2.1. Characterization of SEEU and SETM
The phytochemical characterization of SEEU and SETM is presented in
Table 1 and
Table 2, respectively.
Because part of the extract may be composed of structural materials originating from primary metabolism and not directly related to biological activities [
26], the preparation methodology was based on protein precipitation, a fact that tends to decrease the overall yield of the extractive process but increases the bioactive content, facilitating its binding on the target site. Additionally, ethyl alcohol is used as a solvent because it is accessible, obtained from a renewable source (sugarcane), classified as GRAS (generally recognized as safe), and is suitable for an approach to plant extract production [
27].
In SEEU, flavonoids constitute the most representative class among the compounds identified, which is similar to the findings of Sobral-Souza et al. [
28]. In this study, the authors point out that the extract of
E. uniflora is a promising source of flavonoids, which have a cytoprotective effect due to their antioxidative capacity.
Astragalin (kaempferol-3-O-glucoside) belongs to the class of glycosylated flavonoids and is the primary compound in SETM, as reported elsewhere by Gasparotto Junior et al. [
14]. In this research, the authors evaluated the antihypertensive activity of isoquercitrin and the hydroethanolic extract of
T. majus. Phytochemical identification verified high levels of isoquercitrin and kaempferol glycoside in both the extract and the semi-purified fraction.
Flavonoids act as antioxidants in the inactivation of reactive oxygen and nitrogen species [
29] and exhibit anti-inflammatory, immunomodulatory [
26], and antimicrobial activities [
30], showing broad pharmacological potential. The combined and individual antioxidative capacities of SEEU and SETM were measured using 2,2-diphenyl-1-picryhydrazyl (DPPH), ferric reducing ability of plasma (FRAP), and 2,2-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS
•+) (
Table 3). The theoretical value of the combination was estimated from the individual values and the proportions used to prepare the FFS.
The total amount of phenolic compounds and flavonoids directly influences the antioxidant ability; when the content of these bioactive compounds increases, the reducing potential tends to increase [
31]. Phenol derivatives have antioxidant properties both at the beginning and during the oxidative process by neutralizing or scavenging free radicals [
32], justifying the antioxidative capacity observed in this study, where several bioactive compounds of the phenolic class, such as quercetin, astragalin, and myricetin, were present during phytochemical identification. Furthermore, the scavenging potential of DPPH and FRAP radicals and the reducing potential of FRAP in SEEU and SETM were higher than those previously reported for
E. uniflora [
33] and
T. majus [
34].
During the combined antioxidant analysis of SEEU and SETM, an antagonistic effect for DPPH and synergistic effects for the FRAP and ABTS
•+ methods were observed. The synergistic mechanism of the antioxidative capacity has not yet been elucidated due to the complex nature of the mixtures, mainly of plant extracts. However, some issues can be considered to justify this effect, namely, regeneration of the most effective antioxidant response, formation of stable intermolecular complexes, formation of phenolic products with more significant potential for reduction, and the occurrence of unexpected interactions between the compounds [
35].
Oxidative stress impacts the inflammatory response; therefore, the presence of antioxidants can provide a favorable environment for the healing process [
36]. Plant extracts with antioxidative capacity are promising for incorporation into auxiliary formulations for the healing process and tissue reconstitution. The combination of SEEU and SETM increased the antioxidant effect through FRAP and ABTS
•+ mechanisms, justifying the use of the blend for the development of FFS.
The minimum inhibitory concentration (MIC) of SEEU and SETM (
Table 4), individually and in combination, was evaluated using the checkerboard model.
The hydroalcoholic leaf extracts of
E. uniflora had MIC of 12.5, 15.0, and 17.5 mg mL
−1 for
Staphylococcus aureus,
Escherichia coli, and
Candida albicans, respectively [
31]; the results from this study show a similar range. Few studies have investigated the antimicrobial activity of
T. majus leaf extract, and the MIC has not been evaluated [
37]; however, antimicrobial activity against
S. aureus,
E. coli,
Pseudomonas aeruginosa, and
C. albicans was observed for both aqueous and ethanolic extracts.
The biological activities of extracts are directly influenced by the classes of compounds they contain [
38]. Therefore, the combination of extracts exhibited increased antioxidant and antimicrobial potential against the microorganisms tested. Both SEEU and SETM had an inhibitory effect against the evaluated strains. However, because the Inhibitory Fractional Concentration Index (FICI) value was greater than 0.5, no synergistic effect of enhanced antimicrobial activity was observed. No antagonistic effect was observed (FICI > 4) for the combination. Therefore, considering the presence of a variety of bioactive compounds in SEEU and SETM, their association may exert complementary effects for the treatment of pathologies [
39].
Based on the antioxidant and antimicrobial effects of SEEU and SETM, FFS loaded with SEEU and SETM was bioprospected.
2.2. Characterization of FFS
The experimental approach consisted of simultaneously evaluating the influence of the concentrations of PVP and PVA polymers in the system because these variables can influence each other, and their ideal values may be interdependent [
40].
The organoleptic characteristics allowed for observing the formation of films with an adhesive character, regardless of the concentrations of PVA and PVP (
Table 5). Adherence is an important aspect because it guarantees a prolonged period of contact between the formulation and the skin tissue, increasing the efficiency in delivering the active compounds to the site [
41]. In addition, it reduces the risk of transfer of the active compounds to other individuals or clothes [
3].
The control FFSs were transparent and had a drying time shorter than or close to that of the film loaded with SEEU and SETM, which showed a greenish-yellow color, characteristic of plant materials. Formulations 1 and 2 (F1 and F2, respectively) exhibited a scaly appearance, which can be attributed to the low concentration of PVA in the system. PVA is widely used owing to its high mechanical resistance and elasticity, non-toxicity, and bioadhesion, which constitute better pharmacotechnical parameters for the development of formulations [
23]. The presence of scales in a formulation leads to detachment during movement; therefore, this would not be ideal for an optimized formulation.
The FFS formulations 3, 4, and 5 (F3, F4, and F5, respectively) showed an opaque, non-scaly, flexible, and non-sticky appearance (
Figure A1), ensuring prolonged adhesion to the site and the release of active compounds for a longer period [
42]. When evaluating the effect of the variables (PVA and PVP) on the drying time, no marked differences were observed between the FFS loaded with SEEU and SETM; however, F4 and F5 presented a drying time close and inferior to that of F3.
For physical–chemical characterization, the pH, volume delivered after each actuation, and viscosity of the FFS were evaluated (
Table 6). The presence of extracts reduced the pH in all FFSs compared to that in the controls; however, the formulations maintained values between 4.37 and 5.3, similar to the pH of human skin, indicating suitability for topical application without discomfort [
4,
43].
The FFS was applied using a metered dose valve for delivering a fixed amount of product topically [
3]. For each FFS, the volume was determined 10 times, and the observed standard deviation was acceptable. This is important because it ensures consistency in the delivered dose and, consequently, in the bioactive concentrations [
4].
Delivery volumes of FFS loaded with SEEU and SETM ranged from 0.43 to 0.56 mL, with an increase in PVP concentration leading to a notable decrease in volume (
Figure 1). PVP can increase viscosity [
44] and consequently influence the delivered volume of the formulation because higher amounts of fluid formulations flow more easily [
45].
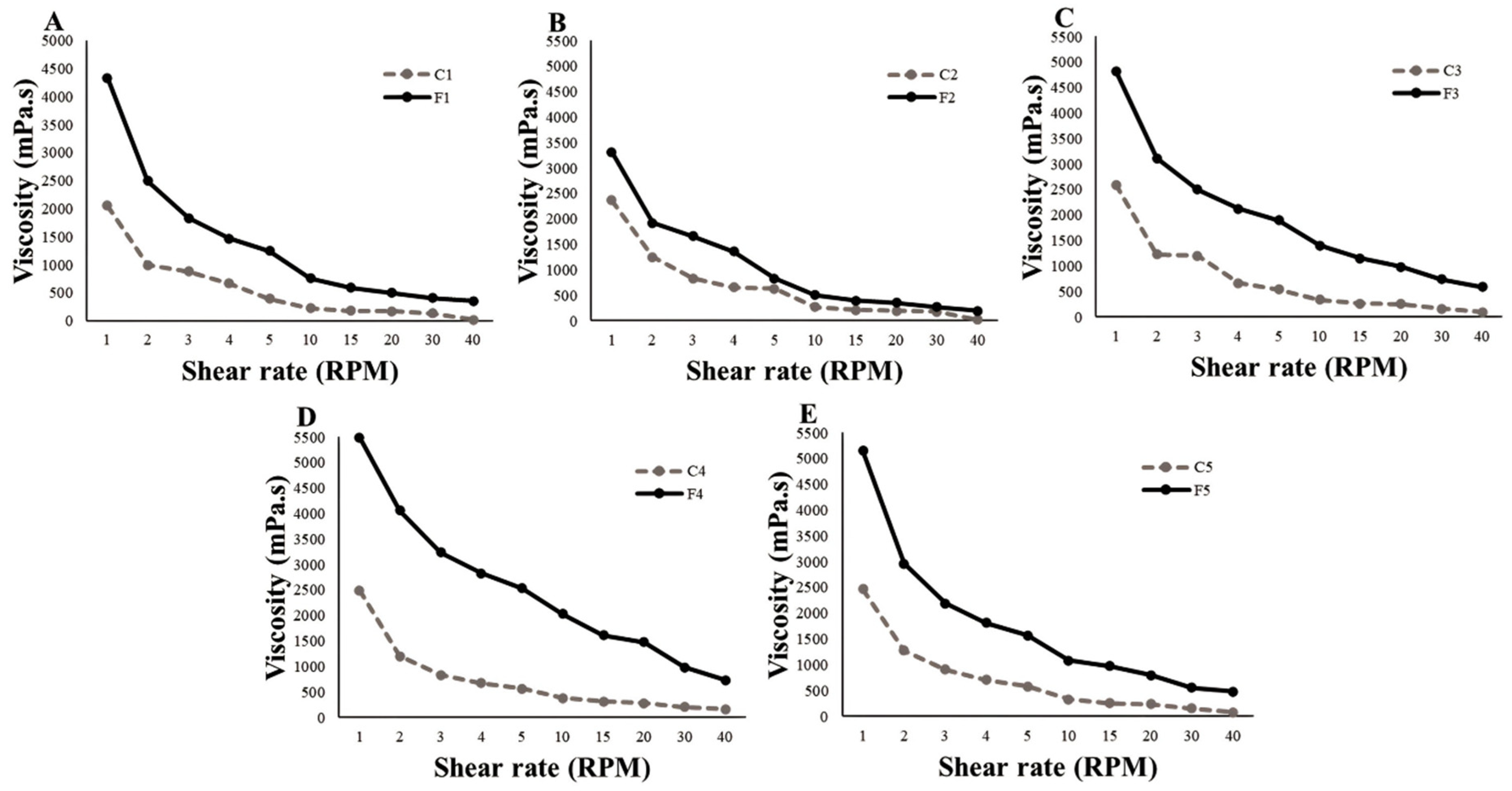
The viscosity and rheological behavior of formulations must be determined, as they are associated with product preparation, transport, storage, and shelf life [
46,
47]. The concentrations of PVA and PVP and the inclusion of SEEU and SETM in the FFS resulted in changes in viscosity and rheological behavior (
Figure 2). This can be observed in the higher values of the initial viscosity (1 RPM) of systems F4 and F5, which contained a higher concentration of polymers.
An increase in the initial viscosity of all FFSs was observed when SEEU and SETM were added; high consistency indices at rest indicate that the system is structured. In general, when the shear force increased, the viscosity decreased; this is characteristic of formulations with pseudoplastic flow. When the shear rate increases, the polymeric structure is organized along the shear direction; therefore, the subsequent shear occurs more quickly and the apparent viscosity decreases [
47].
Formulations with pseudoplastic flow allow for adequate delivery. Despite the high viscosity of the formulations at rest when an expulsion force is applied with high shear rates, the viscosity tends to decrease, which allows the formulation to flow easily out of the bottle dispenser during valve actuation, ensuring ease of application of the product by the patient [
47].
2.3. Characterization of the Optimized FFS
The evaluation of the Fourier transform infrared (FTIR) spectra allows chemical analysis of the raw materials and the interactions between the components of a formulation [
48].
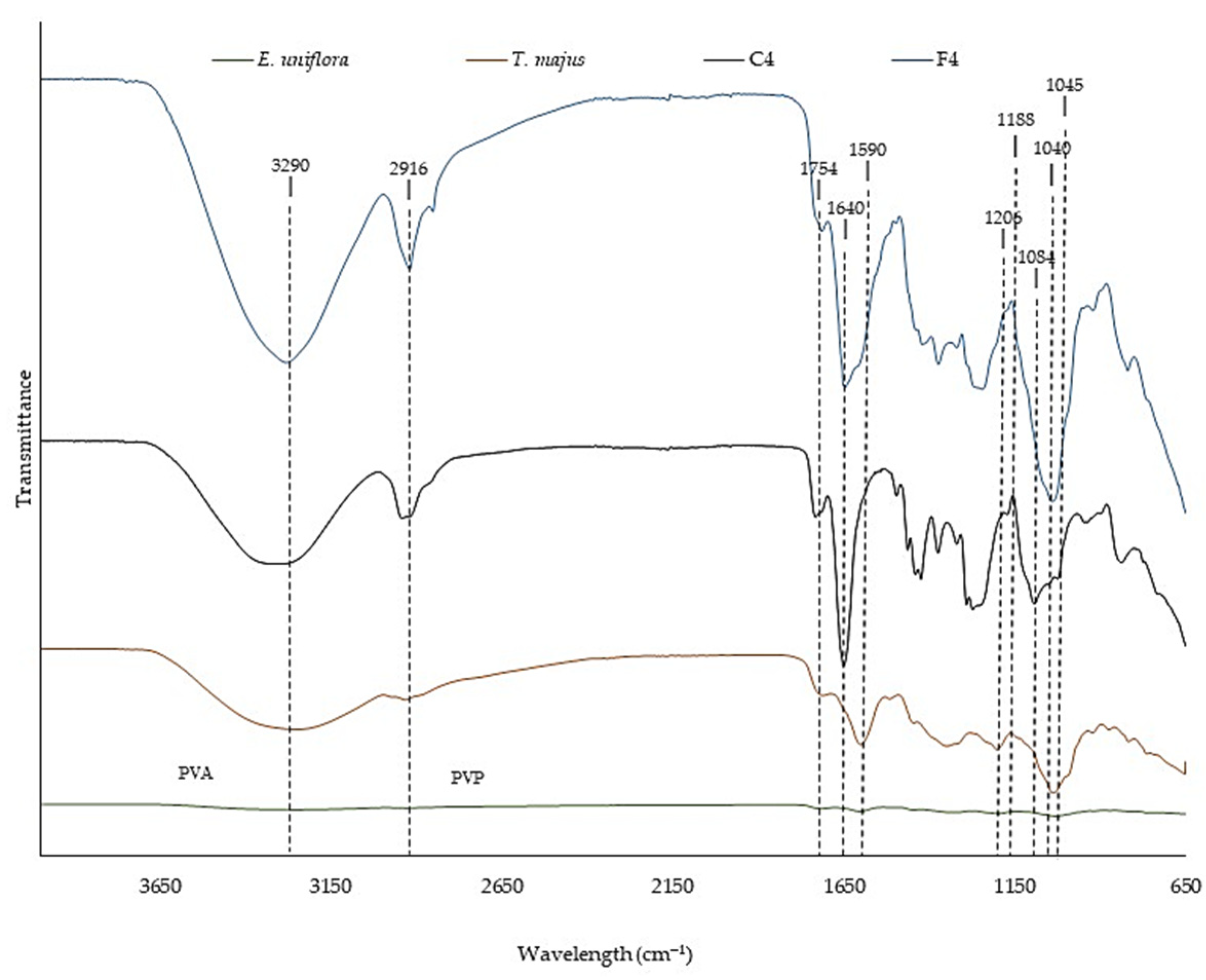
Figure 3 shows the FTIR spectra of SEEU and SETM, control FFS (C4), and FFS loaded with SEEU and SETM (F4).
SEEU and SETM peak at 1754 cm
−1, characteristic of elongation of the carbonyl group, and at 1590 cm
−1, indicative of vibration of the aromatic rings [
49] of phenolic derivatives. In addition, peaks at 1188 cm
−1 and 1040 cm
−1 observed for
E. uniflora and peaks at 1206 cm
−1 and 1045 cm
−1 for
T. majus are characteristic of stretching vibrations of the C-O and C-OH groups, associated with alcohol, ester, ether, and carboxylic acid groups present in phenolic compounds and flavonoids [
50].
The FFS showed a peak at 2916 cm
−1, corresponding to the C-H stretching of PVA and PVP [
51], and at 1640 cm
−1, characteristic of the C=C bond of PVA and PVP [
52]. In C4, a peak was detected at 1084 cm
−1, indicating the elongation of the C-O bond of the polymers [
52].
Incorporating SEEU and SETM into the formulation resulted in changes in signal intensity and slight shifts in some wavelengths due to the interaction between molecules [
50,
52]. In addition, a characteristic broad band of hydroxyl hydrogen bonds was observed at 3290 cm
−1 [
49]. These connections can contribute to the maintenance of the polymeric structure by increasing the resistance of the material [
53].
The interaction between molecules can also be observed in the mechanical properties (
Table 7). The incorporation of SEEU and SETM resulted in a decrease in the tensile stress and elongation of the FFS. The integration of different actives of the crosslinking agents can cause a reduction in the resistance of the films due to the decrease in the binding of the polymeric structure [
54].
The bending strength test without breaking can be used for product characterization and quality control [
55]. It is possible to observe that all FFSs resisted the bending analysis, which can be explained by the properties of resistance and elasticity conferred by PVA [
23].
High values of WS were observed (≥88.31%) and attributed to PVA’s ability to absorb and retain high water amounts on its chains. This feature contributes a lot to maintaining structural moisture, which helps remove the film from the injured skin without damaging it [
1]. On the other hand, water absorption can lead the fibre to swell, promoting a decrease in pores and reducing the water vapor permeation capacity [
56]. Furthermore, the low WVTR values may be related to the low porosity of the films [
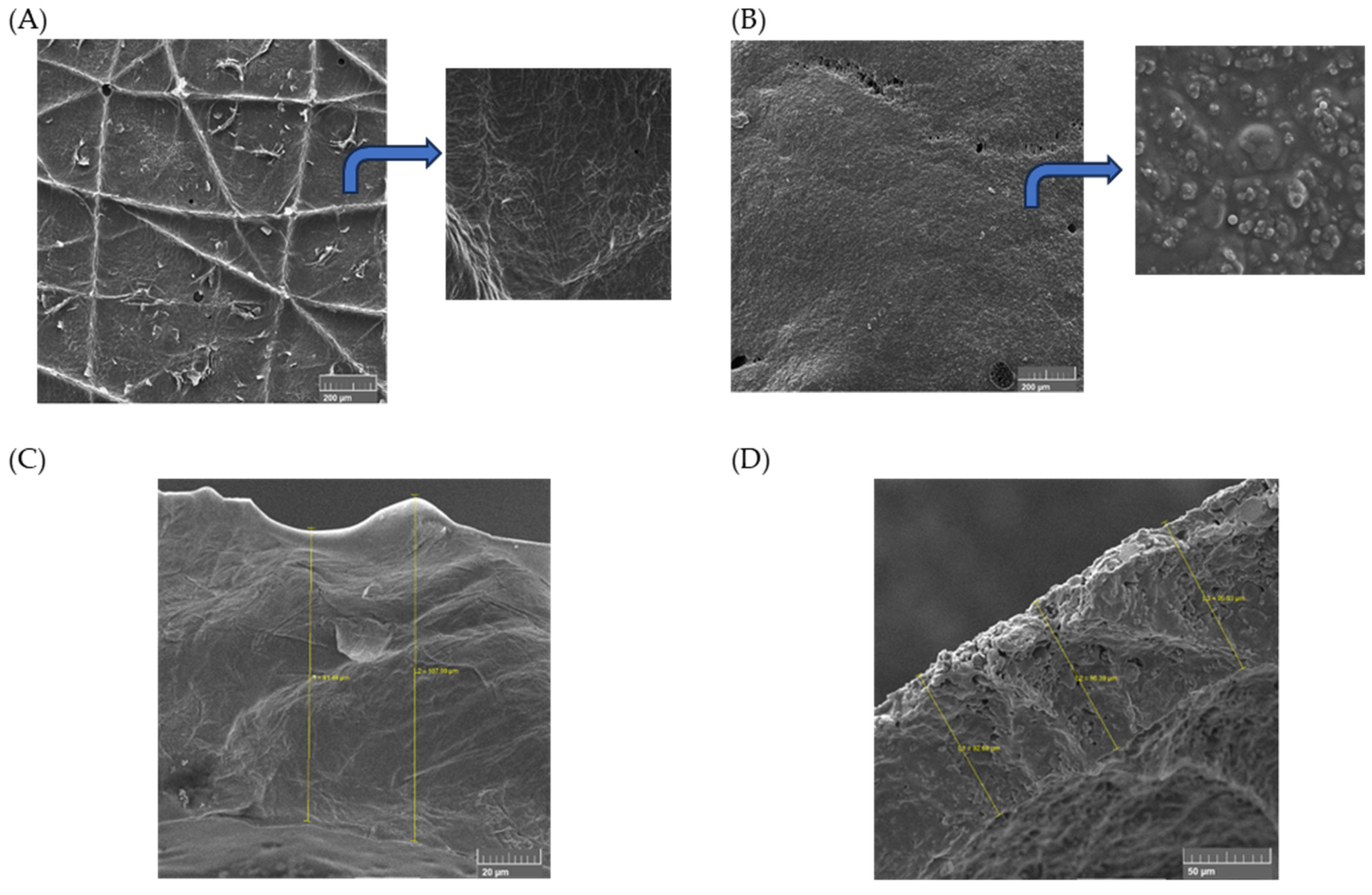
57], demonstrated by scanning electron microscopy (SEM) (
Figure 4).
Scanning electron images represent the surface and cross-sectional views of C4 and F4. The polymeric blend had basically formed a compact and homogeneous film, identifying miscible blending between PVA and PVP (see
Figure 4C) [
58]. The film presented the ability to mold itself to the skin, performing a perfect attachment by adhesion. The thickness of the film varied between 91 and 107 mm, as noted in
Figure 4C. Structural modifications took place in the samples after adding the extracts, as seen in
Figure 4B,D. Polymeric clusters were now present, making the surface rougher than the pristine blend. The film thickness appears to be constant (
Figure 4D).
The incorporation of bioactive compounds into formulations aims to intensify biological functions, such as antioxidant and antimicrobial activities [
59]. The FFS containing SEEU and SETM showed antimicrobial activity, whereas the control FFS did not show any inhibitory activity under the evaluated conditions (
Table 8). The values presented correspond to the amount in mg mL
−1 of FFS necessary for the antimicrobial effect, and F4 contains only 7.81% of SEEU and 3.90% of SETM, concentrations established from the synergism test using the checkerboard model.
In the initial phase of a skin infectious process,
S. aureus is among the dominant organisms involved, whereas
E. coli is found only in the final stages of the process when a chronic wound develops [
60]. Fungi belonging to the genus
Candida can cause infections ranging from those on the skin and mucous membranes to systemic infections, leading to the hematogenous dissemination of the yeast throughout the body [
61].
Phenolic compounds can degrade the bacterial cell wall and cytoplasmic membrane, leading to the exudation of cell components, such as phosphates, ions, purines, and nucleic acids, or the introduction of substances harmful to metabolism, imparting an antibacterial action [
62]. The SEEU and SETM used in this study had high concentrations of phenolic derivatives (93.28% and 98.17%, respectively), which could be related to their antimicrobial activity. Extracts with high concentrations of these compounds are promising candidates for the development of new microbial inhibitors [
63].
Evaluation of the permeation profile helps to analyze the amount of permeated active compounds as a function of time. The evaluation of the optimized FFS loaded with SEEU and SETM (F4) showed practically zero permeation, and after 8 h under the evaluated conditions, only 0.97% of the bioactive compounds had permeated the skin.
Skin permeability is determined by the physicochemical characteristics of the formulation; therefore, certain factors, such as solubility, particle size, and electrical charge, can influence the process. In general, active compounds with solubility close to that of the skin have a higher rate of permeation [
64]. In addition, smaller particles can agglomerate within the grooves present in the stratum corneum and facilitate transport to the deeper layers of the skin [
65]. Positively charged particles can achieve greater permeation because the skin has a negative charge at physiological pH [
66,
67].
The FFS developed in this study aims at a local/topical antimicrobial effect; therefore, low permeation is desirable. However, in the in vitro evaluation, intact pig ear skin was used; the conditions differ from that of skin with an infectious process, where cellular barriers are compromised [
68], resulting in altered permeation rates. Therefore, an in vivo study is necessary to estimate the concentration of bioactive molecules capable of reaching the systemic route and to prove the developed FFS’s safety.
Conventional treatment consists of applying ointments and creams. However, these have several limitations, as multiple daily applications are required, exposing the lesion to infectious agents [
1]. Polymeric films based on biomaterials, on the other hand, can be absorbable, accelerating the tissue recovery process and making the patient return to their routine more quickly [
48].
Additionally, this proposal aims to encourage the production of polymeric bio-dressings and to propose modern solutions for the pharmaceutical industry using environmentally friendly technologies and low-cost raw materials. Still, the prospection of this formulation is an alternative for treating topical infections, which has a widespread appeal aimed at those who do not wish to use synthetic drugs. The scope of this study was limited to the development of the FFS containing the SEEU and SETM combination. In future studies, the physicochemical stability and biodegradability should be evaluated.