3.1. Materials and Animals
Plinabulin (purity ≥ 98%) was purchased from Beijing Solarbio Technology Co., Ltd. (Beijing, China). Propranolol (purity ≥ 99%, internal standard, IS) was obtained from Sigma-Aldrich (St. Louis, MO, USA). HS-15 was purchased from Shanghai BASF Co., Ltd. (Shanghai, China), and propylene glycol was obtained from Beijing Fengli Jingqiu Pharmaceutical Co., Ltd. (Beijing, China) HPLC-grade acetonitrile and HPLC-grade methanol were purchased from Adamas-beta (Shanghai, China). HPLC-grade formic acid was from Fisher, USA. Cyclophosphamide (CY) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Deionized water was from Watsons professional drinking water.
Forty-eight Wistar rats (males, weight 180 ± 20 g) were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. (Shandong, China). The experimental animal production license number is SCXK (Lu) 2022006. All Wistar rats were maintained in the SPF animal room with a temperature of 22 ± 2 °C and a humidity of 60 ± 5% for 12 h light-dark cycles. Animals were allowed to take in rodent food and drink water freely before intravenous administration. The animal study protocol was approved by the Animal Experimental Ethical Committee of Ocean University of China (Permit NO. OUC-AE-2022-109, and the date of approval was 7 March 2022).
3.2. Methods
3.2.1. Establishment of the Leukopenia Rat Model
On the first and third days of the experiment, rats were intraperitoneally injected with the anticancer drug CY at 50, 70, and 90 mg/kg, respectively, to select the appropriate dose for establishing the leukopenia model. Meanwhile, rats in the control group were intraperitoneally injected with the same volume of saline. Blood (20 μL) was collected at 12 h after the last injection, and the white blood cells (WBCs) were counted by an IDEXX ProCyte Dx Hematology Analyzer (Shanghai IDEXX Laboratories Co., Ltd.) (Shanghai, China).
3.2.2. UHPLC-MS/MS Assay
Sample analysis was performed on an Agilent 1290 system consisting of a G7120A high-speed pump, a G7167B auto-sampler, and a G7116B MCT column oven. Plinabulin and IS were detected by Agilent G6460C electrospray ionization (ESI) triple mass spectrometry. The chromatographic column was Capcell Pak C18 (2.0 × 50 mm, 5 μm) at 30 °C. The mobile phase consisted of a mixture of water (A, containing 0.2% (v/v) formic acid) and acetonitrile (B, containing 0.2% (v/v) formic acid) at a flow rate of 0.4 mL/min with a gradient elution procedure as follows: 0–0.5 min, 5% B; 0.5–2.0 min, 5–35% B; 2.0–3.0 min, 35–95% B; 3.0–3.5 min, 95% B; 3.5–3.8 min, 95–5% B; 3.8–5.0 min, 5% B. The volume of injection was 2 μL, and the sample temperature was maintained at 20 °C.
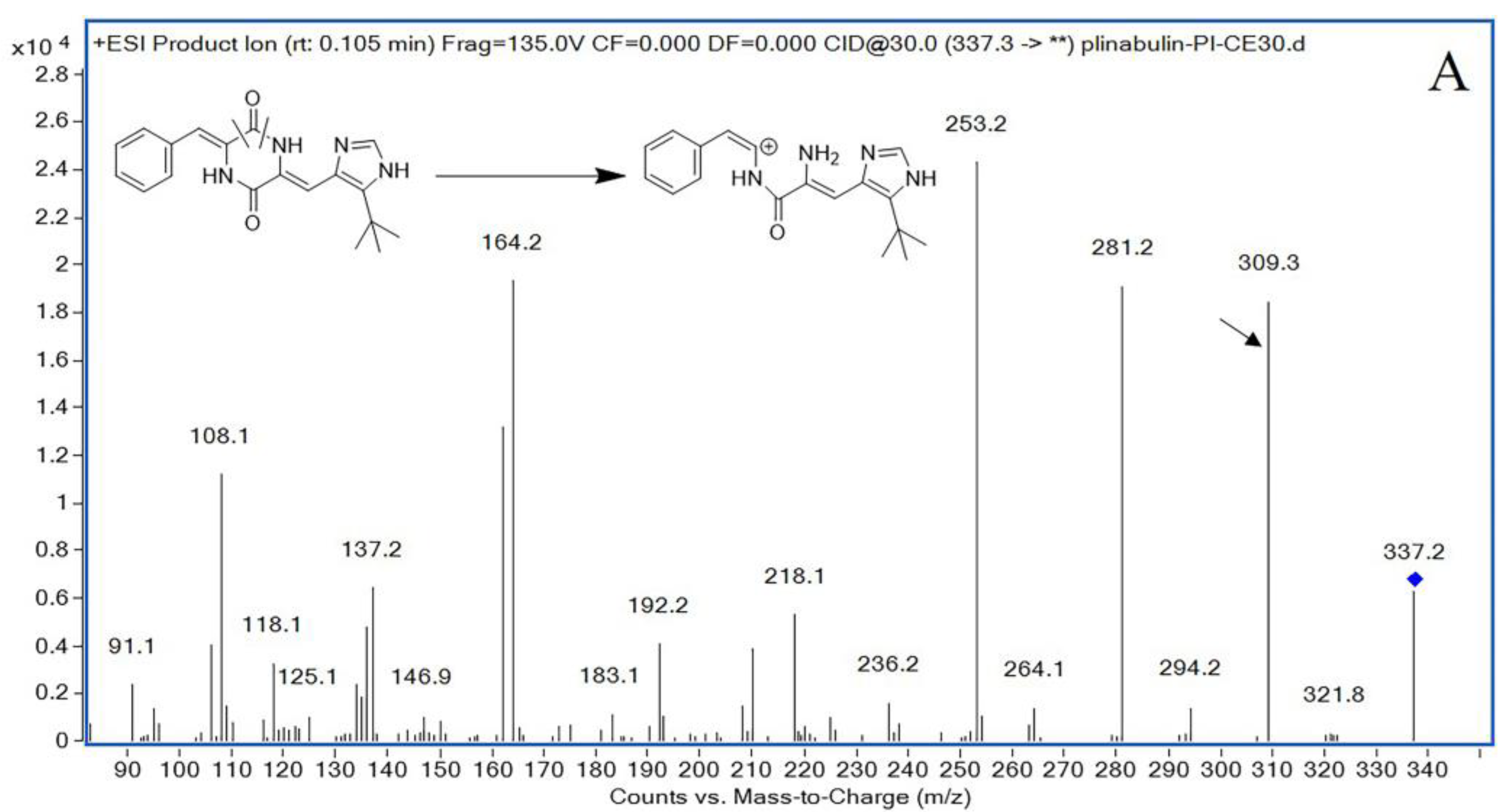
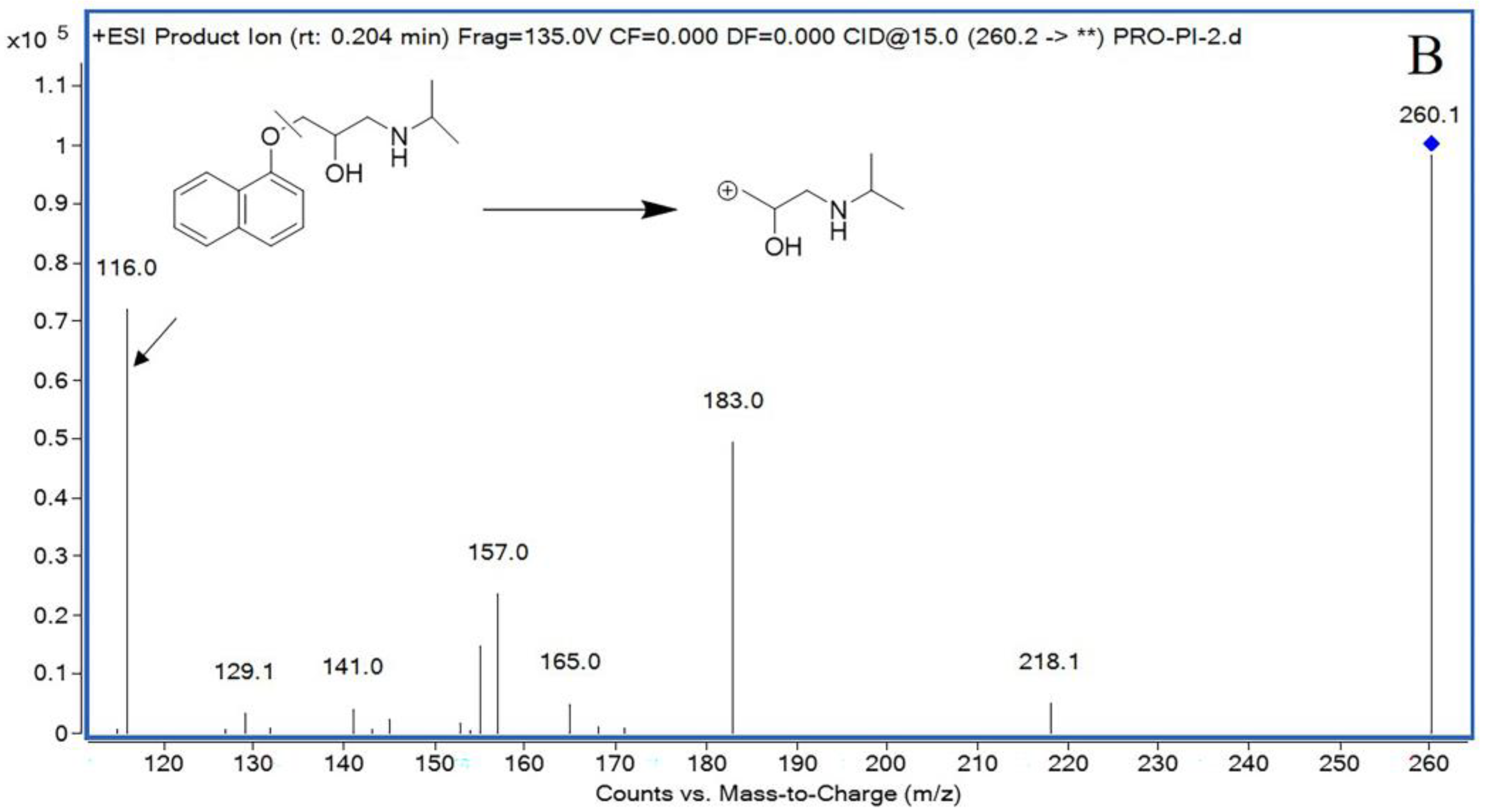
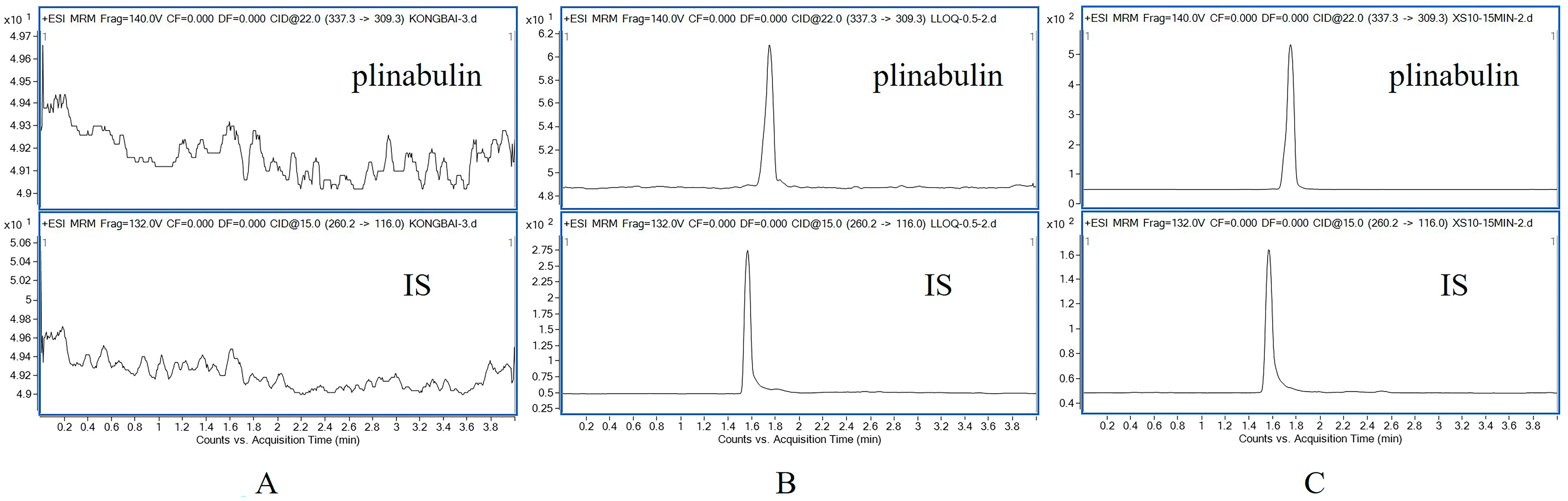
The mass spectrometer was set in a positive ionization pattern under multiple reaction monitoring (MRM) mode, and the optimized mass spectrum conditions were as follows: gas temperature: 350 °C; gas flow rate: 11 L/min; sprayer: 30 psi; and capillary voltage: 4 kV. The fragmentor was optimized to 140 V for plinabulin and 132 V for IS. Plinabulin and IS had collision energies of 22 V and 15 V, respectively. The precursor ion and product ion were m/z 337.3 → 309.3 for plinabulin, and m/z 260.2 → 116.0 for IS, respectively.
3.2.3. Standard and Quality Control (QC) Sample Preparations
Working solutions and QC solutions for plinabulin were prepared by dissolving plinabulin into a freshly prepared stock solution of 10 mg/mL with DMSO (dimethyl sulfoxide) and serially diluting the stock solution with 60% acetonitrile. The most ideal internal standard should be the isotopically labeled plinabulin compound. Due to the double bond in the structure of plinabulin, cis-trans isomerization occurs easily under light conditions [
33]. Isomerization also occurs in isotopically labeled plinabulin and these isomeric compounds are not stable [
34]. Due to its high stability, propranolol has been widely used as a universal internal standard in the analysis of biological samples [
35,
36,
37,
38,
39]. Hence, to eliminate the systematic error of plinabulin content determination, propranolol was chosen as IS for quantification. Besides high stability, propranolol presents chromatographic properties similar to plinabulin, reinforcing its application as IS in the present study. Propranolol solution with a concentration of 20 ng/mL was prepared with acetonitrile and stored at 4 °C until further analysis.
Ten calibration samples and QC samples were prepared by adding 10 μL of a 10-fold concentration of calibrators to 100 μL blank rat plasma. The mixture was vortexed for 30 s, and concentrations of the ten calibration samples of plinabulin in plasma were 0.5, 1, 2, 5, 10, 20, 50, 100, 200, and 500 ng/mL, respectively. QC samples were prepared at four concentrations of 0.5 ng/mL (lower limit of quantification, LLOQ), 1 ng/mL (low-quality control, LQC), 100 ng/mL (middle-quality control, MQC), and 400 ng/mL (high-quality control, HQC) with independent preparation, and then 200 μL acetonitrile containing IS (20 ng/mL) was added to precipitate protein. After vortexing for 1 min and centrifugation at 14,000 rpm for 10 min at 4 °C, 100 μL supernatant was transferred into the auto-sampler vials for UHPLC-MS/MS analysis with 2 μL injection volume.
3.2.4. Plasma Sample Preparation
All rat plasma samples were thawed at room temperature before analysis. To a 100 μL plasma sample was added 10 μL 60% acetonitrile, then 200 μL acetonitrile containing IS (20 ng/mL) was added to precipitate protein. The mixture was vortex-mixed for 1 min and centrifuged at 14,000 rpm for 10 min at 4 °C. Finally, 100 μL supernatant was collected into the auto-sampler vials for UHPLC-MS/MS analysis with 2 μL injection volume.
3.2.5. Method Validation
The method was validated according to the guidelines of ICH M10 Bioanalytical Method Validation and Study Sample Analysis (24 May 2022) [
21].
The selectivity of plinabulin was determined by analyzing the blank rat plasma spiked with plinabulin and IS and rat plasma collected at 15 min after intravenous administration of a 3 mg/kg dose of plinabulin to explore the potential endogenous interference between analytes.
Linearity was evaluated by plotting the plinabulin/IS response ratios versus the concentrations of plinabulin. The calibration curves ranged from 0.5 to 500 ng/mL with ten points fitted y = bx + c by weighting factor 1/x2. Every standard curve solution was freshly prepared in every validation and sample detection. The calibration curves were accepted if R2 > 0.99 (R2, coefficient of determination). The sensitivity was evaluated by LLOQ samples, which was the lowest calibration curve concentration. The RE of LLOQ should be within ±20%, and RSD should not exceed 20%. The signal-to-noise ratio (S/N) was >10.
The accuracy and precision were estimated by six replicates of LLOQ, LQC, MQC, and HQC samples (0.5, 1, 100, and 400 ng/mL, respectively) in intra- and inter-day batches. The accuracy and precision of the inter-day measurements were assessed by investigating three batches of LLOQ, LQC, MQC, and HQC samples on three continuous days. Precision was expressed as a percentage (%) of RSD. The accuracy should be within ±15% for QC samples and within ±20% for LLOQ. The precision should be within 15% for QC samples and within 20% for LLOQ.
The dilution integrity of the bioassay was determined by using blank Wistar rat plasma diluted 20-fold. The RE % and RSD % should be less than 15%.
The extraction recovery was determined by comparing the peak area of plinabulin in the supernatant of rat blank plasma with that of precipitated blank plasma at three QC levels, and each QC sample was set up in 6 replicates. The RSD (%) of the peak area ratio should be less than 15%. The matrix effect was determined by determining the peak area of plinabulin in the supernatant of the precipitated blank rat plasma by acetonitrile (n = 6) with those in 60% acetonitrile at three QC levels. The RSD (%) of the peak area ratio should be less than 15%.
The stability of plinabulin in rat plasma was evaluated by analyzing six replicates of plasma samples at LQC and HQC levels under different storage conditions. The short-term stability was assessed by analyzing the samples in plasma at room temperature for 6 h. LQC and HQC samples were stored at −80 °C for 28 consecutive days to assess long-term stability. The stability of the freeze–thaw cycle was performed by analyzing samples in plasma through three freeze–thaw cycles from −80 °C to room temperature. The stability of processed samples in the auto-sampler was evaluated after maintaining post-preparative samples in the auto-sampler for 24 h. The stability of the samples was evaluated by comparing them with corresponding spiked concentrations of analytes. The RE should be within ±15%, and RSD < 15%.
3.2.6. Pharmacokinetics Study
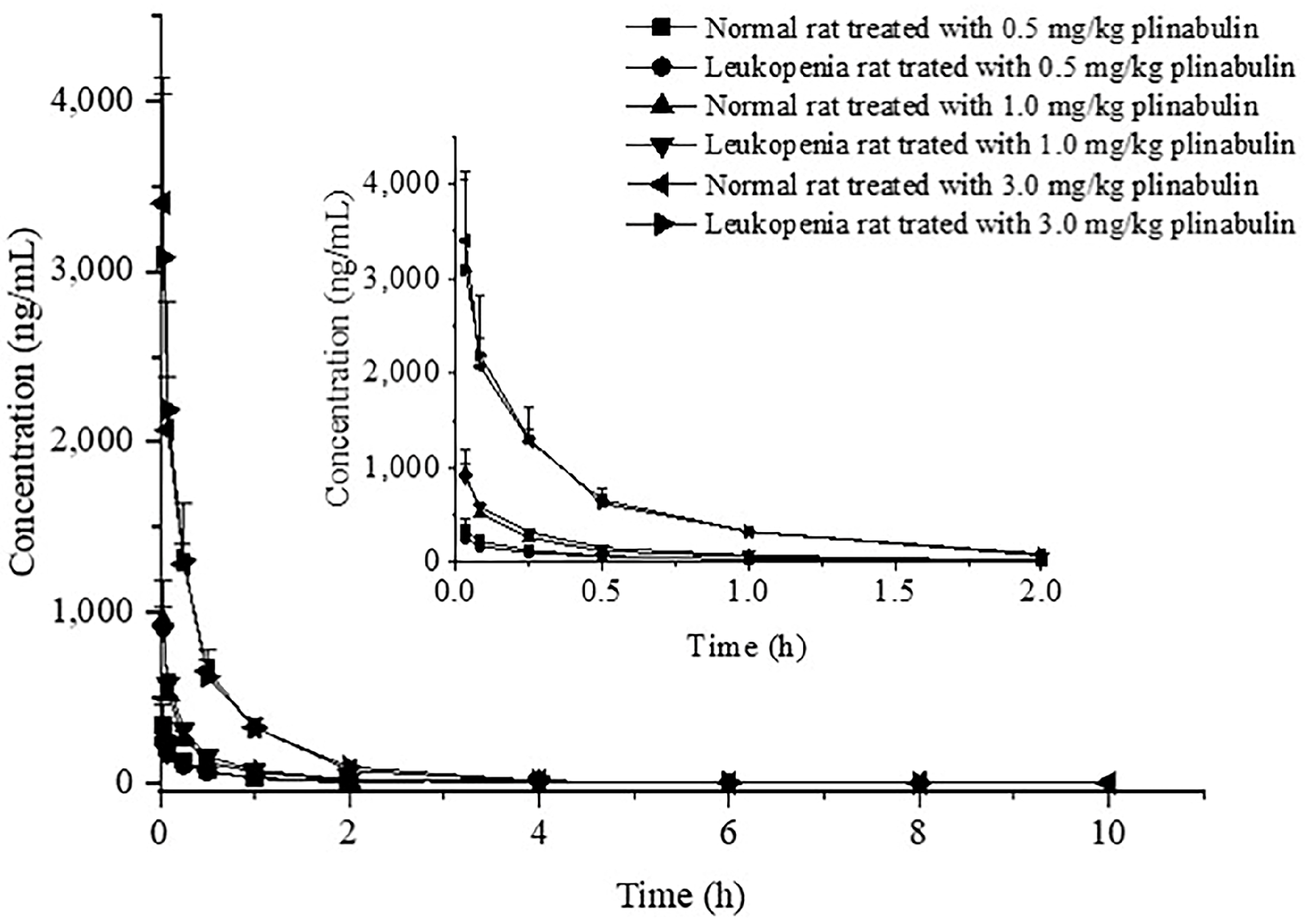
Thirty-six Wistar rats (18 normal rats and 18 leukopenia rats) were randomly divided into six groups (n = 6 per group). They received 0.5, 1, or 3 mg/kg plinabulin intravenously, respectively. The rats were given free water before intravenous administration. A single dose of plinabulin was administered to each group and an equivalent of 0.3 mL blood sample was collected into heparinized Eppendorf tubes by post-orbital venous sinus before dosing and subsequently at 2 min, 5 min, 15 min, 0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h, and 10 h after intravenous administration. Blood samples were centrifuged at 8000 rpm for 10 min at 4 °C, and the supernatants were collected. The plasma samples were stored at −20 °C until further analysis. A 100 μL plasma sample was added with 10 μL 60% acetonitrile, then 200 μL acetonitrile containing IS (20 ng/mL) was added to precipitate protein. The mixture was vortex-mixed for 1 min and centrifuged at 14,000 rpm for 10 min at 4 °C. Finally, the supernatants were collected into the auto-sampler vials for UPLC–MS/MS analysis.