Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery
Abstract
:1. Introduction
2. Anatomy and Physiology
2.1. Choroid
2.2. Sclera
2.3. Suprachoroidal Space
3. Route of Administration
3.1. Topical Administration
3.2. Systemic Administration
3.3. Periocular Injection
3.4. Intravitreal Injection
3.5. Subretinal Injection
4. Suprachoroidal Injection: Rationale
4.1. Advantages over the Intravitreal Injection
4.2. Advantages over Subretinal Injection
4.3. Drug Suspension Size and Formulation Viscosity
4.4. Cost-Effectiveness
5. Suprachoroidal Injection Techniques
6. Biomechanics of Suprachoroidal Injection
6.1. Injection Forces
6.2. Volume and Injections into Multiple Quadrants
6.3. Viscosity and Polymeric Solution Formulations
6.4. Particle Suspensions
6.5. Osmotic Characteristics and Ionic Charges of Formulation
6.6. Compartmentalization and Duration of Injectates in the SCS
6.7. Tailoring Suprachoroidal Drug Delivery
7. Suprachoroidal Injection in Ocular Diseases
7.1. Macular Edema
7.1.1. Suprachoroidal Injection for Macular Edema Secondary to Non-Infectious Uveitis
7.1.2. Suprachoroidal Injection for Diabetic Macular Edema
7.1.3. Suprachoroidal Injection for Macular Edema Secondary to Retina Vein Occlusion
7.1.4. Suprachoroidal Injection for Post-Operative/Pseudophakic Cystoid Macular Edema
7.2. Photoreceptor Loss
7.2.1. Suprachoroidal Injection of AAV Vectors for the Treatment of Inherited and Acquired Retinal Disorders
7.2.2. Suprachoroidal Injection of DNPs for the Treatment of Inherited and Acquired Retinal Disorders
7.2.3. Suprachoroidal Injection for the Treatment Dry-Aged Macular Degeneration and Stargardt’s Macular Dystrophy
7.2.4. Suprachoroidal Injection for the Treatment of Retinitis Pigmentosa
7.2.5. Suprachoroidal Injection for Solar Retinopathy
7.3. Choroidal Neovascularization
7.3.1. Suprachoroidal Injection for Solar Retinopathy
7.3.2. Suprachoroidal Injection for Choroidal Neovascularization Secondary to Neovascular Age-Related Macular Degeneration
7.4. Suprachoroidal Injection for Retinal Detachment
7.5. Suprachoroidal Injection for Uveitis
7.6. Suprachoroidal Injection for Glaucoma
7.7. Suprachoroidal Injection for Uveal Melanoma
7.8. Suprachoroidal Injection for Myopia
7.9. Suprachoroidal Injection for Ocular Inflammatory Diseases
8. Conclusions
- Biomechanical Considerations: Optimizing the chemical and physical properties of the injectate (such as size and viscosity) is crucial for different indications and diseases, depending on the anatomical location. This requires the refinement of current techniques, including injection speed and approach (single quadrant vs. multiple quadrant), to ensure the precise delivery of the correct amount of drug to the appropriate anatomical site for the desired duration of action;
- Need for Further Clinical Studies: More phase 3 clinical trials will be essential for broader clinical adoption. As it stands, most studies of indications other than macular edema are either preclinical or early-stage clinical trials. Comprehensive late-stage clinical research is paramount to assess the efficacy, safety, and applicability of suprachoroidal injections across various ocular and retinal conditions;
- Clinical Translation Challenges: Factors such as drug storage, cost-effectiveness, and efficiency compared to IV injection (the current go-to administration route for posterior segment diseases due to its efficacy) must be carefully considered. Reimbursement considerations also play a vital role in the practical implementation of this technique;
- Transition Challenges: The shift away from IV to SC injections will not be instantaneous. Strong evidence and concerted efforts will be required for clinicians to be willing to adapt to and learn this new technique and to overcome logistical issues such as in-office procedures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No | Disease | Drug | Treatment | Key Findings | Study Design | Phase | Study Title |
|---|---|---|---|---|---|---|---|
| Macular edema secondary to non-infectious uveitis | |||||||
| 1 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) at day 0 and week 12 vs. SC sham injection at day 0 and week 12 | At week 24: -BCVA gain > 15 ETDRS letters: 46.9% (intervention) vs. 15.6% (control) (p < 0.0001) -Early improvement: noticed by week 4 -CST reduction: −153 μm (intervention) vs. −18 μm (control) (p < 0.001) -ME resolution (CST < 300 μm): 53% (intervention) vs. 2% (control) (p < 0.001) -Need for rescue therapy: 13.5% (intervention) vs. 72% (control) -Time to rescue therapy: 89 days (intervention) vs. 36 days (control) -AEs, including IOP elevation: less in intervention group -Cataract AE rates: comparable in both groups -No serious AEs | Randomized, controlled, double-masked, multi-center | Clinical trial (phase III) | Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis (PEACHTREE) [94] |
| 2 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) vs. SC sham injection In patients ≤50 years and >50 years of age | At week 24: -BCVA gain: similar between age groups; greater improvements in intervention group at all visits -CST reduction: change from baseline greater in intervention than sham group in all age groups at all visits -Need for rescue therapy: lower in intervention than sham groups across age groups -Incidences of increased IOP and cataract AEs: similar between intervention and sham groups in all age groups (no statistical analysis) | Randomized, controlled, double-masked, multi-center | Post-hoc analysis of phase III clinical trial | Suprachoroidal triamcinolone acetonide injectable suspension for macular edema associated with uveitis: visual and anatomic outcomes by age [95] |
| 3 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) vs. SC sham injection In patients with concurrent use of systemic corticosteroid or steroid-sparing therapy vs. no use | Among UME patients receiving no steroid-sparing therapy, at week 24: -ETDRS letter change: +15.6 (intervention) vs. +4.9 (control) (p < 0.001) -CST change: −169.8 µm (intervention) vs. −10.3 µm (control) (p < 0.001) -Need for rescue therapy: 14.7% (SCTA) vs. 69.4% (control) Among patients receiving steroid therapy, at week 24: -ETDRS letter change: +9.4 (intervention) vs. −3.2 (control) (p = 0.019) -CST change: −108.3 µm (intervention) vs. −43.5 µm (control) (p = 0.190) -Need for rescue therapy: 10.7% (intervention) vs. 80% (control) -AEs: no serious AEs in either group | Randomized, controlled, double-masked, multi-center | Post-hoc analysis of phase III clinical trial | Suprachoroidal CLS-TA with and without systemic corticosteroid and/or steroid sparing therapy: a post-hoc analysis of the phase 3 PEACHTREE clinical trial [96] |
| 4 | Macular edema secondary to non-infectious uveitis | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) at baseline and week 12 vs. SC injection of sham at baseline and week 12 | Over 48 weeks: -Need for rescue therapy: 39.3% (intervention) vs. 60% (control) -Medium time to rescue therapy: 257 days (intervention) vs. 55.5 days (control) -At least 1 ocular AE: 64.3% (intervention) vs. 60% (control); most common being subcapsular cataract -At least 1 elevated IOP reading (>10 mm Hg): 14.3% (intervention) vs. 0% (control) | Observational extension study of PEACHTREE trial | Parent study was clinical trial (phase III) | Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA) [97] |
| 5 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral SC injection of TA 4 mg (0.1 mL of 40 mg/m) at day 0 and week 12 | At 24 weeks: -BCVA improvement: 68.9 to 75.0 at week 8 and 75.9 at week 24 -CST improvement: 335.9 µm to 284.0 µm at week 24 -IOP elevation > 10 mmHg, >30 mmHg: 15.8%, 5.3% of participants with 87.5% treated with IOP lowering drops -Cataract AEs: 10.5% of participants, only 1 was treatment-related -Treatment related AEs: 18.4% of participants -Serious AEs related to treatment: None | Nonrandomized, single arm, multi-center | Clinical trial (phase III) | Suprachoroidal CLS-TA for non-infectious uveitis: an open-label, safety trial (AZALEA) [93] |
| 6 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral, SC injection TA, 40 mg/mL (4 mg in 100 µL) vs. Single unilateral, SC injection TA, 8 mg/mL (0.8 mg in 100 µL) | -CST reduction: −135 μm at month 1 (p = 0.0056), −164 μm at month 2 (p = 0.0017) (4 mg group); −78 μm at month 2 (0.8 mg group) -ETDRS letter change: +7.7 at month 1 (p = 0.0001), +9.2 at month 2 (p = 0.0004); 65% had improvement of >5 ETDRS letters (4 mg group) -Anterior cell grade: 60% resolution (change to score of 0 for those >0 at baseline) (4 mg group) -Vitreous haze score: 80% improvement (for those >0 at baseline) (4 mg group) -At least 1 AE: 47% (4 mg group) vs. 100% (0.8 mg group), none requiring treatment -Serious AEs related to treatment or increase in IOP: none | Dose randomized, controlled, masked, multi-center | Clinical trial (phase II) | Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis (DOGWOOD) [98] |
| 7 | Macular edema secondary to non-infectious uveitis | TA | Single unilateral SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -CMT reduction: >50% reduction at 1 month (p < 0.001) with a further reduction of 22% by month 3 (p < 0.001) -Resolution of ME: 100% of patients -BCVA gain: significant improvement from baseline at month 1 and month 3 (p < 0.001) -AE: lenticular changes in 5 -No significant difference in IOP by 3 months | Prospective, nonrandomized, interventional study, uni-center | Clinical trial (phase I/II) | Safety and efficacy of suprachoroidal injection of triamcinolone in treating macular edema secondary to noninfectious uveitis [99] |
| 8 | Macular edema secondary to uveitis, vascular disorders and diabetes | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -BCVA gain: significant improvement observed -Early improvement at 1 week -IOP: highest at 6 months in 11–15 mmHg cases and highest at 1 month in 16–20 mmHg cases | Prospective interventional study, uni-center | Clinical trial (phase I/II) | Visual outcome after suprachoroidal injection of triamcinolone acetate in cystoid macular edema of different pathology [100] |
| Diabetic macular edema | |||||||
| 9 | Diabetic macular edema | Aflibercept in combination with TA | Single unilateral IV injection of Aflibercept, 2 mg (0.05 mL) followed by single unilateral SC injection of TA, 4 mg (0.1 mL) in same eye of treatment-naïve participants vs. Single unilateral SC injection of TA, 4 mg (0.1 mL) alone in previously treated participants | -Average number of SCTA injections: 3.3 (monotherapy) vs. 2.6 (combination) At 6 months: -Mean CST reduction: −128 μm (monotherapy) vs. −91 μm (combination) -Mean ETDRS letter change: +1.1 (monotherapy) vs. +8.5 (combination) -AEs: elevated IOP (2 patients), cataracts (3 patients), pain during the procedure (1 patient) | Nonrandomized, open-label, parallel-design, multi-center | Clinical trial (phase I/II) | Suprachoroidal triamcinolone acetonide for diabetic macular edema: the HULK trial [102] |
| 10 | Diabetic macular edema | Aflibercept in combination with TA | Single unilateral IV injection of Aflibercept, 2 mg (0.05 mL) followed by single unilateral SC injection of TA, 4 mg (0.1 mL) in same eye of treatment-naïve participants vs. Single unilateral SC injection of TA, 4 mg (0.1 mL) alone in previously treated participants | -Mean time from last SCTA delivery to final AS-OCT: 4.8 (range: 1.0–9.9) months -Mean SCS width at final AS-OCT: 8.4 µm (combination) vs. 8.1 µm (monotherapy) (p = 0.698) -Mean SCS width: 9.9 µm to 75.1 µm (p < 0.001) immediately before to 30 min after SC injection; normalized to 14.9 µm 1 month after final injection (p = 0.221) -Anatomical differences from baseline: none in both groups | Nonrandomized, controlled | Clinical trial (phase I/II) | Suprachoroidal space alterations following delivery of triamcinolone acetonide: post-hoc analysis of the phase 1/2 HULK study of patients with diabetic macular edema [4] |
| 11 | Diabetic macular edema | Aflibercept in combination with TA | IV injection of Aflibercept, 2 mg (0.05 mL) followed by SC injection of TA, 4 mg (0.1 mL) at months 0 and 3 vs. Monthly IV injection of Aflibercept, 2 mg (0.05 mL) followed by SC injection of sham for 3 months | By week 24: -Mean ETDRS letter change: +11.4 (combination) vs. +13.8 (monotherapy) (p = 0.228) -Mean CST reduction: −212.1 μm (combination) vs. −178.6 μm (monotherapy) (p = 0.089) -Number of treatments required: 2.6 (combination) vs. 4.6 (monotherapy) -AEs: no serious AEs related to treatment in either group | Randomized, controlled, double-masked, parallel design, multi-center | Clinical trial (phase II) | Suprachoroidal CLS-TA plus intravitreal aflibercept for diabetic macular edema: a randomized, double-masked, parallel-design, controlled study (The TYBEE trial) [103] |
| 12 | Diabetic macular edema | TA in combination with IVB | SC injection of TA, 0.1 mL of 40 mg/mL + IVB, 1.25 mg of 0.05 mL; another injection of IVB (same dose) at 1 month and 2 months vs. SC sham injection of TA + IVB, 1.25 mg of 0.05 mL; another injection of IVB (same dose) at 1 month and 2 months | -Mean BCVA change (log MAR): −0.37 ± 0.24 (p < 0.001) (combination) vs. −0.20 ± 0.20 (monotherapy) (p = 0.004) at 12 weeks [between-group analysis (p = 0.014)]; combination group showed improvements from baseline at week 4 (p = 0.046) and 24 weeks later (p < 0.001) -Mean CST reduction: higher in combination group vs. monotherapy group (p = 0.019) -AEs: no serious AEs related to treatment in either group | Randomized, double-masked, parallel design, uni-center | Clinical trial (Phase II/III) | Suprachoroidal injection of triamcinolone acetonide plus intravitreal bevacizumab in diabetic macular edema: a randomized pilot trial [104] |
| 13 | Diabetic macular edema | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) vs. IVB, 2.5 mg/0.01 mL | -Mean ETDRS letter change: +5 after 3 months; higher in the SCTA vs. IVB group (p = 0.002) -Mean CST reduction: at least 10% from baseline after 1 and 3 months; higher in SCTA vs. IVB group (p = 0.01 and p = 0.04, respectively) -Efficacy: 37.5% (SCTA) vs. 29.5% (IVB) (p = 0.03) | Prospective observational study, uni-center | Clinical trial (Phase I/II) | Comparison of suprachoroidal injection of triamcinolone acetonide versus intravitreal bevacizumab in primary diabetic macular odema [106] |
| 14 | Diabetic macular edema | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) vs. IV of TA, 4 mg (0.1 mL of 40 mg/mL) vs. SC injection of TA, 2 mg (0.1 mL of 20 mg/mL) | -Mean BCVA gain: significant improvements in all 3 groups at 1 and 3 months; greatest improvement in 4 mg SCTA group by 1 month, BVCA returned to near baseline values at 6 months, except the 4 mg SCTA group -Mean CMT reduction: significantly decreased in all groups at 1 and 3 months; highest reduction in 4 mg SCTA group; CMT increased again after 3 months and returned to near baseline values at 6 months, except 4 mg SCTA group which maintained a mean reduction of 60.18 µm -AEs: no signs of infection, acute rise of IOP on injection day, nor serious AEs in any group | Prospective, interventional, randomized, uni-center | Clinical trial (Phase II) | Suprachoroidal versus intravitreal triamcinolone acetonide for the treatment of diabetic macular edema [107] |
| 15 | Diabetic macular edema | TA in combination with IVB | Single IVB 1.25 mg + single SC injection of TA, 2 mg vs. Single IVB 1.25 mg only | At 1 month: -Mean CMT reduction: −113 + 10 µm (combination) vs. −81 + 10 µm (monotherapy) (p < 0.001) -AEs: <33% reported mild to moderate pain | Randomized, uni-center | Clinical trial (Phase II) | Comparison of suprachoroidal triamcinolone injection with intravitreal bevacizumab vs. intravitreal bevacizumab only in treatment of refractory diabetic macular edema [105] |
| 16 | Diabetic macular edema (refractory) | TA | Single IV injection of TA, 4 mg (0.1 mL of 40 mg/mL) vs. Single SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BVCA gain: improved compared to baseline in both groups at 6 weeks; no difference between groups at 1 month -Mean CMT reduction: reduced in both groups at 6 weeks; comparable between groups at 1 month -Elevated IOP: higher in the IVTA vs. SCTA group at months 3 and 6 (p < 0.003); -Cataract progression: slower in SCTA vs. IVTA group -Serious AEs: none in either group | Randomized, parallel arm, uni-center | Clinical trial (Phase II) | Comparison between suprachoroidal triamcinolone and intravitreal triamcinolone acetonide in patients of resistant diabetic macular edema [108] |
| 17 | Diabetic macular edema post pars plana vitrectomy (PPV) | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) post PPV | -Mean BVCA gain (log MAR): 0.75 ± 0.40 µm at baseline to 0.40 ± 0.33 µm at 8 weeks (p = 0.003) -Mean CMT reduction: 35% reduction by week 1 (p = 0.003); 43.9% by week 4 (p = 0.003), 45.74% reduction by 8 weeks (p = 0.003) -Retreatment: none required -AEs: no statistically significant change in IOP, no serious AEs | Retrospective, single arm case series | Not applicable | Suprachoroidal injection of triamcinolone acetonide using a custom-made needle to treat diabetic macular edema post pars plana vitrectomy: a case series [109] |
| 18 | Diabetic macular edema (refractory) | TA | Single SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA improvement (logMAR): from 1.193 ± 0.2 at baseline to 0.76 ± 0.3 at 12 months (p-value < 0.001); eyes with more baseline CMT and worse baseline BCVA achieved worse final BCVA at 12 months -Mean CMT reduction: 478.7 ± 170.2µm at baseline to 230.2 ± 47.4 µm at 12 months (p < 0.001) -AEs: mean IOP increased significantly 1 month post injection but returned to baseline levels at month 3 | Prospective, nonrandomized, single arm, uni-center | Clinical trial (Phase I/II) | Effectiveness of suprachoroidal injection of triamcinolone acetonide in resistant diabetic macular edema using a modified microneedle [110] |
| 19 | Diabetic macular edema (refractory) | TA | Single SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | At 3 months: -Mean CMT reduction: from 776.21.0 ± 19.17 to 251.14 ± 6.27 µm (p < 0.001) -Mean BCVA improvement: 0.10 ± 0.005 to 0.37 ± 0.01 (p < 0.001) -Mean IOP: reduced from 13.01 ± 0.10 to 13.26 ± 0.10 mmHg (p < 0.001) -AEs: no serious AEs related to treatment | Nonrandomized, single arm, uni-center | Clinical trial (Phase I/II) | Suprachoroidal injection of triamcinolone acetonide for management of resistant diabetic macular oedema [111] |
| 20 | Diabetic macular edema (refractory) | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean CST reduction: from 612.89 ± 195.58 μm (baseline) to 308.59 ± 56.75 μm at 1 month and 304.89 ± 54.29 μm at 3 months (p < 0.00001) -Mean BCVA improvement: reduced at 3 months (p < 0.05) -Mean IOP: no statistically significant change at 1 or 3 months | Case series, uni-center | Not applicable | To determine the efficacy of suprachoroidal triamcinolone injection for the treatment of refractory diabetic macular edema [112] |
| 21 | Diabetic macular edema (refractory) | TA | Single, unilateral SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean CST reduction: from 636.5 ± 200.11 µm (baseline) to 304.54 ± 67.43 (at 1 month, p < 0.00001) and to 302.66 ± 66.93 µm (at 3 months, p < 0.00001) -Mean BCVA gain: improved from 0.8 ± 0.24 ETDRS letters to 0.47 ± 0.3 (at 1 month, p < 0.05) and to 0.45 + 0.27 (at 3 months, p < 0.05) -Mean IOP: no difference from baseline at 1 and 3 months | Prospective, nonrandomized interventional, uni-center | Clinical trial (Phase I/II) | Efficacy and safety of suprachoroidal triamcinolone acetonide in cases of resistant diabetic macular edema [113] |
| 22 | Diabetic macular edema (refractory) | TA | SC injections of TA, 4 mg (0.1 mL of 40 mg/mL) | -Number of prior injections received: 6.2 (maximum 12, minimum 4) -Mean CST reduction: 612.8 ± 198.3 µm (baseline) to 308.6 ± 62.6 µm (at 1 month) to 302.72 ± 58.64 µm (at 3 months) -Mean BCVA improvement: from 0.8 ± 0.19 (baseline) to 0.49 ± 0.29 (at 1 month) and 0.39 ± 0.20 (at 3 months) -Mean IOP: from 12.32 mmHg (baseline) to 14.82 mmHg (at 1 month) and to 14.48 mmHg (at 3 months) | Retrospective, nonrandomized, single arm, uni-center, case series | Not applicable | Efficacy and safety of suprachoroidal triamcinolone acetonide in cases of resistant diabetic macular edema [114] |
| 23 | Diabetic macular edema (refractory) | TA | Single SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) at baseline and administered every 3 months during the follow-up period (12 months) if intraretinal cysts, intraretinal or subretinal fluid persisted and CMT remained >250 μm | -Number of injections needed: 37.6% eyes required 2 injections; 23.5% of eyes required 3 injections -Mean CMT reduction: significant after 12-months (p < 0.001); positive correlation between final CMT and frequency of injection (p < 0.001) -Mean BVCA improvement: from 1.194 ± 0.1 (baseline) to 0.75 ± 0.2 (at 12 months, p < 0.001) -Mean IOP: reached a maximum value at 1 month; then, gradually declined using topical beta blockers; glaucoma surgery not required in any patients -IS/OS disruption and NSD: associated with worse final BVCA (p < 0.001) -AEs: no systemic or serious AEs related to treatment | Prospective, nonrandomized, single arm, uni-center | Clinical trial (Phase I/II) | Efficacy of suprachoroidal triamcinolone acetonide injection in the treatment of resistant diabetic macular edema [115] |
| 24 | Diabetic macular edema | TA | Posterior subtendon injection of TA (40 mg) in combination with VISCOAT (20 mg sodium chondroitin sulfate + 15 mg sodium hyaluronate) vs. Posterior subtendon injection of TA (40 mg) alone vs. SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA improvement: highest in SCTA group with VISCOAT -Mean CMT reduction: lowest in SCTA group with VISCOAT; reduction from baseline at all follow-up periods in all groups (p < 0.0001), significant reduction at 1, 3 and 6 months in SCTA group formulated with VISCOAT (p < 0.001); no difference in CMT between 1 month, 3 months, and 6 months in SCTA group -Retreatment: lowest frequency in SCTA group with VISCOAT | Prospective, nonrandomized, uni-center | Clinical trial (Phase II) | Suprachoroidal triamcinolone versus posterior subtenon triamcinolone alone or formulated in the management of diabetic macular edema [116] |
| 25 | Diabetic macular edema | AAV8 vector | Comparison of 3 different doses of RGX-314 (AAV8) administered suprachoroidally, one of which will be infused with topical steroid | -Recruitment ongoing Preliminary results (3-month data of 15 patients): -Diabetic retinopathy improvement: 33% in treatment arm had a ≥2 improvement in diabetic retinopathy severity score vs. 0% in in control arm -AEs: no intraocular inflammation observed; common observed AEs not considered treatment-related | Randomized, dose-escalation study | Clinical trial (Phase II) | RGX-314 gene therapy administered in the suprachoroidal space for participants with diabetic retinopathy (DR) without center involved-diabetic macular edema (CI-DME) (ALTITUDE) [117] |
| Macular edema secondary to retinal vein occlusion | |||||||
| 26 | Macular edema secondary to RVO | TA in combination with IV Aflibercept | SC injection of TA, 0.1 mL of 40 mg/mL + 2 mg IV of Aflibercept vs. SC sham injection + 2 mg IV injection of Aflibercept | -Number of retreatments: 23 (combination) vs. 9 (monotherapy) (−61%; p = 0.013), % of participants requiring no re-treatments was increased (78% vs. 30%; p = 0.003) -Mean BCVA imporvement: higher in combination arm vs. monotherapy arm at month 1 (p = 0.20) and month 2 (p = 0.04) -Mean CST reduction: reduced to normal values at month 1 and remained there at months 2 and 3 in combination arm, decreased at month 1 and increased at months 2 and 3 in monotherapy arm -Frequency of CST resolution (CST ≤ 310 μm): higher in combination arm at months 1, 2, and 3 vs. monotherapy arm | Randomized, triple masked, multi-center | Clinical trial (Phase II) | Suprachoroidal triamcinolone acetonide for retinal vein occlusion: results of the TANZANITE study [119] |
| 27 | Macular edema secondary to RVO | TA in combination with IV injection of Aflibercept | Single unilateral, SC injection of TA, 40 mg/mL (4 mg in 100 µL) following a 2 mg IV injection of Aflibercept vs. Single unilateral, SC sham procedure following a 2 mg IV injection of Aflibercept | -Retreatment: not required in 74% (17/23 patients) in combination arm vs. only 17% (4/23 patients) in monotherapy arm -Mean BCVA improvement: higher improvements observed as early as month 1 and maintained through month 3 in combination arm | Extension of TANZANITE trial (randomized, parallel design, triple masked, multi-center) | Clinical trial (Phase II) | TANZANITE extension study in patients with macular edema associated with retinal vein occlusion [120] |
| 28 | Macular edema secondary to RVO | TA in combination with IV of Aflibercept | IV Aflibercept (2 mg/0.05 mL) + SC TA (4 mg/100 µL) injections vs. IV Aflibercept (2 mg/0.05 mL) + sham SC procedure | -Mean BCVA gain: ~50.0% of patients in both groups reported a ≥15 ETDRS letters improvement at 8 week; results comparable between groups -No additional benefit of the combination therapy was observed leading to study discontinuation | Randomized, triple masked, controlled, parallel group, multi-center | Clinical trial (Phase III) | Suprachoroidal injection of triamcinolone acetonide with IVT aflibercept in subjects with macular edema following RVO (SAPPHIRE) [121] |
| 29 | Macular edema secondary to RVO | TA in combination with IV injection of Ranibizumab | IV Ranibizumab (0.5 mg/0.05 mL) + SCTA (4 mg/0.10 mL) vs. IVB (1.25 mg/0.05 mL) + SCTA (4 mg/0.10 mL) vs. IV Ranibizumab (0.5 mg/0.05 mL) + sham SC injection or IVB (1.25 mg/0.05 mL) + sham SC injection | -Early discontinuation due to the SAPPHIRE study outcomes | Randomized, masked, controlled, parallel group, multi-center | Clinical trial (Phase III) | Suprachoroidal injection of triamcinolone acetonide with IVT anti-VEGF in subjects with macular edema following RVO (TOPAZ) [122] |
| 30 | Macular edema secondary to BRVO | TA in combination with IV Ranibizumab | IV injection of 0.05 mL (0.5 mg) of Ranibizumab + SCTA (Group 1) vs. IV injection of 0.05 mL (0.5 mg) of Ranibizumab only (Group 2) Both groups received monthly Ranibizumab injection PRN for 1 year | -Number of injections: 2.47 ± 1.2 (group 2) vs. 4.4 ± 1.5 (group 1) -Mean CMT reduction: significant reduction in both groups at 12 months (p < 0.001); group 2 showed greater reduction than group 1 at 1 month (p = 0.008); after 12 months, CMT was similar in both groups -Recurrent ME: higher in group 1 compared to group 2 -Predictors of final BCVA: baseline CMT and number of injections in group 1; baseline BCVA only predictor in group 2 | Prospective, randomized interventional study, uni-center | Clinical trial (Phase II) | Modified microneedle for suprachoroidal injection of triamcinolone acetonide combined with intravitreal injection of ranibizumab in branch retinal vein occlusion patients [123] |
| 31 | Macular edema secondary to RVO | TA | Single SCinjection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA gain: >15 letter increase in 68.7% participants at week 1, 62.5% at month 1, 50% at month 2 and 50% at month 3 ->70 ETDRS letter score: 81.25% at week 1 75% at month 1, 75% at month 2, 75% at month 3 -Mean CST reduction: associated with improvements in BCVA -Mean IOP: no significant changes, with an increase ranging from 0.75 mmHg at week 1 (p = 0.09) and 0.5 mmHg at 3 months (p = 0.72) | Nonrandomized, open-label, single arm, uni-center | Clinical trial (Phase I/II) | Suprachoroidal triamcinolone acetonide for the treatment of macular edema associated with retinal vein occlusion: a pilot study [124] |
| 32 | Macular edema secondary to RVO | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA gain: significant improvement from baseline at 3 months (p = 0.003) -Mean central retinal thickness reduction: significantly decreased from 342.2 ± 40.2 µm to 289 ± 47.5 µm at 3 months (p = 0.002) | Uni-center, case series | Not applicable | Effect of supra-choroidal triamcinolone injection on best-corrected visual acuity and central retinal thickness in patients with macular edema secondary to retinal vein occlusion [125] |
| 33 | Macular edema secondary to RVO | TA, Aflibercept | Single unilateral, SCinjection of 40 mg/mL (4 mg in 100 µL) TA following a 2 mg IV injection of Aflibercept vs. Single unilateral, SC sham procedure following a 2 mg IV injection of Aflibercept | -Mean vascular choroidal thickness (VCT), stromal choroidal thickness (SCT) and total choroidal thickness (TCT): slight trend toward choroidal thinning in both groups at 3 months, but none reached significance (p = 0.231–0.342) -SCS thickening: 13.4 µm (combination) vs. 5.3 µm (monotherapy) at 3 months (p = 0.130) -SCS expansion: 39.5% eyes demonstrated visible SCS at baseline; significant expansion after SCTA injection (16.2 µm to 27.8 µm at 3 months; p = 0.033) | Randomized, masked, parallel design, multi-center | Clinical trial (Phase II) | Choroidal changes after suprachoroidal injection of triamcinolone in eyes with macular edema secondary to retinal vein occlusion [200] |
| 34 | Macular edema with subfoveal hard exudates (SHE) secondary to central or branch RVO or diffuse DME | TA in combination with IVB | Single injection or IVB (1.25 mg/0.05 mL) + SCTA (4.0 mg/0.1 mL) | -Mean ETDRS letter change: ≥2 lines in 4/6 eyes; remained unchanged in 2 eyes -Mean OCT macular thickness: decreased from 603.5 ± 348.5 μm (baseline) to 276.3 ± 40.7 μm (at 12 months) -Mean OCT macular volume: decreased from 9.44 ± 2.16 μm (baseline) to 7.62 ± 0.55 μm (at 12 months) -SHE resolution: mostly resolved at 1 month and 2 months in all eyes and ME was significantly reduced -AEs: no serious AEs observerd | Prospective, nonrandomized, case series | Not applicable | Suprachoroidal drug infusion for the treatment of severe subfoveal hard exudates [126] |
| 35 | RVO, DME, Vogt Koyanaji Harada Disease | TA | SC injection of TA, 4 mg (0.1 mL of 40 mg/mL | -Results pending study completion | Randomized, parallel assignment, interventional | NA | One year results for suprachoroidal triamcinolone acetonide injection in various retinal diseases [127] |
| Post-operative/pseudophakic cystoid macular edema | |||||||
| 36 | Diabetic macular edema (refractory) | TA | Single SCTA 4 mg (0.1 mL of 40 mg/mL) vs. Single IVTA, 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA gain (logMAR): improved from baseline in both groups; comparable between groups at 1 month (p = 0.605) and 3 months (p = 0.313) -Mean CFT reduction: statistically significant difference in CFT at 3 months, with less reduction in the IVTA group than the SCTA group (p = 0.028) -AEs: mean IOP significantly higher in the IVTA group compared to the SCTA group at 1 month (p = 0.01) and 3 months (p = 0.028) -DME recurrence rate at 1 month: 50% (IVTA group) vs. 0% (SCTA group) -DME recurrence rate at 3 months: 70% (IVTA group) vs. 30.8% (SCTA group) | Randomized, multi-center | Clinical trial (Phase II) | A randomized trial comparing suprachoroidal and intravitreal injection of triamcinolone acetonide in refractory diabetic macular edema due to epiretinal membrane [128] |
| 37 | Pseudophakic cystoid macular edema/cystoid macular edema | TA | SCTA 4 mg (0.1 mL of 40 mg/mL) at day 0 | At 3 months: -Mean CST reduction: from 535.0 ± 157.24 (baseline) to 319.55 ± 127.30 µm (p < 0.001) -Mean BCVA gain: from 1.05 ± 0.41 (baseline) to 0.73 ± 0.41 logMAR (p < 0.001) -Mean IOP: from 15.05 ± 2.54 (baseline) to 15.85 ± 3.60 mm Hg (p = 0.185) -AEs: no serious AEs | Retrospective, non-comparative case-series | Not applicable | A simple technique for suprachoroidal space injection of triamcinolone acetonide in treatment of macular edema [129] |
| 38 | Pseudophakic cystoid macular edema | TA | SCTA 4 mg (0.1 mL of 40 mg/mL) | -Mean BCVA gain (logMAR): 0.3 at week 4; improvements maintained to 6 months -Mean CMT reduction: 473.5 µm (baseline) to 287 µm -Mean IOP: remained within normal limits -AEs: none related to uveitis or glaucoma | Retrospective, single arm case series | Not applicable | Modified inexpensive needle for suprachoroidal triamcinolone acetonide injections in pseudophakic cystoid macular edema [130] |
| 39 | Pseudophakic cystoid macular edema | TA | SCTA of 0.1 mL TA | -Case summary: 52 year old female received SC injection of TA due to inability to afford monthly IV anti-VEGF and IV dexamethasone -Mean IOP: reduced from 19 mmHg to 15 mmHg; persisted without raised spikes until week 24 -BCVA recovery: from 20/80 to 20/50 at week 1, 20/40 at 8 weeks and 20/30 at 24 weeks -OCT findings: significant thickness decrease within 24 h (348 μm); after 8 weeks, improvement in retinal thickness to normal (265 μm), complete anatomical resolution at 24 weeks -AEs: none | Case report | Not applicable | A manually made needle for treating pseudophakic cystoid macular edema by injecting triamcinolone acetonide in the suprachoroidal space: a case report [131] |
| 40 | Central serous chorioretinopathy, Irvine-gass syndrome, pars planitis, cystoid macular edema | TA | SCTA in patients diagnosed with central serous chorioretinopathy and Irving-Gass Syndrome | -Results pending study completion | Randomized, parallel assignment, interventional | Clinical trial (Phase II/III) | Suprachoroidal triamcinolone acetonide injection in two chorioretinal diseases: one year results [132] |
| Photoreceptor loss | |||||||
| 41 | Gene therapy | AAV5 vector | Single SC injection of 100 µL of sc-AAV5-smCBA-hGFP vector at a concentration of 4.5 × 1013 vector genomes/mL in rabbits | -Efficacy of transfection: comparable among all treated eyes by microscopic examination -GFP expression: occurred at the level of the choroid, RPE, photoreceptors and retinal ganglion cells in whole mounts of treated eyes (no direct staining performed); absence of GFP In controls -Inflammatory response: no evidence of inflammation or tissue destruction | Animal experimental study | Preclinical (animal study) | Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter [65] |
| 42 | Gene therapy | AAV2, AAV5 or AAV2 (triple) containing 3 tyrosine-phenylalanine mutations on its capsid surface | SC or vitreal/subretinal injections of AAV2, AAV5, or AAV2 (triple) vector containing 3 tyrosine-phenylalanine mutations on its capsid surface; vector doses were either 1 × 1012 and 4 × 1012, or 1 × 1012 and 9 × 1012 particles/mL). vs. SC delivery of basic saline solution (BSS) | - GFP expression: found in eyes that received both vitrectomy/subretinal and SC injections; strong expression in AAV2(triple) treatment, intermediate expression in AAV2 treatment, minimal expression in AAV5 treatment, no GFP expression in BSS-injected eyes -Transduction profiles: not significantly influenced by vector concentration | Animal experimental study | Preclinical (animal study) | Comparison of suprachoroidal delivery via an Ab-externo approach with the iTrack microcatheter versus vitrectomy and subretinal delivery for 3 different AAV serotypes for gene transfer to the retina [133] |
| 43 | Gene therapy | AAV2 vector | AAV2 administration in mice using intrastromal, intracameral, IV, subretinal, or SC injections | -Transduction: of stroma, ciliary body, retinal ganglion cells, outer retina, and RPE, irrespective of delivery route; transduction of multiple retinal layers without causing retinal detachment | Animal experimental study | Preclinical (animal study) | Comparison of AAV Serotype2 transduction by various delivery routes to the mouse eye [134] |
| 44 | Gene therapy | AAV8, AAV9, AAV2, RGX-314 vectors | SC injections of 2.85 × 1010 gene copies (GCs) of AAV8.GFP, 2.85 × 1010 GCs of AAV9.GFP, 2.0 × 1010 GCs of AAV2.GFP in rats SC or subretinal injection of 1.2 × 108 GCs of RGX-314 in rats SC injection of 50 μL containing 4.75 × 1011 GCs of AAV8.GFP in nonhuman primates and pigs | -GFP expression: widespread throughout the RPE and photoreceptors in rats, nonhuman primates, and pigs; SC and subretinal injection of same vector dose resulted in comparable expression which could be increased by multiple SC vector injections -Transduction of the RPE and photoreceptors: strong after SC injection of AAV9.GFP, similar to AAV8.GFP, but poor after SC injection of AAV2.GFP -Suppression of VEGF-induced vasodilation and vascular leakage: suppression by SC injection of RGX-314 comparable to subretinal injection in rats | Animal experimental study | Preclinical (animal study) | AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression [135] |
| 45 | Gene therapy | AAV2, AAV5 vectors | SC injections of 7.8 × 109 GC of AAV2tYF-CBA-hGFP, 7.8 × 109 GC of AAV2tYF-GRK1-hGFP, 7.8 × 109 GC of AAV5-GRK1-hGFP, or 6.87 × 109 GC of AAV2-CBA-hGFP in Norway Brown rats | -Duration of GFP expression: peak expression observed within 2 weeks, except for AAV2tYF-GRK1-hGFP, which showed further increase between 2 and 4 weeks -Strength of GFP expression: highest and more extensive for AAV2tYF-GRK1-hGFP; AAV2-CBA-hGFP; expression extended approximately 1/4 circumference in the RPE and all layers of the retina; injection of AAV5-GRK1-hGFP resulted in lowest GFP expression at 2 and 4 weeks -Areas of GFP expression: for AAV2tYF-GRK1-GFP and AAV5tYF-GRK1-GFP expression limited to photoreceptors, including inner segments, outer segments, and some cell bodies; extent of GFP expression was around 1/4 and 1/6 eye circumference, respectively | Animal experimental study | Preclinical (animal study) | Transgene expression in RPE and retina after suprachoroidal delivery of AAV vectors [136] |
| 46 | Gene therapy | AAV8 vector | SC, subretinal and IVT injections of AAV8.GFP in primates (7 × 1011 or 7 × 1012 vector genomes per eye using a 700-μm-long 30-gauge microneedle) | -Transduction: diffuse, peripheral transduction of mostly the RPE (SC delivery) vs. robust focal transduction near injection site with some transduction of retinal ganglion neurons (subretinal delivery) vs. only scant peripapillary expression mostly nasal to the optic disc (IV delivery) -Duration of GFP expression: transient, reaching maximal expression at 1 month but decreased by months 2 and 3 for SC AAV8 -Inflammatory response: more local infiltration of retinal microglia, choroidal macrophages, leukocytes and T-cells for SC; subretinal injection showed minimal microglial activation, fewer leukocytes and T-cells compared to SC; IV injection showed minimal microglial activation and almost no leukocytes or T-cells; higher systemic humoral response after IV delivery -Tolerance of injection: comparable between groups | Animal experimental study | Preclinical (animal study) | Suprachoroidal and subretinal injections of AAV using transscleral microneedles for retinal gene delivery in nonhuman primates [24] |
| 47 | Gene therapy | AAV8 vector | SC, subretinal, and IV injections (100 μL) of AAV8 (7 × 1012 or 1012 vector genes per eye) and TA (40 mg) in primates | -Humoral response: minimal antibody response by SC injection with greater responses to GFP; IV injection induced an early and higher robust humoral response to the viral capsid -Cell-mediated immune response: no appreciable T-cell responses to AAV8 capsid after SC injection with some T cell responses to GFP beginning as early as 1 month -Systemic distribution: higher genome copies of vector in spleen and liver for IV injection compared to SC injection or subretinal injection | Animal experimental study | Preclinical (animal study) | Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes [138] |
| 48 | Gene therapy | AAV serotypes | SC injections of 1.0 μL fluorescein sodium (1.0 × 10−6%) or 1 of 3 AAV serotypes via injection of scAAVs-CBA-EGFP solution in mice | -Transduction: occurred in outer retina and the RPE in all 3 AAV serotypes; 3 AAVs displayed varied efficiency and cell specificity; widespread distribution across different layers of the mouse retina -Inflammatory response activation of inflammatory cells depending on the dosage used -AEs: retinal detachment was avoided | Animal experimental study | Preclinical (animal study) | Suprachoroidal injections of AAV for retinal gene delivery in mouse [201] |
| 49 | Gene therapy | AAV1, AAV2, AAV6, AAV8, and AAV9 vectors | SC, subretinal, and IV delivery of AAV1, AAV2, AAV6, AAV8, and AAV9 with GFP in rats vs. Buffer-injected controls for each route of delivery | -Inflammatory response: response induced by AAV2 and AAV6 vectors for all routes of delivery with AAV6 inducing the highest levels when delivered suprachoroidally; AAV1-induced inflammation was highest when delivered suprachoroidally, whereas minimal inflammation was seen with IV delivery; AAV8 and AAV9 induced least amount of inflammation across all delivery routes -Cell-mediated immune response: AAV1, AAV2, and AAV6 each induced adaptive immune cells into neural retina | Animal experimental study | Preclinical (animal study) | The degree of adeno-associated virus-induced retinal inflammation varies based on serotype and route of delivery: intravitreal, subretinal, or suprachoroidal [202] |
| 50 | Gene therapy | AAV2 serotypes (AAV2/1, AAV2/2, AAV2/6, AAV2/8, and AAV2/9) vectors | SC, subretinal, and IV injections of AAV2-viral particles; >3 injections per serotype per route of delivery (5 × 1012 vector genes/mL for a total dose of 5 × 1010 vector genes) in rats | -Transduction: successful in the RPE and outer nuclear layer (ONL) for all serotypes with AAV2/1 subretinal delivery showing the highest transduction efficiency and minimal inner subretinal delivery; SC tropism comparable to subretinal delivery, but wider distribution and greater average ONL transduction efficiency for all serotypes; retinal transduction primarily in inner retina for IV delivery with AAV2/6 showing the highest transduction | Animal experimental study | Preclinical (animal study) | Retinal tropism and transduction of adeno-associated virus varies by serotype and route of delivery (intravitreal, subretinal, or suprachoroidal) in rats [203] |
| 51 | Gene therapy | DNA nanoparticles (DNPs) | Single bilateral SC injection (0.1 mL) of ellipsoid-shaped DNPs, rod-shaped DNPs or saline in non-human primates and rabbits | -Tolerance: well-tolerated in both animal models -Luciferase activity in non-human primates: ellipsoid-shaped DNPs had persistent luciferase activity up to day 22; rod-shaped DNPs showed a significant decrease in choroid and the RPE - Luciferase activity in rabbits: both rod and ellipsoid-shaped DNPs injected in SCS alongside rod-shaped DNPs injected subretinally, exhibited similar luciferase activity after week 1 | Animal experimental study | Preclinical (animal study) | Suprachoroidally delivered DNA nanoparticles transfect chorioretinal cells in non-human primates and rabbits [137] |
| 52 | Gene therapy | DNA nanoparticles (DNPs) | Unilateral SC injection (0.1 mL) of ellipsoid-shaped DNPs, rod-shaped DNPs, or saline in rabbits Unilateral subretinal injection (0.05 mL) of rod-shaped DNPs in rabbits | -Tolerance: well-tolerated, resulted in reversible opening of the SCS -Luciferase activity: high activity in the retina and the RPE; mean luciferase activity comparable between treatment groups -Drug distribution: greater surface area coverage after SC administration | Animal experimental study | Preclinical (animal study) | Suprachoroidally delivered DNA nanoparticles transfect retina and retinal pigment epithelium/choroid in rabbits [139] |
| 53 | Gene therapy | Poly (β-amino ester)s nanoparticles (PBAE NPs) | SC injections of PBAE NPs containing 1 μg of pEGFP-N1 (an expression plasmid in which a cytomegalovirus promoter drives expression of GFP (CMV-GFP)) SC injections of PBAE NPs containing 1 μg of pVEGF SC injections of PBAE NPs containing 1 μg of p3sFlt1Fc or 1 μg of a CMV-Luciferase expression plasmid (pCMV-Luc) (control) followed by intravitreous injection of 100 ng of recombinant VEGF165 2 weeks later All injections occurred in Brown Norway rats | -GFP expression after SC injection of PBAE NPs containing pEGFP-NP: expression in anterior retina around the entire eye circumference; less expression in the RPE and photoreceptors compared to SC injection of 2.85 × 1010 GC of AAV8.GFP, fluorescence not strong enough to be visualized on flat mounts; multiple injections markedly increase expression; expression maintained without substantial decline at least through 8 months -Neovascularization after SC injection of NPs containing VEGF expression plasmid: severe subretinal neovascularization starting in the periphery near injection site and extended posteriorly progressing to subretinal fibrosis -Suppression of VEGF-induced retinal vascular leakage and neovascularization: resulted after SC injection of p3sFlt1Fc NPs | Animal experimental study | Preclinical (animal study) | Suprachoroidal gene transfer with nonviral nanoparticles [140] |
| 54 | Dry age-related macular degeneration (AMD) and Stargardt’s macular dystrophy (SMD) | Adipose tissue-derived mesenchymal stem cell (ADMSC) implantation | SC implantation of adipose tissue-derived mesenchymal stem cell (ADMSC) implantation | -Beneficial outcomes: improvements in visual acuity, visual field, and electroretinogram recordings -AEs: no complications in the 6-month follow-up period -Choroidal thickening: observed on OCT scans, indicating increased choroidal perfusion | Prospective, single arm, uni-center | Clinical trial (Phase I/II) | First Year Results of Suprachoroidal Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Degenerative Macular Diseases [204] |
| 55 | Retinitis pigmentosa patients | Umbilical cord derived mesenchymal stem cell (UCMSC) implantation | SC umbilical cord derived mesenchymal stem cell (UCMSC) implantation | -Mean BCVA and visual field scores: both improved after treatment during the 12-month study period (p < 0.05); negative correlation between BCVA improvement and disease scores and grades -Mean score and mean grade of disease: improved after treatment (p < 0.05) | Prospective, uin-center | Clinical trial (Phase I/II) | Does stem cell implantation have an effect on severity of retinitis pigmentosa: evaluation with a classification system? [143] |
| 56 | Retinitis pigmentosa patients | Umbilical cord derived mesenchymal stem cell (UCMSC) implantation | SC umbilical cord derived mesenchymal stem cell (UCMSC) implantation in patients ≤18 years old | -Mean BCVA gain: improved from baseline (p < 0.05); declined in 56% of 46 eyes during 1st year; no improvement in untreated eyes -Mean visual field: improvement after treatment (p < 0.05); 65% of 46 eyes had improvement at 1 year -Mean CMT reduction: not significant from baseline (p > 0.05) -Mean mfERG: improvement after treatment (p < 0.05) -AEs: none | Prospective, uni-center | Clinical trial (Phase I/II) | Suprachoroidal umbilical cord derived mesenchymal stem cell implantation for the treatment of retinitis pigmentosa in pediatric patients [144] |
| 57 | Photoreceptor loss due to neovascular age-related macular degeneration, DME, RVO | BD311 integration-deficient lentiviral vector (IDLV) expressing VEGFA antibody | Single SC injection of IDLV expressing VEGFA antibody, 500 uL | -Recruiting participants, results pending study completion | Prospective, interventional, single arm | Clinical trial (Phase I) | VEGFA-targeting gene therapy to treat retinal and choroidal neovascularization diseases [164] |
| 58 | Retinitis pigmentosa patients | Umbilical cord derived mesenchymal stem cell (UCMSC) implantation | SC mesenchymal stem cell implanted vs. SC mesenchymal spheroidal stem cell implantation | -Study results pending | Prospective, clinical case series | Not applicable | Spheroidal mesenchymal stem cells in retinitis pigmentosa [145] |
| 59 | Solar retinopathy | TA | SC injection of 0.1 mL TA with a custom-made needle | -Case summary: 17-year-old female with decreased vision due to solar retinopathy received single SCTA injection without any surgical complications -Mean BCVA gain: 0.7 at 1 week, 0.8 as 12 weeks; full recovery by week 4 -AEs: no serious AEs as of 12 weeks -Mean IOP: increased to 28 mmHg in week 7, controlled by topical eye drops (timolol) to 15 mmHg | Case report | Not applicable | Managing solar retinopathy with suprachoroidal triamcinolone acetonide injection in a young girl: a case report [146] |
| Choroidal neovascularization (CNV) | |||||||
| 60 | Choroidal neovascularizaiton | Pazopanib, Bevacizumab, Fusion protein hI-con1 | IVB injection, 2.5 mg vs. IV Pazopanib injection, 1 mg vs. IV 300 μg hI-con1 vs. SC Pazopanib injection, 1 mg vs. 10 vehicle controls (SC+ IV) | -CNV height: smaller in IV pazopanib (90 ± 20 μm) vs. control (180 ± 20 μm; p = 0.009); smaller maximum height in IV Pazopanib (173 ± 43 μm) vs. SC Pazopanib (478 ± 105 μm; p = 0.018); small decrease with IV Bevacizumab vs. controls -CNV surface area: no difference between the 3 treatment groups; with hI-con1 lesions were thinner than controls -Lesion size: smaller for all vs. controls | Animal experimental study | Preclinical trial (animal study) | A pharmacodynamic analysis of choroidal neovascularization in a porcine model using three targeted drugs [151] |
| 61 | Choroidal neovascularizaiton | Bevacizumab | 1250 µg/50 µL SC Bevacizumab | -% bevacizumab recovered: 88.4 ± 0.9% at 15 min, 4.6 ± 0.5% at 1 day, and 0.2 ± 0.1% at 2 days after injection -Drug distribution (at 15 min): 76% in choroid, 13% in sclera and 2.9% in retina, 1.0% in vitreous, 0.5% in aqueous humor, 0.9% in anterior chamber, 0.6% in lens and 0.1% in optic nerve -Drug distribution (at day 1): 34% in choroid, 27% in sclera and 23% in retina, 11% in vitreous, 0.7% in aqueous humor, 1.6% in anterior chamber, 3.8% in lens and 0.3% in optic nerve -Drug distribution (At day 2): 0.5% in choroid, 3.3% in sclera, 0.5% in retina, 55% in vitreous, 3% in aqueous humor, 36% in anterior chamber, 1.1% in lens and 0.6% in optic nerve | Animal experimental study | Preclinical trial (animal study) | Pharmacokinetics and biodistribution of Bevacizumab following suprachoroidal injection into the rabbit eye using a microneedle [152] |
| 62 | Choroidal neovascularizaiton | Bevacizumab | SC injection of Bevacizumab cross-linked with polycaprolactone dimethacrylate and hydroxyethyl methacrylate | -Drug release: sustained manner > 4 months -Bevacizumab’s mechanism of action: not affected in animal models | Animal experimental study | Preclinical trial (animal study) | Light activated, in- situ forming gel for sustained suprachoroidal delivery of Bevacizumab [66] |
| 63 | Choroidal neovascularizaiton | Bevacizumab | SC injection of an in-situ forming hydrogel comprised Bevacizumab and hyaluronic acid (HA) | -Duration of drug release: >6 months -Tolerance: well tolerated by clinical exam, fundus imaging, histological analysis, and IOP measurement | Animal experimental study | Preclinical trial (animal study) | Six-month sustained delivery of anti-VEGF from in-situ forming hydrogel in the suprachoroidal space [80] |
| 64 | Choroidal neovascularizaiton | Acriflavine | Intraocular injection of 100 ng Acriflavine vs. SC injection of 300 ng Acriflavine vs. Topical administration of Acriflavine | -Inner retina fluorescence: SC injection caused fluorescence in quadrant of injection at 1 h with entire retinal and choroid spread by day 1 (detectable for 5 days), strong suppression of CNV at Bruch’s membrane rupture sites at day 7 vs. topical administration caused fluorescence in retina and the RPE within 5 min, detectable for 6–12 h -CNV reduction: dramatic at 14 days after rupture of Bruch’s membrane (SC); also reduced (topical) | Animal experimental study | Preclinical trial (animal study) | The HIF-1 antagonist Acriflavine: visualization in retina and suppression of ocular neovascularization [154] |
| 65 | Choroidal neovascularizaiton | Acriflavine | SC injection of Acriflavine poly lactic-co-glycolic acid formulated micro particle | -CNV suppression: suppressed for at least 9 weeks (IV injection) in mice vs. 18 weeks (SC injection) in rats -Full-field electroretinogram function: modest reduction in IV, no reduction in SC injection; normal electroretinogram scotopic a- and b- wave amplitudes at 28 days with SC injection -Other outcomes: normal retinas, retinal histology, and IOP at 28 days with SC injection; active component of Acriflavine had steady-state levels in the low nM range in RPE/choroid/retina for at least 16 weeks | Animal experimental study | Preclinical trial (animal study) | Sustained delivery of Acriflavine from suprachoroidal space provides long term suppression of choroidal neovascularization [84] |
| 66 | Choroidal neovascularizaiton | VEGFR-1 (sFlt-1)-encoding plasmid | SC injection of soluble VEGFR-1 (sFlt-1)-encoding plasmid into the with an electrical field | -Transduction: at least 1 month of the RPE -AEs: none -Inhibition of CNV: significant levels achieved 15 days after transfection | Animal experimental study | Pre-clinical study (animal study) | Suprachoroidal electrotransfer: a novel gene delivery method to transfect the choroid and the retnia without detaching the retina [88] |
| Neovascular age related macular degeneration | |||||||
| 67 | Neovascular age related macular degeneration | RGX-314 | Cohort 1: SC 2.5 × 1011 RGX genomic copies/eye vs. monthly 0.5 mg SC Ranibizumab Cohort 2: SC 5 × 1011 RGX genomic copies/eye vs. monthly 0.5 mg SC Ranibizumab Cohort 3: SC 5 × 1011 RGX genomic copies/eye vs. SC 5 × 1011 RGX genomic copies/eye in patients who are neutralizing antibody positive | -Tolerance: well tolerated in cohorts 1–3 with no serious AEs -AEs: mild AEs related to treatment occurred in cohorts 1 and 2 through 6 months; 23% mild intraocular inflammation (similar incidence across dose levels), all cases resolved within days to weeks on topical corticosteroid -BCVA and CRT: stable at 6 months in patients dosed with RGX-314 in cohorts 1 and 2 -Anti-VEGF reduction: >70% in cohorts 1 and 2 -Retreatment: 29% (cohort 1) and 40% (cohort 2) received no anti-VEGF injections for over 6 months following RGX-314 injection | Randomized, interventional, open-label | Clinical trial (phase II) | Suprachoroidal delivery of RGX-314 gene therapy for neovascular age related macular degeneration: The phase II AAVIATE study [157] |
| 68 | Neovascular age related macular degeneration | RGX-314 | Cohort 1: SC 2.5 × 1011 RGX genomic copies/eye vs. Monthly 0.5 mg SC Ranibizumab Cohort 2: SC 5 × 1011 RGX genomic copies/eye vs. Monthly 0.5 mg SC Ranibizumab Cohort 3: SC 5 × 1011 RGX genomic copies/eye vs. SC 5 × 1011 RGX genomic copies/eye in patients who are neutralizing antibody positive | -Pending full study results | Randomized, interventional, open-label | Clinical trial (phase II) | RGX-314 gene therapy administered in the suprachoroidal space for participants with neovascular age-related macular degeneration (nAMD) (AAVIATE) [156] |
| 69 | Neovascular age related macular degeneration | Bevacizumab, Triamcinolone | SC Bevacizumab and TA | -AEs: no serious AEs related to treatment -IOP elevation: 4.76% of eyes at 3 months, medically controlled -Nuclear sclerotic cataracts: increase in 10.5% of eyes | Nonrandomized, single arm, interventional, uni-center | Clinical trial (phase I) | Safety of submacular suprachoroidal drug administration via a microcatheter: retrospective analysis of European treatment results [158] |
| 70 | Neovascular age related macular degeneration | Bevacizumab | SC injection of 100 µL Bevacizumab | -AEs: moderate pain only, no serious AEs -IOP: no serious elevation -Rescue therapy: none required for two months | Single-center, open-label | Clinical trial (phase I) | Suprachoroidal microinjection of Bevacizumab is well tolerated in human patients [159] |
| 71 | Neovascular age related macular degeneration | Aflibercept | 4 mg SC Aflibercept vs. SC saline | -Neovascularization reduction: ~4862 ± 192 pixels2 on fluorescein angiography (control) vs. ~3318 ± 353 pixels2 (intervention) based on evaluation of neovascular leak area at week 3 (p < 0.001) | Animal experimental study | Preclinical trial (animal study) | Efficacy of suprachoroidal Aflibercept in a laser induced choroidal neovascularization model [160] |
| 72 | Neovascular age related macular degeneration | CLS011A | 4 mg (100 µL) SC CLS011A | -Tolerance: well-tolerated till day 91 with no signs of toxicity -Drug distribution: not detected at quantifiable levels in plasma or aqueous humor; quantifiable at all times in vitreous humor, retina, and sclera/choroid-RPE -Drug concentration gradient: sclera/choroid-RPE > retina > vitreous humor -Drug elimination: half-life of 102 days, >60% remaining at 3 months | Animal experimental study | Preclinical trial (animal study) | Pharmacokinetics including ocular distribution characteristics of suprachoroidally administered CLS011A in rabbits could be beneficial for wet AMD therapeutic candidate [161] |
| 73 | Neovascular age related macular degeneration | Axitinib | SC 0.03, 0.10, 0.50, and 1.0 mg of Axitinib following IV 2 mg Aflibercept | -Preliminary safety data: no treatment related serious AEs -Final safety data to be released | Interventional, prospective, non-randomized, sequential assignment | Clinical trial (phase I/II) | Safety and tolerability study of suprachoroidal injection of CLS-AX following anti-VEGF therapy in neovascular AMD (OASIS) [162] |
| 74 | Neovascular age-related macular degeneration | Axitinib | Subjects who received SC 0.1 mg, 0.5 mg, and 1.0 mg Axitinib in the parent study will be followed for an additional 12 weeks | -Study ongoing | Observational, prospective cohort | Parent study was a phase II clinical trial | Extension study to evaluate the long-term outcomes of subjects in the CLS-AX CLS1002-101 study [163] |
| 75 | Neovascular age related macular degeneration | Axitinib | 100 µL SC Axitinib 0.03 mg/eye of vs. 100 µL SC Axitinib 0.1 mg/eye Once weekly for two weeks SC 0.2 mg/5 µLs/eye Axitinib in rabbit with laser induced choroidal neovascularization vs. SC saline in rabbit with laser induced choroidal neovascularization 4 mg SC Axitinib in pig with laser induced choroidal neovascularization vs. SC saline in pig with laser induced choroidal neovascularization | -Tolerance: well tolerated -Drug concentration: no Axitinib detected in plasma or aqueous humor; sustained, high exposure through 10-week study, highest in the sclera/choroid/RPE, retina, vitreous -Drug levels in choroid–retina: >1000× higher in humans vs. in-vitro value through 6 months -Drug efficacy: reduced CNV by Axitibin in 40% eyes with clinically important lesions (scores of 3 or 4); general improvement (scores of 0 to 2) by day 21 vs. control group -Fluorescein leakage: reduced at weeks 1 and 2 (p < 0.009 for both) by SC Axitinib vs. control | Animal experimental study | Preclinical trial (animal study) | Suprachoroidal CLS-AX (Axitinib injectable suspension), as a potential long-acting therapy for neovascular age-related macular degeneration (nAMD) [92] |
| 76 | Neovascular age related macular degeneration | Axitinib | 100 µL SC Axitinib 0.03 mg/eye (Group 1) vs. 100 µL SC Axitinib 0.01 (Group 2) | -Tolerance: well tolerated -Drug distribution: sustained and high exposure of Axitinib the RPE-choroid–sclera throughout 10-week study -Drug concentration: 138 ng/g at week 10, 4400 ng/g for Groups 1 and 2 (1153× and 36,667× higher than in-vitro value, respectively); Axitinib not detected in either aqueous humor or plasma -Mean retina drug levels: maximum of 4480 ng/g (Group 1) and 6260 ng/g (Group 2) at 24 h -Mean vitreous drug levels: 4 to 5 orders of magnitude lower than in retina -Estimation of human drug levels: SC 0.1 mg/eye may provide Axitinib levels in choroid–retina > 1000× higher than in-vitro value until 6 months | Animal experimental study | Preclinical trial (animal study) | Pharmacokinetics and ocular tolerability of suprachoroidal CLS-AX (Axitinib injectable suspension) in rabbits [205] |
| 78 | Serous pigment epithelium detachment due to neovascular age related macular degeneration | Bevacizumab | 2 SC injections, 1 month apart, of 0.1 mL Bevacizumab | -BCVA change (logMAR): reduced from 0.604 (baseline) to 0.146667 at 8 weeks (p < 0.05) -Size and height of pigment epithelium detachment on OCT: reduced from 676.8 ± 156.4 μm (baseline) to 108.6 ± 52.4 μm (p < 0.05) at 8 weeks -IOP: rise immediately after injection, normalized with 500 mg oral acetazolamide -AEs: pain -IOP: no change | Prospective, interventional, single group | Clinical trial (phase I) | Role of suprachoroidal anti-VEGF injections in recalcitrant serous pigment epithelium detachment [165] |
| Retinal detachment | |||||||
| 79 | Retinal detachment due to Vogt–Koyanagi Harada Disease | TA | 4 mg SCTA and systemic steroids vs. Systemic steroids only | -BCVA gain: higher in SCTA eyes at 1 month and 3 months (p-value = 0.026) - CFT reduction: CFT higher in control eyes at 1 month and 3 months (p-value 0.028) -Mean IOP: comparable between groups | Prospective, parallel group | Clinical trial (phase II) | Suprachoroidal Triamcinolone Acetonide injection: a novel therapy for serous retinal detachment due to Vogt–Koyanagi Harada Disease [167] |
| 80 | Serous choroidal detachment due to rhegmatogenous retinal detachment | TA | 4 mg SC TA before vitrectomy or scleral buckle surgery | -Fluid reduction: >50% reduction in %50 eyes by day 3 and %20 eyes by day 5 -Treatment response: failed in 30% requiring surgical drainage before proceeding with vitrectomy -AEs: none during procedure -Elevated IOP: transient rise (30 mmHg) in %10 following vitrectomy managed with therapy | Prospective, noncomparative, interventional pilot study | Clinical trial (phase I) | Safety and efficacy of suprachoroidal Triamcinolone Acetonide for the management of serous choroidal detachment prior to rhegmatogenous retinal detachment surgery: a pilot study [166] |
| 81 | Rhegmatogenous retinal detachment | Sodium hyaluronate | Sodium hyaluronate injected into the SCS before retinal hole scleral freezing and laser photocoagulation | -Reattachment: required in 50%; 33% partly anatomically reattached with subretinal fluid; 16.67% failed reattachment and received vitrectomy with silicone oil tamponade or sclera buckling surgery -AEs: no severe AEs related to treatment | Prospective, interventional, single group | Clinical trial (phase I) | Suprachoroidal injection of sodium hyaluronate in the treatment of 12 patients with rhegmatogenous retinal detachment [168] |
| 82 | Retinal detachment | Sodium hyaluronate | Fractions of sodium hyaluronate injected into the SCS | -Buckling effect: short-lived between 12 and 72 h -Drug distribution: sodium hyaluronate present in SCS for >10–14 days -Dosage effect: no significant differences between buckles by sodium hyaluronate (1 and 2%), or by cross-linked sodium hyaluronate | Animal experimental study | Preclinical trial (animal study) | Suprachoroidal injection of sodium hyaluronate as an ‘internal’ buckling procedure [169] |
| 83 | Retinal detachment | Air | 1.5 mL air injected into SCS | -Treatment response: 5 cases responded satisfactorily -AEs: 1 case of vitreous hemorrhage -Relapse: in 2 cases -Further treatment: in 1 case | Case series | Not applicable | Suprachoroidal air injection for detached retina [170] |
| Other diseases | |||||||
| 84 | Non-infectious intermediate, posterior, or pan-uveitis | TA | SC injection of 4 mg TA in 100 μL | -AEs: 38 reported, 89% mild or moderate in severity: uveitis progression (18%), cataract progression (3%) requiring surgery, ocular pain (16%); all systemic AEs unrelated to treatment; no treatment-related increases in IOP -BCVA: improvement in all eyes, >2-line improvement in 4 patients (who did not need additional therapy) through week 26 | Open-label, clinical study | Clinical trial (phase I/II) | Suprachoroidal corticosteroid administration: a novel route for local treatment of non-infectious uveitis [171] |
| 85 | Acute posterior uveitis | TA, Prednisone | Group 1:50 μL injection of balanced salt solution vs. Group 2:1 mg/kg/d oral prednisone for 3 days vs. Group 3:2 mg SC TA injection vs. Group 4:0.1 mg/kg/day oral prednisone for 3 days vs. control eye | -Inflammatory score: on day 1 and 2 only, group 3 had mean cumulative inflammation scores significantly lower than group 1 (p ≤ 0.04); by day 3, group 2 and 3 had lower mean cumulative inflammation scores than group 1 (p < 0.034); group 4 had mean cumulative inflammation scores not significantly different than BSS treated eyes at any time (p > 0.05) -Mean histologic inflammation scores of the anterior and posterior segment: significantly lower in group 3 than eyes in group 1 | Animal experimental study | Preclinical trial (animal study) | Evaluation of suprachoroidal CLS-TA and oral Prednisone in a porcine model of uveitis [172] |
| 86 | Acute posterior uveitis | TA | SC injection of 0.2 mg or 2.0 mg TA vs. IV injection of 0.2 mg or 2.0 mg of TA | -Drug efficacy: comparable reduction in inflammation in posterior segment of both SCTA (0.2 mg and 2 mg) and high-dose IVTA (2.0 mg); low-dose SCTA (0.2 mg) also effective in reducing inflammation but low-dose IVTA (0.2 mg) was not - Vitreous humor cell count and protein concentration: lower in high dose SCTA vs. low dose SCTA and IVTA groups - Aqueous humor protein concentration: no differences between the groups -AEs: none for SCTA group within 3 days -IOP and OCT measurements: similar between SCTA and 2.0 mg IVTA groups | Animal experimental study | Preclinical trial (animal study) | Treatment of acute posterior uveitis in a porcine model by injection of Triamcinolone Acetonide into the suprachoroidal space using microneedles [173] |
| 87 | Panuveitis | TA | 4 mg SCTA vs. 4 mg IVTA vs. SC Injection of vehicle as control | -AEs: none, including rise in IOP -Drug efficacy: higher panuveitis in control and IVTA treated eyes after 24 h; vitritis, aqueous flare, and cellularity less severe in both SCTA and IVTA treated eyes vs. control -Iris vessel dilation and tortuosity: greater reduction in SC group than IVTA group -Inflammatory response: significant reduction in SCTA group; histology showed marked reduction in SCTA and IVTA vs. control | Animal experimental study | Preclinical trial (animal study) | Evaluation of suprachoroidal microinjection of Triamcinolone Acetonide in a model of panuveitis in albino rabbits [174] |
| 88 | Experimental uveitis | TA | 50 μL (2 mg) SCTA vs. Subtenon injection of 20 mg TA | - IOP: acute rise post-injection; higher rise with higher volume (p < 0.0001); equivalent volume of indocyanine green solution led to smaller rise than SCTA -Drug concentration: SCTA group, 1912 ng/mL in posterior vitreous and 400,369 ng/mL in retina; maximum in plasma was 11.6 ng/mL; exposure to posterior retina was 523,910 times greater than that to aqueous and 29,516 times more than systemic TA exposure -Efficacy for lipopolysaccharide-induced uveitis: less aqueous humor cells and lower vitreous opacity scores in 2 mg SCTA group compared to 20 mg subtenon group (p < 0.05) -Inflammatory response: less vitreous inflammation in SCTA group compared to subtenon group (p < 0.0001) -Tolerance: SCTA well tolerated -Drug distribution: excellent penetration into posterior retina in SCTA group vs. subtenon group | Animal experimental study | Preclinical trial (animal study) | Safety and pharmacodynamics of suprachoroidal injection of Triamcinolone Acetonide as a controlled ocular drug release model [67] |
| 89 | Glaucoma | Sulprostone, Brimonidine | Supraciliary injection of 10 μL Sulprostone or 10 μL Brimonidine vs. Topical administration of 0.05 mg/mL Sulprostone, 1 drop or 1.5 mg/mL Brimonidine, 1 drop | -IOP: SC Sulprostone and Brimonidine reduced IOP by 3 mmHg in a dose-dependent mannber over 9 h -Dose sparing: ~100-fold dose sparing vs. topical administration -Safety study: comparable kinetics of IOP elevation immediately after supraciliary and IV injection of placebo formulations | Animal experimental study | Preclinical trial (animal study) | Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles [179] |
| 90 | Glaucoma | Brimonidine | SC Brimonidine-loaded microspheres using poly (lactic acid) microspheres | -IOP: reduce initially by 6 mmHg; then, by progressively smaller amounts for >1 month -Tolerance: overall well, mild conjunctival redness and injection site healing delays -Histological examination: foreign-body reaction to the microspheres | Animal experimental study | Preclinical trial (animal study) | Sustained reduction in intraocular pressure by supraciliary delivery of Brimonidine-loaded poly (lactic acid) microspheres for the treatment of glaucoma [180] |
| 91 | Glaucoma | NA | Hyaluronic acid in situ-forming hydrogel | -IOP: reduced for >1 month, observed for 4 months -AEs: none other than minor hemorrhage and fibrosis at injection site | Animal experimental study | Preclinical trial (animal study) | Drug-free, nonsurgical reduction in intraocular pressure for four months after suprachoroidal injection of hyaluronic acid hydrogel [182] |
| 92 | Glaucoma | NA | In situ-forming polyzwitterion polycarboxybetaine hydrogel | -Tolerance: well-tolerated with minimal inflammatory reaction -Histopathology assessment: SCS expansion with hydrogel -IOP: decreased for 6 weeks, correlated with SCS expansion | Animal experimental study | Preclinical trial (animal study) | Suprachoroidal injection of polyzwitterion hydrogel for treating glaucoma [183] |
| 93 | Uveal melanoma | AU-011 | SC injection of AU-011 followed by photoactivation 1/week for 3 consecutive weeks | -Drug distribution: volume-dependent fashion in the choroid within 30 min. 100 μL distributed to ~75% of posterior globe -Drug clearance: not cleared from choroid for several days -Tumor regression: seen in all rabbits after SC 100 μL AU-011 -Histological evaluation: evidence of tumor responses | Animal experimental study | Preclinical trial (animal study) | Ocular distribution and efficacy after suprachoroidal injection of AU-011 for treatment of ocular melanoma [74] |
| 94 | Uveal melanoma | AU-011 | SC AU-011 injection vs. IV AU-011 injection | -Drug distribution: negligible levels in vitreous, high exposure levels in tumor (up to 48 h) and choroid/retina for SC injection; high exposure in tumor for IV injection (up to 48 h) -Drug concentration: exposure of AU-011 in tumor ~5× higher for SC injection; mean AU-011 concentrations were 12,459 ± 5190 and 1996 ± 421 ng/mL for SC and IV injection, respectively, for 48 h -Positive IHC staining: AU-011 was present in tumor after both SC and IV injection; AU-011 staining observed penetrating throughout tumor for SC injection vs. localizing on the apex or vitreal surface of tumor for IV injection | Animal experimental study | Preclinical trial (animal study) | Ocular distribution and exposure of AU-011 after suprachoroidal or intravitreal administration in an orthotropic rabbit model of human uveal melanoma [184] |
| 95 | Uveal melanoma | Fluorescent particles, Resin beads | SC injection of 10 µm fluorescent particles vs. SC injection of resin beads | -Fundus examination: yellow-tinted area (fluorescent particles) around tumor in posterior pole -Histological examination: polystyrene microspheres or resin beads located in SCS -Inflammatory response: not associated with microspheres -Drug distribution: microbeads present in SCS | Animal experimental study | Preclinical trial (animal study) | Suprachoroidal injection of microspheres with microcatheter in a rabbit model of uveal melanoma [185] |
| 96 | Uveal melanoma | AU-011 | 3 cycles of 3 weekly AU-011 treatments via SC administration with a maximum dose of 80 μg with 2 laser applications | -AE related to treatment: anterior chamber inflammation (24%), conjunctival hyperemia (12%), eye pain (12%), punctate keratitis (12%) -No dose-limiting toxicities, treatment-related serious or grade 3/4 AEs, vitritis or vision loss; 2 subjects had 5 serious AEs unrelated to treatment | Randomized, multi-center | Clinical trial (phase II) | A phase II trial of AU-011, an investigational, virus-like drug conjugate (VDC) for the treatment of primary indeterminate lesions and small choroidal melanoma (IL/CM) using suprachoroidal administration [187] |
| 97 | Myopia | Biologic agents | NA | NA | NA | NA | Suprachoroidal injection of biological agents may have a potential role in the prevention of progression and complications in high myopia [190] |
| 98 | Ocular inflammatory diseases | Ketorolac | Intracameral injection of Ketorolac 250 μg/0.05 mL (Group A) vs. IV Ketorolac 250 μg/0.05 mL (Group B) vs. SC Ketorolac 250 μg/0.05 mL (Group C) | -Maximum drug concentration in vitreous: 0.378 ± 0.19 μg/mL at 0.5 h (Group A) vs. 156.2 ± 20.74 μg/mL at 0.5 h (Group B) vs. 0.873 ± 0.34 μg/mL at 0.5 h (Group C) -Maximum drug concentration in retina-choroid: 3.15 ± 0.49 μg/g at 0.5 h (Group A); 208.0 ± 21.67 μg/g at 1 h (Group B); 56.71 ± 22.64 μg/g at 0.5 h (Group C)-Drug concentration: <2 μg/g in retina-choroid in group C; plasma concentration < 0.4 μg/mL in all 3 group-Area under the curve: 866.1 ± 52.67 μg/g·h in retina-choroid in group B vs. 77.10 ± 25.90 μg/g·h in group C (p < 0.01) -Drug elimination: half-life was 3.09 h (Group B) vs. 1.19 h (Group C) (p< 0.01); Ketorolac in retina-choroid until 24 h (Group B) and 8 h (Group C) | Animal experimental study | Preclinical trial (animal study) | Pharmacokinetic comparison of Ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits [194] |
| 99 | Ocular inflammatory diseases | Ketorolac | SC 3 mg Ketorolac vs. SC 6 mg Ketorolac vs. Control (left eye) | -Electroretinography results: no abnormal changes in rod cell response, maximum rod cell or cone cell mixing reaction, oscillation potential, cone cell response, waveform, amplitude, and potential of 30 Hz scintillation response at 1, 2, and 4 weeks, comparable with control eye -Light microscopy: normal histology in each retinal layer at 4 weeks | Animal experimental study | Preclinical trial (animal study) | Suprachoroidal injection of Ketorolac Tromethamine does not cause retinal damage [195] |
References
- Wu, K.Y.; Joly-Chevrier, M.; Akbar, D.; Tran, S.D. Overcoming Treatment Challenges in Posterior Segment Diseases with Biodegradable Nano-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 1094. [Google Scholar] [CrossRef]
- Ghate, D.; Edelhauser, H.F. Ocular Drug Delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lampen, S.I.R.; Khurana, R.N.; Noronha, G.; Brown, D.M.; Wykoff, C.C. Suprachoroidal Space Alterations Following Delivery of Triamcinolone Acetonide: Post-Hoc Analysis of the Phase 1/2 HULK Study of Patients with Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retina 2018, 49, 692–697. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Hariprasad, S.M.; Albini, T.A.; Dutta, S.K.; John, D.; Padula, W.V.; Harrison, D.; Joseph, G. Suprachoroidal Injection of Triamcinolone Acetonide Injectable Suspension for the Treatment of Macular Edema Associated with Uveitis in the United States: A Cost-Effectiveness Analysis. Value Health 2022, 25, 1705–1716. [Google Scholar] [CrossRef]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef]
- Naftali Ben Haim, L.; Moisseiev, E. Drug Delivery via the Suprachoroidal Space for the Treatment of Retinal Diseases. Pharmaceutics 2021, 13, 967. [Google Scholar] [CrossRef]
- Margolis, R.; Spaide, R.F. A Pilot Study of Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Normal Eyes. Am. J. Ophthalmol. 2009, 147, 811–815. [Google Scholar] [CrossRef]
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kaplan, H.J. Anatomy and Function of the Eye. In Chemical Immunology and Allergy; Niederkorn, J.Y., Kaplan, H.J., Eds.; KARGER: Basel, Switzerland, 2007; pp. 4–10. ISBN 978-3-8055-8187-5. [Google Scholar]
- Vurgese, S.; Panda-Jonas, S.; Jonas, J.B. Scleral Thickness in Human Eyes. PLoS ONE 2012, 7, e29692. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef]
- Ciulla, T.; Yeh, S. Microinjection via the Suprachoroidal Space: A Review of a Novel Mode of Administration. Am. J. Manag. Care 2022, 28, S243–S252. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Butterworth, J.; Malecaze, F.; Calvas, P. Axial Length of Myopia: A Review of Current Research. Ophthalmologica 2011, 225, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic Aspects of Retinal Drug Delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Emi, K.; Pederson, J.E.; Toris, C.B. Hydrostatic Pressure of the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 1989, 30, 233–238. [Google Scholar]
- Krohn, J.; Bertelsen, T. Light Microscopy of Uveoscleral Drainage Routes after Gelatine Injections into the Suprachoroidal Space. Acta Ophthalmol. Scand. 1998, 76, 521–527. [Google Scholar] [CrossRef]
- Krohn, J.; Bertelsen, T. Corrosion Casts of the Suprachoroidal Space and Uveoscleral Drainage Routes in the Human Eye. Acta Ophthalmol. Scand. 1997, 75, 32–35. [Google Scholar] [CrossRef]
- Chiang, B.; Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Circumferential Flow of Particles in the Suprachoroidal Space Is Impeded by the Posterior Ciliary Arteries. Exp. Eye Res. 2016, 145, 424–431. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and Obstacles of Ocular Pharmacokinetics and Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Wu, K.Y.; Ashkar, S.; Jain, S.; Marchand, M.; Tran, S.D. Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma. Polymers 2023, 15, 1373. [Google Scholar] [CrossRef]
- Carnahan, M.C.; Goldstein, D.A. Ocular Complications of Topical, Peri-Ocular, and Systemic Corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef]
- Tyagi, P.; Kadam, R.S.; Kompella, U.B. Comparison of Suprachoroidal Drug Delivery with Subconjunctival and Intravitreal Routes Using Noninvasive Fluorophotometry. PLoS ONE 2012, 7, e48188. [Google Scholar] [CrossRef] [PubMed]
- Yiu, G.; Chung, S.H.; Mollhoff, I.N.; Nguyen, U.T.; Thomasy, S.M.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal and Subretinal Injections of AAV Using Transscleral Microneedles for Retinal Gene Delivery in Nonhuman Primates. Mol. Ther. Methods Clin. Dev. 2020, 16, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A Comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefánsson, E. Aqueous Eye Drops Containing Drug/Cyclodextrin Nanoparticles Deliver Therapeutic Drug Concentrations to Both Anterior and Posterior Segment. Acta Ophthalmol. 2022, 100, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Yokoi, N.; Shimazaki, J.; Hori, Y.; Tsubota, K.; on behalf of the Japan Dry Eye Society. Adherence to Eye Drops Usage in Dry Eye Patients and Reasons for Non-Compliance: A Web-Based Survey. J. Clin. Med. 2022, 11, 367. [Google Scholar] [CrossRef]
- Foley, L.; Larkin, J.; Lombard-Vance, R.; Murphy, A.W.; Hynes, L.; Galvin, E.; Molloy, G.J. Prevalence and Predictors of Medication Non-Adherence among People Living with Multimorbidity: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e044987. [Google Scholar] [CrossRef]
- Holló, G. The Side Effects of the Prostaglandin Analogues. Expert Opin. Drug Saf. 2007, 6, 45–52. [Google Scholar] [CrossRef]
- Santulli, R.J.; Kinney, W.A.; Ghosh, S.; Decorte, B.L.; Liu, L.; Tuman, R.W.A.; Zhou, Z.; Huebert, N.; Bursell, S.E.; Clermont, A.C.; et al. Studies with an Orally Bioavailable Alpha V Integrin Antagonist in Animal Models of Ocular Vasculopathy: Retinal Neovascularization in Mice and Retinal Vascular Permeability in Diabetic Rats. J. Pharmacol. Exp. Ther. 2008, 324, 894–901. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Miyashita, H.; Yamaguchi, M. Exploration of Orally Available Calpain Inhibitors. Part 3: Dipeptidyl Alpha-Ketoamide Derivatives Containing Pyridine Moiety. Bioorg. Med. Chem. 2006, 14, 5691–5698. [Google Scholar] [CrossRef]
- Kampougeris, G.; Antoniadou, A.; Kavouklis, E.; Chryssouli, Z.; Giamarellou, H. Penetration of Moxifloxacin into the Human Aqueous Humour after Oral Administration. Br. J. Ophthalmol. 2005, 89, 628–631. [Google Scholar] [CrossRef]
- Sakamoto, H.; Sakamoto, M.; Hata, Y.; Kubota, T.; Ishibashi, T. Aqueous and Vitreous Penetration of Levofloxacin after Topical and/or Oral Administration. Eur. J. Ophthalmol. 2007, 17, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki, Y. Molecular Design for Enhancement of Ocular Penetration. J. Pharm. Sci. 2008, 97, 2462–2496. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future Perspective in Topical Glaucoma Therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K.; Argüeso, P. Role of Mucins in the Function of the Corneal and Conjunctival Epithelia. Int. Rev. Cytol. 2003, 231, 1–49. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Transscleral Drug Delivery for Posterior Segment Disease. Adv. Drug Deliv. Rev. 2001, 52, 37–48. [Google Scholar] [CrossRef]
- Hosseini, K.; Matsushima, D.; Johnson, J.; Widera, G.; Nyam, K.; Kim, L.; Xu, Y.; Yao, Y.; Cormier, M. Pharmacokinetic Study of Dexamethasone Disodium Phosphate Using Intravitreal, Subconjunctival, and Intravenous Delivery Routes in Rabbits. J. Ocul. Pharmacol. Ther. 2008, 24, 301–308. [Google Scholar] [CrossRef]
- Weijtens, O.; Feron, E.J.; Schoemaker, R.C.; Cohen, A.F.; Lentjes, E.G.; Romijn, F.P.; van Meurs, J.C. High Concentration of Dexamethasone in Aqueous and Vitreous after Subconjunctival Injection. Am. J. Ophthalmol. 1999, 128, 192–197. [Google Scholar] [CrossRef]
- Kim, S.H.; Csaky, K.G.; Wang, N.S.; Lutz, R.J. Drug Elimination Kinetics Following Subconjunctival Injection Using Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Pharm. Res. 2008, 25, 512–520. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Noonan, J.S. Permeability of Cornea, Sclera, and Conjunctiva: A Literature Analysis for Drug Delivery to the Eye. J. Pharm. Sci. 1998, 87, 1479–1488. [Google Scholar] [CrossRef]
- Modi, Y.S.; Tanchon, C.; Ehlers, J.P. Comparative Safety and Tolerability of Anti-VEGF Therapy in Age-Related Macular Degeneration. Drug Saf. 2015, 38, 279–293. [Google Scholar] [CrossRef]
- Massa, H.; Nagar, A.M.; Vergados, A.; Dadoukis, P.; Patra, S.; Panos, G.D. Intravitreal Fluocinolone Acetonide Implant (ILUVIEN®) for Diabetic Macular Oedema: A Literature Review. J. Int. Med. Res. 2019, 47, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Adelman, R.A.; Parnes, A.J.; Bopp, S.; Saad Othman, I.; Ducournau, D. Strategy for the Management of Macular Edema in Retinal Vein Occlusion: The European VitreoRetinal Society Macular Edema Study. BioMed Res. Int. 2015, 2015, 870987. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, X.; Jiao, L.; Tang, L. Intravitreal Corticosteroids for Diabetic Macular Edema: A Network Meta-Analysis of Randomized Controlled Trials. Eye Vis. 2021, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.M.; Hariprasad, S.M.; Ciulla, T.A. Treatment Burden in Neovascular AMD:Visual Acuity Outcomes Are Associated with Anti-VEGF Injection Frequency. Ophthalmic Surg. Lasers Imaging Retina 2017, 48, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Bracha, P.; Pollack, J.; Williams, D.F. Real-World Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema in the United States. Ophthalmol. Retina 2018, 2, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.; Pollack, J.S.; Williams, D.F. Visual Acuity Outcomes and Anti-VEGF Therapy Intensity in Macular Oedema Due to Retinal Vein Occlusion: A Real-World Analysis of 15 613 Patient Eyes. Br. J. Ophthalmol. 2021, 105, 1696–1704. [Google Scholar] [CrossRef]
- Chin, H.-S.; Park, T.-S.; Moon, Y.-S.; Oh, J.-H. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 2005, 25, 556–560. [Google Scholar] [CrossRef]
- Multicenter Uveitis Steroid Treatment Trial Research Group. The Multicenter Uveitis Steroid Treatment (MUST) Trial: Rationale, Design and Baseline Characteristics. Am. J. Ophthalmol. 2010, 149, 550–561.e10. [Google Scholar] [CrossRef]
- Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group. Association between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs. Systemic Anti-Inflammatory Therapy and Visual Acuity at 7 Years Among Patients with Intermediate, Posterior, or Panuveitis. JAMA 2017, 317, 1993–2005. [Google Scholar] [CrossRef]
- Holekamp, N.M.; Campochiaro, P.A.; Chang, M.A.; Miller, D.; Pieramici, D.; Adamis, A.P.; Brittain, C.; Evans, E.; Kaufman, D.; Maass, K.F.; et al. Archway Randomized Phase 3 Trial of the Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2022, 129, 295–307. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Genentech: Press Releases|Friday, 28 January 2022. Available online: https://www.gene.com/media/press-releases/14943/2022-01-28/fda-approves-genentechs-vabysmo-the-firs (accessed on 6 July 2023).
- Genentech: Press Releases|Friday, 22 October 2021. Available online: https://www.gene.com/media/press-releases/14935/2021-10-22/fda-approves-genentechs-susvimo-a-first- (accessed on 6 July 2023).
- Pitkänen, L.; Ruponen, M.; Nieminen, J.; Urtti, A. Vitreous Is a Barrier in Nonviral Gene Transfer by Cationic Lipids and Polymers. Pharm. Res. 2003, 20, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Peeters, L.; Sanders, N.N.; Braeckmans, K.; Boussery, K.; Van de Voorde, J.; De Smedt, S.C.; Demeester, J. Vitreous: A Barrier to Nonviral Ocular Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3553–3561. [Google Scholar] [CrossRef] [PubMed]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-Mediated Retinal Transduction from the Vitreous. Mol. Ther. J. Am. Soc. Gene Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef]
- Kansara, V.; Muya, L.; Wan, C.-R.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-HRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- REGENXBIO Announces Additional Positive Interim Phase I/IIa and Long-Term Follow-Up Data of RGX-314 for the Treatment of Wet AMD|Regenxbio Inc. Available online: https://ir.regenxbio.com/news-releases/news-release-details/regenxbio-announces-additional-positive-interim-phase-iiia-and/ (accessed on 6 July 2023).
- Jung, J.H.; Chae, J.J.; Prausnitz, M.R. Targeting Drug Delivery within the Suprachoroidal Space. Drug Discov. Today 2019, 24, 1654–1659. [Google Scholar] [CrossRef]
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of Suprachoroidal Drug Delivery: From Benchtop to Clinical Investigation in Ocular Therapies. Expert Opin. Drug Deliv. 2021, 18, 777–788. [Google Scholar] [CrossRef]
- Peden, M.C.; Min, J.; Meyers, C.; Lukowski, Z.; Li, Q.; Boye, S.L.; Levine, M.; Hauswirth, W.W.; Ratnakaram, R.; Dawson, W.; et al. Ab-Externo AAV-Mediated Gene Delivery to the Suprachoroidal Space Using a 250 Micron Flexible Microcatheter. PLoS ONE 2011, 6, e17140. [Google Scholar] [CrossRef]
- Tyagi, P.; Barros, M.; Stansbury, J.W.; Kompella, U.B. Light Activated, In Situ Forming Gel for Sustained Suprachoroidal Delivery of Bevacizumab. Mol. Pharm. 2013, 10, 2858–2867. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and Pharmacodynamics of Suprachoroidal Injection of Triamcinolone Acetonide as a Controlled Ocular Drug Release Model. J. Control. Release 2015, 203, 109–117. [Google Scholar] [CrossRef]
- Kansara, V.S.; Hancock, S.E.; Muya, L.W.; Ciulla, T.A. Suprachoroidal Delivery Enables Targeting, Localization and Durability of Small Molecule Suspensions. J. Control. Release 2022, 349, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.; Ciulla, T. Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated with Noninfectious Uveitis: An In-Depth Look at Efficacy and Safety. Am. J. Manag. Care 2023, 29, S19–S28. [Google Scholar] [PubMed]
- Fisher, N.; Yoo, J.; Hancock, S.E.; Andino, R.V. A Novel Technique to Characterize Key Fluid Mechanic Properties of the SC Injection Procedure in an In Vivo Model; Clear Association for Research in Vision and Ophthalmology Annual Meeting: Atlanta, GA, USA, 2018. [Google Scholar]
- Fisher, N.; Wan, C. Suprachoroidal Delivery with the SCS Microinjector™: Characterization of Operational Forces. Investig. Ophthalmol. Vis. Sci. 2020, 61, 24. [Google Scholar]
- Moisseiev, E.; Loewenstein, A.; Yiu, G. The Suprachoroidal Space: From Potential Space to a Space with Potential. Clin. Ophthalmol. 2016, 10, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Liu, J.; Li, X.; Ma, Q.; Shen, M.; Cheng, L. Real-Time Monitoring of Suprachoroidal Space (SCS) Following SCS Injection Using Ultra-High Resolution Optical Coherence Tomography in Guinea Pig Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3623–3634. [Google Scholar] [CrossRef]
- Savinainen, A.; Grossniklaus, H.; Kang, S.; Rasmussen, C.; Bentley, E.; Krakova, Y.; Struble, C.B.; Rich, C. Ocular Distribution and Efficacy after Suprachoroidal Injection of AU-011 for Treatment of Ocular Melanoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3615. [Google Scholar]
- Chiang, B.; Venugopal, N.; Grossniklaus, H.E.; Jung, J.H.; Edelhauser, H.F.; Prausnitz, M.R. Thickness and Closure Kinetics of the Suprachoroidal Space Following Microneedle Injection of Liquid Formulations. Investig. Ophthalmol. Vis. Sci. 2017, 58, 555–564. [Google Scholar] [CrossRef]
- Jung, J.H.; Park, S.; Chae, J.J.; Prausnitz, M.R. Collagenase Injection into the Suprachoroidal Space of the Eye to Expand Drug Delivery Coverage and Increase Posterior Drug Targeting. Exp. Eye Res. 2019, 189, 107824. [Google Scholar] [CrossRef]
- Nork, T.M.; Katz, A.W.; Kim, C.B.Y.; Rasmussen, C.A.; Wabers, H.D.; Bentley, E.; Struble, C.B.; Savinainen, A. Distribution of Aqueous Solutions Injected Suprachoroidally (SC) in Rabbits. Investig. Ophthalmol. Vis. Sci. 2020, 61, 320. [Google Scholar]
- Oshika, T.; Bissen-Miyajima, H.; Fujita, Y.; Hayashi, K.; Mano, T.; Miyata, K.; Sugita, T.; Taira, Y. Prospective Randomized Comparison of DisCoVisc and Healon5 in Phacoemulsification and Intraocular Lens Implantation. Eye 2010, 24, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Oh, K.H.; Edelhauser, H.F.; Prausnitz, M.R. Formulation to Target Delivery to the Ciliary Body and Choroid via the Suprachoroidal Space of the Eye Using Microneedles. Eur. J. Pharm. Biopharm. 2015, 95, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, S.S.; Chung, H.; Hejri, A.; Prausnitz, M.R. Six-Month Sustained Delivery of Anti-VEGF from in-Situ Forming Hydrogel in the Suprachoroidal Space. J. Control. Release 2022, 352, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Wang, K.; Ethier, C.R.; Prausnitz, M.R. Clearance Kinetics and Clearance Routes of Molecules From the Suprachoroidal Space After Microneedle Injection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 545–554. [Google Scholar] [CrossRef]
- Mustafa, M.B.; Tipton, D.L.; Barkley, M.D.; Russo, P.S.; Blum, F.D. Dye Diffusion in Isotropic and Liquid-Crystalline Aqueous (Hydroxypropyl)Cellulose. Macromolecules 1993, 26, 370–378. [Google Scholar] [CrossRef]
- FITC-Dextran Fluorescein Isothiocyanate Dextran. 2011. Available online: https://www.semanticscholar.org/paper/FITC-Dextran-Fluorescein-isothiocyanate-dextran/01c8e9539524bc604f6b45a6bbb06dd03507d82f (accessed on 30 May 2023).
- Hackett, S.F.; Fu, J.; Kim, Y.C.; Tsujinaka, H.; Shen, J.; Lima E Silva, R.; Khan, M.; Hafiz, Z.; Wang, T.; Shin, M.; et al. Sustained Delivery of Acriflavine from the Suprachoroidal Space Provides Long Term Suppression of Choroidal Neovascularization. Biomaterials 2020, 243, 119935. [Google Scholar] [CrossRef]
- Edwards, A.; Prausnitz, M.R. Fiber Matrix Model of Sclera and Corneal Stroma for Drug Delivery to the Eye. AIChE J. 1998, 44, 214–225. [Google Scholar] [CrossRef]
- Jung, J.H.; Desit, P.; Prausnitz, M.R. Targeted Drug Delivery in the Suprachoroidal Space by Swollen Hydrogel Pushing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2069–2079. [Google Scholar] [CrossRef]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular Drug Delivery Targeted by Iontophoresis in the Suprachoroidal Space Using a Microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- Touchard, E.; Berdugo, M.; Bigey, P.; El Sanharawi, M.; Savoldelli, M.; Naud, M.-C.; Jeanny, J.-C.; Behar-Cohen, F. Suprachoroidal Electrotransfer: A Nonviral Gene Delivery Method to Transfect the Choroid and the Retina without Detaching the Retina. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Verhoeven, R.S.; Burke, B.; Struble, C.B.; Patel, S.R. Intraocular Distribution and Targeting of Triamcinolone Acetonide Suspension Administered Into the Suprachoroidal Space. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5259. [Google Scholar]
- Muya, L.; Kansara, V.; Cavet, M.E.; Ciulla, T. Suprachoroidal Injection of Triamcinolone Acetonide Suspension: Ocular Pharmacokinetics and Distribution in Rabbits Demonstrates High and Durable Levels in the Chorioretina. J. Ocul. Pharmacol. Ther. 2022, 38, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Phadke, A.; Kansara, V.; Boyer, D.; Rivera, J.; Marlor, C.; Podos, S.; Wiles, J.; McElheny, R.; Ciulla, T.A.; et al. Ocular Pharmacokinetics and Safety of Suprachoroidal A01017, Small Molecule Complement Inhibitor, Injectable Suspension in Rabbits. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3694. [Google Scholar]
- Kaiser, P.K.; Ciulla, T.; Kansara, V. Suprachoroidal CLS-AX (Axitinib Injectable Suspension), as a Potential Long-Acting Therapy for Neovascular Age-Related Macular Degeneration (NAMD). Investig. Ophthalmol. Vis. Sci. 2020, 61, 3977. [Google Scholar]
- Henry, C.R.; Shah, M.; Barakat, M.R.; Dayani, P.; Wang, R.C.; Khurana, R.N.; Rifkin, L.; Yeh, S.; Hall, C.; Ciulla, T. Suprachoroidal CLS-TA for Non-Infectious Uveitis: An Open-Label, Safety Trial (AZALEA). Br. J. Ophthalmol. 2022, 106, 802–806. [Google Scholar] [CrossRef]
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020, 127, 948–955. [Google Scholar] [CrossRef]
- Henry, C.R.; Kapik, B.; Ciulla, T.A. Suprachoroidal Triamcinolone Acetonide Injectable Suspension for Macular Edema Associated with Uveitis: Visual and Anatomic Outcomes by Age. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3206-A0432. [Google Scholar]
- Merrill, P.T.; Henry, C.R.; Nguyen, Q.D.; Reddy, A.; Kapik, B.; Ciulla, T.A. Suprachoroidal CLS-TA with and without Systemic Corticosteroid and/or Steroid-Sparing Therapy: A Post-Hoc Analysis of the Phase 3 PEACHTREE Clinical Trial. Ocul. Immunol. Inflamm. 2021, 1–8. [Google Scholar] [CrossRef]
- Khurana, R.N.; Merrill, P.; Yeh, S.; Suhler, E.; Barakat, M.R.; Uchiyama, E.; Henry, C.R.; Shah, M.; Wang, R.C.; Kapik, B.; et al. Extension Study of the Safety and Efficacy of CLS-TA for Treatment of Macular Oedema Associated with Non-Infectious Uveitis (MAGNOLIA). Br. J. Ophthalmol. 2022, 106, 1139–1144. [Google Scholar] [CrossRef]
- Yeh, S.; Kurup, S.K.; Wang, R.C.; Foster, C.S.; Noronha, G.; Nguyen, Q.D.; Do, D.V.; DOGWOOD Study Team. Suprachoroidal injection of triamcinolone acetonide, CLS-TA, for macular edema due to noninfectious uveitis: A Randomized, Phase 2 Study (DOGWOOD). Retina 2019, 39, 1880–1888. [Google Scholar] [CrossRef] [PubMed]
- Hanif, J.; Iqbal, K.; Perveen, F.; Arif, A.; Iqbal, R.N.; Jameel, F.; Hanif, K.; Seemab, A.; Khan, A.Y.; Ahmed, M. Safety and Efficacy of Suprachoroidal Injection of Triamcinolone in Treating Macular Edema Secondary to Noninfectious Uveitis. Cureus 2021, 13, e20038. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.S.; Rehman, R.; Nazir, S.; Sharif, N.; Chaudhari, M.Z.; Saleem, S. Visual Outcome after Suprachoroidal Injection of Triamcinolone Acetate in Cystoid Macular Edema of Different Pathology. Pak. J. Med. Health Sci. 2022, 16, 164. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Qiu, W. Intravitreal Anti-Vascular Endothelial Growth Factor, Laser Photocoagulation, or Combined Therapy for Diabetic Macular Edema: A Systematic Review and Network Meta-Analysis. Front. Endocrinol. 2023, 14, 1096105. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Khurana, R.N.; Lampen, S.I.R.; Noronha, G.; Brown, D.M.; Ou, W.C.; Sadda, S.R.; HULK Study Group. Suprachoroidal Triamcinolone Acetonide for Diabetic Macular Edema: The HULK Trial. Ophthalmol. Retina 2018, 2, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retina 2021, 5, 60–70. [Google Scholar] [CrossRef]
- Fazel, F.; Malekahmadi, M.; Feizi, A.; Oliya, B.; Tavakoli, M.; Fazel, M. Suprachoroidal Injection of Triamcinolone Acetonide plus Intravitreal Bevacizumab in Diabetic Macular Edema: A Randomized Pilot Trial. BMC Ophthalmol. 2023, 23, 40. [Google Scholar] [CrossRef]
- Shahid, M.H.; Rashid, F.; Tauqeer, S.; Ali, R.; Farooq, M.T.; Aleem, N. Comparison of Suprachoroidal Triamcinolone Injection with Intravitreal Bevacizumab Vs Intravitreal Bevacizumab Only in Treatment of Refractory Diabetic Macular Edema. Pak. J. Med. Health Sci. 2022, 16, 301. [Google Scholar] [CrossRef]
- Anwar, F.; Khan, A.A.; Majhu, T.M.; Javaid, R.M.M.; Ghaffar, M.T.; Bokhari, M.H. Comparison of Suprachoroidal Injection of Triamcinolone Acetonide Versus Intravitreal Bevacizumab in Primary Diabetic Macular Odema. Pak. J. Med. Health Sci. 2022, 16, 304. [Google Scholar] [CrossRef]
- Zakaria, Y.G.; Salman, A.G.; Said, A.M.A.; Abdelatif, M.K. Suprachoroidal versus Intravitreal Triamcinolone Acetonide for the Treatment of Diabetic Macular Edema. Clin. Ophthalmol. 2022, 16, 733–746. [Google Scholar] [CrossRef]
- Shaikh, K.; Ahmed, N.; Kazi, U.; Zia, A.; Aziz, M.Z. Comparison between Suprachoroidal Triamcinolone and Intravitreal Triamcinolone Acetonide in Patients of Resistant Diabetic Macular Edema. Pak. J. Ophthalmol. 2023, 39. [Google Scholar] [CrossRef]
- Marashi, A.; Zazo, A. Suprachoroidal Injection of Triamcinolone Acetonide Using a Custom-Made Needle to Treat Diabetic Macular Edema Post Pars Plana Vitrectomy: A Case Series. J. Int. Med. Res. 2022, 50, 03000605221089807. [Google Scholar] [CrossRef] [PubMed]
- Nawar, A.E. Effectiveness of Suprachoroidal Injection of Triamcinolone Acetonide in Resistant Diabetic Macular Edema Using a Modified Microneedle. Clin. Ophthalmol. 2022, 16, 3821–3831. [Google Scholar] [CrossRef] [PubMed]
- Ateeq, A.; Majid, S.; Memon, N.A.; Hayat, N.; Somroo, A.Q.; Fattah, A. Suprachoroidal Injection of Triamcinolone Acetonide for Management of Resistant Diabetic Macular Oedema. JPMA J. Pak. Med. Assoc. 2023, 73, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Chandni, A.; Bhatti, S.R.; Khawer, F.; Ijaz, M.; Rafique, D.; Choudhry, T.A. To Determine the Efficacy of Suprachoroidal Triamcinolone Injection for the Treatment of Refractory Diabetic Macular Edema. Pak. J. Med. Health Sci. 2022, 16, 400. [Google Scholar] [CrossRef]
- Tayyab, H.; Ahmed, C.N.; Sadiq, M.A.A. Efficacy and Safety of Suprachoroidal Triamcinolone Acetonide in Cases of Resistant Diabetic Macular Edema. Pak. J. Med. Sci. 2020, 36, 42–47. [Google Scholar] [CrossRef]
- Ahmad, Y.; Memon, S.U.H.; Bhatti, S.A.; Nawaz, F.; Ahmad, W.; Saleem, M. Efficacy and Safety of Suprachoroidal Triamcinolone Acetonide in Cases of Resistant Diabetic Macular Edema. Pak. J. Med. Health Sci. 2023, 17, 324. [Google Scholar] [CrossRef]
- Butt, S.; Iqbal, R.; Siddiq, S.; Waheed, K.; Javed, T. Efficacy of Suprachoroidal Triamcinolone Acetonide Injection in the Treatment of Resistant Diabetic Macular Edema. Biol. Clin. Sci. Res. J. 2023, 2023, 202. [Google Scholar] [CrossRef]
- Tharwat, E.; Ahmed, R.E.H.; Eltantawy, B.; Ezzeldin, E.R.; Elgazzar, A.F. Suprachoroidal Triamcinolone versus Posterior Subtenon Triamcinolone Either Alone or Formulated in the Management of Diabetic Macular Edema. Int. Ophthalmol. 2022. [Google Scholar] [CrossRef]
- Dhoot, D.S. Suprachoroidal Delivery of RGX-314 for Diabetic Retinopathy: The Phase II ALTITUDE™ Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1152. [Google Scholar]
- Veiga Reis, F.; Dalgalarrondo, P.; da Silva Tavares Neto, J.E.; Wendeborn Rodrigues, M.; Scott, I.U.; Jorge, R. Combined Intravitreal Dexamethasone and Bevacizumab Injection for the Treatment of Persistent Diabetic Macular Edema (DexaBe Study): A Phase I Clinical Study. Int. J. Retina Vitr. 2023, 9, 13. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Wykoff, C.C.; Brown, D.M.; Boyer, D.S.; Barakat, M.; Taraborelli, D.; Noronha, G.; Tanzanite Study Group. Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmol. Retina 2018, 2, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Clearside Biomedical’s TANZANITE Extension Study in Patients with Macular Edema Associated with Retinal Vein Occlusion Presented at the 40th Annual Macula Society Meeting|Clearside Biomedical, Inc.-IR Site. Available online: https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedicals-tanzanite-extension-study-patients-macular (accessed on 15 July 2023).
- Clearside Biomedical, Inc. SAPPHIRE: A Randomized, Masked, Controlled Trial to Study the Safety and Efficacy of Suprachoroidal CLS-TA in Conjunction with Intravitreal Aflibercept in Subjects with Retinal Vein Occlusion; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2021. [Google Scholar]
- Clearside Biomedical, Inc. A Randomized, Masked, Controlled Trial to Study the Safety and Efficacy of Suprachoroidal CLS-TA in Combination with an Intravitreal Anti-VEGF Agent in Subjects with Retinal Vein Occlusion; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2021. [Google Scholar]
- Nawar, A.E. Modified Microneedle for Suprachoroidal Injection of Triamcinolone Acetonide Combined with Intravitreal Injection of Ranibizumab in Branch Retinal Vein Occlusion Patients. Clin. Ophthalmol. 2022, 16, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.M.; Azmeh, A.M.; Alhalabi, N.M. Suprachoroidal Triamcinolone Acetonide for the Treatment of Macular Edema Associated with Retinal Vein Occlusion: A Pilot Study. BMC Ophthalmol. 2023, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Muslim, I.; Chaudhry, N.; Javed, R.M.M. Effect of Supra-Choroidal Triamcinolone Injection on Best-Corrected Visual Acuity and Central Retinal Thickness in Patients with Macular Edema Secondary to Retinal Vein Occlusion. Pak. J. Ophthalmol. 2022, 38. [Google Scholar] [CrossRef]
- Rizzo, S.; Ebert, F.G.; Bartolo, E.D.; Barca, F.; Cresti, F.; Augustin, C.; Augustin, A. Suprachoroidal drug Infusion for the Treatment of Severe Subfoveal Hard Exudates. Retina 2012, 32, 776. [Google Scholar] [CrossRef]
- Abdelshafy, A. One Year Results for Suprachoroidal Triamcinolone Acetonide Injection in Various Retinal Diseases; Benha University: Benha, Egypt, 2022. [Google Scholar]
- Abdelshafy Tabl, A.; Tawfik Soliman, T.; Anany Elsayed, M.; Abdelshafy Tabl, M. A Randomized Trial Comparing Suprachoroidal and Intravitreal Injection of Triamcinolone Acetonide in Refractory Diabetic Macular Edema Due to Epiretinal Membrane. J. Ophthalmol. 2022, 2022, 7947710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-D.; Che, D.-Y.; Zhu, D.-Q. A Simple Technique for Suprachoroidal Space Injection of Triamcinolone Acetonide in Treatment of Macular Edema. Int. J. Ophthalmol. 2022, 15, 2017–2021. [Google Scholar] [CrossRef]
- Oli, A.; Waikar, S. Modified Inexpensive Needle for Suprachoroidal Triamcinolone Acetonide Injections in Pseudophakic Cystoid Macular Edema. Indian J. Ophthalmol. 2021, 69, 765–767. [Google Scholar] [CrossRef]
- Marashi, A.; Zazo, A. A Manually Made Needle for Treating Pseudophakic Cystoid Macular Edema by Injecting Triamcinolone Acetonide in the Suprachoroidal Space: A Case Report. Am. J. Ophthalmol. Case Rep. 2022, 25, 101254. [Google Scholar] [CrossRef]
- Abdelshafy, A. Suprachoroidal Triamcinolone Acetonide Injection in Two Chorioretinal Diseases: One Year Results; Benha University: Benha, Egypt, 2022. [Google Scholar]
- Martorana, G.; Levine, M.; Peden, M.; Boye, S.; Lukowski, Z.; Min, J.; Meyers, C.; Boye, S.; Sherwood, M. Comparison of Suprachoroidal Delivery via an Ab-Externo Approach with the ITrack Microcatheter versus Vitrectomy and Subretinal Delivery for 3 Different AAV Serotypes for Gene Transfer to the Retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1931. [Google Scholar]
- Woodard, K.T.; Vance, M.; Gilger, B.; Samulski, R.J.; Hirsch, M. 544. Comparison of AAV Serotype2 Transduction by Various Delivery Routes to the Mouse Eye. Mol. Ther. 2016, 24, S217–S218. [Google Scholar] [CrossRef]
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; Silva, R.L.E.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-Vectored Suprachoroidal Gene Transfer Produces Widespread Ocular Transgene Expression. J. Clin. Investig. 2019, 129, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Shen, J.; Hackett, S.; Campochiaro, P.A. Transgene Expression in RPE and Retina after Suprachoroidal Delivery of AAV Vectors. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4490. [Google Scholar]
- Kansara, V.; Yoo, J.; Cooper, M.J.; Laird, O.S.; Taraborelli, D.; Moen, R.; Noronha, G. Suprachoroidally Delivered Non-Viral DNA Nanoparticles Transfect Chorioretinal Cells in Non-Human Primates and Rabbits. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2909. [Google Scholar]
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host Immune Responses after Suprachoroidal Delivery of AAV8 in Nonhuman Primate Eyes. Hum. Gene Ther. 2021, 32, 682–693. [Google Scholar] [CrossRef]
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal Gene Transfer with Nonviral Nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, X.; Chen, Y.; Chen, D. Interaction Between Mesenchymal Stem Cells and Retinal Degenerative Microenvironment. Front. Neurosci. 2021, 14, 617377. [Google Scholar] [CrossRef]
- Habot-Wilner, Z.; Noronha, G.; Wykoff, C.C. Suprachoroidally Injected Pharmacological Agents for the Treatment of Chorio-Retinal Diseases: A Targeted Approach. Acta Ophthalmol. 2019, 97, 460–472. [Google Scholar] [CrossRef]
- Öner, A.; Kahraman, N.S. Does Stem Cell Implantation Have an Effect on Severity of Retinitis Pigmentosa: Evaluation with a Classification System? Open J. Ophthalmol. 2021, 11, 36–48. [Google Scholar] [CrossRef]
- Oner, A.; Kahraman, N.S. Suprachoroidal Umbilical Cord Derived Mesenchymal Stem Cell Implantation for the Treatment of Retinitis Pigmentosa in Pediatric Patients. Am. J. Stem Cell Res. 2023, 5, 1–7. [Google Scholar]
- Özkan, B. Suprachoroidal Spheroidal Mesenchymal Stem Cell Implantation in Retinitis Pigmentosa: Clinical Results of 6 Months Follow-Up. 2023. Available online: clinicaltrials.gov (accessed on 6 July 2023).
- Marashi, A.; Baba, M.; Zazo, A. Managing Solar Retinopathy with Suprachoroidal Triamcinolone Acetonide Injection in a Young Girl: A Case Report. J. Med. Case Rep. 2021, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Gohil, R.; Crosby-Nwaobi, R.; Forbes, A.; Burton, B.; Hykin, P.; Sivaprasad, S. Caregiver Burden in Patients Receiving Ranibizumab Therapy for Neovascular Age Related Macular Degeneration. PLoS ONE 2015, 10, e0129361. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; George, P.P.; Hoon, H.B.; Han, L.T.; Onn, Y.S. Burden of Wet Age-Related Macular Degeneration and Its Economic Implications in Singapore in the Year 2030. Ophthalmic Epidemiol. 2016, 23, 232–237. [Google Scholar] [CrossRef]
- Sampat, K.M.; Garg, S.J. Complications of Intravitreal Injections. Curr. Opin. Ophthalmol. 2010, 21, 178–183. [Google Scholar] [CrossRef]
- Fallico, M.; Maugeri, A.; Lotery, A.; Longo, A.; Bonfiglio, V.; Russo, A.; Avitabile, T.; Pulvirenti, A.; Furino, C.; Cennamo, G.; et al. Intravitreal Anti-Vascular Endothelial Growth Factors, Panretinal Photocoagulation and Combined Treatment for Proliferative Diabetic Retinopathy: A Systematic Review and Network Meta-Analysis. Acta Ophthalmol. 2021, 99, e795–e805. [Google Scholar] [CrossRef]
- Tran, J.; Craven, C.; Wabner, K.; Schmit, J.; Matter, B.; Kompella, U.; Grossniklaus, H.E.; Olsen, T.W. A Pharmacodynamic Analysis of Choroidal Neovascularization in a Porcine Model Using Three Targeted Drugs. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3732–3740. [Google Scholar] [CrossRef]
- Mansoor, S.; Patel, S.R.; Tas, C.; Pacha-Ravi, R.; Kompella, U.B.; Edelhauser, H.F.; Prausnitz, M.R. Pharmacokinetics and Biodistribution of Bevacizumab Following Suprachoroidal Injection into the Rabbit Eye Using a Microneedle. Investig. Ophthalmol. Vis. Sci. 2012, 53, 498. [Google Scholar]
- Olsen, T.W.; Feng, X.; Wabner, K.; Csaky, K.; Pambuccian, S.; Cameron, J.D. Pharmacokinetics of Pars Plana Intravitreal Injections versus Microcannula Suprachoroidal Injections of Bevacizumab in a Porcine Model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4749–4756. [Google Scholar] [CrossRef]
- Zeng, M.; Shen, J.; Liu, Y.; Lu, L.Y.; Ding, K.; Fortmann, S.D.; Khan, M.; Wang, J.; Hackett, S.F.; Semenza, G.L.; et al. The HIF-1 Antagonist Acriflavine: Visualization in Retina and Suppression of Ocular Neovascularization. J. Mol. Med. 2017, 95, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.W.; Sanderson, S.; Feng, X.; Hubbard, W.C. Porcine Sclera: Thickness and Surface Area. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2529–2532. [Google Scholar]
- AbbVie. A Phase 2, Randomized, Dose-Escalation, Ranibizumab-Controlled Study to Evaluate the Efficacy, Safety, and Tolerability of RGX-314 Gene Therapy Delivered Via One or Two Suprachoroidal Space (SCS) Injections in Participants with Neovascular Age-Related Macular Degeneration (NAMD) (AAVIATE); AbbVie: Wellington, New Zealand, 2023. [Google Scholar]
- Khanani, A.M. Suprachoroidal Delivery of RGX-314 Gene Therapy for Neovascular AMD: The Phase II AAVIATE™ Study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1497. [Google Scholar]
- Tetz, M.; Rizzo, S.; Augustin, A.J. Safety of Submacular Suprachoroidal Drug Administration via a Microcatheter: Retrospective Analysis of European Treatment Results. Ophthalmologica 2012, 227, 183–189. [Google Scholar] [CrossRef]
- Morales-Canton, V.; Fromow-Guerra, J.; Salinas Longoria, S.; Romero Vera, R.; Widmann, M.; Patel, S.; Yerxa, B. Suprachoroidal Microinjection of Bevacizumab Is Well Tolerated in Human Patients. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3299. [Google Scholar]
- Patel, S.R.; Kissner, J.; Farjo, R.; Zarnitsyn, V.; Noronha, G. Efficacy of Suprachoroidal Aflibercept in a Laser Induced Choroidal Neovascularization Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 286. [Google Scholar]
- Kissner, J.; Patel, S.R.; Prusakiewicz, J.J.; Alton, D.; Bikzhanova, G.; Geisler, L.; Burke, B.; Noronha, G. Pharmacokinetics including Ocular Distribution Characteristics of Suprachoroidally Administered CLS011A in Rabbits Could Be Beneficial for a Wet AMD Therapeutic Candidate; ASSOC Research Vision Ophthalmology Inc.: Seattle, WA, USA, 2016. [Google Scholar]
- Clearside Biomedical, Inc. OASIS: Open-Label, Dose-Escalation, Phase 1/2a Study of the Safety and Tolerability of Suprachoroidally Administered CLS-AX Following Intravitreal Anti-VEGF Therapy in Subjects with Neovascular Age-Related Macular Degeneration; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2022. [Google Scholar]
- Clearside Biomedical, Inc. Extension Study to Evaluate the Long-Term Outcomes of Subjects Following CLS-AX Administration for Age-Related Macular Degeneration in the CLS-AX CLS1002-101 Study; Clearside Biomedical, Inc.: Alpharetta, GA, USA, 2023. [Google Scholar]
- Shanghai BDgene Co., Ltd. A Safety and Efficacy Study of VEGFA-Targeting Gene Therapy to Treat Refractory Retinal and Choroidal Neovascularization Diseases; Shanghai BDgene Co., Ltd.: Shanghai, China, 2022. [Google Scholar]
- Datta, D.; Khan, P.; Khan, L.; Singh, A. Role of Suprachoroidal Anti-VEGF Injections in Recalcitrant Serous Pigment Epithelium Detachment. Ophthalmol. Res. Int. J. 2023, 18, 1–10. [Google Scholar] [CrossRef]
- Kohli, G.M.; Shenoy, P.; Halim, D.; Nigam, S.; Shetty, S.; Talwar, D.; Sen, A. Safety and Efficacy of Suprachoroidal Triamcinolone Acetonide for the Management of Serous Choroidal Detachment Prior to Rhegmatogenous Retinal Detachment Surgery: A Pilot Study. Indian J. Ophthalmol. 2022, 70, 1302–1306. [Google Scholar] [CrossRef]
- Tabl, A.A.; Elsayed, M.A.; Tabl, M.A. Suprachoroidal Triamcinolone Acetonide Injection: A Novel Therapy for Serous Retinal Detachment Due to Vogt-Koyanagi Harada Disease. Eur. J. Ophthalmol. 2022, 32, 3482–3488. [Google Scholar] [CrossRef]
- Gao, Y.; An, J.; Zeng, Z.; Lou, H.; Wu, G.; Lu, F. Suprachoroidal injection of sodium hyaluronate in the treatment of 12 patients with rhegmatogenous retinal detachment. Chin. J. Ocul. Fundus Dis. 2019, 6, 274–278. [Google Scholar]
- Mittl, R.N.; Tiwari, R. Suprachoroidal Injection of Sodium Hyaluronate as an “internal” Buckling Procedure. Ophthalmic Res. 1987, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Suprachoroidal Air Injection for Detached Retina. Br. J. Ophthalmol. 1952, 36, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.A.; Do, D.; Noronha, G.; Kissner, J.M.; Srivastava, S.K.; Nguyen, Q.D. Suprachoroidal Corticosteroid Administration: A Novel Route for Local Treatment of Noninfectious Uveitis. Transl. Vis. Sci. Technol. 2016, 5, 14. [Google Scholar] [CrossRef]
- Noronha, G.; Blackwell, K.; Gilger, B.C.; Kissner, J.; Patel, S.R.; Walsh, K.T. Evaluation of Suprachoroidal CLS-TA and Oral Prednisone in a Porcine Model of Uveitis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3110. [Google Scholar]
- Gilger, B.C.; Abarca, E.M.; Salmon, J.H.; Patel, S. Treatment of Acute Posterior Uveitis in a Porcine Model by Injection of Triamcinolone Acetonide into the Suprachoroidal Space Using Microneedles. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Carvalho, R.; Mundwiler, K.; Meschter, C.; Verhoeven, R. Evaluation of Suprachoroidal Microinjection of Triamcinolone Acetonide in a Model of Panuveitis in Albino Rabbits. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2927. [Google Scholar]
- Ghate, D.; Edelhauser, H.F. Barriers to Glaucoma Drug Delivery. J. Glaucoma 2008, 17, 147–156. [Google Scholar] [CrossRef]
- Konstas, A.G.; Stewart, W.C.; Topouzis, F.; Tersis, I.; Holmes, K.T.; Stangos, N.T. Brimonidine 0.2% given Two or Three Times Daily versus Timolol Maleate 0.5% in Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 2001, 131, 729–733. [Google Scholar] [CrossRef]
- Robin, A.L.; Novack, G.D.; Covert, D.W.; Crockett, R.S.; Marcic, T.S. Adherence in Glaucoma: Objective Measurements of Once-Daily and Adjunctive Medication Use. Am. J. Ophthalmol. 2007, 144, 533–540. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Glynn, R.J.; Monane, M.; Everitt, D.E.; Gilden, D.; Smith, N.; Avorn, J. Treatment for Glaucoma: Adherence by the Elderly. Am. J. Public Health 1993, 83, 711–716. [Google Scholar] [CrossRef]
- Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Delivery of Antiglaucoma Drugs to the Supraciliary Space Using Microneedles. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7387–7397. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Kim, Y.C.; Doty, A.C.; Grossniklaus, H.E.; Schwendeman, S.P.; Prausnitz, M.R. Sustained Reduction of Intraocular Pressure by Supraciliary Delivery of Brimonidine-Loaded Poly(Lactic Acid) Microspheres for the Treatment of Glaucoma. J. Control. Release 2016, 228, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Einmahl, S.; Savoldelli, M.; D’Hermies, F.; Tabatabay, C.; Gurny, R.; Behar-Cohen, F. Evaluation of a Novel Biomaterial in the Suprachoroidal Space of the Rabbit Eye. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1533–1539. [Google Scholar]
- Chae, J.J.; Jung, J.H.; Zhu, W.; Gerberich, B.G.; Bahrani Fard, M.R.; Grossniklaus, H.E.; Ethier, C.R.; Prausnitz, M.R. Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel. Adv. Sci. 2021, 8, 2001908. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; He, B.; Yu, B.; Yang, J.; Xing, X.; Liu, W. Suprachoroidal Injection of Polyzwitterion Hydrogel for Treating Glaucoma. Biomater. Adv. 2022, 142, 213162. [Google Scholar] [CrossRef]
- Savinainen, A.; Grossniklaus, H.E.; King, S.; Wicks, J.; Rich, C.C. Ocular Distribution and Exposure of AU-011 after Suprachoroidal or Intravitreal Administration in an Orthotopic Rabbit Model of Human Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 2861. [Google Scholar]
- Kang, S.J.; Patel, S.R.; Berezovsky, D.E.; Zhang, Q.; Yang, H.; Grossniklaus, H.E. Suprachoroidal Injection of Microspheres with Microcatheter in a Rabbit Model of Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1459. [Google Scholar]
- Mruthyunjaya, P.; Schefler, A.C.; Kim, I.K.; Bergstrom, C.; Demirci, H.; Tsai, T.; Bhavsar, A.R.; Capone, A.; Marr, B.; McCannel, T.A.; et al. A Phase 1b/2 Open-Label Clinical Trial to Evaluate the Safety and Efficacy of AU-011 for the Treatment of Choroidal Melanoma. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4025. [Google Scholar]
- Demirci, H.; Narvekar, A.; Murray, C.; Rich, C. 842P A Phase II Trial of AU-011, an Investigational, Virus-like Drug Conjugate (VDC) for the Treatment of Primary Indeterminate Lesions and Small Choroidal Melanoma (IL/CM) Using Suprachoroidal Administration. Ann. Oncol. 2022, 33, S934. [Google Scholar] [CrossRef]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and Adverse Events of Plaque Brachytherapy for Ocular Melanoma. J. Contemp. Brachyther. 2019, 11, 392–397. [Google Scholar] [CrossRef]
- Aura Biosciences. A Phase 2 Open-Label, Ascending Single and Repeat Dose Escalation Trial of Belzupacap Sarotalocan (AU-011) via Suprachoroidal Administration in Subjects with Primary Indeterminate Lesions and Small Choroidal Melanoma; Aura Biosciences: Boston, MA, USA, 2023. [Google Scholar]
- Venkatesh, P.; Takkar, B. Suprachoroidal Injection of Biological Agents May Have a Potential Role in the Prevention of Progression and Complications in High Myopia. Med. Hypotheses 2017, 107, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Morgan, I.G.; Ohno-Matsui, K.; Saw, S.-M. Myopia. Lancet 2012, 379, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.I.; Fiscella, R. Topical Nonsteroidal Anti-Inflammatory Drugs for Ophthalmic Use: A Safety Review. Drug Saf. 2002, 25, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Kadam, R.S.; Lee, V.H.L. Recent Advances in Ophthalmic Drug Delivery. Ther. Deliv. 2010, 1, 435–456. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Lu, Q.; Zeng, H.; Liu, S.; Yue, Y.; Cheng, H.; Liu, Y.; Xue, M. Pharmacokinetic Comparison of Ketorolac after Intracameral, Intravitreal, and Suprachoroidal Administration in Rabbits. Retina 2012, 32, 2158–2164. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ma, Y.; Liu, K.; Wang, M. Suprachoroidal Injection of Ketorolac Tromethamine Does Not Cause Retinal Damage. Neural Regen. Res. 2012, 7, 2770–2777. [Google Scholar] [CrossRef]
- Maldonado, R.M.; Vianna, R.N.G.; Cardoso, G.P.; de Magalhães, A.V.; Burnier, M.N. Intravitreal Injection of Commercially Available Ketorolac Tromethamine in Eyes with Diabetic Macular Edema Refractory to Laser Photocoagulation. Curr. Eye Res. 2011, 36, 768–773. [Google Scholar] [CrossRef]
- Giannantonio, C.; Papacci, P.; Purcaro, V.; Cota, F.; Tesfagabir, M.G.; Molle, F.; Lepore, D.; Baldascino, A.; Romagnoli, C. Effectiveness of Ketorolac Tromethamine in Prevention of Severe Retinopathy of Prematurity. J. Pediatr. Ophthalmol. Strabismus 2011, 48, 247–251. [Google Scholar] [CrossRef]
- Margalit, E.; Boysen, J.L.; Zastrocky, J.P.; Katz, A. Use of Intraocular Ketorolac Tromethamine for the Treatment of Chronic Cystoid Macular Edema. Can. J. Ophthalmol. J. Can. Ophtalmol. 2010, 45, 409–410. [Google Scholar] [CrossRef]
- Kim, S.J.; Toma, H.S. Inhibition of Choroidal Neovascularization by Intravitreal Ketorolac. Arch. Ophthalmol. 2010, 128, 596–600. [Google Scholar] [CrossRef]
- Willoughby, A.S.; Vuong, V.S.; Cunefare, D.; Farsiu, S.; Noronha, G.; Danis, R.P.; Yiu, G. Choroidal Changes after Suprachoroidal Injection of Triamcinolone in Eyes with Macular Edema Secondary to Retinal Vein Occlusion. Am. J. Ophthalmol. 2018, 186, 144–151. [Google Scholar] [CrossRef]
- Tian, B.; Xie, J.; Su, W.; Sun, S.; Su, Q.; Gao, G.; Lin, H. Suprachoroidal Injections of AAV for Retinal Gene Delivery in Mouse. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1177. [Google Scholar]
- Wiley, L.A.; Boyce, T.M.; Meyering, E.E.; Ochoa, D.; Sheehan, K.M.; Stone, E.M.; Mullins, R.F.; Tucker, B.A.; Han, I.C. The Degree of Adeno-Associated Virus-Induced Retinal Inflammation Varies Based on Serotype and Route of Delivery: Intravitreal, Subretinal, or Suprachoroidal. Hum. Gene Ther. 2023, 34, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Han, I.C.; Cheng, J.L.; Burnight, E.R.; Ralston, C.L.; Fick, J.L.; Thomsen, G.J.; Tovar, E.F.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; et al. Retinal Tropism and Transduction of Adeno-Associated Virus Varies by Serotype and Route of Delivery (Intravitreal, Subretinal, or Suprachoroidal) in Rats. Hum. Gene Ther. 2020, 31, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, N.S.; Gonen, Z.B.; Sevim, D.G.; Oner, A. First Year Results of Suprachoroidal Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Degenerative Macular Diseases. Int. J. Stem Cells 2021, 14, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Muya, L.; Kansara, V.; Ciulla, T. Pharmacokinetics and Ocular Tolerability of Suprachoroidal CLS-AX (Axitinib Injectable Suspension) in Rabbits. Investig. Ophthalmol. Vis. Sci. 2020, 61, 4925. [Google Scholar]

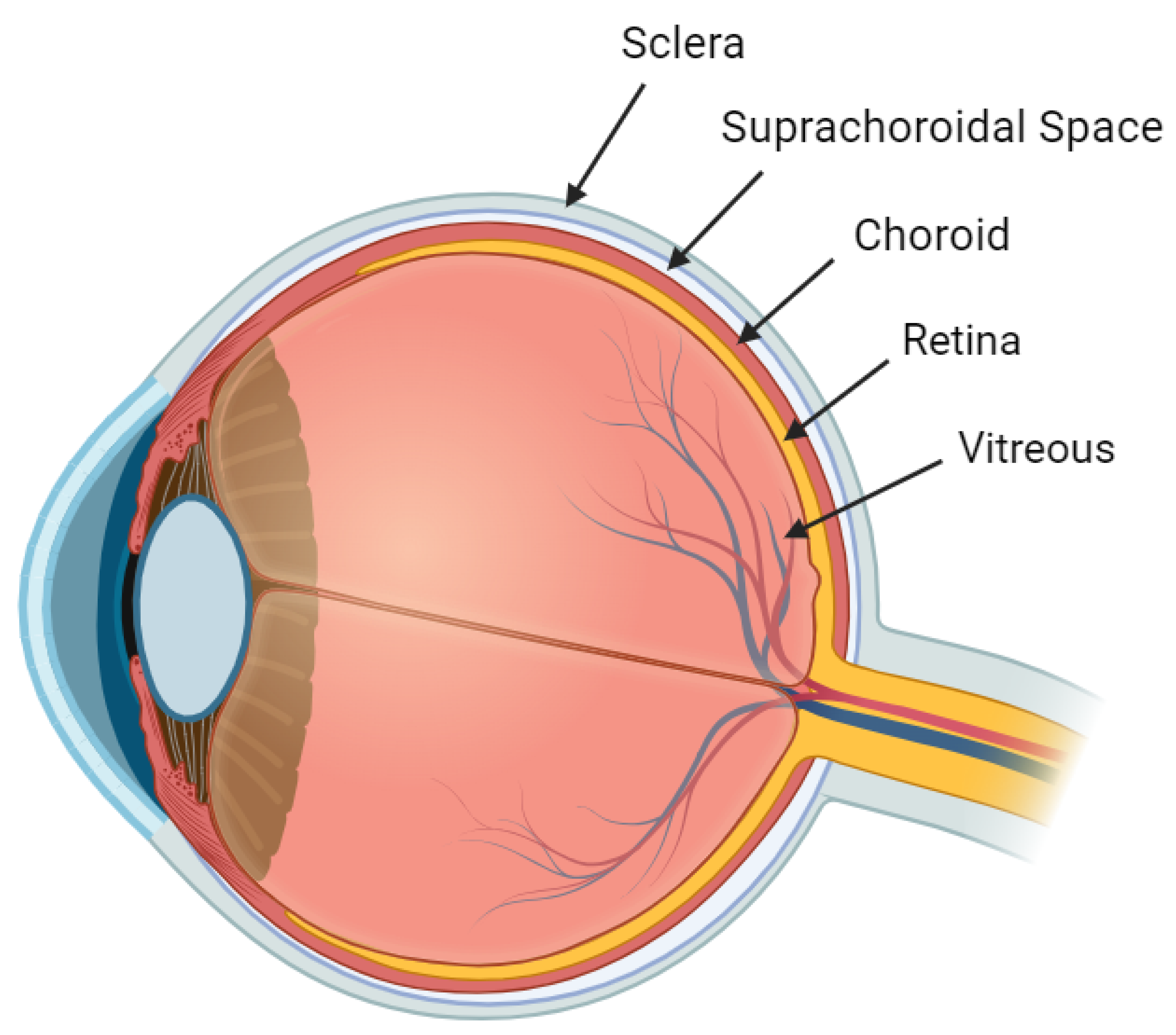
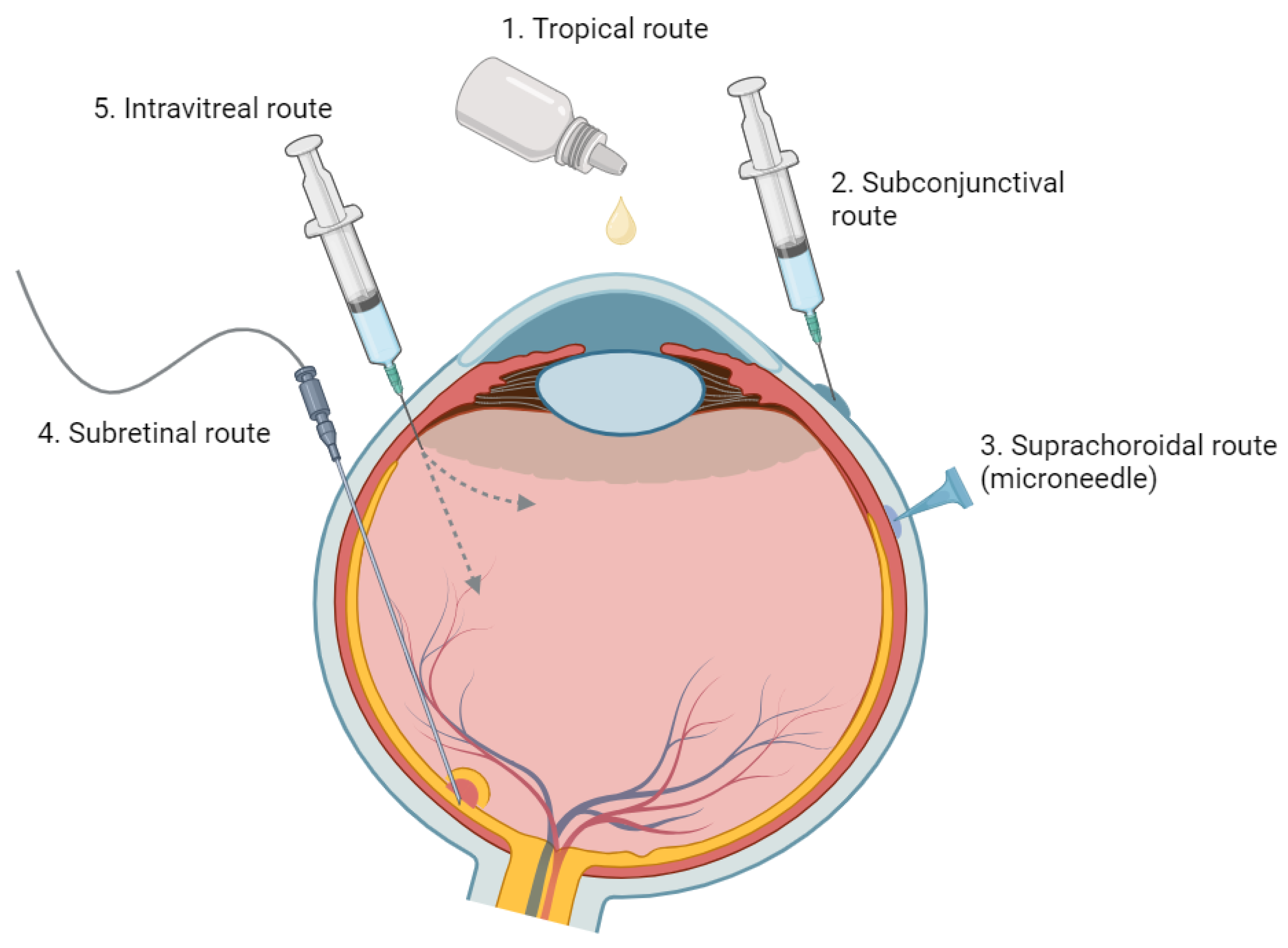

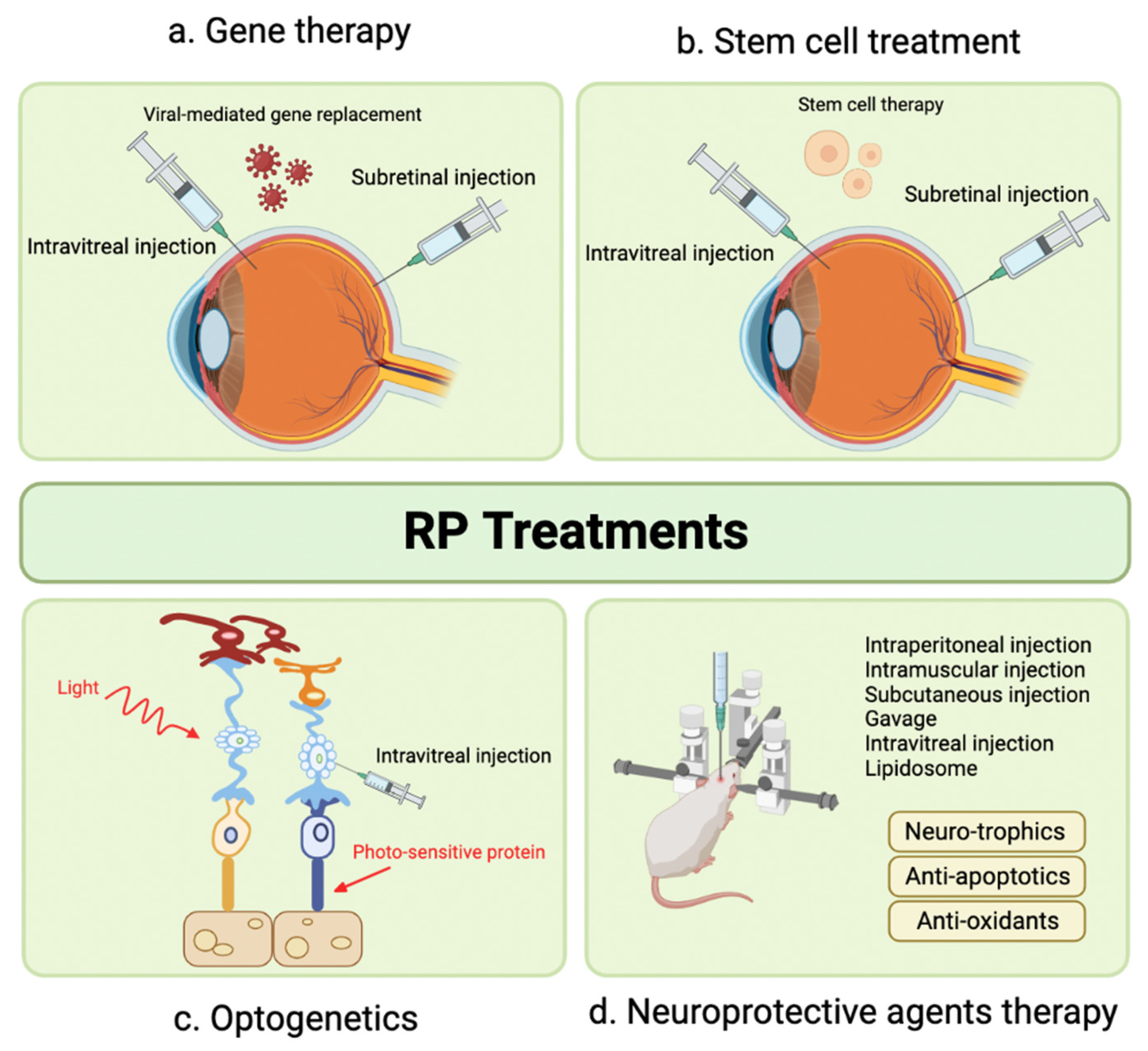
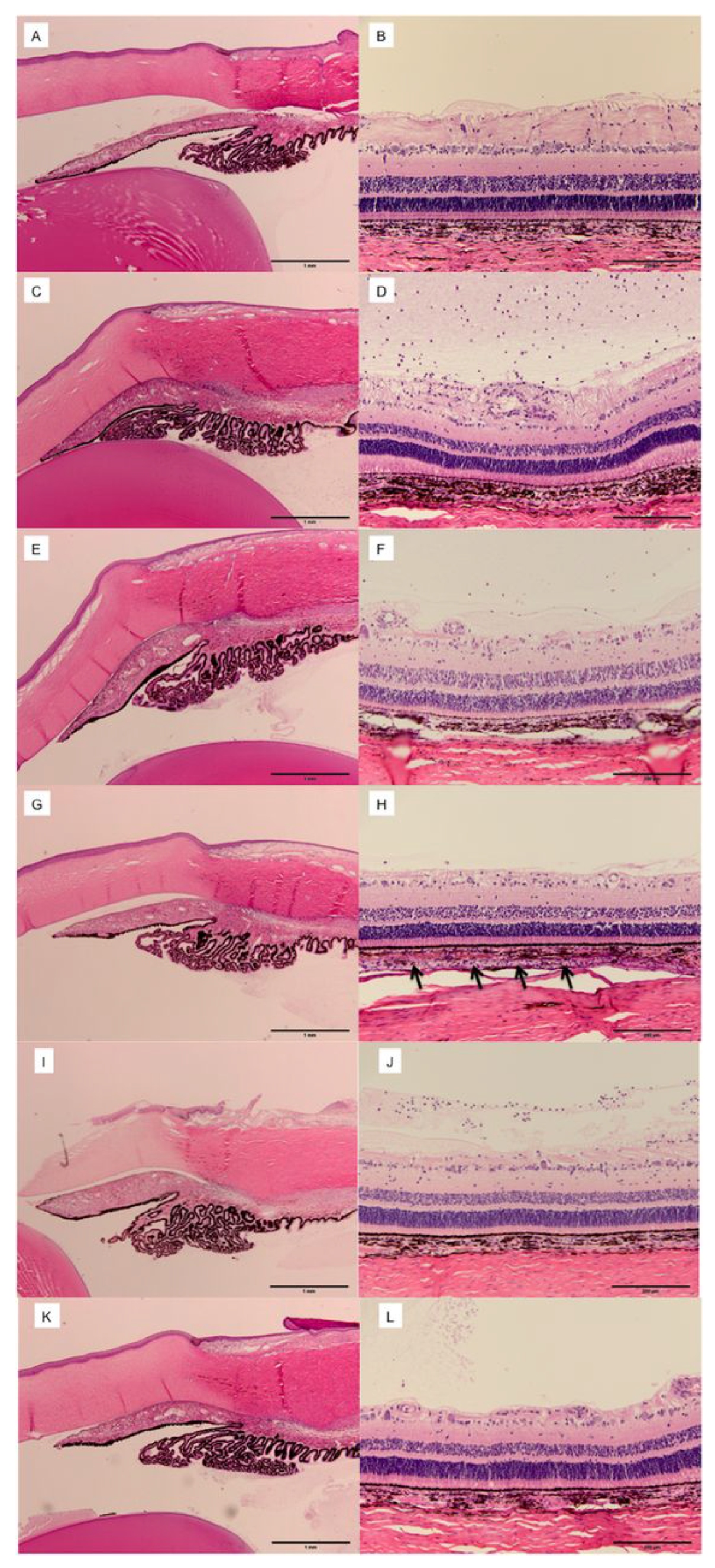

| Injection Method | Advantages | Disadvantages |
|---|---|---|
| Topical Eye Drops [22] | Prevalent, well-known method | Low bioavailability to posterior segment tissues |
| Non-invasive method for ocular drug delivery | Short duration of action, requiring frequent administration | |
| Relies on patient’s compliance | ||
| Local complications (ocular surface irritation, cataracts, ocular hypertension, periocular aesthetic issues) | ||
| Systemic Drug Administration | Noninvasive and potentially patient-preferred | High doses often required due to reduced accessibility to targeted ocular tissues |
| Usable as standalone or in combination with topical delivery | Potential systemic side effects due to high dosage, necessitating safety and toxicity considerations | |
| Effective bioavailability is challenging due to blood–ocular barriers | ||
| Intravitreal Injection [23] | Office-based, outpatient procedure | Requires frequent in-office visits |
| High bioavailability (bypass corneal and blood–retinal barriers) | Potential for severe complications (Endophthalmitis, retinal detachment, vitreous hemorrhage) | |
| Fewer systemic side effects compared to oral or IV administration | Local complications (increased IOP, cataract formation) | |
| Rapid therapeutic onset | Possible post-injection floaters | |
| Systemic absorption and side effects can still occur | ||
| Subretinal Injection [24] | Targeted treatment for the RPE and outer retina | Invasive procedure, requires vitrectomy |
| Reduced immune reactions for gene therapy using viral vectors (due to injection in an immune-privileged site) | Limited distribution of injectate within subretinal space; effects confined to injection site |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.Y.; Fujioka, J.K.; Gholamian, T.; Zaharia, M.; Tran, S.D. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals 2023, 16, 1241. https://doi.org/10.3390/ph16091241
Wu KY, Fujioka JK, Gholamian T, Zaharia M, Tran SD. Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals. 2023; 16(9):1241. https://doi.org/10.3390/ph16091241
Chicago/Turabian StyleWu, Kevin Y., Jamie K. Fujioka, Tara Gholamian, Marian Zaharia, and Simon D. Tran. 2023. "Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery" Pharmaceuticals 16, no. 9: 1241. https://doi.org/10.3390/ph16091241
APA StyleWu, K. Y., Fujioka, J. K., Gholamian, T., Zaharia, M., & Tran, S. D. (2023). Suprachoroidal Injection: A Novel Approach for Targeted Drug Delivery. Pharmaceuticals, 16(9), 1241. https://doi.org/10.3390/ph16091241









