Evaluating Thromboprophylaxis Strategies for High-Risk Pregnancy: A Current Perspective
Abstract
1. Introduction
2. Methods
3. Which Women Are “At Risk” and Why?
3.1. Previous Thromboembolic Episode
3.2. Family History of Venous Thromboembolism
3.3. Smoking Prior to or during Pregnancy
3.4. Obesity
3.5. Age
3.6. Parity with Three or More Pregnancies
3.7. Anemia
3.8. Varicose Veins
3.9. Preeclampsia
3.10. Weight Gain
3.11. Stillbirth
3.12. Preterm Delivery
3.13. Cesarean Section
3.14. Peripartum Hemorrhage
3.15. Postpartum Infection
3.16. Transfusion
3.17. In Vitro Fertilization
3.18. Ovarian Hyperstimulation Syndrome
3.19. Antepartum Immobilization
4. When to Use Antithrombotic Prophylaxis
5. How Important Is the Presence of Thrombophilia and Why?
6. What Is the Form of Anticoagulant Thromboprophylaxis and Which Dose Should Be Used?
6.1. Unfractionated Heparin
6.1.1. Pharmacokinetics in Pregnancy
6.1.2. Dosages
6.2. Low-Molecular-Weight Heparin
6.2.1. Pharmacokinetics in Pregnancy
6.2.2. Enoxaparin
6.2.3. Dalteparin
6.2.4. Tinzaparin
6.2.5. Protamine Sulfate to Reverse Anticoagulation with Low-Molecular-Weight Heparin and Unfractionated Heparin
6.3. Fondaparinux
6.4. Danaparoid
6.5. Vitamin K antagonists
- -
- In patients requiring long-term treatment with VKA who are attempting pregnancy, it is suggested to substitute LMWH for VKA only when pregnancy is achieved. LMWH should be administered in adjusted doses or 75% of therapeutic doses;
- -
- Another option is to switch to UFH or LMWH until the 13th week of gestation, with substitution by VKA until close to delivery when UFH or LMWH is restored;
- -
- In pregnant patients with previous VTE, postpartum thromboprophylaxis with prophylactic or intermediate dose of LMWH or VKA targeted at the International Normalized Ratio (INR) 2.0–3.0 is recommended;
- -
- In pregnant patients without a history of VTE, homozygous for factor V Leiden or the prothrombin G20210A mutation and with a positive family history for VTE, except antepartum prophylactic- or intermediate-dose LMWH, postpartum thromboprophylaxis with continuing LMWH or VKA targeted at INR 2.0–3.0 is recommended;
- -
- In pregnant patients without personal and family history of VTE, homozygous for factor V Leiden or the prothrombin G20210A mutation, postpartum thromboprophylaxis for 6 weeks with prophylactic- or intermediate-dose LMWH or VKA targeted at INR 2.0–3.0 is suggested;
- -
- In asymptomatic pregnant patients with all other thrombophilic states and a positive family history for VTE, postpartum thromboprophylaxis with prophylactic or intermediate dose of LMWH or, in those who do not have PC or PS deficiency, VKA targeted at INR 2.0–3.0 is suggested [68].
6.6. Direct Oral Anticoagulants
7. Further Aspects Related to Thromboprophylaxis That Are Useful for Clinical Practice
7.1. How to Monitor the Effectiveness of Anticoagulant Thromboprophylaxis
7.2. How to Manage Cesarean Section in Patients with Anticoagulant Thromboprophylaxis
7.3. How to Manage Neuraxial Anesthesia in Patients Using Anticoagulants
- -
- To evaluate the need for thromboprophylaxis as soon as the pregnancy is confirmed, consider the presence of the prothrombotic risk factors related to the comorbidities and circumstances of a previous VTE episode;
- -
- In patients with previous idiopathic, recurrent or hormone-related VTE, use ante- and also postpartum prophylaxis;
- -
- In patients with VTE associated with a major reversible risk factor/without thrombophilic state, use postpartum thromboprophylaxis;
- -
- In pregnant patients with prothrombotic risk factors undergoing cesarean section, consider postpartum thromboprophylaxis;
- -
- Control changes in the health condition of the patient and modify the actual thromboprophylaxis on an individual basis;
- -
- Evaluate the presence of bleeding symptoms and allergic reaction, regularly assess platelet count, function of antithrombin, renal parameters and liver function tests;
- -
- In pregnant women with extreme body weight, renal impairment, recurrence of VTE or in the suspicion of noncompliance, evaluate anti-Xa activity of LMWH;
- -
- To take control over prothrombotic comorbidities and complications developed during pregnancy, the multidisciplinary approach is preferred;
- -
- Along with anticoagulant thromboprophylaxis, use nonpharmacologic prophylaxis, such as intermittent pneumatic compression or elastic stockings.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACCP | American College of Chest Physicians |
| ACOG | American College of Obstetricians and Gynecologists |
| AHA/ASA | American Heart Association and American Stroke Association |
| AOR | Adjusted odds ratio |
| aPTT | Activated partial thromboplastin time |
| ASA | Acetylsalicylic acid |
| ASH | American Society of Hematology |
| ASMBS | American Society for Metabolic and Bariatric Surgery |
| CI | Confidence interval |
| Dll4 | Delta-like ligand 4 |
| DOACs | Direct oral anticoagulants |
| DVT | Deep vein thrombosis |
| EG | Endothelial glycocalyx |
| ELAM-1 | Endothelial cell-leukocyte adhesion molecule 1 |
| ESMO | European Society for Medical Oncology |
| FGR | Fetal growth restriction |
| FII | Coagulation factor II |
| FV | Coagulation factor V |
| FVII | Coagulation factor VII |
| FVIII | Coagulation factor VIII |
| FIX | Coagulation factor IX |
| FX | Coagulation factor X |
| FXI | Coagulation factor XI |
| FXII | Coagulation factor XII |
| FXIII | Coagulation factor XIII |
| GARFIELD | Global Anticoagulant Registry in the FIELD |
| GTH | Society of Thrombosis and Haemostasis Research/Gesellschaft für Thrombose und Hämostaseforschung |
| hCG | Human chorionic gonadotropin |
| HIT | Heparin-induced thrombocytopenia |
| HR | Hazard ratio |
| ICAM-1 | Intercellular adhesion molecule 1 |
| INR | International Normalized Ratio |
| IRR | Incidence rate ratio |
| IVF | In vitro fertilization |
| LMWH | Low-molecular-weight heparin |
| NB | Neuraxial blockade |
| NETs | Neutrophil extracellular traps |
| OHSS | Ovarian hyperstimulation syndrome |
| PAI-1 | Plasminogen activator inhibitor 1 |
| PC | Protein C |
| PCOS | Polycystic ovarian syndrome |
| PE | Pulmonary embolism |
| PIC | Plasmin–α2 plasmin inhibitor complex |
| PS | Protein S |
| RANZOG | The Royal Australian and New Zealand College of Obstetricians and Gynaecologists |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| ROTEM | Rotational thromboelastometry |
| QCG | Queensland Clinical Guidelines |
| SC | Subcutaneously |
| SFOG | Swedish Society of Obstetrics and Gynecology |
| SLE | Systemic lupus erythematosus |
| SOGC | Society of Obstetricians and Gynecologists of Canada |
| TAT | Thrombin–antithrombin complex |
| TEG | Thromboelastography |
| TM | Thrombomodulin |
| tPA | Tissue plasminogen activator |
| tPAI-C | Tissue plasminogen activator/plasminogen activator inhibitor compound |
| UFH | Unfractionated heparin |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VKA | Vitamin K antagonists |
| VTE | Venous thromboembolism |
| vWF | Von Willebrand factor |
References
- Galanaud, J.-P.; Laroche, J.-P.; Righini, M. The history and historical treatments of deep vein thrombosis. J. Thromb. Haemost. 2013, 11, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, B.; Rott, H.; Zotz, R.; Hart, C. Venous Thromboembolism Issues in Women. Hamostaseologie 2022, 42, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Felis, S.; Marchese, B.; Gavini, I. The Latest Evidence of Risks and Management of Venous Thromboembolism in Pregnancy: A Comprehensive Review. J. Blood Disord. Transfus. 2023, S2, 006. [Google Scholar]
- Eke, A.C.; Gebreyohannes, R.D.; Scantamburlo Fernandes, M.F.; Pillai, V.C. Physiologic Changes During Pregnancy and Impact on Small-Molecule Drugs, Biologic (Monoclonal Antibody) Disposition, and Response. J. Clin. Pharmacol. 2023, 63 (Suppl. S1), S34–S50. [Google Scholar] [CrossRef] [PubMed]
- Tlamcani, I.; Mouh, N.E.; Amrani, K.E.; Hassani, M.A. Pregnancy and Hemostasis: From Physiology to Pathological States. Clin. Res. Hematol. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Y.; Wu, M.; Xiang, Z. Changes of new coagulation markers in healthy pregnant women and establishment of reference intervals in Changsha. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Leffert, L.; Butwick, A.; Carvalho, B.; Arendt, K.; Bates, S.M.; Friedman, A.; Horlocker, T.; Houle, T.; Landau, R.; Dubois, H.; et al. The Society for Obstetric Anesthesia and Perinatology Consensus Statement on the Anesthetic Management of Pregnant and Postpartum Women Receiving Thromboprophylaxis or Higher Dose Anticoagulants. Anesth. Analg. 2018, 126, 928–944. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Collis, R.E.; Collins, P.W. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br. J. Anaesth. 2012, 109, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, L. Uteroplacental Circulation in Normal Pregnancy and Preeclampsia: Functional Adaptation and Maladap-tation. Int. J. Mol. Sci. 2021, 22, 8622. [Google Scholar] [CrossRef]
- Kevane, B.; Ní Áinle, F.N. Prevention, diagnosis, and management of PE and DVT in pregnant women. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Hu, X.; Peng, X.; Zhang, Z. Incidence of venous thromboembolism during pregnancy and the puerperium: A systematic review and meta-analysis. J. Matern.-Fetal Neonatal Med. 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Maughan, B.C.; Marin, M.; Han, J.; Gibbins, K.J.; Brixey, A.G.; Caughey, A.B.; Kline, J.A.; Jarman, A.F. Venous Thromboem-bolism During Pregnancy and the Postpartum Period: Risk Factors, Diagnostic Testing and Treatment. Obstet. Gynecol. Surv. 2022, 77, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Montinari, M.R.; Minelli, S.; De Caterina, R. Eighty years of oral anticoagulation: Learning from history. Vascul. Pharmacol. 2021, 141, 106918. [Google Scholar] [CrossRef]
- Rath, W.; von Tempelhoff, G.-F.; Tsikouras, P. How to Reduce Maternal Mortality from Venous Thromboembolism. Clin. Appl. Thromb. Hemost. 2018, 24, 6S–7S. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.; Martinelli, I.; Rossi, E.; Battaglioli, T.; Za, T.; Mannucci, P.M.; Leone, G. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br. J. Haematol. 2006, 135, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Galambosi, P.J.; Ulander, V.M.; Kaaja, R.J. The incidence and risk factors of recurrent venous thromboembolism during pregnancy. Thromb. Res. 2014, 134, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Novak, A.; Šabović, M.; Kozak, M. Prophylactic Dose of Dalteparin in Pregnant Women with History of Venous Thromboembolisms and/or Thrombophilia: Real-World Data. Angiology 2023, 74, 783–789. [Google Scholar] [CrossRef]
- Pabinger, I.; Grafenhofer, H.; Kyrle, P.A.; Quehenberger, P.; Mannhalter, C.; Lechner, K.; Kaider, A. Temporary increase in the risk for recurrence during pregnancy in women with a history of venous Thromboembolism. Blood 2002, 100, 1060–1062. [Google Scholar] [CrossRef]
- Alsheef, M.A.; Alabbad, A.M.; Albassam, R.A.; Alarfaj, R.M.; Zia Zaidi, A.R.; Alarfaj, O.A.; Ayyash, M.; Abu-Shaheen, A. Predictors of pregnancy-associated venous thromboembolism: A case-control study. Front. Cardiovasc. Med. 2022, 9, 920089. [Google Scholar] [CrossRef]
- Jerjes-Sánchez, C.; Rodriguez, D.; Farjat, A.E.; Kayani, G.; MacCallum, P.; Lopes, R.D.; Turpie, A.G.G.; Weitz, J.I.; Haas, S.; Ageno, W.; et al. Pregnancy-Associated Venous Thromboembolism: Insights from GARFIELD-VTE. TH Open 2021, 5, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Grouzi, E.; Pouliakis, A.; Aktypi, A.; Christoforidou, A.; Kotsi, P.; Anagnostou, G.; Foifa, A.; Papadakis, E. Pregnancy and thrombosis risk for women without a history of thrombotic events: A retrospective study of the real risks. Thromb. J. 2022, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Nanne Croles, F.; Nasserinejad, K.; Duvekot, J.J.; Kruip, M.J.; Meijer, K.; Wg Leebeek, F. Pregnancy, thrombophilia, and the risk of a first venous thrombosis: Systematic review and bayesian meta-analysis. BMJ 2017, 359, j4452. [Google Scholar] [CrossRef] [PubMed]
- Al-Nasser, B. Influence of Tobacco Smoking on Perioperative Risk of Venous Thromboembolism. Turk. J. Anaesthesiol. Reanim. 2020, 48, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.M.; Bellone, J.M.; Hornsby, L.B.; Treadway, S.; Phillippe, H.M. Pregnancy-Related Venous Thromboembolism. J. Pharm. Pract. 2014, 27, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Bauersachs, R.; Scholz, U.; Zotz, R.; Bergmann, F.; Rott, H.; Linnemann, B. Prevention of venous thromboembolism during pregnancy and the puerperium with a special focus on women with hereditary thrombophilia or prior VTE—Position Paper of the Working Group in Women’s Health of the Society of Thrombosis and Haemostasis (GTH). Hamostaseologie 2020, 40, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Hron, G.; Bialonczyk, C.; Hirschl, M.; Minar, E.; Wagner, O.; Heinze, G.; Kyrle, P.A. Overweight, obesity, and the risk of recurrent venous thromboembolism. Arch. Intern. Med. 2008, 168, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Glise Sandblad, G.; Lundberg, C.E.; Hellsén, G.; Hansson, P.O.; Adiels, M.; Rosengren, A. Prepregnancy overweight and obesity and long-term risk of venous thromboembolism in women. Sci. Rep. 2023, 13, 14597. [Google Scholar] [CrossRef]
- Nurmohamed, M.T.; Büller, H.R.; ten Cate, J.W. Physiological changes due to age. Implications for the prevention and treatment of thrombosis in older patients. Drugs Aging 1994, 5, 20–33. [Google Scholar] [CrossRef]
- Varrias, D.; Spanos, M.; Kokkinidis, D.G.; Zoumpourlis, P.; Kalaitzopoulos, D.R. Venous Thromboembolism in Pregnancy: Challenges and Solutions. Vasc. Health Risk Manag. 2023, 19, 469–484. [Google Scholar] [CrossRef]
- De Barros, V.I.P.L.V.; Kondo Igai, A.M.; Spadotto Baptista, F.; de Figueiredo Lemos Bortolotto, M.R.; Verzinhasse Peres, S.; Pulcinelli Vieira Francisco, R. Venous thromboembolism risk score during hospitalization in pregnancy: Results of 10694 prospective evaluations in a clinical trial. Clinics 2023, 78, 100230. [Google Scholar] [CrossRef]
- Middeldorp, S.; Naue, C.; Köhler, C. Thrombophilia, Thrombosis and Thromboprophylaxis in Pregnancy: For What and in Whom? Hamostaseologie 2022, 42, 54–64. [Google Scholar] [CrossRef]
- Crowley, M.P.; Noone, C.; Higgins, J.R.; O’Shea, S. A Multicentre Study of Thromboprophylaxis in Pregnancy. Ir. Med. J. 2017, 110, 567. [Google Scholar] [PubMed]
- Mokhtari, M.; Nasri, K.; Tara, F.; Zarean, E.; Hantoushzadeh, S.; Radmehr, M.; Kashanian, M. A Survey of Venous Throm boembolism (VTE) Prophylaxis in Obstetrics Patients in Iran. J. Fam. Reprod. Health 2019, 13, 21–25. [Google Scholar]
- Wang, Z.L.; Geng, H.Z.; Zhao, X.L.; Zhu, Q.Y.; Lin, J.H.; Zou, L.; Mi, Y.; Hu, Y.L.; Fan, S.R.; Chen, X.; et al. Survey of related factors of maternal venous thromboembolism in nine hospitals of China. Zhonghua Fu Chan Ke Za Zhi 2020, 55, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-G.; Lee, J.H.; Bang, S.-M. Incidence of Pregnancy-Associated Venous Thromboembolism: Second Nationwide Study. Thromb. Haemost. 2023, 123, 904–910. [Google Scholar] [CrossRef]
- Lok, W.Y.; Kong, C.W.; To, W.W.K. A local risk score model for venous thromboembolism prophylaxis for caesarean section in Chinese women and comparison with international guidelines. Taiwan J. Obstet. Gynecol. 2019, 58, 520–525. [Google Scholar] [CrossRef]
- Ezeh, E.; Katabi, A.; Khawaja, I. Iron Deficiency Anemia as a Rare Risk Factor for Recurrent Pulmonary Embolism and Deep Vein Thrombosis. Cureus 2021, 13, e13721. [Google Scholar] [CrossRef] [PubMed]
- Pathophysiology and Principles of Management of Varicose Veins. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534256/ (accessed on 22 January 2024).
- Deep Vein Thrombosis (Clinical). Available online: https://www.lecturio.com/concepts/deep-vein-thrombosis-clinical/ (accessed on 22 January 2024).
- Jacobs, B.N.; Andraska, E.A.; Obi, A.T.; Wakefield, T.W. Pathophysiology of varicose veins. J. Vasc. Surg. Venous Lymphat. Disord. 2017, 5, 460–467. [Google Scholar] [CrossRef]
- Choy, K.; Emmett, S.; Wong, A. Venous thromboembolism prophylaxis in pregnancy: Are we adequately identifying and managing risks? Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 915–920. [Google Scholar] [CrossRef]
- Lussana, F.; Coppens, M.; Cattaneo, M.; Middeldorp, S. Pregnancy-related venous thromboembolism: Risk and the effect of thromboprophylaxis. Thromb. Res. 2012, 129, 673–680. [Google Scholar] [CrossRef]
- Sultan, A.A.; Tata, L.J.; West, J.; Fiaschi, L.; Fleming, K.M.; Nelson-Piercy, C.; Grainge, M.J. Risk factors for first venous thromboembolism around pregnancy: A population-based cohort study from the United Kingdom. Blood 2013, 121, 3953–3961. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, S.; Maguire, P.B.; Szklanna, P.B.; Weiss, L.; Ewins, K.; O’Doherty, R.; Angelov, D.; Áinle, F.N.; Kevane, B. Pathophysiology of the Venous Thromboembolism Risk in Preeclampsia. Hamostaseologie 2020, 40, 594–604. [Google Scholar] [CrossRef]
- Egan, K.; Kevane, B.; Áinle, F.N. Elevated venous thromboembolism risk in preeclampsia: Molecular mechanisms and clinical impact. Biochem. Soc. Trans. 2015, 43, 696–701. [Google Scholar] [CrossRef]
- Hernández González, L.L.; Pérez-Campos Mayoral, L.; Hernández-Huerta, M.T.; Mayoral Andrade, G.; Martínez Cruz, M.; Ramos-Martínez, E.; Pérez-Campos Mayoral, E.; Cruz Hernández, V.; García, I.A.; Matias-Cervantes, C.A.; et al. Targeting Neutrophil Extracellular Trap Formation: Exploring Promising Pharmacological Strategies for the Treatment of Preeclampsia. Pharmaceuticals 2024, 17, 605. [Google Scholar] [CrossRef] [PubMed]
- Scheres, L.J.J.; Lijfering, W.M.; Groenewegen, N.F.M.; Koole, S.; de Groot, C.J.M.; Middeldorp, S.; Cannegieter, S.C. Hypertensive Complications of Pregnancy and Risk of Venous Thromboembolism. Hypertension 2020, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Havers-Borgersen, E.; Butt, J.H.; Johansen, M.; Petersen, O.B.; Ekelund, C.K.; Rode, L.; Olesen, J.B.; Køber, L.; Fosbøl, E.L. Preeclampsia and Long-Term Risk of Venous Thromboembolism. JAMA Netw. Open 2023, 6, e2343804. [Google Scholar] [CrossRef] [PubMed]
- Hague, W.M.; Dekker, G.A. Risk factors for thrombosis in pregnancy. Best. Pract. Res. Clin. Haematol. 2003, 16, 197–210. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, J.; Dong, L.; Zhou, Z.; Zhou, T.; Zhao, X.; Che, R.; Han, Z.; Hua, X. Association Between Maternal Weight Gain in Different Periods of Pregnancy and the Risk of Venous Thromboembolism: A Retrospective Case-Control Study. Front. Endocrinol. 2022, 13, 858868. [Google Scholar] [CrossRef]
- Rybstein, M.D.; DeSancho, M.T. Risk factors for and clinical management of venous thromboembolism during pregnancy. Clin. Adv. Hematol. Oncol. 2019, 17, 396–404. [Google Scholar]
- Raia-Barjat, T.; Edebiri, O.; Chauleur, C. Venous Thromboembolism Risk Score and Pregnancy. Front. Cardiovasc. Med. 2022, 9, 863612. [Google Scholar] [CrossRef] [PubMed]
- Stillbirth or Pre-Term Birth Outcomes Linked to Elevated Risk of Blood Clots after Pregnancy. Available online: https://www.prnewswire.com/news-releases/stillbirth-or-pre-term-birth-outcomes-linked-to-elevated-risk-of-blood-clots-after-pregnancy-201053771.html (accessed on 11 February 2024).
- Blondon, M.; Quon, B.S.; Harrington, L.B.; Bounameaux, H.; Smith, N.L. Association between newborn birth weight and the risk of postpartum maternal venous thromboembolism: A population-based case-control study. Circulation 2015, 131, 1471–1476, discussion 1476. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Fernández-Alonso, A.M.; Rodríguez, I.; Garrigosa, L.; Caño, A.; Carretero, P.; Vizcaíno, A.; Rocío Gonzalez-Ramirez, A. Postcesarean Thromboprophylaxis with Two Different Regimens of Bemiparin. Obstet. Gynecol. Int. 2011, 2011, 548327. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, C.; Zhong, M.; Yang, F.; Chen, H.; Kong, W.; Lv, P.; Chen, W.; Yao, Y.; Cao, Q.; et al. Changes in Coagulation and Fibrinolysis in Post-Cesarean Section Parturients Treated with Low Molecular Weight Heparin. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620978809. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.F.; Skjeldestad, F.E.; Sandset, P.M. Ante- and postnatal risk factors of venous thrombosis: A hospital-based case–control study. J. Thromb. Haemost. 2008, 6, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Sirilak, T.; Kanjanarat, P.; Nochaiwong, S.; Katip, W. Incidence of postpartum infections and outcomes associated with antibiotic prophylaxis after normal vaginal birth. Front. Med. 2022, 9, 939421. [Google Scholar] [CrossRef] [PubMed]
- Devis, P.; Knuttinen, M.G. Deep venous thrombosis in pregnancy: Incidence, pathogenesis and endovascular management. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. S3), S309–S319. [Google Scholar] [CrossRef] [PubMed]
- Gris, J.-C.; Guillotin, F.; Chéa, M.; Bourguignon, C.; Bouvier, S. The Risk of Thrombosis Around Pregnancy: Where Do We Stand? Front. Cardiovasc. Med. 2022, 9, 901869. [Google Scholar] [CrossRef]
- Gurunath, S.; Vinekar, S.; Biliangady, R. Assisted Reproductive Techniques in a Patient with History of Venous Thromboembolism: A Case Report and Review of Literature. J. Hum. Reprod. Sci. 2018, 11, 193–197. [Google Scholar] [CrossRef]
- Sayyadi, A.; Mahdavi, M.; Dalfardi, B.; Robati, F.K.; Shafiepour, M. Right atrial thrombus and pulmonary thromboembolism related to ovarian hyperstimulation syndrome: A case report and literature review. Clin. Case Rep. 2023, 11, e7018. [Google Scholar] [CrossRef]
- Mitchell-Jones, N.; McEwan, M.; Johnson, M. Management of venous thromboembolism secondary to ovarian hyperstimulation syndrome: A case report documenting the first use of a superior vena caval filter for upper limb venous thromboembolism in pregnancy, and the difficulties and complications relating to anticoagulation in antenatal and peri-partum periods. Obstet. Med. 2016, 9, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Kearsley, R.; Stocks, G. Venous thromboembolism in pregnancy diagnosis, management, and treatment. BJA Educ. 2021, 21, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, P.G.; Westerlund, E.; Hellgren, M. Swedish obstetric thromboprophylaxis guideline: Background and update. J. Obstet. Gynaecol. 2023, 43, 2241527. [Google Scholar] [CrossRef] [PubMed]
- Queensland Clinical Guidelines. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0011/140024/g-vte.pdf (accessed on 11 February 2024).
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.-M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e691S–e736S. [Google Scholar] [CrossRef] [PubMed]
- Liew, N.C.; Alemany, G.V.; Angchaisuksiri, P.; Bang, S.-M.; Choi, G.; De Silva, D.A.; Hong, J.M.; Lee, L.; Li, Y.J.; Rajamoney, G.N.; et al. Asian venous thromboembolism guidelines: Updated recommendations for the prevention of venous thromboembolism. Int. Angiol. 2017, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.M.V.; da Fonseca Cerqueira, M.M.B.; Junqueira, P.L.; Gomez, M.T. Thromboprophylaxis during the Pregnancy-Puerperal Cycle—Literature Review. Rev. Bras. Ginecol. Obstet. 2020, 42, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-S.; Rey, E.; Kent, N.E.; VTE in Pregnancy Guideline Working Group. Venous thromboembolism and antithrombotic therapy in pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 527–553. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Middeldorp, S.; Rodger, M.; James, A.H.; Greer, I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 92–128. [Google Scholar] [CrossRef]
- ASH VTE Guidelines: Pregnancy. Available online: https://www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/pregnancy (accessed on 20 February 2024).
- ACOG Recommended Thromboprophylaxis for Pregnancies Complicated by Inherited Thrombophilias. Available online: https://www.ersebeszet.com/wp-content/uploads/2015/08/ACOG-thromboprophylaxis-recommendations.pdf (accessed on 20 February 2024).
- Wiegers, H.M.G.; Middeldorp, S. Contemporary best practice in the management of pulmonary embolism during pregnancy. Ther. Adv. Respir. Dis. 2020, 14, 1753466620914222. [Google Scholar] [CrossRef]
- Skeith, L. Prevention and management of venous thromboembolism in pregnancy: Cutting through the practice variation. Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 559–569. [Google Scholar] [CrossRef] [PubMed]
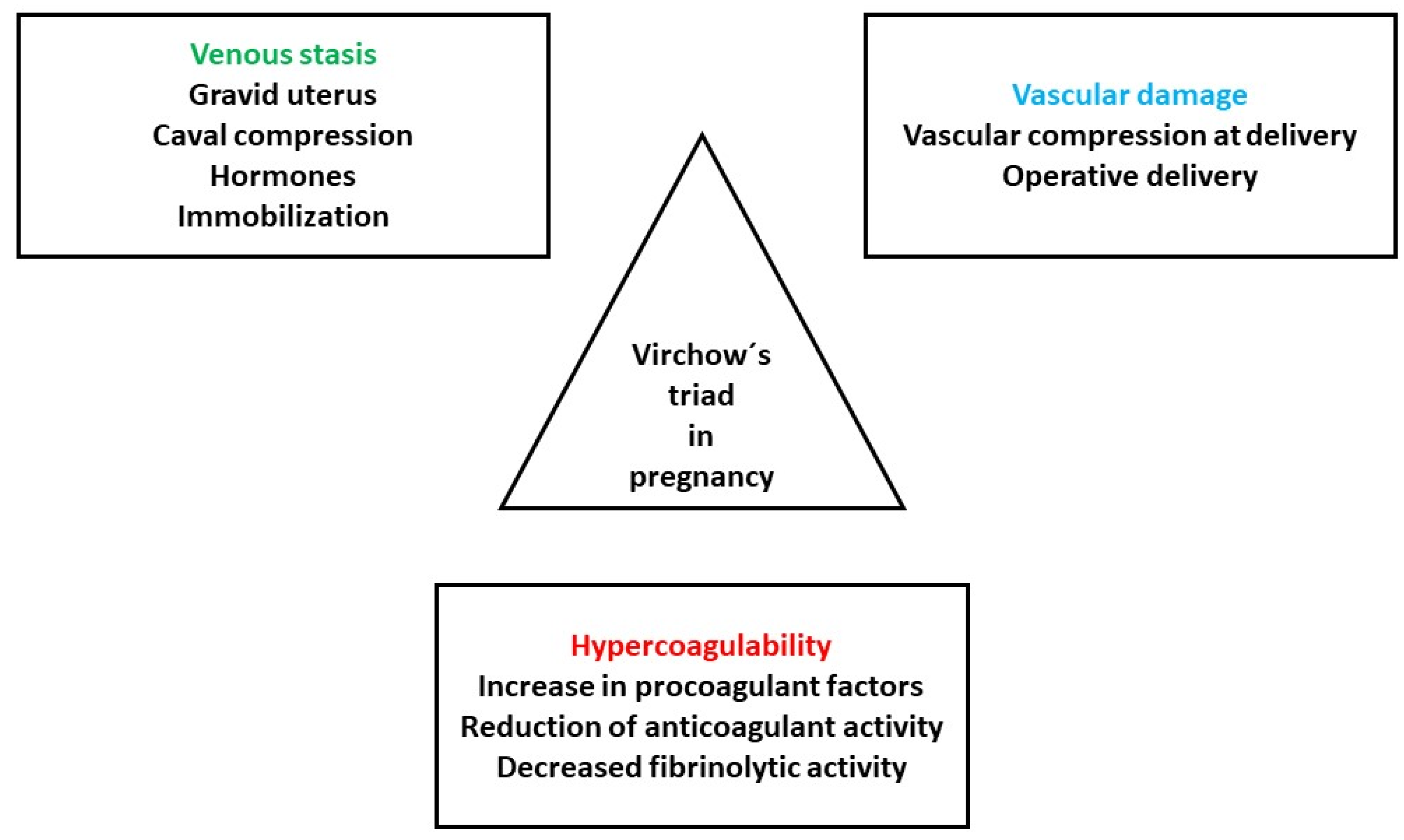
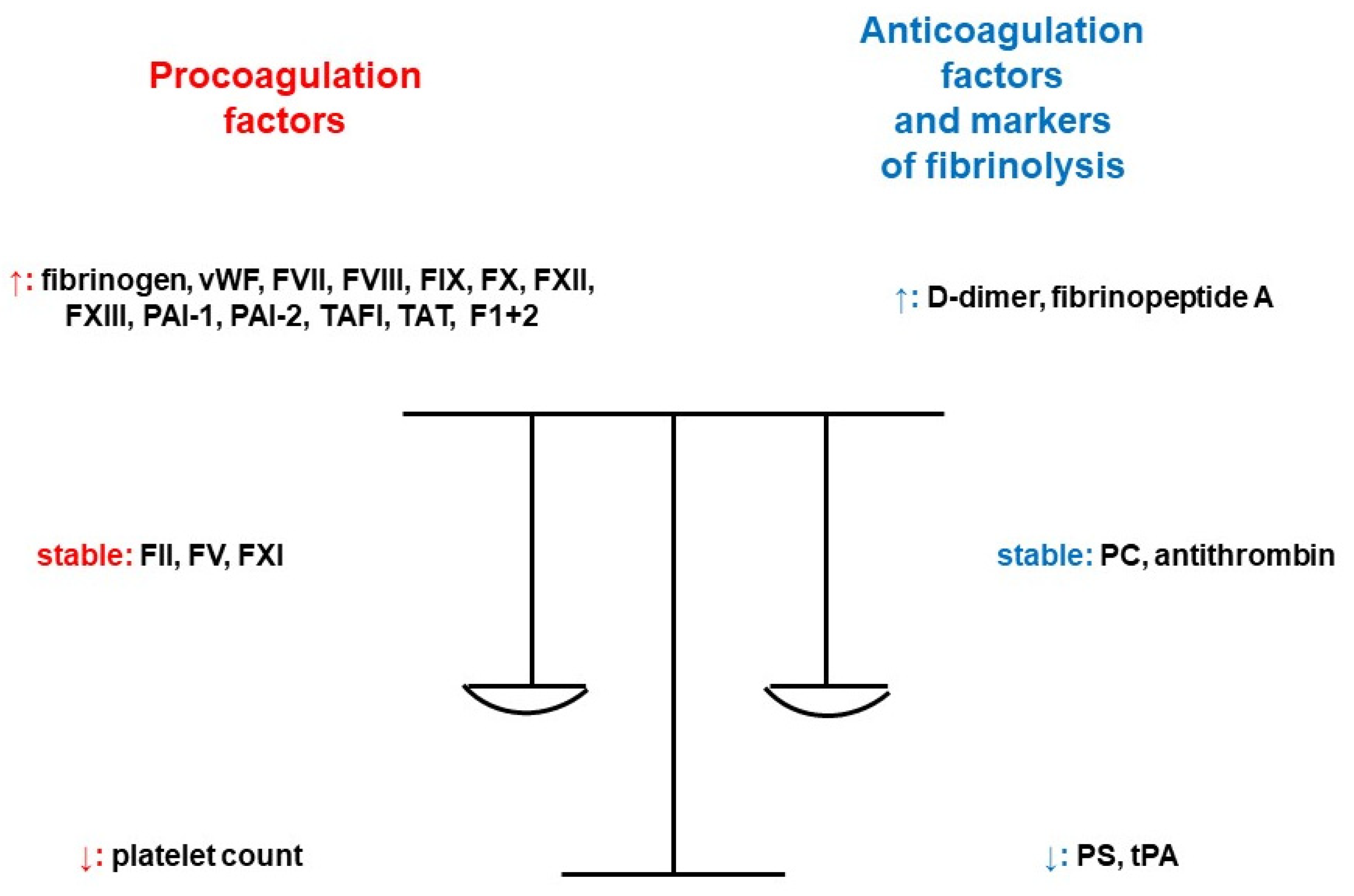
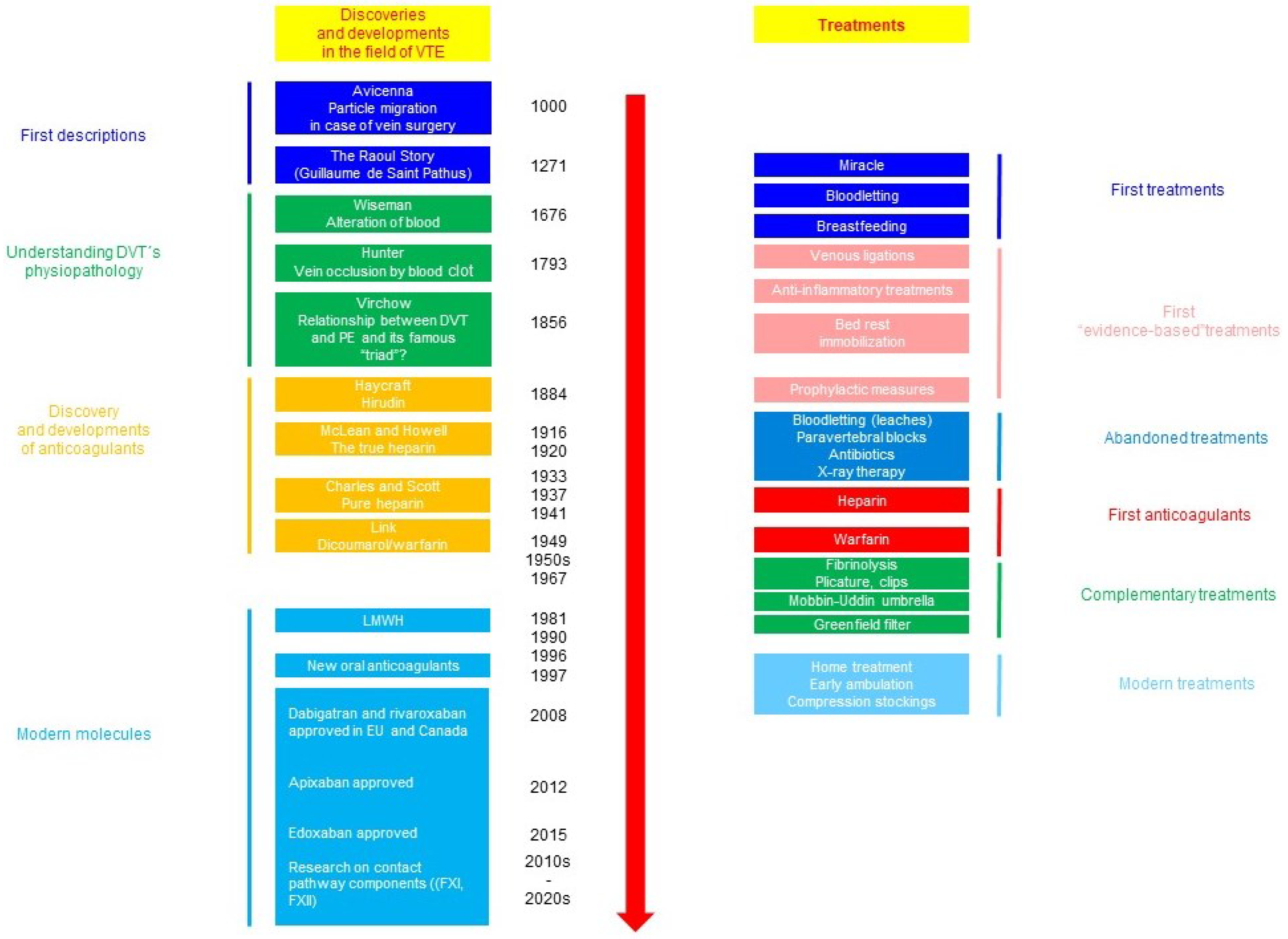
| Very High Risk Factors | Guideline | Recommendation |
|---|---|---|
| Chronic anticoagulation Antithrombin deficiency Repeated episodes of VTE Antiphospholipid syndrome with VTE COVID-19 pneumonia requiring oxygen | Swedish Society of Obstetrics and Gynecology (SFOG) | Antepartum thromboprophylaxis from the time of detection of pregnancy up to at least six weeks postpartum |
| High Risk Factors | Guideline | Recommendation |
| Previous VTE except an episode associated with major surgery | Royal College of Obstetricians and Gynecologists (RCOG) | Antenatal thromboprophylaxis with LMWH |
| Therapeutic anticoagulation before pregnancy | Queensland Clinical Guidelines (QCG) | Antenatal therapeutic anticoagulation with continuation up to 6 weeks after delivery |
| Previous VTE combined with high-risk thrombophilic state (antiphospholipid syndrome, antithrombin/PC/PS deficiency, homozygous form of factor V Leiden mutation, homozygous form of prothrombin mutation, compound heterozygous form of factor V Leiden and prothrombin mutation) Recurrent unprovoked VTE VTE during pregnancy | ||
| Previous VTE episode not provoked by surgical intervention Recurrent provoked episode of VTE Active inflammatory or autoimmune disorder Comorbidity (cancer, heart failure, nephrotic syndrome, type I diabetes mellitus complicated by nephropathy, sickle cell disease) | Antenatal thromboprophylaxis with LMWH from the first trimester continuing up to postpartum period | |
| Antenatal admission to the hospital OHSS during the first trimester Surgery in the course of pregnancy or postpartum period Severe hyperemesis or dehydration with the need for intravenous fluid | LMWH thromboprophylaxis during stay in the hospital or until this state is resolved | |
| Thrombophilic state with/without family history of VTE | Consideration of thromboprophylaxis during pregnancy and postpartum period | |
| Previous VTE Antiphospholipid syndrome without previous VTE OHSS | SFOG | Antepartum thromboprophylaxis from the time of detection of pregnancy up to at least six weeks postpartum |
| Immobilization (strict bed rest lasting 1 week) Postpartum hemorrhage with blood loss of ≥1 L with the need for surgery Postpartum infection Preeclampsia with IUGR Previous VTE Antithrombin deficiency Factor V Leiden mutation Prothrombin G20210A mutation Systemic lupus erythematosus (SLE) Sickle cell disease Blood transfusion Heart disease | American College of Chest Physicians (ACCP) |
|
| Intermediate Risk Factors | Guideline | Recommendation |
| Hospital admission Single prior VTE in relation to major surgery High-risk thrombophilic state (antithrombin, PS or PC deficiency, compound or homozygous state for low-risk thrombophilia) Comorbidities (cancer, active SLE, inflammatory bowel disease, inflammatory polyarthropathy, heart failure, type I diabetes mellitus associated with nephropathy, nephrotic syndrome, sickle cell disease, intravenous use of drugs) Surgical procedure OHSS during the first trimester | RCOG | Antepartum thromboprophylaxis with LMWH should be considered |
| Minor Risk Factors | Guideline | Recommendation |
| BMI > 30 kg/m2 Multiple pregnancies Postpartum hemorrhage > 1 L Smoking > 10 cigarettes/day IUGR Preeclampsia PC deficiency PS deficiency | ACCP | Please, see the recommendations for major risk factors for VTE |
| Further Risk Factors | Guideline | Recommendation |
| QCG | ||
| Prenatal risk factors | Risk score | Recommendation |
| Family history of unprovoked/estrogen-associated VTE | 1 | Score = 3: Thromboprophylaxis with LMWH from 28 weeks of gestation Score ≥ 4: Thromboprophylaxis with LMWH from the time of evaluation |
| Episode of VTE provoked by surgical intervention | 3 | |
| Age > 35 years | 1 | |
| Parity ≥ 1 | 1 | |
| Smoking | ||
| Gross varicose veins | 1 | |
| BMI 30−39 kg/m2 | 1 | |
| BMI ≥ 40 kg/m2 | 2 | |
| IVF/assisted reproductive technology | 1 | |
| Multiple pregnancies | 1 | |
| Preeclampsia | 1 | |
| Immobility | 1 | |
| Systemic infection | 1 | |
| Diabetes mellitus | 1 | |
| Postnatal risk factors | Risk score | Recommendation |
| Postnatal risk score = antenatal risk factors score + postnatal risk factors score 2 points: Thromboprophylaxis until discharge ≥3 points: thromboprophylaxis lasting 7 days/longer if ongoing risk | ||
| Cesarean section | 3 | |
| Elective cesarean section | 1 | |
| Labor lasting > 24 h | 1 | |
| Operative vaginal birth | 1 | |
| Preterm birth | 1 | |
| Peripartum hemorrhage > 1 L or requiring transfusion | 1 | |
| Stillbirth | 1 | |
| Cesarean hysterectomy | 3 | |
| SFOG | ||
| Risk factor | Risk score | Recommendation |
| Heterozygous factor V Leiden | 1 | 1 point: lifestyle advice 2 points: postpartum thromboprophylaxis at least 7 days and short-term, prophylaxis during the presence of temporary risk factor 3 points: postpartum thromboprophylaxis for at least six weeks |
| Heterozygous prothrombin mutation | 1 | |
| Age > 40 years | 1 | |
| BMI 30–40 | 1 | |
| VTE episode among first-grade relatives aged < 50 years | ||
| Inflammatory bowel disease | 1 | |
| Homocysteine > 8 mmol/L in the course of pregnancy | 1 | |
| Comorbidities (cancer and its treatment, SLE, heart disease, sickle cell disease, essential thrombocytosis) | 1 | |
| PS deficiency | 2 | |
| PC deficiency | 2 | |
| Fracture cast, immobilization due to strict bed rest or COVID-19 infection | 2 | |
| BMI > 40 | 2 | |
| Homozygous factor V Leiden mutation | 3 | |
| Homozygous prothrombin mutation | 3 | |
| Double mutation | 3 | |
| Postnatal risk factors | Risk score | |
| Preeclampsia | 1 | |
| Placental abruption | 1 | |
| Cesarean section | 1 | |
| Blood transfusion | 1 | |
| Stillbirth | 1 | |
| Severe infection/sepsis | 1 |
| Situation | American College of Obstetricians and Gynecologists (ACOG) | ACCP | American Society of Hematology (ASH) | The Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZOG) | Society of Obstetricians and Gynecologists of Canada (SOGC) |
|---|---|---|---|---|---|
| History of idiopathic VTE or VTE with hormones (estrogen) | Pharmacologic thromboprophylaxis of VTE during pregnancy and postpartum period | Pharmacologic thromboprophylaxis of VTE during pregnancy and postpartum period | Pharmacologic thromboprophylaxis of VTE during pregnancy and postpartum period | Pharmacologic thromboprophylaxis of VTE during pregnancy and postpartum period | Pharmacologic thromboprophylaxis of VTE during pregnancy and postpartum period |
| Previous VTE associated with a major reversible risk factor/ without thrombophilic state | Postpartum period: pharmacologic thromboprophylaxis in the presence of additional risk factors (cesarean section family history, etc.) | Postpartum period: pharmacologic thromboprophylaxis | Postpartum period: pharmacologic thromboprophylaxis | Postpartum period: pharmacologic thromboprophylaxis | |
| Previous recurrent VTE | Pharmacologic thromboprophylaxis during pregnancy and postpartum period | Pharmacologic thromboprophylaxis during pregnancy and postpartum period | Pharmacologic thromboprophylaxis during pregnancy and postpartum period | Pharmacologic thromboprophylaxis during pregnancy and postpartum period | |
| Woman receiving anticoagulants who becomes pregnant | Pregnancy: adjusted dose of LMWH/UFH Postpartum period: therapeutic anticoagulation with VKA/ LMWH | Pregnancy: LMWH in therapeutic dose or 75% of the dose Postpartum period: therapeutic anticoagulation with VKA/ LMWH | Pregnancy: LMWH in one dose or twice a day Postpartum period: UFH/LMWH/fondaparinux fondaparinux not recommended during breastfeeding (2C), VKA | Pregnancy: Therapeutic anticoagulation Postpartum period: Return to prepregnancy anticoagulation | |
| Doses of LMWH | Prophylactic, intermediate or adjusted dose during pregnancy and postpartum period | Prophylactic or intermediate dose during pregnancy and postpartum period | Pregnancy: Standard dose Postpartum period: Standard or intermediate dose | Prophylactic, intermediate or adjusted dose during pregnancy and postpartum period | Prophylactic, intermediate or adjusted dose during pregnancy and postpartum period |
| ASH | SOGC | RCOG | ACOG | ACCP | Society of Thrombosis and Haemostasis Research/Gesellschaft für Thrombose und Hämostaseforschung (GTH) | |
|---|---|---|---|---|---|---|
| Heterozygous form of factor V Leiden or prothrombin mutation | ||||||
| Antepartum | No | No | Yes, if additional risk factors from * with a total score of 3 are present—thromboprophylaxis throughout the pregnancy; if further risk factors from * with a total score of 2 are present, thromboprophylaxis from 28 weeks of gestation should be evaluated (D) | Surveillance or prophylactic LMWH/UFH | No (2C) | +/− |
| Postpartum | No | Thromboprophylaxis in the presence of 2 risk factors: BMI ≥ 30 kg/m2, (II-2B), smoking > 10 cigarettes/day (II-2B), preeclampsia (II-2B), preterm delivery (III-B), IUGR (II-2B), placenta previa (II-2B), emergency cesarean section (II-2B), blood loss of >1 L or need for transfusion (II-2B), stillbirth (III-B), comorbidity (cardiac disease, varicose veins, SLE, inflammatory disease, sickle cell disease, gestational diabetes mellitus (III-B). The length of such thromboprophylaxis should be 6 weeks (II-3B) | Thromboprophylaxis for at least 10 days after delivery if additional risk factor from * with a total score of 1 is present; If a woman has a positive family history of VTE, thromboprophylaxis ought to last for six weeks (D) | Surveillance or anticoagulation if there are further risk factors (first-degree relative with thromboembolic episode aged <50 years, obesity, prolonged immobilization) | Yes, if there is positive family history of VTE with prophylactic or intermediate dose of LMWH/ VKA) targeted at International Normalized Ratio (INR) 2–3 (2C) | +/− in the case of negative family history, otherwise yes |
| PC/PS deficiency | ||||||
| Antepartum | No | No prophylaxis with LMWH should be evaluated (D) | Thromboprophylactic LMWH/UFH | Surveillance | No (2C) | Yes |
| Postpartum | Yes, for women with a family history of VTE | Surveillance or thromboprophylaxis in the presence of 2 risk factors: BMI ≥ 30 kg/m2 (II-2B), smoking > 10 cigarettes/day (II-2B), preeclampsia 838 (II-2B), IUGR (II-2B), placenta previa (II-2B), emergency cesarean section (II-2B), blood loss of >1 L or need for transfusion (II-2B), preterm delivery (III-B), stillbirth (III-B), comorbidity cardiac disease, varicose veins, SLE, inflammatory disease, sickle cell disease, gestational diabetes mellitus (III-B). The length of such thromboprophylaxis should be 6 weeks (II-3B) | Yes with LMWH (D) | Surveillance or anticoagulation in the presence of further risk factors (first-degree relative with episode of VTE aged <50 years obesity, prolonged immobilization) | Prophylactic or intermediate dose of LMWH if a family history of VTE is positive (2C) | Yes |
| Compound heterozygosity | ||||||
| Antepartum | Yes | Yes with LMWH (IIIB) should be evaluated (D) | Thrombo-prophylaxis with LMWH | Prophylactic LMWH/UFH | No (2C) | Yes |
| Postpartum | Yes | Yes with LMWH (II-3B) | Yes with LMWH (D) | Anticoagulation | Yes, if there is positive family history of VTE with prophylactic or intermediate dose of LMWH/ VKA targeted at INR 2–3 (2C) | Yes |
| Homozygous form of factor V Leiden or prothrombin mutation | ||||||
| Antepartum | Yes for factor V Leiden mutation, for the prothrombin mutation only in the case of positive family history of VTE | Yes, LMWH (II-2A for factor V Leiden mutation, IIIB for prothrombin G20210A mutation) | Thromboprophylaxis with LMWH should be evaluated (D) | Prophylactic LMWH/ UFH | Prophylactic or intermediate dose of LMWH if a family history of VTE is positive (2B) | Yes |
| Postpartum | Yes | Yes, LMWH (II-2B) with the length of thromboprophylaxis 6 weeks (II-3B) | Yes, LMWH (D) | Anticoagulation | Yes, with prophylactic or intermediate dose of LMWH/ VKA targeted at INR 2–3 (2B) | Yes |
| Antithrombin deficiency | ||||||
| Antepartum | Yes, in the case of positive family history of VTE | Yes, LMWH (IIIB) | Yes, from at least 28 weeks of gestation; if further risk factor from those in * with a total score of 1 is present, beginning from the first trimester (D) | Prophylactic LMWH/UFH | No (2C) | Yes |
| Postpartum | Yes in the case of positive family history of VTE | Yes, LMWH (II-2B) lasting for 6 weeks after delivery (II-3B) | Yes, LMWH (D) | Anticoagulation | Yes with prophylactic or intermediate dose of LMWH/ VKA targeted at INR 2–3 (2C) | Yes |
| Aspect | Consideration |
|---|---|
| Context |
|
| Animal origin of heparin |
|
| Contraindication |
|
| Caution |
|
| Risk factors of bleeding |
|
| Regimen | Dosage |
|---|---|
| Prophylactic | 1st trimester: 5000–7500 IU subcutaneously (SC) twice daily 2nd trimester: 7500–10,000 IU SC twice daily 3rd trimester: 10,000 IU twice daily |
| Adjusted (according to aPTT) | 10,000 IU or more, SC twice daily—adjusted for aPTT to be between 1.5–2.5 times control 6 h after administration |
| Weight (kg) | Standard Prophylactic Dosage (IU SC) |
|---|---|
| <50 50–90 91–130 131–170 >170 | Consider reduced dosage 5000 twice a day 7500 twice a day |
| Weight (kg) | High prophylactic dosage (IU) * |
| <50 50–130 ≥130 | Consider reduced dosage 7500 twice a day 7500 thrice a day |
| Enoxaparin | ||||||
|---|---|---|---|---|---|---|
| Weight (kg) | Dosage (RCOG) | Dosage (SFOG) | Dosage (SOGC) | Dosage (QCG) | Dosage (ASH, ACCP) | Dosage (ACOG) |
| <50 kg | 20 mg/day | Very high risk of VTE (measurable anti-Xa activity before the next dose (≥0.05–0.1 IU/mL)): 20 mg twice a day | Prophylactic dose: 40 mg/day (30 mg twice a day) - obese patients: 60 mg/day | 40 mg/day | ||
| 50–90 | 40 mg/day | Very high risk of VTE: 40 mg twice a day | 40 mg/day | 40 mg/day | ||
| 91–130 | 60 mg/day | Very high risk of VTE: 60 mg twice a day | 60 mg/day | |||
| 131–170 | 80 mg/day | 80 mg/day | ||||
| >170 | 0.6 mg/kg/day | 0.5 mg/kg/day | ||||
| High prophylactic dose for patients weighing 50–90 kg | 40 mg twice a day | Intermediate dose *: 40 mg twice a day | High prophylactic dose for patients weighing < 50 kg%: 40 mg/day High prophylactic dose for patients weighing 50–130 kg%: 80 mg/day High prophylactic dose for patients weighing > 131 kg%: 60 mg twice a day | Intermediate dose &: 40 mg twice a day or 80 mg/day Weight-adjusted dose (ACCP): 1 mg/kg twice a day | Intermediate dose+: 40 mg twice a day | |
| Dalteparin | ||||||
| Weight (kg) | Dosage (RCOG) | Dosage (SFOG) | Dosage (SOGC) | Dosage (QCG) | Dosage (ASH, ACCP) | Dosage (ACOG) |
| <50 | 2500 IU/day | Very high risk of VTE: 2500 IU twice a day | Prophylactic dose: 5000 IU/day - >20 weeks of gestation: 5000 IU twice a day - obesity: 7500 IU/day | 2500 IU/day | 5000 IU/day | |
| 50–90 | 5000 IU/day | Very high risk of VTE: 5000 IU twice a day | 5000 IU/day | 5000 IU/day | ||
| 91–130 | 7500 IU/day | Very high risk of VTE: 7500 IU twice a day | 7500 IU/day | |||
| 131–170 | 10,000 IU/day | 10,000 IU/day | ||||
| >170 | 75 IU/kg/day | 75 IU/kg/day | ||||
| High prophylactic dose for patients weighing 50–90 kg | 5000 IU twice a day | Intermediate dose *: 100 IU/kg/day or 5000 IU twice a day | High prophylactic dose for patients weighing <50 kg%: 2500 IU twice a day High prophylactic dose for patients weighing 50–130 kg%: 5000 IU twice a day High prophylactic dose for patients weighing >131 kg%: 7500 IU twice a day | Intermediate dose &: 5000 IU twice a day or 10,000 IU/day | Intermediate dose+: 5000 IU twice a day | |
| <90 kg: 5000 IU/day | Weight-adjusted dose (ACCP): 200 IU/kg or 100 IU/kg twice a day | |||||
| >90 kg: 7500 IU/day | ||||||
| Tinzaparin | ||||||
| Weight (kg) | Dosage (RCOG) | Dosage (SFOG) | Dosage (SOGC) | Dosage (QCG) | Dosage (ASH, ACCP) | Dosage (ACOG) |
| <50 | 3500 IU/day | Very high risk of VTE: 2500 IU twice a day | 4500 IU/day - obesity: 75 IU/kg/ day | |||
| 50–90 | 4500 IU/day | Very high risk of VTE: 4500 IU twice a day | 4500 IU/day | |||
| 91–130 | 7000 IU/day | Very high risk of VTE: 4500 + 8000 IU/day | 75 IU/kg/day in the case of extreme body weight | |||
| 131–170 | 9000 IU/day | |||||
| >170 | 75 IU/kg/day | |||||
| Intermediate dose *: 4500 IU twice a day or 9000 IU/day | Intermediate dose &: 10,000 IU/day | |||||
| <90 kg: 4500 IU/day | Weight-adjusted dose (ACCP): 175 IU/kg/day | |||||
| >90 kg: 8000 IU/day | ||||||
| Nadroparin | ||||||
| Weight (kg) | Dosage (RCOG) | Dosage (SFOG) | Dosage (SOGC) | Dosage (QCG) | Dosage (ASH, ACCP) | Dosage (ACOG) |
| Prophylactic dose: 2850 IU/day | Prophylactic dose: 2850 IU/day | |||||
| Drug | Aspect | Consideration |
|---|---|---|
| LMWH | Fetus |
|
| Safety |
| |
| Monitoring |
| |
| Recommendation |
| |
| UFH | Fetus |
|
| Safety |
| |
| Monitoring |
| |
| Recommendation |
| |
| Warfarin | Consideration |
|
| Fondaparinux | Consideration |
|
| Danaparoid | Consideration |
|
| DOACs | Consideration |
|
| Acetylsalicylic acid (ASA) | Consideration |
|
| Method | Application | Limitation |
|---|---|---|
| aPTT |
|
|
| anti-Xa activity |
|
|
| Point-of-care: Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) |
|
|
| Feature | ASH (2018) | RCOG (2015) | SOGC (2014) | ACCP (2012) | ANZJOG (2012) |
|---|---|---|---|---|---|
| Elective cesarean delivery | In patients with no or 1 risk factor (excluding thrombophilic state or previous VTE), no antepartum and postpartum, thromboprophylaxis is suggested | no | no | no (1B) | no |
| Emergent cesarean delivery | All patients who had cesarean section ought to be considered (C) for thromboprophylaxis with LMWH lasting for 10 days after delivery except women undergoing an elective cesarean delivery, who ought to be considered for prophylaxis for 10 days following delivery if they have any further risk factors (C) | no | Thromboprophylaxis with LMWH/ UFH for ≥5 days until recovery of full mobility (evidence level 1 with agreement of all authors of the guideline) | ||
| Elective cesarean delivery and risk factors | Not stated | See above (consider prophylaxis with LMWH for 10 days following delivery) (C) | Postpartum prophylaxis ought to be considered in the presence of 2 risk factors (emergency cesarean section is 1 risk factor) (II-2B), LMWH up to 2 weeks if 2 risk factors are present * | In patients with 1 major or ≥2 minor risk factors, prophylactic LMWH or mechanic prophylaxis in the case of contraindication to anticoagulants during stay in the hospital is recommended (2B) & | ≥1 major and ≥2 minor risk factors: thromboprophylaxis for ≥5 days or until fully mobile @ 1 major and 2 minor risk factors: consider graduated compression stockings@ |
| Emergent cesarean delivery and risk factors | Not stated | See above (C) | Postpartum prophylaxis in the presence of any 3 or more risk factors (elective cesarean section is 1) (II-2B), LMWH up to 2 weeks if 1 risk factor is present * | Presence of at least 1 major risk factor or at least 2 minor risk factors (planned cesarean section) or 1 minor risk factor in the case of an emergency cesarean delivery & | Thromboprophylaxis with LMWH or UFH for at least 5 days or longer until restoration of full mobility (group consensus of all authors, level of evidence 1) @ |
| Drug | Intrapartum | Postpartum | |
|---|---|---|---|
| Elective | Urgent and emergent | ||
| Subcutaneous UFH | Low-dose UFH thromboprophylaxis 5000 IU twice a day or 5000 IU thrice a day: pause for 4–6 h before NB or assessment of coagulation status (IIa C-EO) Intermediate-dose UFH thromboprophylaxis (7500 IU twice a day or 10,000 IU twice a day): pause 12 h and assessment of coagulation parameters before NB (IIa C-EO) High-dose UFH (individual dose >10,000 IU or >20,000 IU daily dose): pause 24 h before NB and assessment of coagulation parameters (IIa C-EO) | Low-dose UFH thromboprophylaxis: pause 4–6 h following the last dose before NB or assessment of coagulation parameters; in urgent situation, with greater risk of general anesthesia in the comparison with NB, neuraxial procedure is possible (IIa C-EO) Intermediate-dose UFH thromboprophylaxis: pause 12 h following the last dose before NB and assessment of coagulation status - In urgent situation, neuraxial procedure rather than general anesthesia is preferred (IIa C-EO) High-dose UFH: if it is ≥24 h since the last dose, and normal coagulation parameters were obtained (normal aPTT or undetectable antiXa activity), NB is possible (IIb C-EO) | UFH thromboprophylaxis: pause ≥ 1 h after NB and after catheter removal before UFH administration - Indwelling catheters might be maintained with low dose of UFH (5000 IU twice a day) - Pause non-steroidal anti-inflammatory drugs, not acetaminophen until catheter removal, if using thromboprophylaxis |
| Intravenous UFH | Stop infusion for 4–6 h and then assess coagulation status before NB (IIa C-EO) | Pause ≥ 1 h after NB before restarting anticoagulation (IIb C-EO) | |
| LMWH | Low-dose LMWH thromboprophylaxis (enoxaparin ≤ 40 mg once a day or 30 mg twice a day, or dalteparin 5000 IU once a day): pause ≥ 12 h before placing NB (I C-EO) Intermediate-dose LMWH thromboprophylaxis (enoxaparin > 40 mg once a day or 30 mg twice a day and <1 mg/kg twice a day or 1.5 mg/kg once a day or dalteparin > 5000 IU once a day and <120 IU/kg twice a day or 200 IU/kg once a day): potential pause 12–24 h before NB (IIb C-EO) Higher dose of LMWH (enoxaparin 1 mg/kg twice a day or 1.5 mg/kg once a day; dalteparin 120 IU/kg twice a day or 200 IU/kg once a day): pause ≥ 24 h before NB (I C-EO) | Low-dose LMWH thromboprophylaxis if pause ≥ 12 h, low risk of NB (I C-EO); if pause < 12 h before NB, insufficient data to recommend NB (IIb C-EO) - In high-risk situation, risk of general anesthesia is greater than NB (IIb C-EO) Intermediate-dose LMWH thromboprophylaxis: insufficient data to specify pause 12–24 h before NB (IIb C-EO) Higher dose of LMWH: if pause ≥ 24 h, low risk for NB (I C-EO); if pause < 24 h, insufficient data to recommend NB (IIb C-EO) | Low-dose LMWH thromboprophylaxis pause ≥ 12 h after NB and ≥4 h after catheter removal before restarting LMWH - Indwelling catheters might be maintained with low dose of LMWH: - Removal can take place ≥ 12 h after a last LMWH dose, and next dose of LMWH can be administered in ≥4 h (I C-EO) - Pause non-steroidal anti-inflammatory drugs, not acetaminophen, until removal if using thromboprophylaxis (IIa C-EO) Higher dose of LMWH: pause ≥ 24 h after NB and ≥4 h after catheter removal before restarting LMWH thromboprophylaxis (I C-EO) |
| SOGC | QCG | ACCP | ANZJOG | |
|---|---|---|---|---|
| Last dose to neuraxial block | Hours to wait | |||
| LMWH | 10–12 | 12 | 24 (1B) | 12 |
| UFH | Maximally 10,000 IU/day; no pause unless abnormal coagulation parameters are obtained | 4 (not in the case of dosages >5000 IU twice or thrice a day) | 24 in patients using adjusted dose of subcutaneous UFH (twice daily) (1B) | |
| Neuraxial block to next dose | Hours to wait | |||
| LMWH | 6–8 >24 if bleeding during NB | 4 | ||
| UFH | 1–8 | 1 (not in the case of dosages >5000 IU twice or thrice a day) | ||
| Last dose to catheter removal | Hours to wait | |||
| LMWH | 10–12 | 12 | 12 | |
| UFH | 4 | 4 (not in the case of dosages >5000 IU twice or three times a day) | ||
| Catheter removal to the next dose | Hours to wait | |||
| LMWH | >4 | 4 | ||
| UFH | 1 (not in the case of dosages >5000 IU twice or three times a day) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stančiaková, L.; Brisudová, K.; Škorňová, I.; Bolek, T.; Samoš, M.; Biringer, K.; Staško, J.; Sokol, J. Evaluating Thromboprophylaxis Strategies for High-Risk Pregnancy: A Current Perspective. Pharmaceuticals 2024, 17, 773. https://doi.org/10.3390/ph17060773
Stančiaková L, Brisudová K, Škorňová I, Bolek T, Samoš M, Biringer K, Staško J, Sokol J. Evaluating Thromboprophylaxis Strategies for High-Risk Pregnancy: A Current Perspective. Pharmaceuticals. 2024; 17(6):773. https://doi.org/10.3390/ph17060773
Chicago/Turabian StyleStančiaková, Lucia, Kristína Brisudová, Ingrid Škorňová, Tomáš Bolek, Matej Samoš, Kamil Biringer, Ján Staško, and Juraj Sokol. 2024. "Evaluating Thromboprophylaxis Strategies for High-Risk Pregnancy: A Current Perspective" Pharmaceuticals 17, no. 6: 773. https://doi.org/10.3390/ph17060773
APA StyleStančiaková, L., Brisudová, K., Škorňová, I., Bolek, T., Samoš, M., Biringer, K., Staško, J., & Sokol, J. (2024). Evaluating Thromboprophylaxis Strategies for High-Risk Pregnancy: A Current Perspective. Pharmaceuticals, 17(6), 773. https://doi.org/10.3390/ph17060773







