosis Cancer: Methods and Protocol; Humana Press: New York, NY, USA, 2015; Volume 1219, pp. 1–9. [Google Scholar]
- Mazumder, S.; Plesca, D.; Almasan, A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. In Apoptosis Cancer: Methods and Protocol; Humana Press: Totowa, NJ, USA, 2008; Volume 414, pp. 13–21. [Google Scholar]
- Ibrahim, T.S.; Bokhtia, R.M.; Al-Mahmoudy, A.M.; Taher, E.S.; AlAwadh, M.A.; Elagawany, M.; Abdel-Aal, E.H.; Panda, S.; Gouda, A.M.; Asfour, H.Z. Design, synthesis and biological evaluation of novel 5-((substituted quinolin-3-yl/1-naphthyl) methylene)-3-substituted imidazolidin-2, 4-dione as HIV-1 fusion inhibitors. Bioorg. Chem. 2020, 99, 103782. [Google Scholar] [CrossRef]
- Maier, J.K.; Labute, P. Assessment of fully automated antibody homology modeling protocols in molecular operating environment. Proteins: Struct. Funct. Bioinform. 2014, 82, 1599–1610. [Google Scholar] [CrossRef]
- Park, J.H.; Liu, Y.; Lemmon, M.A.; Radhakrishnan, R. Erlotinib binds both inactive and active conformations of the EGFR tyrosine kinase domain. Biochem. J. 2012, 448, 417. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mahmoud, M.A.; Mostafa, Y.A.; Raslan, A.E.; Youssif, B.G. Novel piperine-carboximidamide hybrids: Design, synthesis, and antiproliferative activity via a multi-targeted inhibitory pathway. J. Enzym. Inhib. Med. Chem. 2023, 38, 376–386. [Google Scholar] [CrossRef]
- Miller, D.S.; Voell, S.A.; Sosič, I.; Proj, M.; Rossanese, O.W.; Schnakenburg, G.; Gütschow, M.; Collins, I.; Steinebach, C. Encoding BRAF inhibitor functions in protein degraders. RSC Med. Chem. 2022, 13, 731–736. [Google Scholar] [CrossRef]
- Bakchi, B.; Krishna, A.D.; Sreecharan, E.; Ganesh, V.B.J.; Niharika, M.; Maharshi, S.; Puttagunta, S.B.; Sigalapalli, D.K.; Bhandare, R.R.; Shaik, A.B. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: A medicinal chemist’s perspective. J. Mol. Struct. 2022, 1259, 132712. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef] [PubMed]
- Daoud, N.E.-H.; Borah, P.; Deb, P.K.; Venugopala, K.N.; Hourani, W.; Alzweiri, M.; Bardaweel, S.K.; Tiwari, V. ADMET profiling in drug discovery and development: Perspectives of in silico, in vitro and integrated approaches. Curr. Drug Metab. 2021, 22, 503–522. [Google Scholar] [CrossRef]
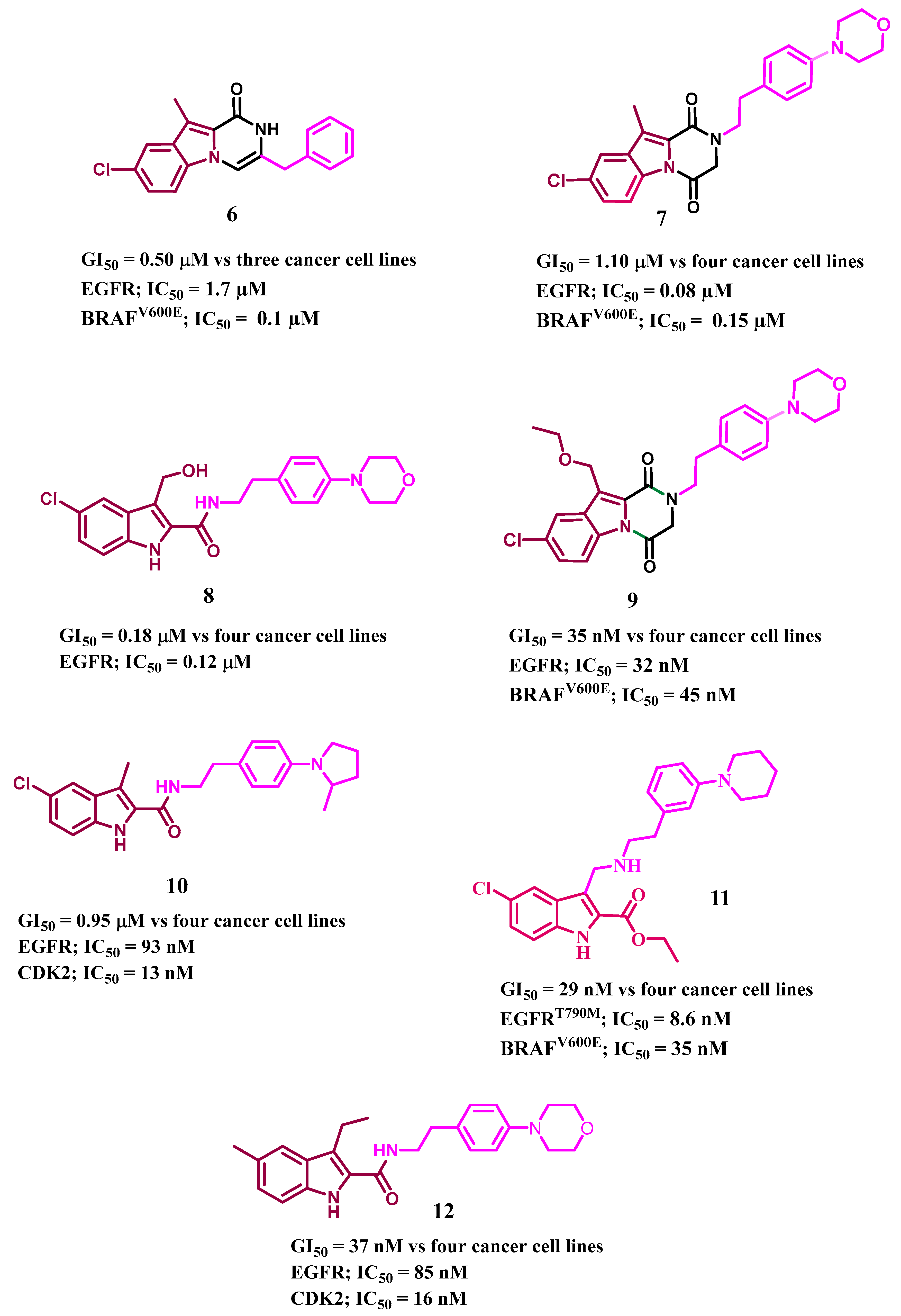

 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comp. | R1 | R2 | X | Cell Viability % | Antiproliferative Activity IC50 ± SEM (nM) | ||||
| A-549 | MCF-7 | Panc-1 | HT-29 | Average (GI50) | |||||
| IV | -- | -- | -- | 92 | 102 ± 10 | 106 ± 10 | 104 ± 10 | 104 ± 10 | 104 |
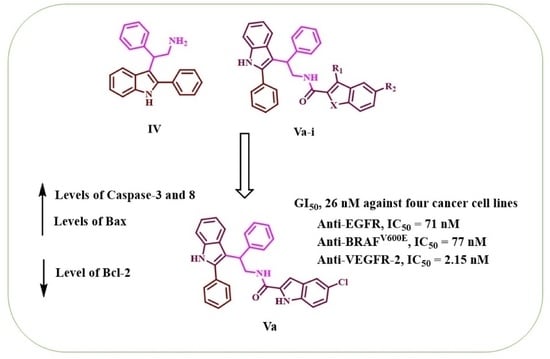
| Va | H | Cl | NH | 91 | 25 ± 2 | 28 ± 2 | 26 ± 2 | 26 ± 2 | 26 |
| Vb | CH3 | Cl | NH | 88 | 58 ± 5 | 61 ± 6 | 58± 5 | 59 ± 5 | 59 |
| Vc | CH2CH3 | Cl | NH | 91 | 54 ± 5 | 57 ± 5 | 56± 5 | 55 ± 5 | 56 |
| Vd | CH2CH3 | Br | NH | 89 | 64 ± 6 | 68 ± 6 | 66 ± 6 | 66 ± 6 | 66 |
| Ve | CH2OH | Cl | NH | 90 | 42 ± 4 | 46 ± 4 | 44 ± 4 | 45 ± 4 | 44 |
| Vf | Ph | Cl | NH | 91 | 46 ± 4 | 49 ± 4 | 48 ± 4 | 48 ± 4 | 48 |
| Vg | CH=CH-OCH3 | Cl | NH | 89 | 30 ± 2 | 33 ± 3 | 30 ± 2 | 30 ± 2 | 31 |
| Vh | CH2OCH2CH3 | Cl | NH | 90 | 34 ± 3 | 38 ± 3 | 36 ± 3 | 38 ± 3 | 37 |
| Vi | CH2CH3 | Cl | O | 89 | 86 ± 8 | 89 ± 8 | 85 ± 8 | 85 ± 8 | 86 |
| Erlotinib | -- | -- | -- | ND | 30 ± 3 | 40 ± 3 | 30 ± 3 | 30 ± 3 | 33 |
| Compd. | EGFR Inhibition IC50 ± SEM (nM) | BRAFV600E Inhibition IC50 ± SEM (nM) | VEGFR-2 Inhibition IC50 (nM) |
|---|---|---|---|
| Va | 71 ± 6 | 77 ± 6 | 2.15 ± 0.20 |
| Ve | 94 ± 7 | 97 ± 8 | 1.10 ± 0.08 |
| Vf | 103 ± 8 | 107 ± 9 | 2.50 ± 0.20 |
| Vg | 79 ± 6 | 83 ± 6 | 1.60 ± 0.10 |
| Vh | 85 ± 7 | 89 ± 7 | 3.25 ± 0.25 |
| Erlotinib | 80 ± 5 | 60 ± 5 | -- |
| Sorafenib | -- | -- | 0.17 ± 0.01 |
| Compd. No. | Caspase-3 | Caspase-8 | Bax | Bcl-2 | ||||
|---|---|---|---|---|---|---|---|---|
| Conc (pg/mL) | Fold Change | Conc (ng/mL) | Fold Change | Conc (pg/mL) | Fold Change | Conc (ng/mL) | Fold Reduction | |
| Va | 726± 6 | 11 | 3.50 | 35 | 410 | 45 | 0.75 | 7 |
| Ve | 462 ± 4 | 7 | -- | -- | -- | -- | -- | -- |
| Vg | 528 ± 5 | 8 | 2.20 | 22 | 320 | 35 | 0.85 | 6 |
| Vh | 460 ± 4 | 7 | -- | -- | -- | -- | -- | -- |
| Doxorubicin | 505 ± 4 | 7.5 | 1.80 | 18 | 280 | 31 | 0.90 | 6 |
| Control | 66 | 1 | 0.10 | 1 | 9 | 1 | 5 | 1 |
| Compd. | MOE Score kcal/mol | Hydrogen Bond Interactions | Hydrophobic Interactions | Other Interactions |
|---|---|---|---|---|
| Erlotinib | −10.70 | Met769 | Leu694, Leu820, Val702, Gly722, Thr766, Thr830 | Leu694 |
| IV | −7.79 | Met769 Thr830 | Leu820, Val702, Phe699, Asp831 | Glu738 (ionic) Asp831 (ionic) Val702 (pi-H) |
| Va | −10.52 | Asp831 | Gly722, Thr766, Pro770, Glu780Leu694, Leu820, Val702 | Gly772 (pi-H) |
| Vb | −8.89 | Asp831 Asp776 | Thr766, Pro770, Glu780, Leu694, Leu820, Val702, Gly722 | Cys773 (pi-H) |
| Vc | −9.38 | Asp831 Arg817 | Leu694, Leu820, Val702, Gly722, Thr766, Pro770, Glu780, His781 | -------------------- |
| Vd | −9.35 | Leu764 Asp831 | Leu820, Val702, Gly722, Thr766, Leu694, Asp776, Glu780 | Leu820 (pi-H) |
| Ve | −9.89 | Asp831 | Glu780, Leu694, Leu820, Val702, Gly722, Thr766, Asp776, | Leu694 (pi-H) |
| Vf | −9.90 | Asp831 Asp776 | Leu694, Leu820, Val702, Gly722, Thr766, Asp776, Glu780 | Val702 (pi-H) |
| Vg | −10.05 | Asp831 | Leu694, Leu820, Val702, Gly722, Thr766, Asp776, Glu780 | ------------------ |
| Vh | −10.13 | Asp831 Arg817 | Thr766, Asp776, Glu780, Leu694, Leu820, Val702, Gly722 | Gly695, Val702 (pi-H) |
| Vi | −9.58 | ---------- | Leu694, Leu820, Val702, Gly722, Thr766, Asp776, Glu780 | Gly772 (pi-H) |
| Compd. | MOE Score kcal/mol | Hydrogen Bond Interactions | Hydrophobic Interactions | Other Interactions |
|---|---|---|---|---|
| Vemurafenib | −11.78 | Thr529 Gln530 Cys532 Asp594 Gly596 | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Lys483 (ionic) |
| Va | −7.97 | Thr529 | Phe583, Cys532, Thr592, val471, Lys483, Leu514 | -------------------- |
| Ve | −4.21 | Leu505 Thr508 Lys483 | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | -------------------- |
| Vf | −4.30 | ---------- | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Val471 (pi-H) Leu514 (pi-H) Phe583 (pi-pi) |
| Vg | −7.32 | ---------- | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514 | Val471 (pi-H) |
| Vh | −7.44 | Asp594 | Trp531, Phe583, Cys532, Ile463, Thr592, val471, Lys483, Leu514, Gly596 | Val471 (pi-H) Ile527 (pi-H) |
| Compd. | MOE Score kcal/mol | Hydrogen Bond Interactions | Hydrophobic Interactions | Pi-H Interactions |
|---|---|---|---|---|
| Sorafenib | −10.73 | Cys919, Glu885 | Val916, Leu889, Leu840, Asp1046, Cys1045 and Phe1047 | Phe1047 |
| Va | −9.61 | Glu885, Asp1046 | Leu889, Asp814, Asp1046, Glu885 and Leu886 | ----------- |
| Ve | −9.77 | Glu885, Asp1046 | Leu889, Asp814, Asp1046, Glu885 and Leu886, Cys1045 and His1026 | Cys1045 |
| Vf | −8.18 | Glu885, Asp1046 | Leu889, Asp814, Asp1046, Glu885 and Leu886, Cys1045 and Phe1047 | Cys1045 |
| Vg | −9.07 | Cys1045, Asp1046 | Leu889, Asp814, Asp1046, Glu885 and Leu886, Cys1045 and Phe1047 | Cys1045 |
| Vh | −9.09 | Glu885, Asp1046, Cys1045 | Leu889, Asp814, Asp1046, Glu885 and Leu886, Cys1045 and Phe1047 | ------------- |
| Compd. | MW | nROTB | HBA | HBD | Violations | MR | TPSA | Log P |
|---|---|---|---|---|---|---|---|---|
| IV | 312 | 4 | 1 | 2 | 0 | 100 | 41.81 | 4.25 |
| Va | 490 | 7 | 1 | 3 | 1 | 147 | 60.68 | 6.22 |
| Vb | 504 | 7 | 1 | 3 | 2 | 152 | 60.68 | 6.61 |
| Vc | 518 | 8 | 1 | 3 | 2 | 157 | 60.68 | 6.79 |
| Vd | 563 | 8 | 1 | 3 | 2 | 160 | 60.68 | 6.91 |
| Ve | 520 | 8 | 2 | 4 | 2 | 154 | 80.91 | 5.72 |
| Vf | 566 | 8 | 1 | 3 | 2 | 173 | 60.68 | 7.59 |
| Vg | 546 | 9 | 2 | 3 | 2 | 164 | 69.91 | 6.54 |
| Vh | 548 | 10 | 2 | 3 | 2 | 163 | 69.91 | 6.5 |
| Vi | 519 | 8 | 2 | 2 | 2 | 155 | 58.03 | 7.12 |
| Compd. | GI Abs. | BBB | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|---|
| IV | High | ++ | - | + | --- | --- | + | - |
| Va | Low | ++ | --- | + | + | + | + | ++ |
| Vb | Low | ++ | --- | + | + | + | + | + |
| Vc | Low | ++ | --- | + | - | + | + | + |
| Vd | Low | ++ | --- | + | + | + | - | + |
| Ve | Low | ++ | --- | - | - | + | + | - |
| Vf | Low | ++ | --- | + | - | + | + | - |
| Vg | Low | ++ | --- | - | - | + | + | ++ |
| Vh | Low | + | --- | - | --- | + | + | + |
| Vi | Low | ++ | --- | + | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wahaibi, L.H.; Mohammed, A.F.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G.M. Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents. Pharmaceuticals 2023, 16, 1039. https://doi.org/10.3390/ph16071039
Al-Wahaibi LH, Mohammed AF, Abdelrahman MH, Trembleau L, Youssif BGM. Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents. Pharmaceuticals. 2023; 16(7):1039. https://doi.org/10.3390/ph16071039
Chicago/Turabian StyleAl-Wahaibi, Lamya H., Anber F. Mohammed, Mostafa H. Abdelrahman, Laurent Trembleau, and Bahaa G. M. Youssif. 2023. "Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents" Pharmaceuticals 16, no. 7: 1039. https://doi.org/10.3390/ph16071039
APA StyleAl-Wahaibi, L. H., Mohammed, A. F., Abdelrahman, M. H., Trembleau, L., & Youssif, B. G. M. (2023). Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides as Potential Multi-Target Antiproliferative Agents. Pharmaceuticals, 16(7), 1039. https://doi.org/10.3390/ph16071039