Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis
Abstract
:1. Introduction
2. Results
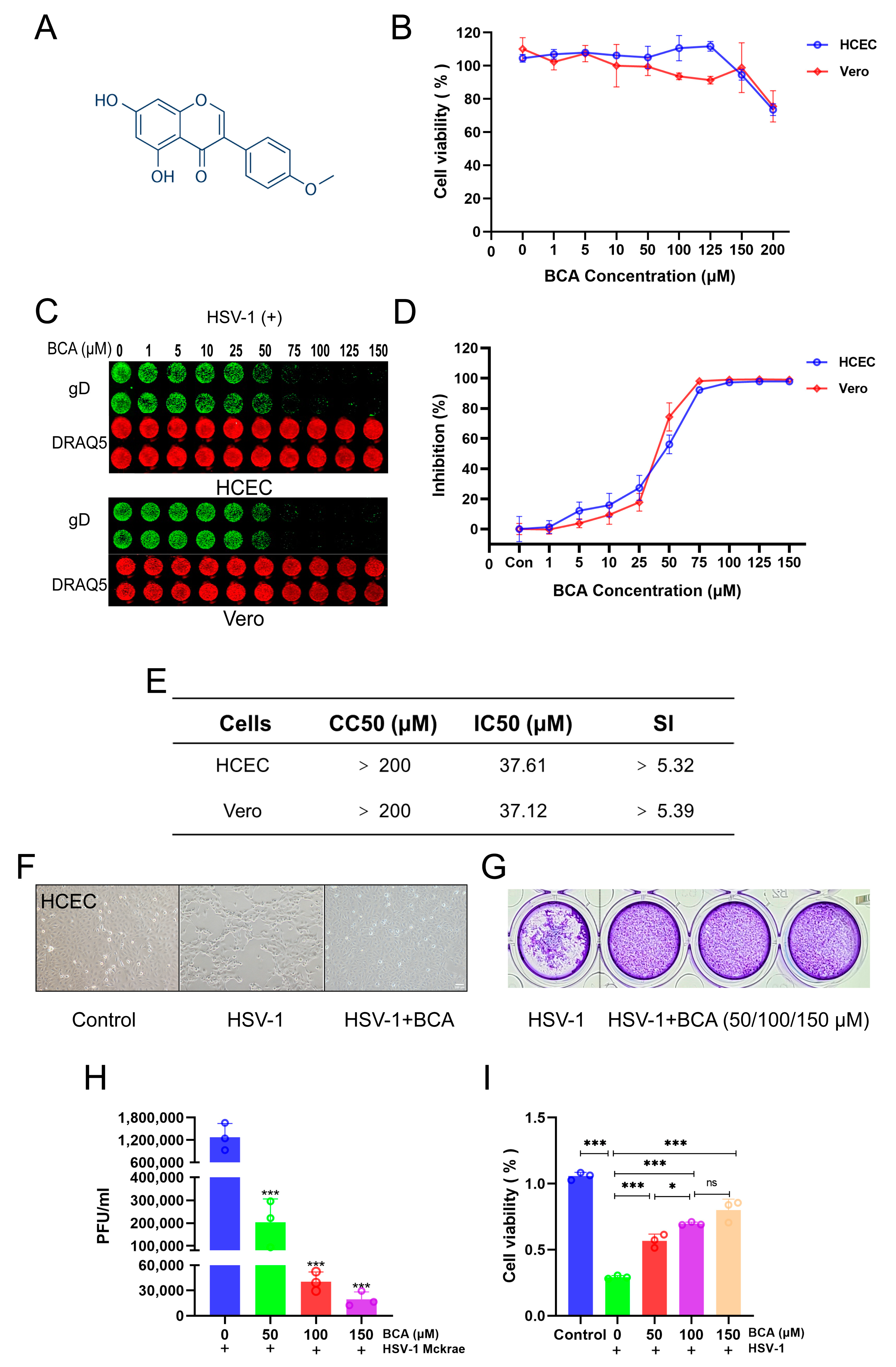
2.1. BCA Inhibited HSV-1 Replication In Vitro
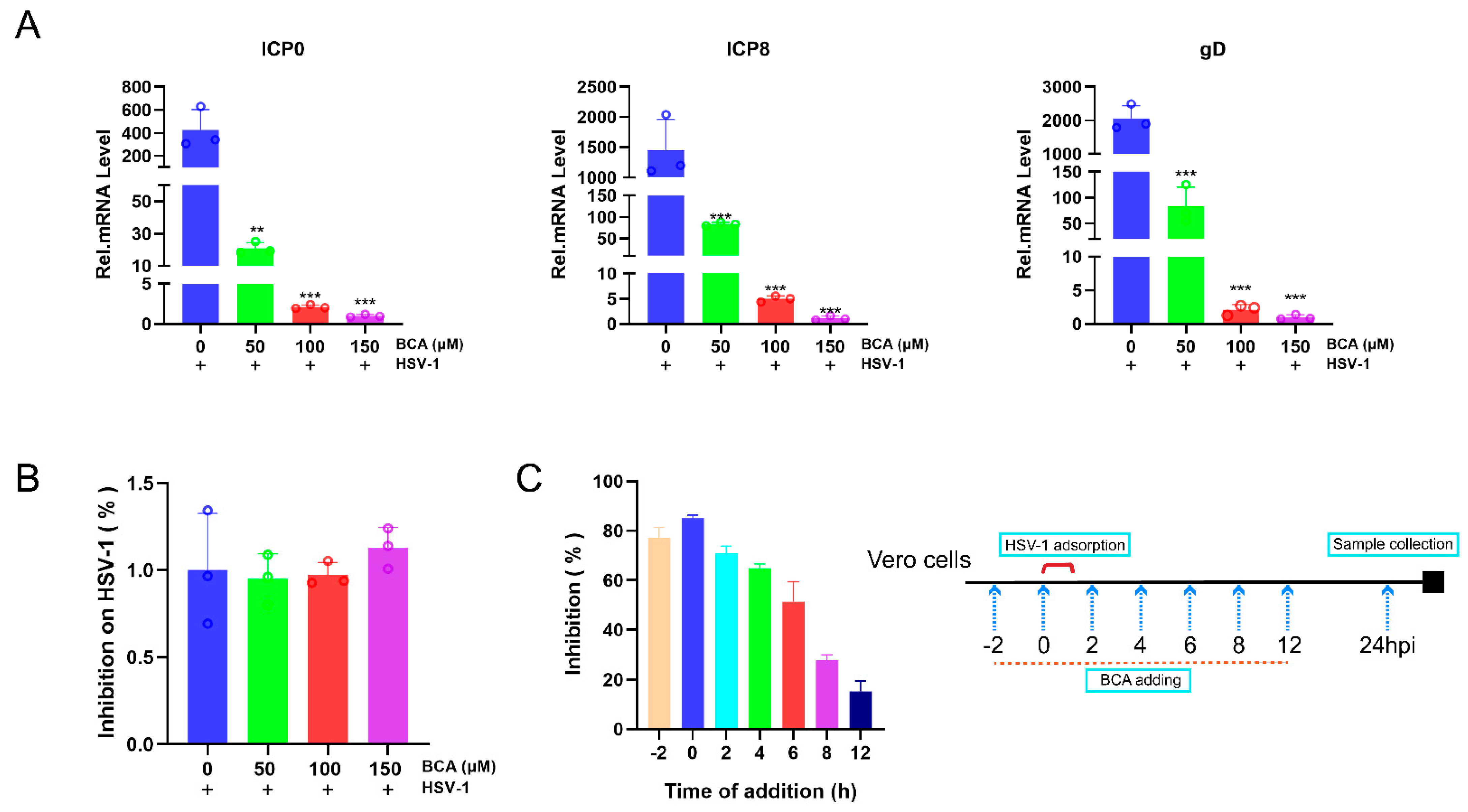
2.2. BCA Suppressed the Expression of HSV-1-Immediate–Early (IE), -Early (E) and -Late (L) Genes and Blocked HSV-1 at an Early Stage
2.3. The Inhibition of HSV-1 Infection by BCA Was Independent on Interferons (IFNs) and BCA Reduced the Overproduction of Inflammatory Cytokines in Corneal Epithelial Cells
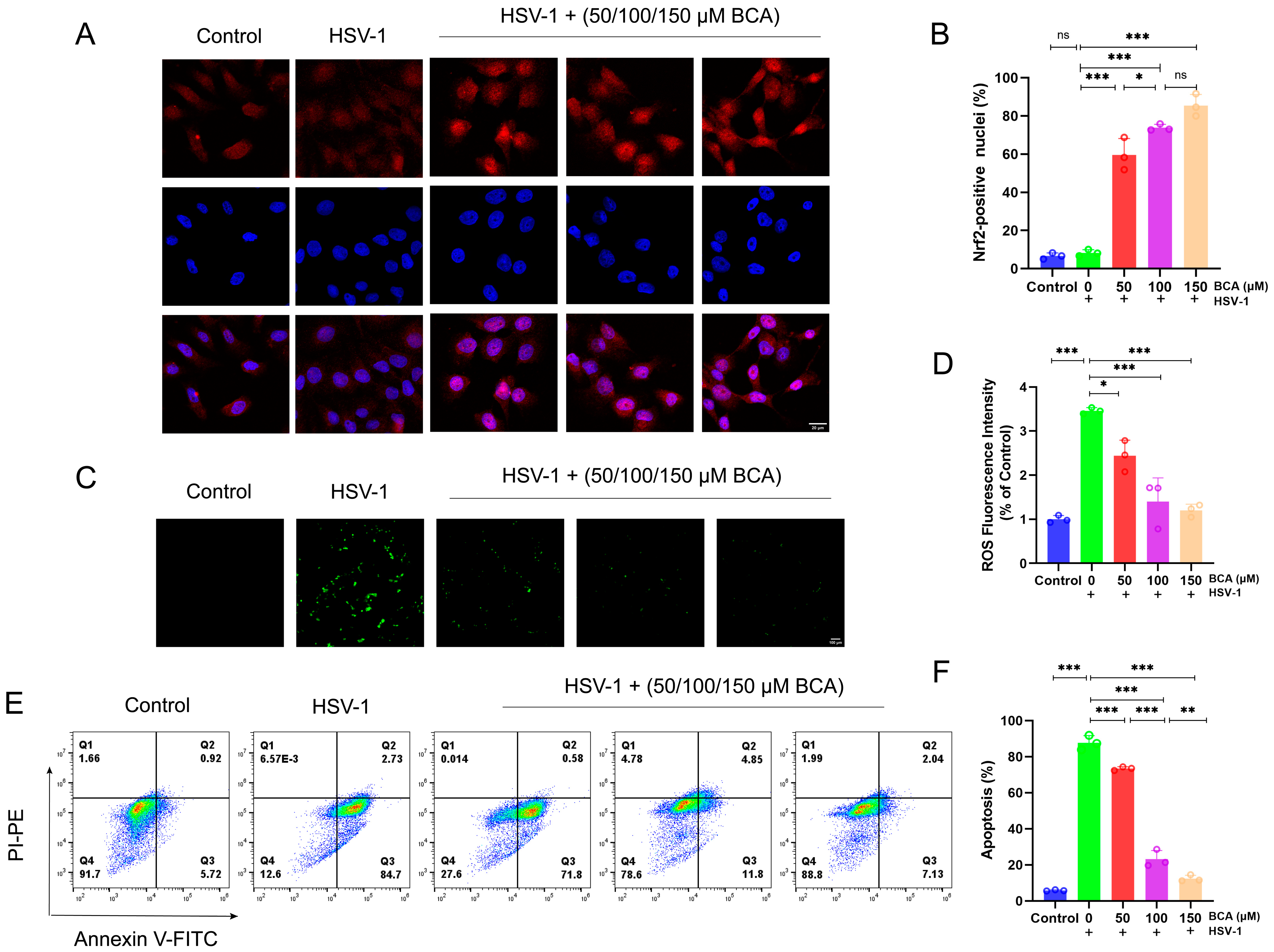
2.4. BCA Alleviated Oxidative Stress and Apoptosis in Corneal Epithelial Cells Infected by HSV-1
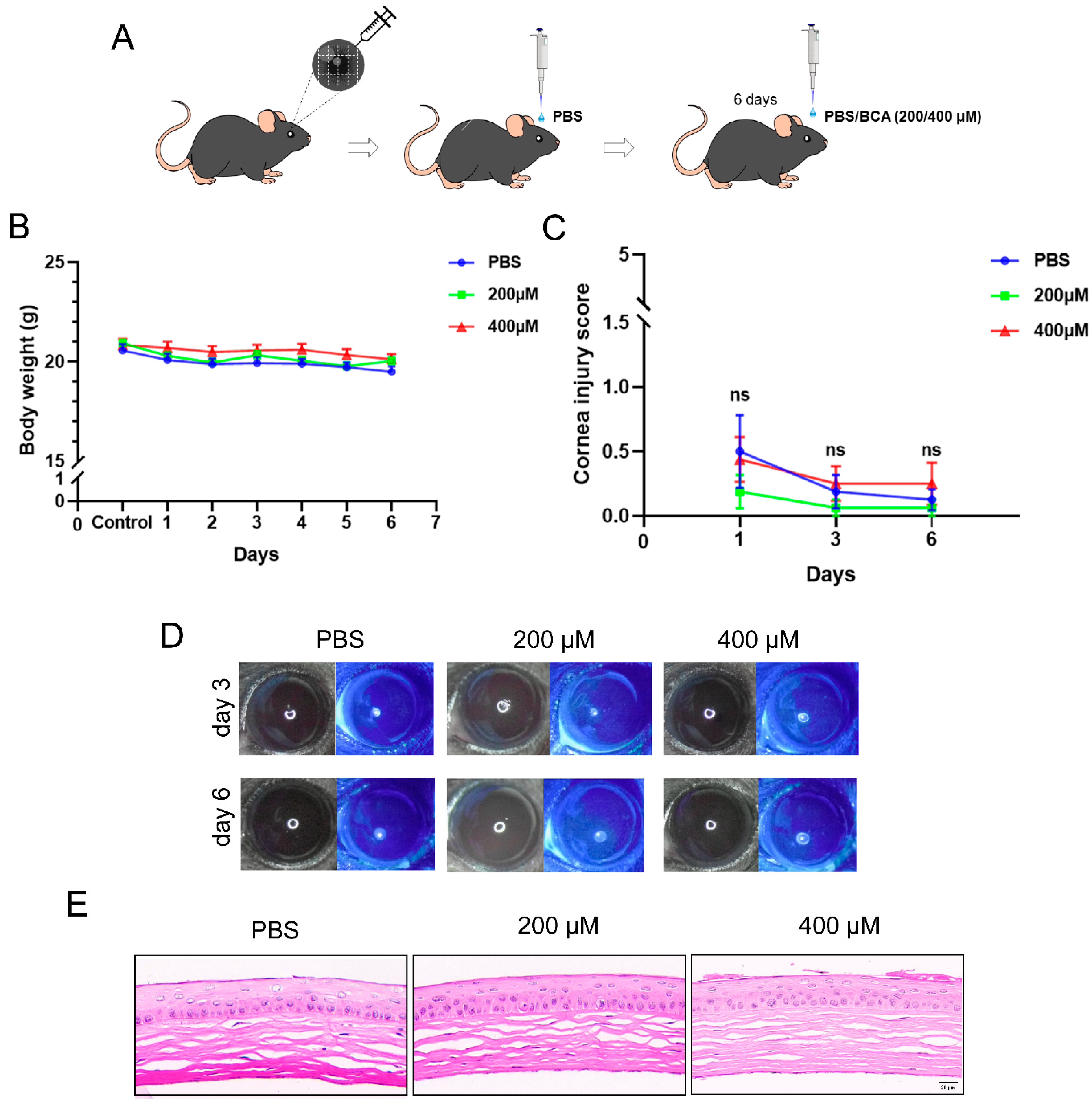
2.5. Safety Assessment of Topical BCA on the Cornea in Mice
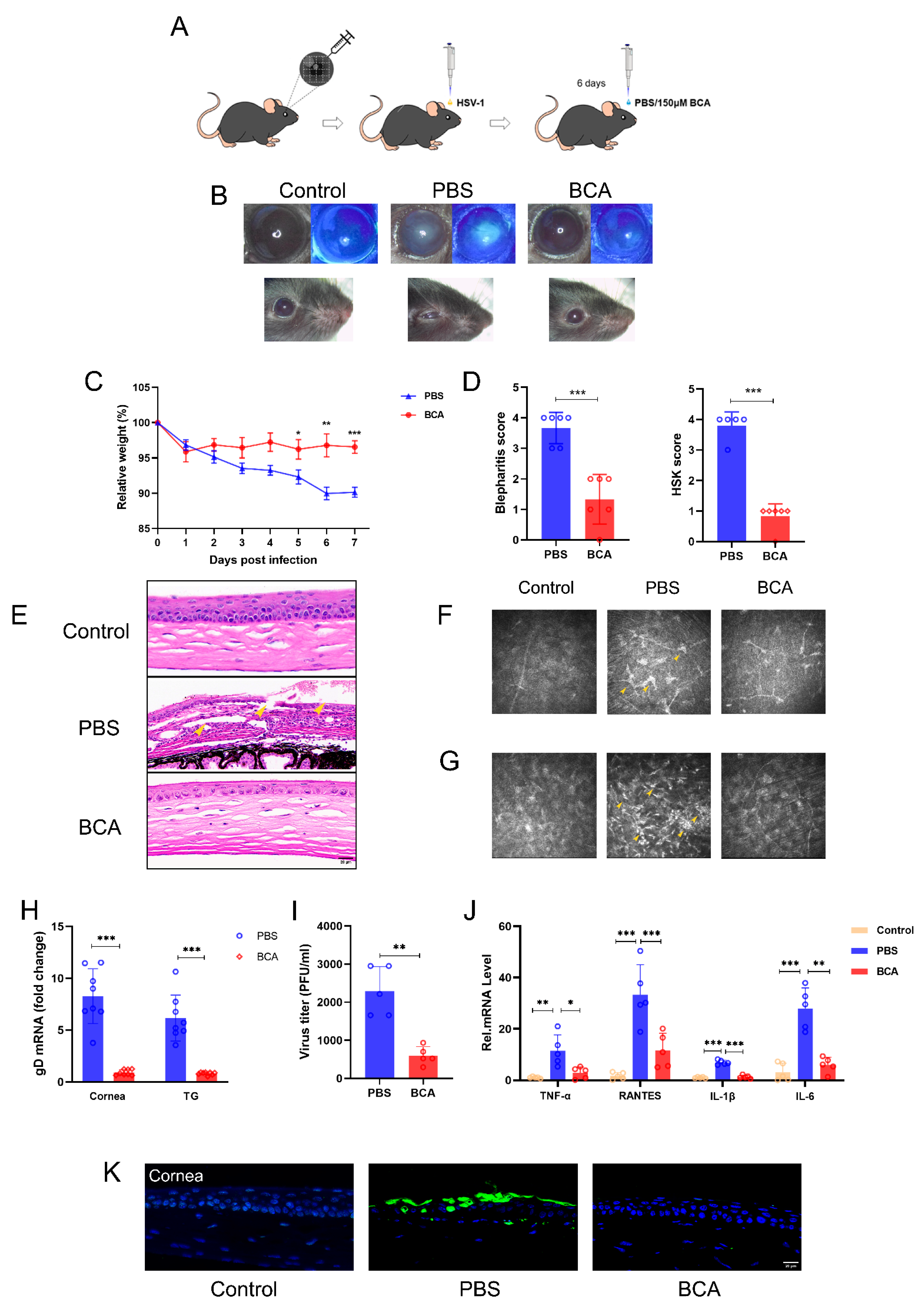
2.6. BCA Treatment Ameliorates HSK Severity in Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Viruses and Reagents
4.2. Cytotoxicity Assay
4.3. In-Cell Western Assay
4.4. Antiviral Activity
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Plaque Reduction Assay
4.7. Time-of-Drug-Addition Assay
4.8. Virucidal Assay
4.9. Transfection
4.10. Immunofluorescence
4.11. Measurement of ROS
4.12. Analysis of Apoptosis
4.13. Mice
4.14. Corneal Toxicity of BCA in a Mouse Model
4.15. Herpes Simplex Keratitis Mouse Model
4.16. TUNEL Assay
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, I.; Wilson, D.W. HSV-1 Cytoplasmic Envelopment and Egress. Int. J. Mol. Sci. 2020, 21, 5969. [Google Scholar] [CrossRef] [PubMed]
- Khadr, L.; Harfouche, M.; Omori, R.; Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J. The Epidemiology of Herpes Simplex Virus Type 1 in Asia: Systematic Review, Meta-analyses, and Meta-regressions. Clin. Infect. Dis. 2018, 68, 757–772. [Google Scholar] [CrossRef] [PubMed]
- WHO. Herpes Simplex Virus. 2017. Available online: https://www.who.int/en/news-room/fact-sheets/detail/herpes-simplex-virus#hsv1 (accessed on 20 June 2023).
- Tognarelli, E.I.; Palomino, T.F.; Corrales, N.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Herpes Simplex Virus Evasion of Early Host Antiviral Responses. Front. Cell. Infect. Microbiol. 2019, 9, 127. [Google Scholar] [CrossRef]
- Rowe, A.M.; Leger, A.S.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Alimbarova, L.; Egorova, A.; Riabova, O.; Monakhova, N.; Makarov, V. A proof-of-concept study for the efficacy of dispirotripiperazine PDSTP in a rabbit model of herpes simplex epithelial keratitis. Antivir. Res. 2022, 202, 105327. [Google Scholar] [CrossRef]
- Knickelbein, J.E.; Hendricks, R.L.; Charukamnoetkanok, P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv. Ophthalmol. 2009, 54, 226–234. [Google Scholar] [CrossRef]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef]
- Holland, E.J.; Fingeret, M.; Mah, F.S. Use of Topical Steroids in Conjunctivitis: A Review of the Evidence. Cornea 2019, 38, 1062–1067. [Google Scholar] [CrossRef]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alter-natives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Xu, C.; Zhou, H. Pathogenesis of Herpes Stromal Keratitis: Immune Inflammatory Response Mediated by Inflammatory Regulators. Front. Immunol. 2020, 11, 766. [Google Scholar] [CrossRef]
- Sendra, V.G.; Tau, J.; Zapata, G.; Vitar, R.M.L.; Illian, E.; Chiaradía, P.; Berra, A. Polluted Air Exposure Compromises Corneal Immunity and Exacerbates Inflammation in Acute Herpes Simplex Keratitis. Front. Immunol. 2021, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.-M.; Agelidis, A.M.; Shukla, D. Pathogenesis of herpes simplex keratitis: The host cell response and ocular surface sequelae to infection and inflammation. Ocul. Surf. 2019, 17, 40–49. [Google Scholar] [CrossRef]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total. Environ. 2020, 722, 137907. [Google Scholar] [CrossRef] [PubMed]
- Marucci, C.; Fumagalli, G.; Calogero, F.; Silvani, A.; Christodoulou, M.S.; Martinet, N.; Passarella, D. Natural Products and Cancer Stem Cells. Curr. Pharm. Des. 2015, 21, 5547–5557. [Google Scholar] [CrossRef] [PubMed]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef]
- Wang, J.; He, C.; Wu, W.Y.; Chen, F.; Wu, Y.Y.; Li, W.Z.; Chen, H.Q.; Yin, Y.Y. Biochanin A protects dopaminergic neurons against lipopolysaccharide-induced damage and oxidative stress in a rat model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2015, 138, 96–103. [Google Scholar] [CrossRef]
- Sangeetha, M.; Chamundeeswari, D.; Babu, C.S.; Rose, C.; Gopal, V. Attenuation of oxidative stress in arthritic rats by ethanolic extract of Albizia procera benth bark through modulation of the expression of inflammatory cytokines. J. Ethnopharmacol. 2020, 250, 112435. [Google Scholar] [CrossRef]
- Sithisarn, P.; Michaelis, M.; Schubert-Zsilavecz, M.; Cinatl, J., Jr. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir. Res. 2013, 97, 41–48. [Google Scholar] [CrossRef]
- Qin, J.; Guo, C.; Yang, L.; Liang, X.; Jiao, A.; Lai, K.P.; Yang, B. Bioinformatics and in-silico findings reveal medical features and pharmacological targets of biochanin A against colorectal cancer and COVID-19. Bioengineered 2021, 12, 12461–12469. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Dybas, J.M.; Hughes, J.; Weitzman, M.D.; Boutell, C. The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection. Virus Res. 2020, 285, 198015. [Google Scholar] [CrossRef] [PubMed]
- Weerasooriya, S.; DiScipio, K.A.; Darwish, A.S.; Bai, P.; Weller, S.K. Herpes simplex virus 1 ICP8 mutant lacking annealing activity is deficient for viral DNA replication. Proc. Natl. Acad. Sci. USA 2019, 116, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.C.; Starkey, J.; Zhang, D.; Sarfo, A.; Chadha, P.; Wills, J.W.; Han, J. Glycoprotein D of HSV-1 is dependent on tegument protein UL16 for packaging and contains a motif that is differentially required for syncytia formation. Virology 2019, 527, 64–76. [Google Scholar] [CrossRef]
- Mathew, S.S.; Bryant, P.W.; Burch, A.D. Accumulation of oxidized proteins in Herpesvirus infected cells. Free. Radic. Biol. Med. 2010, 49, 383–391. [Google Scholar] [CrossRef]
- Ma, N.; Yang, X.; Qi, C.; Yu, Q.; Zhu, C.; Ren, H. Farrerol Enhances Nrf2-Mediated Defense Mechanisms against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial Cells by Activating Akt and MAPK. Oxidative Med. Cell. Longev. 2021, 2021, 8847844. [Google Scholar] [CrossRef] [PubMed]
- Uchino, Y.; Kawakita, T.; Miyazawa, M.; Ishii, T.; Onouchi, H.; Yasuda, K.; Ogawa, Y.; Shimmura, S.; Ishii, N.; Tsubota, K. Oxidative stress induced inflammation initiates functional decline of tear production. PLoS ONE 2012, 7, e45805. [Google Scholar] [CrossRef] [PubMed]
- Goodkin, M.L.; Epstein, S.; Asbell, P.A.; Blaho, J.A. Nuclear translocation of NF-kappaB precedes apoptotic poly(ADP-ribose) polymerase cleavage during productive HSV-1 replication in corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2007, 48, 4980–4988. [Google Scholar] [CrossRef]
- Hillenaar, T.; van Cleynenbreugel, H.; Verjans, G.M.; Wubbels, R.J.; Remeijer, L. Monitoring the Inflammatory Process in Herpetic Stromal Keratitis: The Role of In Vivo Confocal Microscopy. Ophthalmology 2012, 119, 1102–1110. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, M.; Wu, L. In vivo Confocal Microscopy of Posner-Schlossman Syndrome: Comparison with herpes simplex keratitis, HLA-B27 anterior uveitis and acute attack of primary angle closure. Sci. Rep. 2017, 7, 9832. [Google Scholar] [CrossRef]
- Krawczyk, A.; Arndt, M.A.E.; Grosse-Hovest, L.; Weichert, W.; Giebel, B.; Dittmer, U.; Hengel, H.; Jäger, D.; Schneweis, K.E.; Eis-Hübinger, A.M.; et al. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. USA 2013, 110, 6760–6765. [Google Scholar] [CrossRef]
- van de Sand, L.; Bormann, M.; Schmitz, Y.; Heilingloh, C.S.; Witzke, O.; Krawczyk, A. Antiviral Active Compounds Derived from Natural Sources against Herpes Simplex Viruses. Viruses 2021, 13, 1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, H.; Bin Wei, B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J. Zhejiang Univ. Sci. B 2017, 18, 277–288. [Google Scholar] [CrossRef]
- Rolinski, J.; Hus, I. Immunological Aspects of Acute and Recurrent Herpes Simplex Keratitis. J. Immunol. Res. 2014, 2014, 513560. [Google Scholar] [CrossRef] [PubMed]
- Remeijer, L.; Osterhaus, A.; Verjans, G. Human herpes simplex virus keratitis: The pathogenesis revisited. Ocul. Immunol. Inflamm. 2004, 12, 255–285. [Google Scholar] [CrossRef]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bai, X.C.; Chen, Z.J. Structures and Mechanisms in the cGAS-STING Innate Immunity Pathway. Immunity 2020, 53, 43–53. [Google Scholar] [CrossRef]
- Chen, B.; Rao, X.; Wang, X.; Luo, Z.; Wang, J.; Sheng, S.; Liu, Y.; Zhang, N.; Jin, S.; Chen, H.; et al. cGAS-STING Signaling Pathway and Liver Disease: From Basic Research to Clinical Practice. Front. Pharmacol. 2021, 12, 719644. [Google Scholar] [CrossRef]
- Krawczyk, E.; Kangas, C.; He, B. HSV Replication: Triggering and Repressing STING Functionality. Viruses 2023, 15, 226. [Google Scholar] [CrossRef]
- Ma, X.; Wu, W.; Liang, W.; Takahashi, Y.; Cai, J.; Ma, J.X. Modulation of cGAS-STING signaling by PPARα in a mouse model of ischemia-induced retinopathy. Proc. Natl. Acad. Sci. USA 2022, 119, e2208934119. [Google Scholar] [CrossRef]
- Azher, T.N.; Yin, X.-T.; Stuart, P.M. Understanding the Role of Chemokines and Cytokines in Experimental Models of Herpes Simplex Keratitis. J. Immunol. Res. 2017, 2017, 7261980. [Google Scholar] [CrossRef]
- Sutter, J.; Bruggeman, P.J.; Wigdahl, B.; Krebs, F.C.; Miller, V. Manipulation of Oxidative Stress Responses by Non-Thermal Plasma to Treat Herpes Simplex Virus Type 1 Infection and Disease. Int. J. Mol. Sci. 2023, 24, 4673. [Google Scholar] [CrossRef]
- Elias, T.; Lee, L.H.; Rossi, M.; Caruso, F.; Adams, S.D. In Vitro Analysis of the Antioxidant and Antiviral Activity of Embelin against Herpes Simplex Virus-1. Microorganisms 2021, 9, 434. [Google Scholar] [CrossRef]
- Wan, S.; Zhou, Y.; Huang, Q.; Yang, Y. Dot1l Aggravates Keratitis Induced by Herpes Simplex Virus Type 1 in Mice via p38 MAPK-Mediated Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 6612689. [Google Scholar] [CrossRef]
- Sanfilippo, C.M.; Blaho, J.A. ICP0 Gene Expression Is a Herpes Simplex Virus Type 1 Apoptotic Trigger. J. Virol. 2006, 80, 6810–6821. [Google Scholar] [CrossRef]
- Qian, H.; Atherton, S.S. Apoptosis and increased expression of Fas ligand after uniocular anterior chamber (AC) inoculation of HSV-1. Curr. Eye Res. 2003, 26, 195–203. [Google Scholar] [CrossRef]
- Miles, D.; Athmanathan, S.; Thakur, A.; Willcox, M. A novel apoptotic interaction between HSV-1 and human corneal epithelial cells. Curr. Eye Res. 2003, 26, 165–174. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, Z.; Li, Y.; Wang, H.; Liu, H. Upregulation of nuclear factor E2-related factor 2 (Nrf2) represses the replication of herpes simplex virus type 1. Virol. J. 2022, 19, 23. [Google Scholar] [CrossRef]
- Wyler, E.; Franke, V.; Menegatti, J.; Kocks, C.; Boltengagen, A.; Praktiknjo, S.; Walch-Rückheim, B.; Bosse, J.; Rajewsky, N.; Grässer, F.; et al. Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program. Nat. Commun. 2019, 10, 4878. [Google Scholar] [CrossRef]
- Liang, F.; Cao, W.; Huang, Y.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors 2019, 45, 563–574. [Google Scholar] [CrossRef]
- Sarkar, C.; Chaudhary, P.; Jamaddar, S.; Janmeda, P.; Mondal, M.; Mubarak, M.S.; Islam, M.T. Redox Activity of Flavonoids: Impact on Human Health, Therapeutics, and Chemical Safety. Chem. Res. Toxicol. 2022, 35, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, T.; Liu, X.; Cai, L.; Qi, J.; Zhang, P.; Li, Y. Biochanin A protects lipopolysaccharide/D-galactosamine-induced acute liver injury in mice by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Int. Immunopharmacol. 2016, 38, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Garbutcheon-Singh, K.B.; Carnt, N.; Pattamatta, U.; Samarawickrama, C.; White, A.; Calder, V. A Review of the Cytokine IL-17 in Ocular Surface and Corneal Disease. Curr. Eye Res. 2019, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Felix, F.B.; Vago, J.P.; Fernandes, D.D.O.; Martins, D.G.; Moreira, I.Z.; Gonçalves, W.A.; Costa, W.C.; Araújo, J.M.D.; Queiroz-Junior, C.M.; Campolina-Silva, G.H.; et al. Biochanin A Regulates Key Steps of Inflammation Resolution in a Model of Antigen-Induced Arthritis via GPR30/PKA-Dependent Mechanism. Front. Pharmacol. 2021, 12, 662308. [Google Scholar] [CrossRef] [PubMed]
- Giménez, F.; Suryawanshi, A.; Rouse, B.T. Pathogenesis of herpes stromal keratitis—A focus on corneal neovascularization. Prog. Retin. Eye Res. 2013, 33, 1–9. [Google Scholar] [CrossRef]
- Krenn, L.; Paper, D. Inhibition of angiogenesis and inflammation by an extract of red clover (Trifolium pratense L.). Phytomedicine 2009, 16, 1083–1088. [Google Scholar] [CrossRef]
- Whatmore, J.L.; Swann, E.; Barraja, P.; Newsome, J.J.; Bunderson, M.; Beall, H.D.; Tooke, J.E.; Moody, C.J. Comparative study of isoflavone, quinoxaline and oxindole families of anti-angiogenic agents. Angiogenesis 2002, 5, 45–51. [Google Scholar] [CrossRef]
- Jamali, A.; Hu, K.; Sendra, V.G.; Blanco, T.; Lopez, M.J.; Ortiz, G.; Qazi, Y.; Zheng, L.; Turhan, A.; Harris, D.L.; et al. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep. 2020, 32, 108099. [Google Scholar] [CrossRef]
- Knipe, D.M.; Spang, A.E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J. Virol. 1982, 43, 314–324. [Google Scholar] [CrossRef]
- Hafidh, R.R.; Abdulamir, A.S.; Abu Bakar, F.; Sekawi, Z.; Jahansheri, F.; Jalilian, F.A. Novel antiviral activity of mung bean sprouts against respiratory syncytial virus and herpes simplex virus-1: An in vitro study on virally infected Vero and MRC-5 cell lines. BMC Complement. Altern. Med. 2015, 15, 179. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, N.; Zheng, D.; You, Q.; Chen, T.; Jiang, J.; Shen, W.; Zhang, D.; Liu, J.; Chen, D.; Hu, K. Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis. Pharmaceuticals 2023, 16, 1240. https://doi.org/10.3390/ph16091240
Zhou N, Zheng D, You Q, Chen T, Jiang J, Shen W, Zhang D, Liu J, Chen D, Hu K. Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis. Pharmaceuticals. 2023; 16(9):1240. https://doi.org/10.3390/ph16091240
Chicago/Turabian StyleZhou, Nan, Deyuan Zheng, Qiao You, Taige Chen, Jiaxuan Jiang, Wenhao Shen, Di Zhang, Junpeng Liu, Deyan Chen, and Kai Hu. 2023. "Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis" Pharmaceuticals 16, no. 9: 1240. https://doi.org/10.3390/ph16091240
APA StyleZhou, N., Zheng, D., You, Q., Chen, T., Jiang, J., Shen, W., Zhang, D., Liu, J., Chen, D., & Hu, K. (2023). Therapeutic Potential of Biochanin A in Herpes Simplex Keratitis. Pharmaceuticals, 16(9), 1240. https://doi.org/10.3390/ph16091240




