The Anti-Thrombotic Effects of PCSK9 Inhibitors
Abstract
1. Introduction
2. Methods
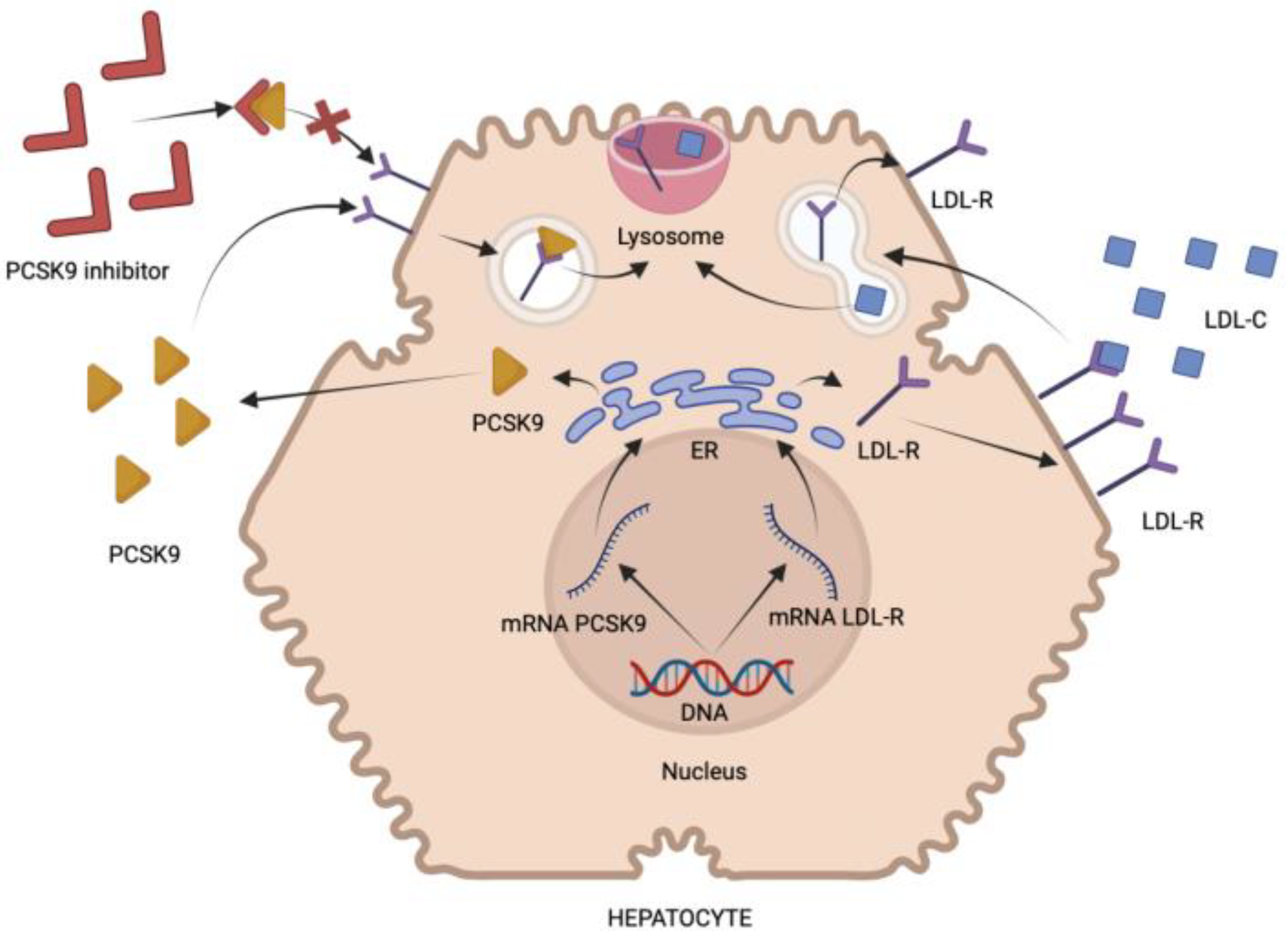
3. Proprotein Convertase Subtilis Kexin-9
4. Platelet Activation Markers and PCSK9 Inhibitors
5. Endothelial Dysfunction Markers and PCSK9 Inhibitors
6. Inflammation Markers and PCSK9 Inhibitors
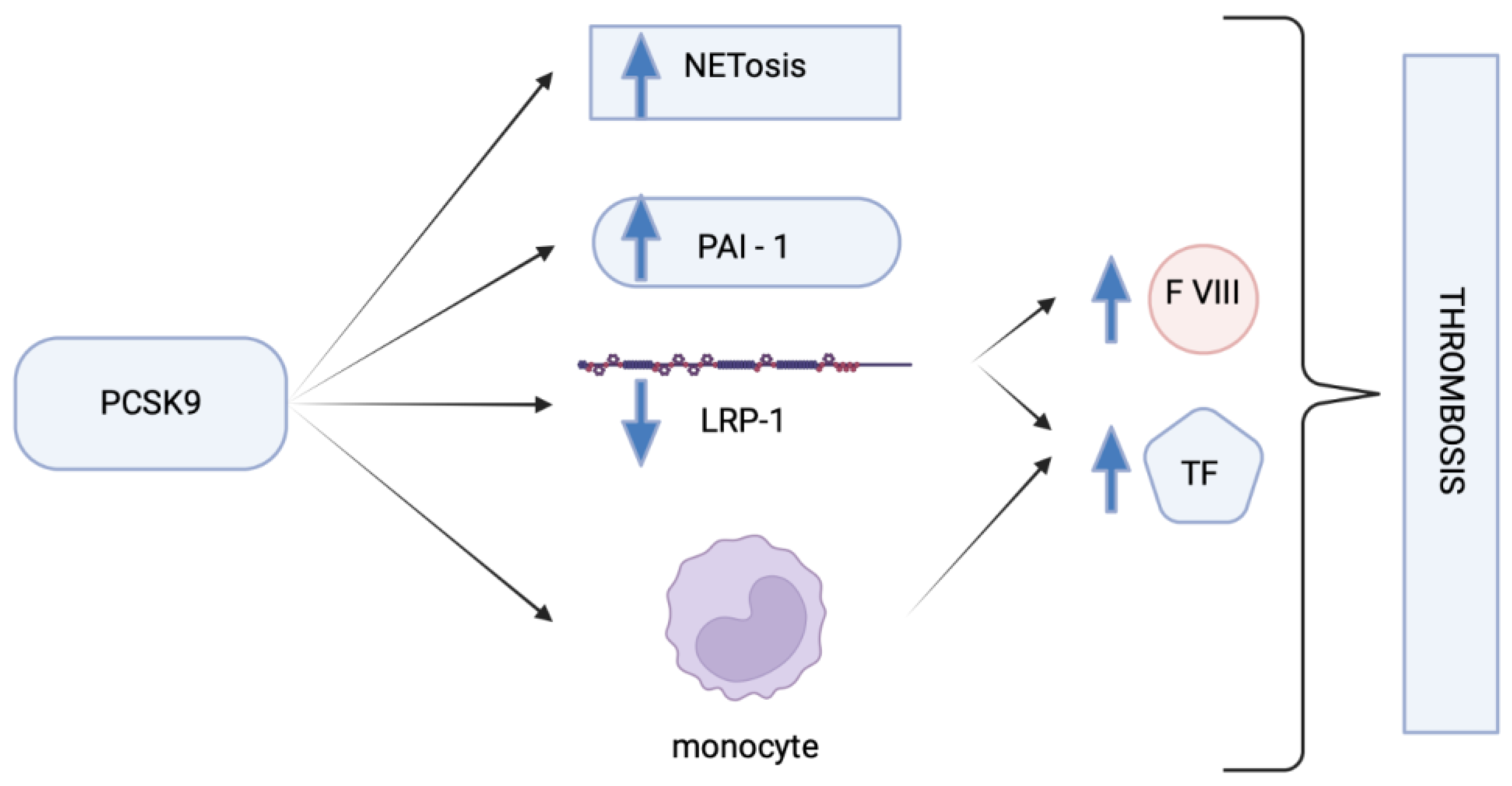
7. Coagulation Factors and PCSK9 Inhibitors
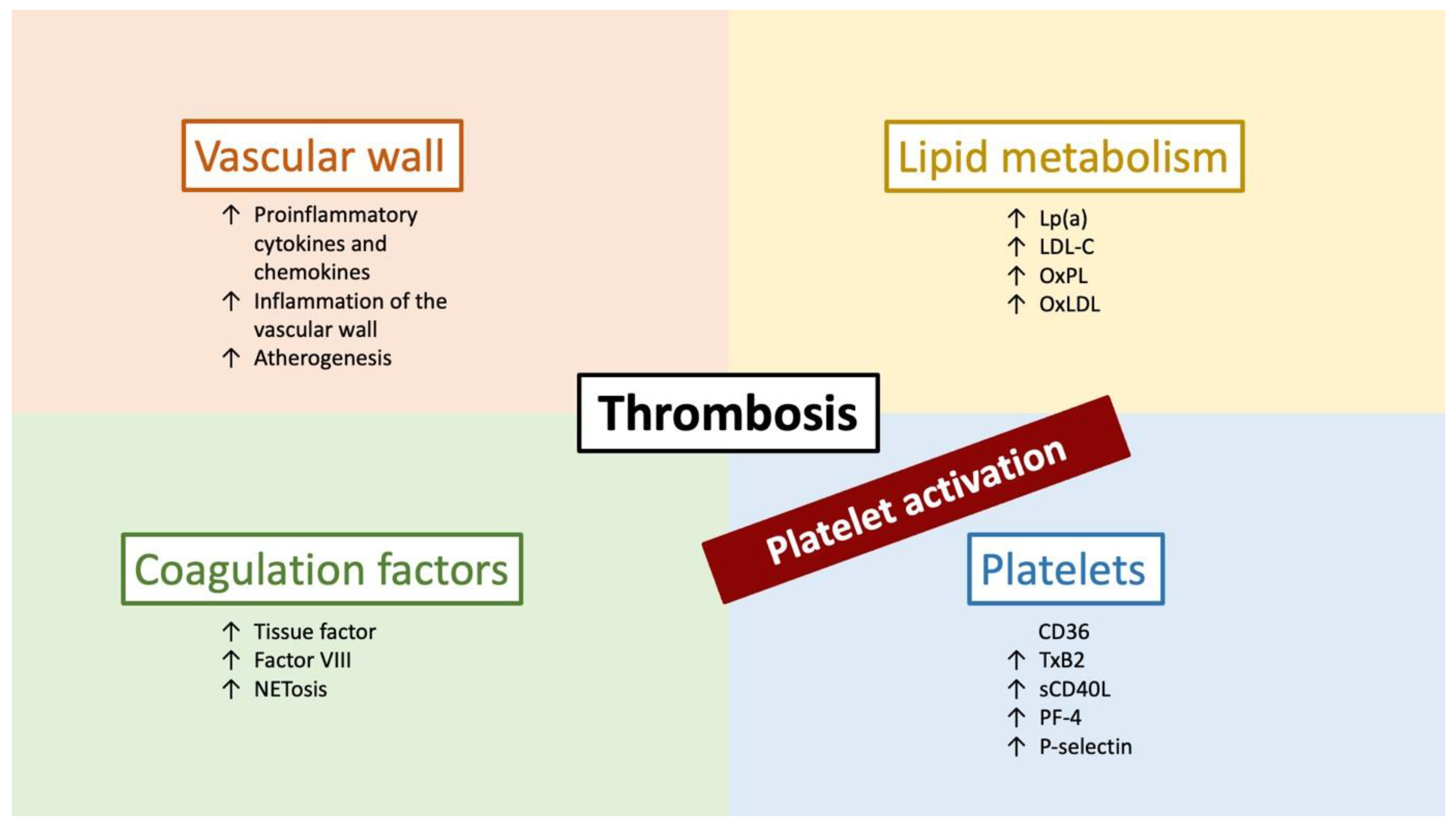
8. Other Possible Implications of PCSK9 Inhibitors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, J.L.; Brown, M.S. Regulation of low-density lipoprotein receptors: Implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation 1987, 76, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Badimon, J.J.; Rosenson, R.S. Beginning to understand high-density lipoproteins. Endocrinol. Metab. Clin. North Am. 2014, 43, 913–947. [Google Scholar] [CrossRef]
- Elshourbagy, N.A.; Meyers, H.V.; Abdel-Meguid, S.S. Cholesterol: The Good, the Bad, and the Ugly—Therapeutic Targets for the Treatment of Dyslipidemia. Med. Princ. Pract. 2013, 23, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Cybulska, B.; Kłosiewicz-Latoszek, L.; Penson, P.E.; Banach, M. What do we know about the role of lipoprotein(a) in atherogenesis 57 years after its discovery? Prog. Cardiovasc. Dis. 2020, 63, 219–227. [Google Scholar] [CrossRef]
- Kiechl, S.; Willeit, J. The Mysteries of Lipoprotein(a) and Cardiovascular Disease Revisited. J. Am. Coll. Cardiol. 2010, 55, 2168–2170. [Google Scholar] [CrossRef]
- Segrest, J.P.; Jones, M.K.; De Loof, H.; Dashti, N. Structure of apolipoprotein B-100 in low density lipoproteins. J. Lipid Res. 2001, 42, 1346–1367. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Anderson, R.G.; Basu, S.K.; Goldstein, J.L. Recycling of cell-surface receptors: Observations from the LDL receptor system. Cold Spring Harb. Symp. Quant. Biol. 1982, 46 Pt 2, 713–721. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Zhang, D.W.; Garuti, R.; Tang, W.J.; Cohen, J.C.; Hobbs, H.H. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 13045–13050. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Breslow, J.L. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc. Natl. Acad. Sci. USA 2004, 101, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, W.; Lu, M.; Wang, Y. Association Between Circulating Proprotein Convertase Subtilisin/Kexin Type 9 and Major Adverse Cardiovascular Events, Stroke, and All-Cause Mortality: Systemic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 617249. [Google Scholar] [CrossRef]
- Ugovšek, S.; Šebeštjen, M. Non-Lipid Effects of PCSK9 Monoclonal Antibodies on Vessel Wall. J. Clin. Med. 2022, 11, 3625. [Google Scholar] [CrossRef]
- Zulkapli, R.; Yusof, M.Y.P.M.; Abd Muid, S.; Wang, S.M.; Firus Khan, A.Y.; Nawawi, H. A Systematic Review on Attenuation of PCSK9 in Relation to Atherogenesis Biomarkers Associated with Natural Products or Plant Bioactive Compounds in In Vitro Studies: A Critique on the Quality and Imprecision of Studies. Int. J. Environ. Res. Public Health 2022, 19, 12878. [Google Scholar] [CrossRef]
- Seidah, N.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, R.; Basta, G.; Neglia, D.; De Caterina, R.; Del Turco, S.; Caselli, C. PCSK9 and atherosclerosis: Looking beyond LDL regulation. Eur. J. Clin. Investig. 2021, 51, e13459. [Google Scholar] [CrossRef]
- Leblond, F.; Seidah, N.G.; Précourt, L.-P.; Delvin, E.; Dominguez, M.; Levy, E. Regulation of the proprotein convertase subtilisin/kexin type 9 in intestinal epithelial cells. Am. J. Physiol. Liver Physiol. 2009, 296, G805–G815. [Google Scholar] [CrossRef][Green Version]
- Luquero, A.; Badimon, L.; Borrell-Pages, M. PCSK9 Functions in Atherosclerosis Are Not Limited to Plasmatic LDL-Cholesterol Regulation. Front. Cardiovasc. Med. 2021, 8, 639727. [Google Scholar] [CrossRef]
- Rohrbach, S.; Li, L.; Novoyatleva, T.; Niemann, B.; Knapp, F.; Molenda, N.; Schulz, R. Impact of PCSK9 on CTRP9-Induced Metabolic Effects in Adult Rat Cardiomyocytes. Front. Physiol. 2021, 12, 593862. [Google Scholar] [CrossRef]
- Langhi, C.; Le May, C.; Gmyr, V.; Vandewalle, B.; Kerr-Conte, J.; Krempf, M.; Pattou, F.; Costet, P.; Cariou, B. PCSK9 is expressed in pancreatic δ-cells and does not alter insulin secretion. Biochem. Biophys. Res. Commun. 2009, 390, 1288–1293. [Google Scholar] [CrossRef]
- O’Connell, E.M.; Lohoff, F.W. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in the Brain and Relevance for Neuropsychiatric Disorders. Front. Neurosci. 2020, 14, 609. [Google Scholar] [CrossRef]
- Artunc, F. Kidney-derived PCSK9—A new driver of hyperlipidemia in nephrotic syndrome? Kidney Int. 2020, 98, 1393–1395. [Google Scholar] [CrossRef]
- Persson, L.; Cao, G.; Ståhle, L.; Sjöberg, B.G.; Troutt, J.S.; Konrad, R.J.; Gälman, C.; Wallén, H.; Eriksson, M.; Hafström, I.; et al. Circulating Proprotein Convertase Subtilisin Kexin Type 9 Has a Diurnal Rhythm Synchronous with Cholesterol Synthesis and Is Reduced by Fasting in Humans. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Ruscica, M.; Coggi, D.; Bonomi, A.; Amato, M.; Frigerio, B.; Sansaro, D.; Ravani, A.; Veglia, F.; Capra, N.; et al. Sex-specific predictors of PCSK9 levels in a European population: The IMPROVE study. Atherosclerosis 2020, 309, 39–46. [Google Scholar] [CrossRef]
- Galema-Boers, A.M.H.; Mulder, J.W.C.M.; Steward, K.; Roeters van Lennep, J.E. Sex differences in efficacy and safety of PCSK9 monoclonal antibodies: A real-world registry. Atherosclerosis 2023, S0021-9150(23)00115-6. [Google Scholar] [CrossRef]
- Cui, Q.; Ju, X.; Yang, T.; Zhang, M.; Tang, W.; Chen, Q.; Hu, Y.; Haas, J.V.; Troutt, J.S.; Pickard, R.T.; et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis 2010, 213, 632–636. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; Döring, Y.; van der Vorst, E.P.C. PCSK9: A Multi-Faceted Protein That Is Involved in Cardiovascular Biology. Biomedicines 2021, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, Y.G.L.; Day, R.; Seidah, N.G. The M2 Module of the Cys-His-rich Domain (CHRD) of PCSK9 Protein Is Needed for the Extracellular Low-density Lipoprotein Receptor (LDLR) Degradation Pathway. J. Biol. Chem. 2012, 287, 43492–43501. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, G.; Afonyushkin, T.; Binder, C.J. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J. Thromb. Haemost. 2018, 16, 418–428. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Navarese, E.P.; Kolodziejczak, M.; Winter, M.-P.; Alimohammadi, A.; Lang, I.M.; Buffon, A.; Lip, G.Y.; Siller-Matula, J.M. Association of PCSK9 with platelet reactivity in patients with acute coronary syndrome treated with prasugrel or ticagrelor: The PCSK9-REACT study. Int. J. Cardiol. 2017, 227, 644–649. [Google Scholar] [CrossRef]
- Qi, Z.; Hu, L.; Zhang, J.; Yang, W.; Liu, X.; Jia, D.; Yao, Z.; Chang, L.; Pan, G.; Zhong, H.; et al. PCSK9 (Proprotein Convertase Subtilisin/Kexin 9) Enhances Platelet Activation, Thrombosis, and Myocardial Infarct Expansion by Binding to Platelet CD36. Circulation. 2021, 143, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Baratta, F.; Castellani, V.; Bartimoccia, S.; Nocella, C.; D’Erasmo, L.; Cocomello, N.; Barale, C.; Scicali, R.; Di Pino, A.; et al. Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Reduce Platelet Activation Modulating ox-LDL Pathways. Int. J. Mol. Sci. 2021, 22, 7193. [Google Scholar] [CrossRef]
- Cammisotto, V.; Pastori, D.; Nocella, C.; Bartimoccia, S.; Castellani, V.; Marchese, C.; Scavalli, A.S.; Ettorre, E.; Viceconte, N.; Violi, F.; et al. PCSK9 Regulates Nox2-Mediated Platelet Activation via CD36 Receptor in Patients with Atrial Fibrillation. Antioxidants 2020, 9, 296. [Google Scholar] [CrossRef]
- El-Seweidy, M.M.; Sarhan Amin, R.; Husseini Atteia, H.; El-Zeiky, R.R.; Al-Gabri, N.A. Dyslipidemia induced inflammatory status, platelet activation and endothelial dysfunction in rabbits: Protective role of 10-Dehydrogingerdione. Biomed. Pharmacother. 2019, 110, 456–464. [Google Scholar] [CrossRef]
- Demers, A.; Samami, S.; Lauzier, B.; Des Rosiers, C.; Ngo Sock, E.; Ong, H.; Mayer, G. PCSK9 Induces CD36 Degradation and Affects Long-Chain Fatty Acid Uptake and Triglyceride Metabolism in Adipocytes and in Mouse Liver. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2517–2525. [Google Scholar] [CrossRef]
- Gaudet, D.; Kereiakes, D.J.; McKenney, J.M.; Roth, E.M.; Hanotin, C.; Gipe, D.; Du, Y.; Ferrand, A.-C.; Ginsberg, H.N.; Stein, E.A. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am. J. Cardiol. 2014, 114, 711–715. [Google Scholar] [CrossRef]
- Marston, N.A.; Gurmu, Y.; Melloni, G.E.M.; Bonaca, M.; Gencer, B.; Sever, P.S.; Pedersen, T.R.; Keech, A.C.; Roselli, C.; Lubitz, S.A.; et al. The Effect of PCSK9 (Proprotein Convertase Subtilisin/Kexin Type 9) Inhibition on the Risk of Venous Thromboembolism. Circulation 2020, 141, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Oleaga, C.; Eren, M.; Amaral, A.P.; Shang, M.; Lux, E.; Khan, S.S.; Shah, S.J.; Omura, Y.; Pamir, N.; et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci. Rep. 2021, 11, 430. [Google Scholar] [CrossRef]
- Puccini, M.; Landmesser, U.; Rauch, U. Pleiotropic Effects of PCSK9: Focus on Thrombosis and Haemostasis. Metabolites 2022, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Kei, A.; Rizos, C.V.; Elisaf, M.S. Effects of PCSK9 Inhibitors on Other than Low-Density Lipoprotein Cholesterol Lipid Variables. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 3–12. [Google Scholar] [CrossRef]
- Pęczek, P.; Leśniewski, M.; Mazurek, T.; Szarpak, L.; Filipiak, K.J.; Gąsecka, A. Antiplatelet Effects of PCSK9 Inhibitors in Primary Hypercholesterolemia. Life 2021, 11, 466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Wang, Y.; Xu, Z.; Li, G. Investigation of highly expressed PCSK9 in atherosclerotic plaques and ox-LDL-induced endothelial cell apoptosis. Mol. Med. Rep. 2017, 16, 1817–1825. [Google Scholar] [CrossRef]
- Paciullo, F.; Momi, S.; Gresele, P. PCSK9 in Haemostasis and Thrombosis: Possible Pleiotropic Effects of PCSK9 Inhibitors in Cardiovascular Prevention. Thromb. Haemost. 2019, 119, 359–367. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Petersen-Uribe, A.; Kremser, M.; Rohlfing, A.K.; Castor, T.; Kolb, K.; Dicenta, V.; Emschermann, F.; Li, B.; Borst, O.; Rath, D.; et al. Platelet-Derived PCSK9 Is Associated with LDL Metabolism and Modulates Atherothrombotic Mechanisms in Coronary Artery Disease. Int. J. Mol. Sci. 2021, 22, 11179. [Google Scholar] [CrossRef] [PubMed]
- Cammisotto, V.; Baratta, F.; Simeone, P.G.; Barale, C.; Lupia, E.; Galardo, G.; Santilli, F.; Russo, I.; Pignatelli, P. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis. Antioxidants 2022, 11, 569. [Google Scholar] [CrossRef]
- Barale, C.; Bonomo, K.; Frascaroli, C.; Morotti, A.; Guerrasio, A.; Cavalot, F.; Russo, I. Platelet function and activation markers in primary hypercholesterolemia treated with anti-PCSK9 monoclonal antibody: A 12-month follow-up. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 282–291. [Google Scholar] [CrossRef]
- Li, S.; Zhu, C.-G.; Guo, Y.-L.; Xu, R.-X.; Zhang, Y.; Sun, J.; Li, J.-J. The relationship between the plasma PCSK9 levels and platelet indices in patients with stable coronary artery disease. J. Atheroscler. Thromb. 2015, 22, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Nocella, C.; Farcomeni, A.; Bartimoccia, S.; Santulli, M.; Vasaturo, F.; Carnevale, R.; Menichelli, D.; Violi, F.; Pignatelli, P.; et al. Relationship of PCSK9 and Urinary Thromboxane Excretion to Cardiovascular Events in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1455–1462. [Google Scholar] [CrossRef]
- El-Seweidy, M.M.; Amin, R.S.; Atteia, H.H.; El-Zeiky, R.R.; Al-Gabri, N.A. New Insight on a Combination of Policosanol and 10-Dehydrogingerdione Phytochemicals as Inhibitors for Platelet Activation Biomarkers and Atherogenicity Risk in Dyslipidemic Rabbits: Role of CETP and PCSK9 Inhibition. Appl. Biochem. Biotechnol. 2018, 186, 805–815. [Google Scholar] [CrossRef]
- Koskinas, K.C.; Windecker, S.; Buhayer, A.; Gencer, B.; Pedrazzini, G.; Mueller, C.; Cook, S.; Muller, O.; Matter, C.M.; Räber, L.; et al. Design of the randomized, placebo-controlled evolocumab for early reduction of LDL-cholesterol levels in patients with acute coronary syndromes (EVOPACS) trial. Clin. Cardiol. 2018, 41, 1513–1520. [Google Scholar] [CrossRef]
- Di Minno, A.; Orsini, R.C.; Chiesa, M.; Cavalca, V.; Calcaterra, I.; Tripaldella, M.; Anesi, A.; Fiorelli, S.; Eligini, S.; Colombo, G.I.; et al. Treatment with PCSK9 Inhibitors in Patients with Familial Hypercholesterolemia Lowers Plasma Levels of Platelet-Activating Factor and Its Precursors: A Combined Metabolomic and Lipidomic Approach. Biomedicines 2021, 9, 1073. [Google Scholar] [CrossRef]
- Marques, P.; Domingo, E.; Rubio, A.; Martinez-Hervás, S.; Ascaso, J.F.; Piqueras, L.; Real, J.T.; Sanz, M.-J. Beneficial effects of PCSK9 inhibition with alirocumab in familial hypercholesterolemia involve modulation of new immune players. Biomed. Pharmacother. 2022, 145, 112460. [Google Scholar] [CrossRef]
- Werner, C.; Hoffmann, M.M.; Winkler, K.; Böhm, M.; Laufs, U. Risk prediction with proprotein convertase subtilisin/kexin type 9 (PCSK9) in patients with stable coronary disease on statin treatment. Vascul Pharmacol. 2014, 62, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, M.-M.; Jin, J.-L.; Cao, Y.-X.; Guo, Y.-L.; Wu, N.-Q.; Zhu, C.-G.; Dong, Q.; Sun, J.; Xu, R.-X.; et al. Association of circulating PCSK9 concentration with cardiovascular metabolic markers and outcomes in stable coronary artery disease patients with or without diabetes: A prospective, observational cohort study. Cardiovasc. Diabetol. 2020, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, X.; Chen, R.; Li, J.; Zhou, J.; Liu, C.; Zhou, P.; Wang, Y.; Chen, Y.; Zhao, H.; et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc. Diabetol. 2022, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Montecucco, F.; Nanchen, D.; Carbone, F.; Klingenberg, R.; Vuilleumier, N.; Aghlmandi, S.; Heg, D.; Räber, L.; Auer, R.; et al. Prognostic value of PCSK9 levels in patients with acute coronary syndromes. Eur. Heart J. 2016, 37, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 673–2678. [Google Scholar] [CrossRef]
- Maulucci, G.; Cipriani, F.; Russo, D.; Casavecchia, G.; Di Staso, C.; Di Martino, L.; Ruggiero, A.; Di Biase, M.; Brunetti, N.D. Improved endothelial function after short-term therapy with evolocumab. J. Clin. Lipidol. 2018, 12, 669–673. [Google Scholar] [CrossRef]
- Leucker, T.M.; Gerstenblith, G.; Schär, M.; Brown, T.T.; Jones, S.R.; Afework, Y.; Weiss, R.G.; Hays, A.G. Evolocumab, a PCSK9-Monoclonal Antibody, Rapidly Reverses Coronary Artery Endothelial Dysfunction in People Living with HIV and People With Dyslipidemia. J. Am. Heart Assoc. 2020, 9, e016263. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Mager, A.; Leshem-Lev, D.; Lev, E.; Kornowski, R.; Eisen, A. The Effect of Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors on Circulating Endothelial Progenitor Cells in Patients with Cardiovascular Disease. Cardiovasc. Drugs Ther. 2022, 36, 85–92. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Cheng, Z.; Lv, Z.; Luo, S.; Xia, Y. PCSK9 Promotes Endothelial Dysfunction During Sepsis Via the TLR4/MyD88/NF-κB and NLRP3 Pathways. Inflammation 2022, 46, 115–128. [Google Scholar] [CrossRef]
- Sposito, A.C.; Breder, I.; Barreto, J.; Breder, J.; Bonilha, I.; Lima, M.; Oliveira, A.; Wolf, V.; Luchiari, B.; Carmo, H.R.D.; et al. Evolocumab on top of empagliflozin improves endothelial function of individuals with diabetes: Randomized active-controlled trial. Cardiovasc. Diabetol. 2022, 21, 147. [Google Scholar] [CrossRef]
- Karagiannis, A.D.; Liu, M.; Toth, P.P.; Zhao, S.; Agrawal, D.K.; Libby, P.; Chatzizisis, Y.S. Pleiotropic Anti-atherosclerotic Effects of PCSK9 Inhibitors from Molecular Biology to Clinical Translation. Curr. Atheroscler. Rep. 2018, 20, 20. [Google Scholar] [CrossRef]
- Kosowski, M.; Basiak, M.; Hachuła, M.; Okopień, B. Impact of Alirocumab on Release Markers of Atherosclerotic Plaque Vulnerability in Patients with Mixed Hyperlipidemia and Vulnerable Atherosclerotic Plaque. Medicina 2022, 58, 969. [Google Scholar] [CrossRef]
- Di Minno, A.; Gentile, M.; Iannuzzo, G.; Calcaterra, I.; Tripaldella, M.; Porro, B.; Cavalca, V.; Di Taranto, M.D.; Tremoli, E.; Fortunato, G.; et al. Endothelial Function Improvement in Patients with Familial Hypercholesterolemia Receiving PCSK-9 Inhibitors on Top of Maximally Tolerated Lipid Lowering Therapy. Thromb. Res. 2020, 194, 229–236. [Google Scholar] [CrossRef]
- Metzner, T.; Leitner, D.R.; Dimsity, G.; Gunzer, F.; Opriessnig, P.; Mellitzer, K.; Beck, A.; Sourij, H.; Stojakovic, T.; Deutschmann, H.; et al. Short-Term Treatment with Alirocumab, Flow-Dependent Dilatation of the Brachial Artery and Use of Magnetic Resonance Diffusion Tensor Imaging to Evaluate Vascular Structure: An Exploratory Pilot Study. Biomedicines 2022, 10, 152. [Google Scholar] [CrossRef]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef]
- Ricci, C.; Ruscica, M.; Camera, M.; Rossetti, L.; Macchi, C.; Colciago, A.; Zanotti, I.; Lupo, M.G.; Adorni, M.P.; Cicero, A.F.G.; et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci. Rep. 2018, 8, 2267. [Google Scholar] [CrossRef]
- Hoogeveen, R.M.; Opstal, T.S.J.; Kaiser, Y.; Stiekema, L.C.A.; Kroon, J.; Knol, R.J.J.; Bax, W.A.; Verberne, H.J.; Cornel, J.H.; Stroes, E.S.G. PCSK9 Antibody Alirocumab Attenuates Arterial Wall Inflammation Without Changes in Circulating Inflammatory Markers. JACC Cardiovasc. Imaging 2019, 12, 2571–2573. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Wu, W.; Geng, P.; Zhu, J.; Li, J.; Zhang, L.; Chen, W.; Zhang, D.; Lu, Y.; Xu, X. KLF2 regulates eNOS uncoupling via Nrf2/HO-1 in endothelial cells under hypoxia and reoxygenation. Chem. Biol. Interact. 2019, 305, 105–111. [Google Scholar] [CrossRef]
- Zulkapli, R.; Muid, S.A.; Wang, S.M.; Nawawi, H. PCSK9 Inhibitors Reduce PCSK9 and Early Atherogenic Biomarkers in Stimulated Human Coronary Artery Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 5098. [Google Scholar] [CrossRef]
- Baruch, A.; Mosesova, S.; Davis, J.D.; Budha, N.; Vilimovskij, A.; Kahn, R.; Peng, K.; Cowan, K.J.; Harris, L.P.; Gelzleichter, T.; et al. Effects of RG7652, a Monoclonal Antibody Against PCSK9, on LDL-C, LDL-C Subfractions, and Inflammatory Biomarkers in Patients at High Risk of or With Established Coronary Heart Disease (from the Phase 2 EQUATOR Study). Am. J. Cardiol. 2017, 119, 1576–1583. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Aday, A.W.; Rose, L.M.; Ridker, P.M. Residual Inflammatory Risk on Treatment with PCSK9 Inhibition and Statin Therapy. Circulation 2018, 138, 141–149. [Google Scholar] [CrossRef]
- East, C.; Bass, K.; Mehta, A.; Rahimighazikalayed, G.; Zurawski, S.; Bottiglieri, T. Alirocumab and Lipid Levels, Inflammatory Biomarkers, Metabolomics, and Safety in Patients Receiving Maintenance Dialysis: The ALIrocumab in DIALysis Study (A Phase 3 Trial to Evaluate the Efficacy and Safety of Biweekly Alirocumab in Patients on a Stable Dialysis Regimen). Kidney Med. 2022, 4, 100483. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.-G.; Xu, R.-X.; Li, S.; Guo, Y.-L.; Sun, J.; Li, J.-J. Relation of circulating PCSK9 concentration to fibrinogen in patients with stable coronary artery disease. J. Clin. Lipidol. 2014, 8, 494–500. [Google Scholar] [CrossRef]
- Boyd, J.H.; Fjell, C.D.; Russell, J.A.; Sirounis, D.; Cirstea, M.S.; Walley, K.R. Increased Plasma PCSK9 Levels Are Associated with Reduced Endotoxin Clearance and the Development of Acute Organ Failures during Sepsis. J. Innate Immun. 2016, 8, 211–220. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Deng, X.; Fan, Y.; Shahanawaz, J.; Reis, R.J.S.; Varughese, K.I.; Sawamura, T.; Mehta, J.L. Cross-talk between LOX-1 and PCSK9 in vascular tissues. Cardiovasc. Res. 2015, 107, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Tibolla, G.; Pirillo, A.; Cipollone, F.; Mezzetti, A.; Pacia, S.; Corsini, A.; Catapano, A.L. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 2012, 220, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Marcinkiewicz, J.; Zaid, A.; Gauthier, D.; Poirier, S.; Lazure, C.; Seidah, N.G.; Prat, A. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012, 125, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, J.; Wang, W.; Wang, M.; Qi, Y.; Zhao, F.; Sun, J.; Liu, J.; Li, Y.; Zhao, D. Association between plasma PCSK9 levels and 10-year progression of carotid atherosclerosis beyond LDL-C: A cohort study. Int. J. Cardiol. 2016, 215, 293–298. [Google Scholar] [CrossRef]
- Moens, S.J.B.; Neele, A.E.; Kroon, J.; van der Valk, F.M.; Bossche, J.V.D.; Hoeksema, M.A.; Hoogeveen, R.M.; Schnitzler, J.G.; Baccara-Dinet, M.T.; Manvelian, G.; et al. PCSK9 monoclonal antibodies reverse the proinflammatory profile of monocytes in familial hypercholesterolaemia. Eur. Heart J. 2017, 38, 1584–1593. [Google Scholar] [CrossRef]
- Wu, N.Q.; Shi, H.W.; Li, J.J. Proprotein Convertase Subtilisin/Kexin Type 9 and Inflammation: An Updated Review. Front. Cardiovasc. Med. 2022, 9, 763516. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Koutagiar, I.; Skoumas, I.; Terentes-Printzios, D.; Zacharis, E.; Kolovou, G.; Stamatelopoulos, K.; Rallidis, L.; Katsiki, N.; Bilianou, H.; et al. Long-Term Administration of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors Reduces Arterial FDG Uptake. JACC Cardiovasc. Imaging 2019, 12, 2573–2574. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Wang, J.; Guo, C.; Kleiman, K.; Meng, H.; Knight, J.S.; Eitzman, D.T. Proprotein convertase subtilisin/kexin type 9 (PCSK9) Deficiency is Protective Against Venous Thrombosis in Mice. Sci. Rep. 2017, 7, 14360. [Google Scholar] [CrossRef]
- Jin, L.; Batra, S.; Jeyaseelan, S. Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathog. 2017, 13, e1006637. [Google Scholar] [CrossRef]
- Schuster, S.; Rubil, S.; Endres, M.; Princen, H.M.G.; Boeckel, J.N.; Winter, K.; Werner, C.; Laufs, U. Anti-PCSK9 antibodies inhibit pro-atherogenic mechanisms in APOE*3Leiden.CETP mice. Sci. Rep. 2019, 9, 11079. [Google Scholar] [CrossRef]
- Park, K.-H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Basiak, M.; Hachula, M. Effect of PCSK9 Inhibitors on Hemostasis in Patients with Isolated Hypercholesterolemia. J. Clin. Med. 2022, 11, 2542. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.; Iriondo, M.; Manzano, C.; Fullaondo, A.; Villar, I.; Ruiz-Irastorza, G.; Zubiaga, A.M.; Estonba, A. LDLR and PCSK9 Are Associated with the Presence of Antiphospholipid Antibodies and the Development of Thrombosis in aPLA Carriers. PLoS ONE 2016, 11, e0146990. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Li, Y.F.; Guo, Y.G.; Chen, M.M.; Jiang, Z.L.; Song, J.Y. Positive correlation between plasma PCSK9 and tissue factors levels in patients with angiographically diagnosed coronary artery disease and diabetes mellitus. J. Geriatr. Cardiol. 2016, 13, 312–315. [Google Scholar]
- Hamik, A.; Setiadi, H.; Bu, G.; McEver, R.P.; Morrissey, J.H. Down-regulation of monocyte tissue factor mediated by tissue factor pathway inhibitor and the low density lipoprotein receptor-related protein. J. Biol. Chem. 1999, 274, 4962–4969. [Google Scholar] [CrossRef]
- Scalise, V.; Sanguinetti, C.; Neri, T.; Cianchetti, S.; Lai, M.; Carnicelli, V.; Celi, A.; Pedrinelli, R. PCSK9 Induces Tissue Factor Expression by Activation of TLR4/NFkB Signaling. Int. J. Mol. Sci. 2021, 22, 12640. [Google Scholar] [CrossRef]
- Scalise, V.; Lombardi, S.; Sanguinetti, C.; Nieri, D.; Pedrinelli, R.; Celi, A.; Neri, T. A novel prothrombotic role of proprotein convertase subtilisin kexin 9: The generation of procoagulant extracellular vesicles by human mononuclear cells. Mol. Biol. Rep. 2022, 49, 4129–4134. [Google Scholar] [CrossRef]
- Basiak, M.; Kosowski, M.; Cyrnek, M.; Bułdak, Ł.; Maligłówka, M.; Machnik, G.; Okopień, B. Pleiotropic Effects of PCSK-9 Inhibitors. Int. J. Mol. Sci. 2021, 22, 3144. [Google Scholar] [CrossRef]
- Jenkins, P.V.; Rawley, O.; Smith, O.P.; O’Donnell, J.S. Elevated factor VIII levels and risk of venous thrombosis. Br. J. Haematol. 2012, 157, 653–663. [Google Scholar] [CrossRef]
- Rietveld, I.M.; Lijfering, W.M.; le Cessie, S.; Bos, M.H.A.; Rosendaal, F.R.; Reitsma, P.H.; Cannegieter, S.C. High levels of coagulation factors and venous thrombosis risk: Strongest association for factor VIII and von Willebrand factor. J. Thromb. Haemost. 2019, 17, 99–109. [Google Scholar] [CrossRef]
- Bovenschen, N.; Mertens, K.; Hu, L.; Havekes, L.M.; van Vlijmen, B.J. LDL receptor cooperates with LDL receptor-related protein in regulating plasma levels of coagulation factor VIII in vivo. Blood 2005, 106, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Canuel, M.; Sun, X.; Asselin, M.C.; Paramithiotis, E.; Prat, A.; Seidah, N.G. Proprotein convertase subtilisin/kexin type 9 (PCSK9) can mediate degradation of the low density lipoprotein receptor-related protein 1 (LRP-1). PLoS ONE 2013, 8, e64145. [Google Scholar] [CrossRef]
- Paciullo, F.; Petito, E.; Falcinelli, E.; Gresele, P.; Momi, S. Pleiotropic effects of PCSK9-inhibition on hemostasis: Anti-PCSK9 reduce FVIII levels by enhancing LRP1 expression. Thromb. Res. 2022, 213, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Wu, J.-L.; Ji, F.; Kang, W.; Bian, X.; Chen, H.; Chan, L.-S.; Luk, S.T.Y.; Tong, S.; Xu, J.; et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat. Commun. 2022, 13, 3971. [Google Scholar] [CrossRef] [PubMed]
- Pitteri, S.J.; Kelly-Spratt, K.S.; Gurley, K.E.; Kennedy, J.; Buson, T.B.; Chin, A.; Wang, H.; Zhang, Q.; Wong, C.-H.; Chodosh, L.A.; et al. Tumor microenvironment-derived proteins dominate the plasma proteome response during breast cancer induction and progression. Cancer Res. 2011, 71, 5090–5100. [Google Scholar] [CrossRef]
- Zia, S.; Batool, S.; Shahid, R. Could PCSK9 be a new therapeutic target of Eugenol? In vitro and in silico evaluation of hypothesis. Med. Hypotheses 2020, 136, 109513. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Morishima, K.; Okamoto, H.; Ishibashi, S. Possible involvement of PCSK9 overproduction in hyperlipoproteinemia associated with hepatocellular carcinoma: A case report. J. Clin. Lipidol. 2016, 10, 1045–1049. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Fang, Y.; Zou, Y.; Song, D.; Zhang, S.; Cai, Y. Proprotein Convertase Subtilisin/Kexin Type 9 Promotes Gastric Cancer Metastasis and Suppresses Apoptosis by Facilitating MAPK Signaling Pathway Through HSP70 Up-Regulation. Front. Oncol. 2021, 10, 609663. [Google Scholar] [CrossRef]
- Yuan, J.; Cai, T.; Zheng, X.; Ren, Y.; Qi, J.; Lu, X.; Chen, H.; Lin, H.; Chen, Z.; Liu, M.; et al. Potentiating CD8+ T cell antitumor activity by inhibiting PCSK9 to promote LDLR-mediated TCR recycling and signaling. Protein Cell 2021, 12, 240–260. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chowdhury, A.; Chaudhury, K.; Shukla, P.C. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188581. [Google Scholar] [CrossRef]
- Xie, M.; Yu, X.; Chu, X.; Xie, H.; Zhou, J.; Zhao, J.; Su, C. Low baseline plasma PCSK9 level is associated with good clinical outcomes of immune checkpoint inhibitors in advanced non-small cell lung cancer. Thorac. Cancer 2022, 13, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.X.; Bai, J.W.; Zhang, P.F.; Zhang, Y.Z. PCSK9 regulates apoptosis in human neuroglioma u251 cells via mitochondrial signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 2787–2794. [Google Scholar] [PubMed]
- Bai, J.; Na, H.; Hua, X.; Wei, Y.; Ye, T.; Zhang, Y.; Jian, G.; Zeng, W.; Yan, L.; Tang, Q. A retrospective study of NENs and miR-224 promotes apoptosis of BON-1 cells by targeting PCSK9 inhibition. Oncotarget 2017, 8, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
- Oza, P.P.; Kashfi, K. The evolving landscape of PCSK9 inhibition in cancer. Eur. J. Pharmacol. 2023, 949, 175721. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Nik, M.E.; Jaafari, M.R.; Banach, M.; Sahebkar, A. Effects of immunisation against PCSK9 in mice bearing melanoma. Arch. Med. Sci. 2019, 16, 189–199. [Google Scholar] [CrossRef]
- He, M.; Hu, J.; Fang, T.; Tang, W.; Lv, B.; Yang, B.; Xia, J. Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol. Med. 2021, 19, 90–103. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Lee, S.P.; Linsley, P.S.; Gersuk, V.; Yeh, M.M.; Chen, Y.-Y.; Peng, Y.-J.; Dutta, M.; Mascarinas, G.; Molla, B.; et al. Pcsk9 Deletion Promotes Murine Nonalcoholic Steatohepatitis and Hepatic Carcinogenesis: Role of Cholesterol. Hepatol. Commun. 2022, 6, 780–794. [Google Scholar] [CrossRef]
- Molina, S.; Castet, V.; Fournier-Wirth, C.; Pichard-Garcia, L.; Avner, R.; Harats, D.; Roitelman, J.; Barbaras, R.; Graber, P.; Ghersa, P.; et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J. Hepatol. 2007, 46, 411–419. [Google Scholar] [CrossRef]
- Caron, J.; Pène, V.; Tolosa, L.; Villaret, M.; Luce, E.; Fourrier, A.; Heslan, J.; Saheb, S.; Bruckert, E.; Gómez-Lechón, M.; et al. Low-density lipoprotein receptor-deficient hepatocytes differentiated from induced pluripotent stem cells allow familial hypercholesterolemia modeling, CRISPR/Cas-mediated genetic correction, and productive hepatitis C virus infection. Stem Cell Res. Ther. 2019, 10, 221. [Google Scholar] [CrossRef]
- Grin, P.M.; Dwivedi, D.J.; Chathely, K.M.; Trigatti, B.L.; Prat, A.; Seidah, N.G.; Liaw, P.C.; Fox-Robichaud, A.E. Low-density lipoprotein (LDL)-dependent uptake of Gram-positive lipoteichoic acid and Gram-negative lipopolysaccharide occurs through LDL receptor. Sci. Rep. 2018, 8, 10496. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Grin, P.M.; Khan, M.; Prat, A.; Zhou, J.; Fox-Robichaud, A.E.; Seidah, N.G.; Liaw, P.C. Differential Expression of PCSK9 Modulates Infection, Inflammation, and Coagulation in a Murine Model of Sepsis. Shock 2016, 46, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Vecchié, A.; Bonaventura, A.; Meessen, J.; Novelli, D.; Minetti, S.; Elia, E.; Ferrara, D.; Ansaldo, A.M.; Scaravilli, V.; Villa, S.; et al. PCSK9 is associated with mortality in patients with septic shock: Data from the ALBIOS study. J. Intern. Med. 2021, 289, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, W.; Burgner, D.; Tonkin, A.; Zhu, C.; Sun, C.; Magnussen, C.G.; Ernst, M.E.; Breslin, M.; Nicholls, S.J.; et al. The Association Between PCSK9 Inhibitor Use and Sepsis: A Systematic Review and Meta-Analysis of 20 Double-Blind, Randomized, Placebo-Controlled Trials. Am. J. Med. 2023, 136, 558–567.e20. [Google Scholar] [CrossRef] [PubMed]
| Study/Authors | Number of Patients and Their Characteristics | Treatment | Result |
|---|---|---|---|
| Li et al., 2015 [49] | 330, with CAD | N/A | PCSK9 levels are positively associated with the platelet count and plateletcrit, while no correlation with MPV and PDW |
| Pastori et al., 2017 [50] | 907, with atrial fibrillation | N/A | PCSK9 levels are connected to increased risk of cardiovascular events, PCSK9 levels are correlating with thromboxane B2 levels |
| PCSK9—REACT study, 2017 [31] | 178, with acute coronary syndrome | N/A | Increased PCSK9 levels are associated with higher platelet reactivity |
| Elseweidy et al. [35] | Animal model | Policosanol, 10-dehydrogingerdione | Decreased PCSK9, platelet activation and inflammation markers (sCD40L, sP-selectin, interferon-gamma) |
| Elseweidy et al. [51] | Animal model | 10-dehydrogingerdione | Decreased interferon-gamma, sCD40L, sP-selectin correlated with PCSK9 suppression |
| EVOPACS study, 2018 [52] | 308 | PCSK9 inhibitors | Plasma levels of PCSK9 correlate with increased platelet count and platelet activation |
| Barale et al., 2020 [48] | 24 | PCSK9 inhibitors | Platelet activation markers—sCD40L, platelet factor 4, and sP-selectin, significantly decreased after treatment with PCSK9 inhibitors. These markers correlate with PCSK9. |
| Cammisotto et al., 2020 [34] | 88, with atrial fibrillation | N/A | Markers of platelet activation and oxidative stress correlate, and changes were amplified by adding LDL |
| Cammisotto et al., 2021 [33] | 80, heterozygous familial hypercholesterolemia | PCSK9 inhibitors | Treatment reduces platelet activation modulating NOX2 activity and, in turn, ox-LDL formation |
| Qi et al., 2021 [32] | N/A | PCSK9 inhibitors | PCSK9 in plasma enhances platelet activation and thrombosis by binding to CD36. PCSK9 inhibitors or aspirin abolish the enhancing effects of PCSK9, supporting the use of aspirin in patients with a high plasma level of PCSK9. |
| Di Minno et al., 2021 [53] | 25, with familial hypercholesterolemia | PCSK9 inhibitors | Reduction in platelet-activating factors after treatment. |
| Marques et al., 2022 [54] | 14, with familial hypercholesterolemia | PCSK9 inhibitors | PCSK9 inhibition impairs systemic inflammation and endothelial dysfunction by constraining leukocyte-endothelium interactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péč, M.J.; Benko, J.; Jurica, J.; Péčová, M.; Samec, M.; Hurtová, T.; Bolek, T.; Galajda, P.; Péč, M.; Samoš, M.; et al. The Anti-Thrombotic Effects of PCSK9 Inhibitors. Pharmaceuticals 2023, 16, 1197. https://doi.org/10.3390/ph16091197
Péč MJ, Benko J, Jurica J, Péčová M, Samec M, Hurtová T, Bolek T, Galajda P, Péč M, Samoš M, et al. The Anti-Thrombotic Effects of PCSK9 Inhibitors. Pharmaceuticals. 2023; 16(9):1197. https://doi.org/10.3390/ph16091197
Chicago/Turabian StylePéč, Martin Jozef, Jakub Benko, Jakub Jurica, Monika Péčová, Marek Samec, Tatiana Hurtová, Tomáš Bolek, Peter Galajda, Martin Péč, Matej Samoš, and et al. 2023. "The Anti-Thrombotic Effects of PCSK9 Inhibitors" Pharmaceuticals 16, no. 9: 1197. https://doi.org/10.3390/ph16091197
APA StylePéč, M. J., Benko, J., Jurica, J., Péčová, M., Samec, M., Hurtová, T., Bolek, T., Galajda, P., Péč, M., Samoš, M., & Mokáň, M. (2023). The Anti-Thrombotic Effects of PCSK9 Inhibitors. Pharmaceuticals, 16(9), 1197. https://doi.org/10.3390/ph16091197






