Neuroprotective Effects of Albizia lebbeck (L.) Benth. Leaf Extract against Glutamate-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Microglial Cells
Abstract
1. Introduction
2. Results
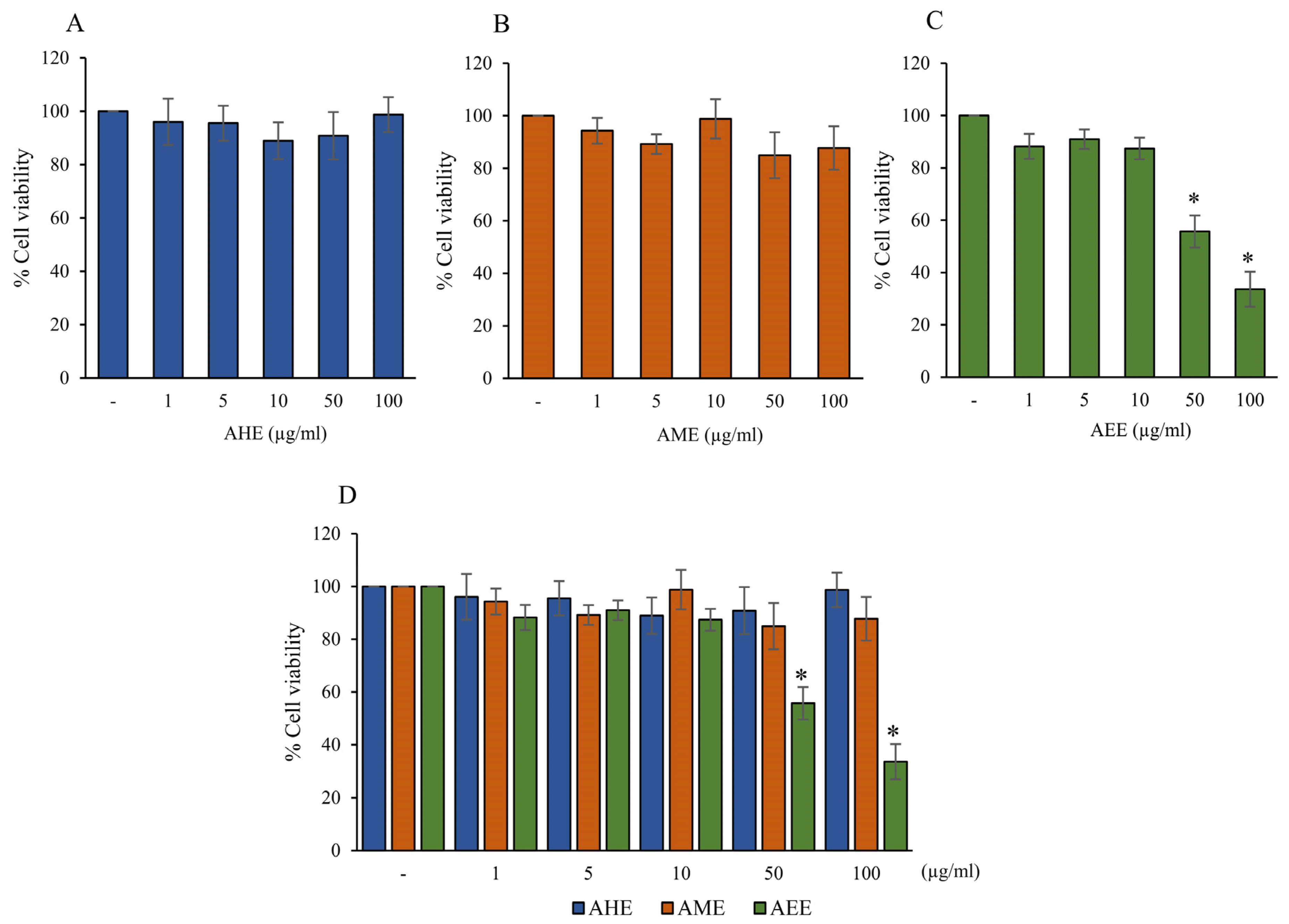
2.1. The Cytotoxicity of Different Solvent Extracts from A. lebbeck Leaf on Cell Viability of HMC3 Cells
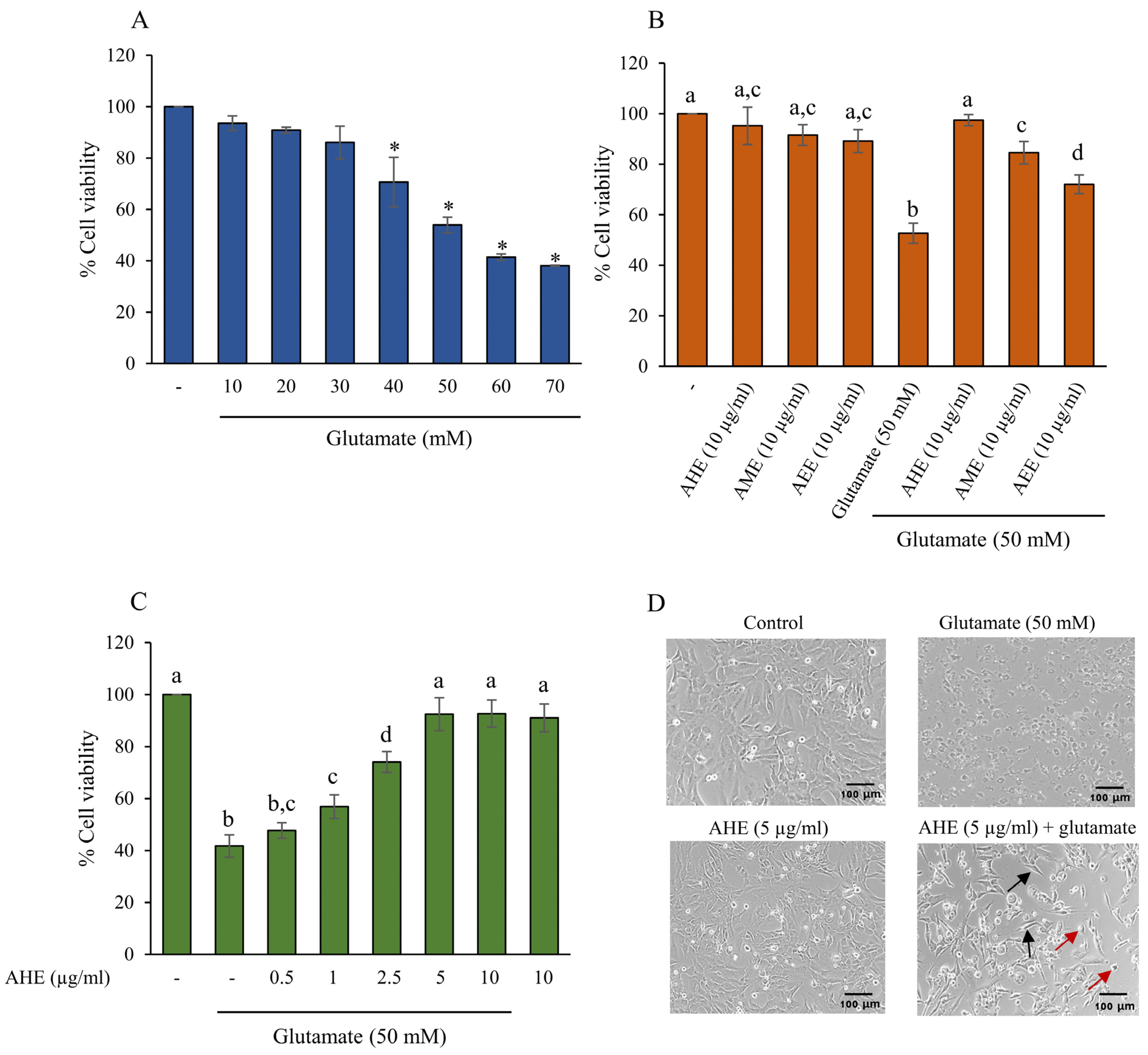
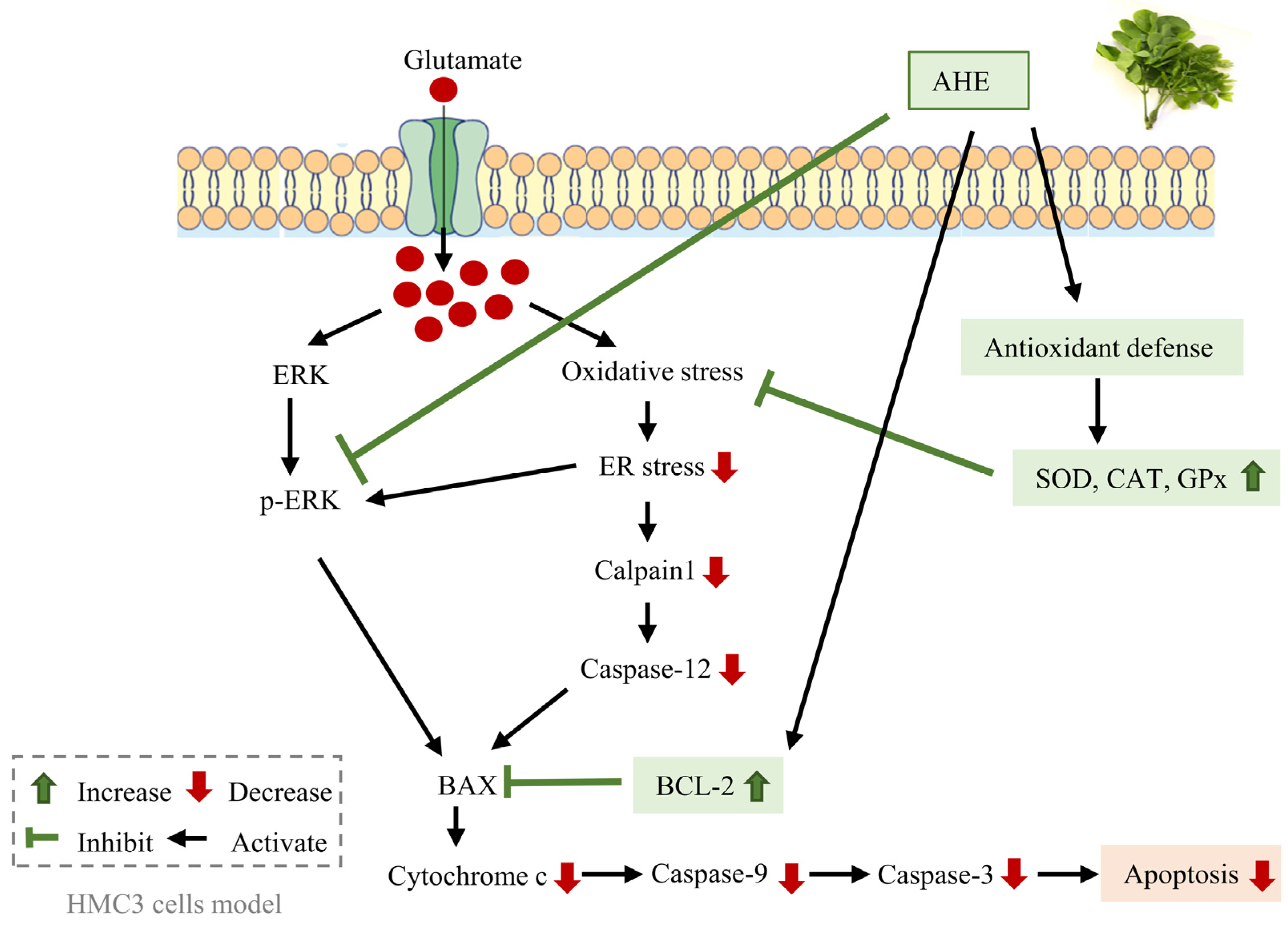
2.2. The Protective Effect of A. lebbeck Leaf Extracts on Glutamate-Induced Toxicity in HMC3 Cells
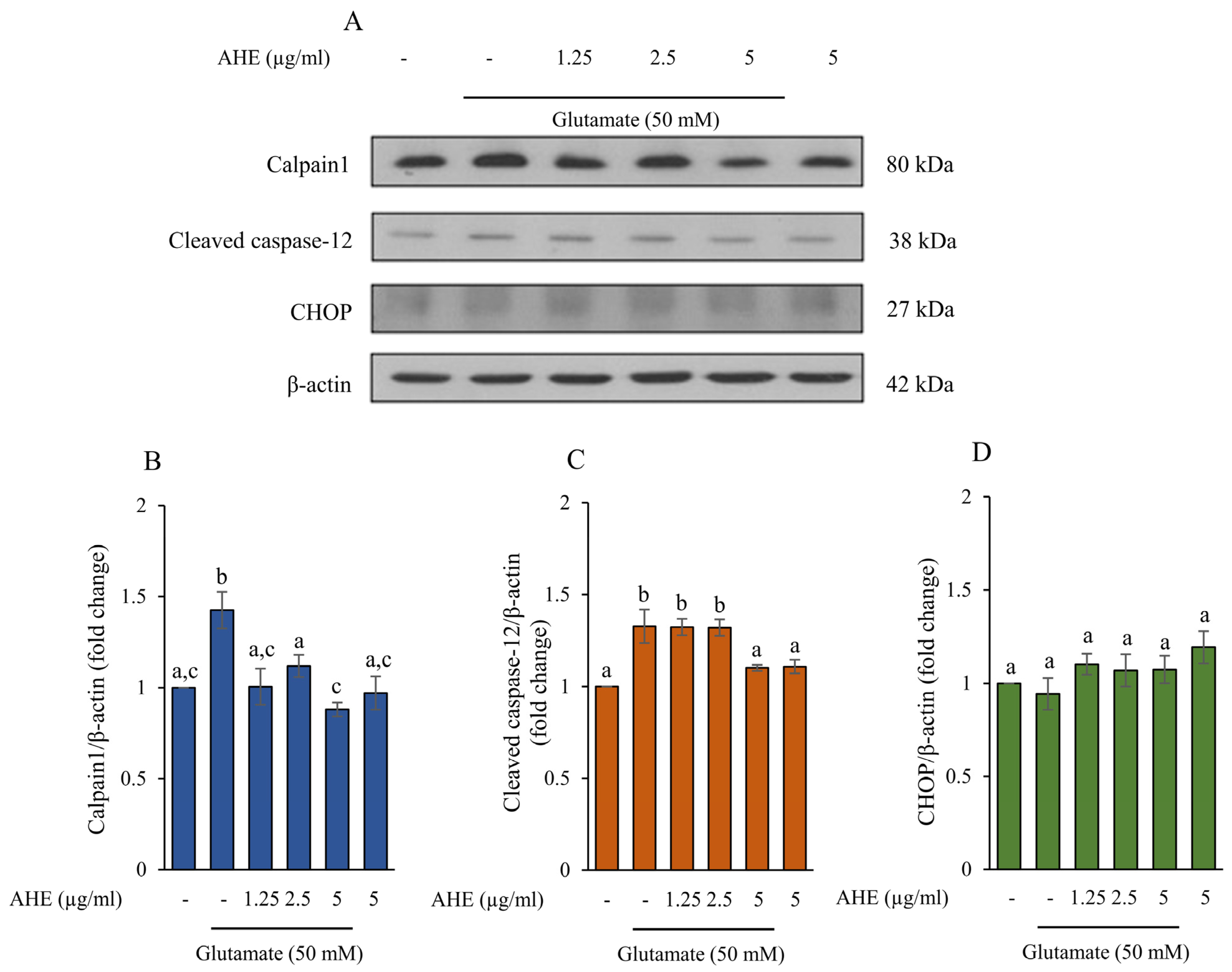
2.3. AHE Suppresses Glutamate-Induced ER Stress in HMC3 Cells
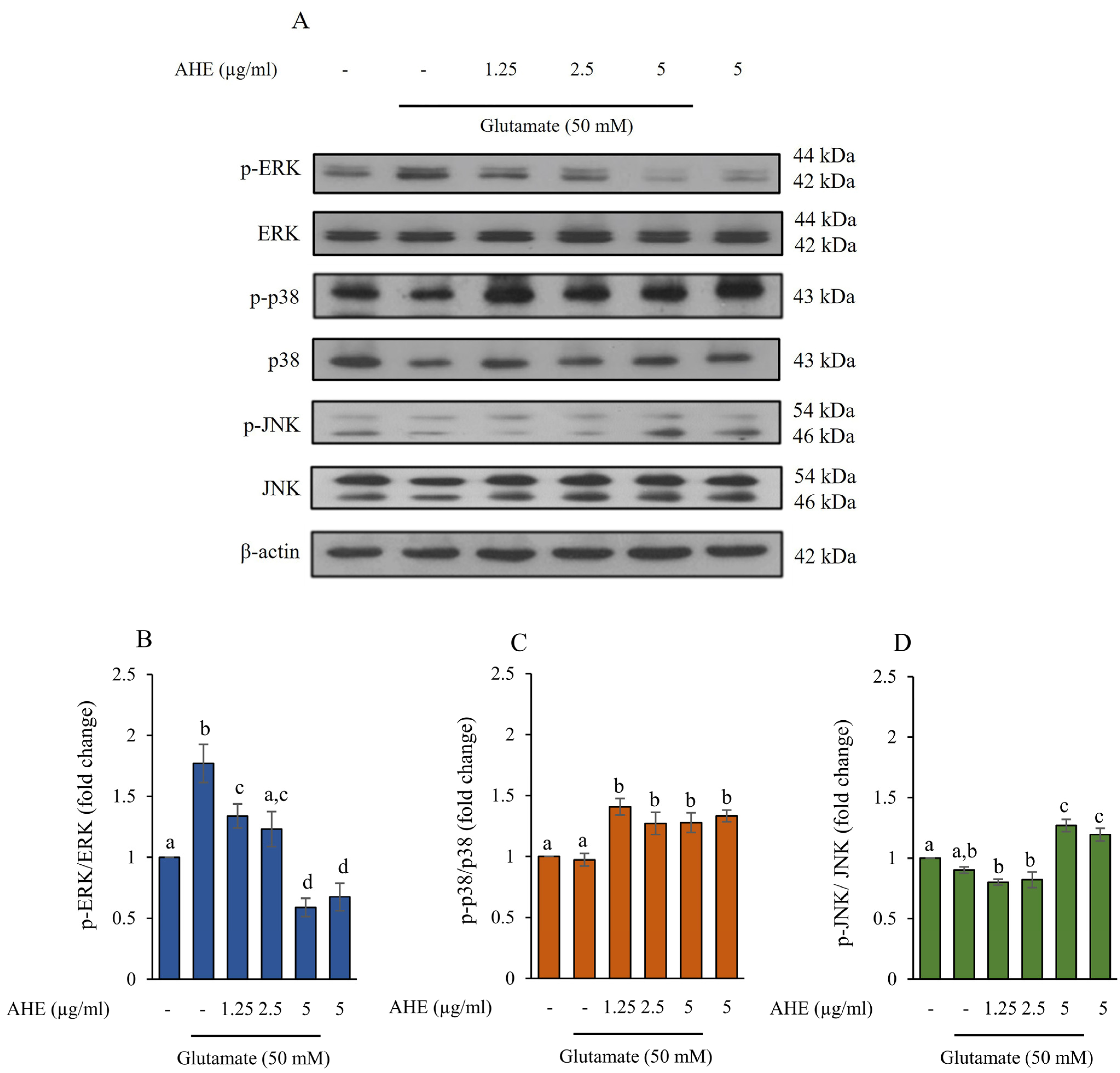
2.4. Effect of AHE on MAPKs Activation in HMC3 Cells
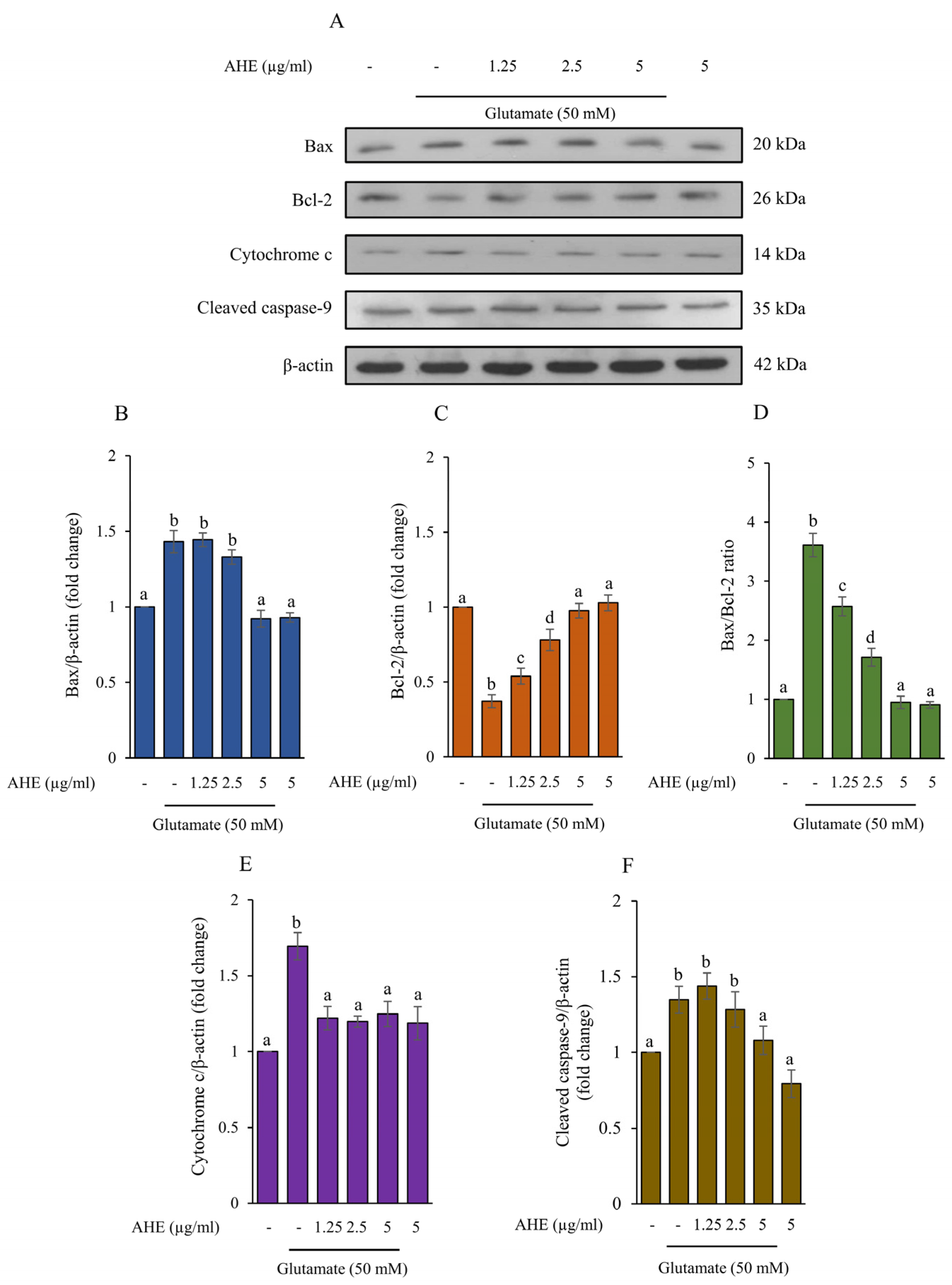
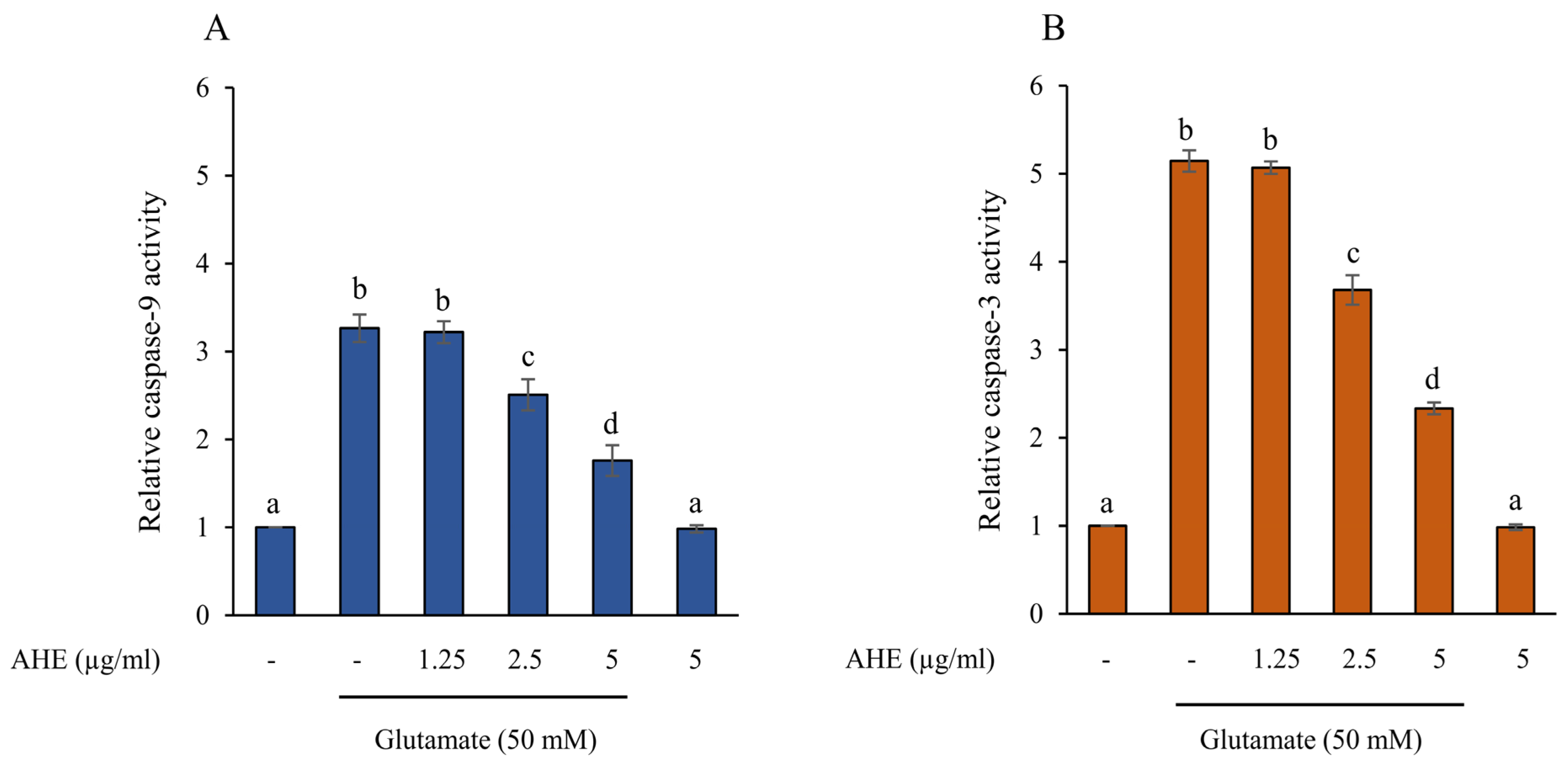
2.5. AHE Inhibits Glutamate-Induced Apoptosis and Caspase Activities in HMC3 Cells
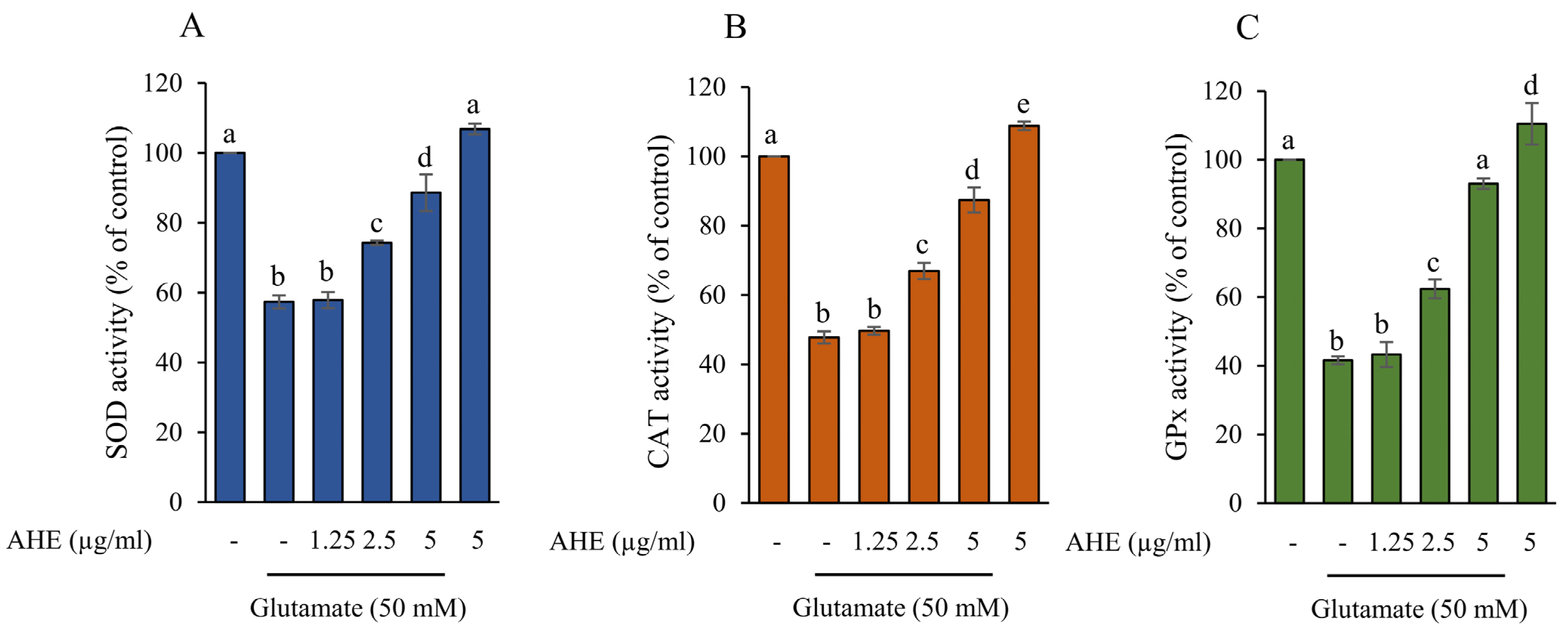
2.6. AHE Promotes Antioxidant Enzymes Activity in HMC3 Cells
2.7. Carotenoids and Flavonoids in AHE
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Collection and Preparation
4.3. Sample Extraction
4.4. Cell Culture
4.5. Cytotoxicity of the Extracts and Glutamate in HMC3 Cells
4.6. Western Blot Analysis
4.7. Measurement of Caspase-9 and Caspase-3 Activities by Assay Kit
4.8. Determination of Antioxidant Activity by Assay Kits
4.9. Carotenoid Content
4.10. Flavonoid Content
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fereshtehnejad, S.M.; Vosoughi, K.; Heydarpour, P.; Sepanlou, S.G.; Farzadfar, F.; Tehrani-Banihashemi, A.; Malekzadeh, R.; Sahraian, M.A.; Vollset, S.E.; Naghavi, M.; et al. Burden of neurodegenerative diseases in the Eastern Mediterranean Region, 1990–2016: Findings from the Global Burden of Disease Study 2016. Eur. J. Neurol. 2019, 26, 1252–1265. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular mechanisms of glutamate toxicity in Parkinson’s disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef]
- Hsa, E.; Ya, K. Glutamate excitotoxicity and neurodegeneration. J. Mol. Genet. 2014, 8, 1–4. [Google Scholar]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases-What is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-triggered glutamate excitotoxicity from the perspective of glial cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef]
- Czapski, G.A.; Strosznajder, J.B. Glutamate and GABA in microglia-neuron cross-talk in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 11677. [Google Scholar] [CrossRef]
- Takeuchi, H. Neurotoxicity by microglia: Mechanisms and potential therapeutic strategy. Clin. Exp. Neuroimmunol. 2010, 1, 12–21. [Google Scholar] [CrossRef]
- Dello Russo, C.; Cappoli, N.; Coletta, I.; Mezzogori, D.; Paciello, F.; Pozzoli, G.; Navarra, P.; Battaglia, A. The human microglial HMC3 cell line: Where do we stand? A systematic literature review. J. Neuroinflamm. 2018, 15, 259. [Google Scholar] [CrossRef]
- Liu, H.; Leak, R.; Hu, X. Neurotransmitter receptors on microglia. BMJ 2016, 1, e000012. [Google Scholar] [CrossRef]
- Barger, S.W.; Goodwin, M.E.; Porter, M.M.; Beggs, M.L. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J. Neurochem. 2007, 101, 1205–1213. [Google Scholar] [CrossRef]
- Serafini, G.; Rihmer, Z.; Amore, M. The role of glutamate excitotoxicity and neuroinflammation in depression and suicidal behavior: Focus on microglia cells. Neuroimmunol. Neuroinflamm. 2015, 2, 127–130. [Google Scholar] [CrossRef]
- Domercq, M.; Vázquez-Villoldo, N.; Matute, C. Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 2013, 7, 49. [Google Scholar] [CrossRef]
- Jangra, A.; Verma, M.; Kumar, D.; Chandrika, C.; Rachamalla, M.; Dey, A.; Dua, K.; Jha, S.K.; Ojha, S.; Alexiou, A.; et al. Targeting endoplasmic reticulum stress using natural products in neurological disorders. Neurosci. Biobehav. Rev. 2022, 141, 104818. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Bahar, E.; Kim, H.; Yoon, H. ER stress-mediated signaling: Action potential and Ca2+ as key players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef]
- Remondelli, P.; Renna, M. The endoplasmic reticulum unfolded protein response in neurodegenerative disorders and its potential therapeutic significance. Front. Mol. Neurosci. 2017, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Sprenkle, N.T.; Sims, S.G.; Sánchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef]
- Wei, S.G.; Yu, Y.; Weiss, R.M.; Felder, R.B. Endoplasmic reticulum stress increases brain MAPK signaling, inflammation and renin-angiotensin system activity and sympathetic nerve activity in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H871–H880. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 2150–2163. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Yang, E.J.; Choi, S.M.; Kim, S.H.; Baek, M.G.; Jiang, J.H. Effects of bee venom on glutamate-induced toxicity in neuronal and glial cells. Evid. Based Complement. Altern. Med. 2012, 2012, 368196. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.; Wang, Y.; Kentor, R.; Burke, N.; Watkins, S.; Kress, G.; Reynolds, I.; Klann, E.; Angiolieri, M.R.; Johnson, J.W.; et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000, 275, 12200–12206. [Google Scholar] [CrossRef]
- Choi, B.H.; Hur, E.M.; Lee, J.H.; Jun, D.J.; Kim, K.T. Protein kinase Cdelta-mediated proteasomal degradation of MAP kinase phosphatase-1 contributes to glutamate-induced neuronal cell death. J. Cell Sci. 2006, 119, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-J.; Chiu, G.N.C. Role of oxidative stress, endoplasmic reticulum stress and ERK activation in triptolide-induced apoptosis. Int. J. Oncol. 2013, 42, 1605–1612. [Google Scholar] [CrossRef]
- Shirisha, K.; Priyanka, B.; Habibur, R.; Dipankar, B.; Fulchan, A. Review on Albizia lebbeck (L.) Benth: A plant possessing diverse pharmacological activities. Res. J. Pharmacogn. Phytochem. 2013, 5, 263–268. [Google Scholar]
- Praengam, K.; Muangnoi, C.; Charoenkiatkul, S.; Thiyajai, P.; Tuntipopipat, S. Antioxidant and anti-inflammatory activity of aqueous fraction from Albizia lebbeck leaves. Int. Food Res. J. 2017, 24, 1174–1185. [Google Scholar]
- Shirode, D.S.; Powar, P.; Jain, B.B. Determination of in vitro antioxidant capacity of Albizia lebbeck leaves. Int. J. Chem. Sci. 2018, 2, 1–4. [Google Scholar]
- Vasanthi, P.; Ganapathy, M.; Evanjelene, V.K.; Ayyavuv, N.; Angamuthu, J. Phytochemical screening and antioxidant activity of extracts of the leaf and bark of Albizzia lebbeck (Benth). AJMP 2014, 2, 26–31. [Google Scholar]
- Meshram, G.G.; Kumar, A.; Rizvi, W.; Tripathi, C.D.; Khan, R.A. Evaluation of the anti-inflammatory activity of the aqueous and ethanolic extracts of the leaves of Albizzia lebbeck in rats. J. Tradit. Complement. Med. 2016, 6, 172–175. [Google Scholar] [CrossRef]
- Saleem, U.; Raza, Z.; Anwar, F.; Ahmad, B.; Hira, S.; Ali, T. Experimental and computational studies to characterize and evaluate the therapeutic effect of Albizia lebbeck (L.) seeds in Alzheimer’s disease. Medicina 2019, 55, 184. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Raza, Z.; Anwar, F.; Chaudary, Z.; Ahmad, B. Systems pharmacology based approach to investigate the in-vivo therapeutic efficacy of Albizia lebbeck (L.) in experimental model of Parkinson’s disease. BMC Complement Altern. Med. 2019, 19, 352. [Google Scholar] [CrossRef]
- Ahmed, D.; Kumar, V.; Verma, A.; Gupta, P.S.; Kumar, H.; Dhingra, V.; Mishra, V.; Sharma, M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement. Altern. Med. 2014, 14, 243. [Google Scholar] [CrossRef]
- Resmi, C.; Venukumar, M.; Latha, M. Antioxidant activity of Albizzia lebbeck (Linn.) Benth. in alloxan diabetic rats. Indian J. Physiol. Pharmacol. 2006, 50, 297–302. [Google Scholar]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate—Induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef]
- Merighi, A.; Lossi, L. Endoplasmic reticulum stress signaling and neuronal cell death. Int. J. Mol. Sci. 2022, 23, 15186. [Google Scholar] [CrossRef] [PubMed]
- Rakkhittawattana, V.; Panichayupakaranant, P.; Prasanth, M.I.; Brimson, J.M.; Tencomnao, T. Rhinacanthin-C but not -D extracted from Rhinacanthus nasutus (L.) Kurz offers neuroprotection via ERK, CHOP, and LC3B pathways. Pharmaceuticals 2022, 15, 627. [Google Scholar] [CrossRef] [PubMed]
- Sukprasansap, M.; Chanvorachote, P.; Tencomnao, T. Cyanidin-3-glucoside activates Nrf2-antioxidant response element and protects against glutamate-induced oxidative and endoplasmic reticulum stress in HT22 hippocampal neuronal cells. BMC Complement. Med. Ther. 2020, 20, 46. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Fibuch, E.E.; Mao, L. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007, 100, 1–11. [Google Scholar] [CrossRef]
- Park, J.-S.; Park, J.-H.; Kim, K.-Y. Neuroprotective effects of myristargenol A against glutamate-induced apoptotic HT22 cell death. RSC Adv. 2019, 9, 31247–31254. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, P.J.; Fang, B.; Park, S.; Saravanakumar, K.; Irfan, N.; Pham, C.H.; Yoo, G.; Yoo, H.M.; Cho, N. Neuroprotective effects of phenolic glycosides from Populus tomentiglandulosa roots in HT22 mouse hippocampal neuronal cells. J. Mol. Struct. 2023, 1276, 134685. [Google Scholar] [CrossRef]
- Lee, H.J.; Spandidos, D.A.; Tsatsakis, A.; Margina, D.; Izotov, B.N.; Yang, S.H. Neuroprotective effects of Scrophularia buergeriana extract against glutamate-induced toxicity in SH-SY5Y cells. Int. J. Mol. Med. 2019, 43, 2144–2152. [Google Scholar] [CrossRef]
- Terzioğlu Bebitoğlu, B.; Oğuz, E.; Gökçe, A. Effect of valproic acid on oxidative stress parameters of glutamate-induced excitotoxicity in SH-SY5Y cells. Exp. Ther. Med. 2020, 20, 1321–1328. [Google Scholar] [CrossRef]
- Pap, R.; Pandur, E.; Jánosa, G.; Sipos, K.; Nagy, T.; Agócs, A.; Deli, J. Lutein decreases inflammation and oxidative stress and prevents iron accumulation and lipid peroxidation at glutamate-induced neurotoxicity. Antioxidants 2022, 11, 2269. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative stress in the brain: Basic concepts and treatment strategies in stroke. Antioxidants. 2021, 10, 1886. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, M.-Z.; Yang, Z.-Y.; Jin, W.-L. Microglia in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 270–280. [Google Scholar]
- Muddapu, V.R.; Dharshini, S.A.P.; Chakravarthy, V.S.; Gromiha, M.M. Neurodegenerative diseases—Is metabolic deficiency the root cause? Front. Neurosci. 2020, 14, 213. [Google Scholar] [CrossRef]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef]
- Kara, M.; Oztas, E. Endoplasmic reticulum stress-mediated cell death. In Programmed Cell Death, 1st ed.; Gali-Muhtasib, H., Rahal, O.N., Eds.; IntechOpen Limited: London, UK, 2020; pp. 1–14. [Google Scholar]
- Lee, D.; Choi, H.G.; Hwang, J.H.; Shim, S.H.; Kang, K.S. Neuroprotective effect of tricyclic pyridine alkaloids from Fusarium lateritium SSF2, against glutamate-induced oxidative stress and apoptosis in the HT22 hippocampal neuronal cell line. Antioxidants 2020, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, H.; Zhang, Y.; Wang, X.; Yu, L.; Xu, W.; Xu, W.; Lin, Y. Paeoniflorin exerts neuroprotective effects against glutamate-induced PC12 cellular cytotoxicity by inhibiting apoptosis. Int. J. Mol. Med. 2017, 40, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Unsicker, K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010, 277, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kang, K.S.; Choi, Y.K. Protective effect of casuarinin against glutamate-induced apoptosis in HT22 cells through inhibition of oxidative stress-mediated MAPK phosphorylation. Bioorg. Med. Chem. Lett. 2017, 27, 5109–5113. [Google Scholar] [CrossRef] [PubMed]
- Umesh, P.; Swapnesh, A.; Kailaspati, P.; Patil, M.; Wagh, R. Free radical scavenging activity of methanolic and aqueous extract of Albizia lebbeck leaves. J. Pharm. Biosci. 2018, 6, 9–12. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Kumar, D.J.; Hegde, M.L.; Rao, K.S. Carotenoids as novel therapeutic molecules against neurodegenerative disorders: Chemistry and molecular docking analysis. Int. J. Mol. Sci. 2019, 20, 5553. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-Inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Park, H.A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-apoptotic effects of carotenoids in neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Baek, S.Y.; Kim, M.R. Neuroprotective effect of carotenoid-rich Enteromorpha prolifera extract via TrkB/Akt pathway against oxidative stress in hippocampal neuronal cells. Mar. Drugs. 2020, 18, 372. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, H.-N.; Kim, C.Y.; Seo, M.-D.; Baek, S.-H. Synergistic protection by isoquercitrin and quercetin against glutamate-induced oxidative cell death in HT22 cells via activating Nrf2 and HO-1 signaling pathway: Neuroprotective principles and mechanisms of Dendropanax morbifera leaves. Antioxidants 2021, 10, 554. [Google Scholar] [CrossRef]
- Yang, E.-J.; Kim, G.-S.; Kim, J.; Song, K.-S. Protective effects of onion-derived quercetin on glutamate-mediated hippocampal neuronal cell death. Pharmacogn. Res. 2013, 9, 302–308. [Google Scholar]
- Avoseh, O.N.; Mtunzi, F.M.; Ogunwande, I.A.; Ascrizzi, R.; Guido, F. Albizia lebbeck and Albizia zygia volatile oils exhibit anti-nociceptive and anti-inflammatory properties in pain models. J. Ethnopharmacol. 2021, 268, 113676. [Google Scholar] [CrossRef] [PubMed]
- Sridonpai, P.; Kongprapun, P.; Sungayuth, N.; Sukprasansap, M.; Chimkerd, C.; Judprasong, K. Nutritive values and phytochemical compositions of edible indigenous plants in Thailand. Front. Sustain. Food Syst. 2022, 6, 870147. [Google Scholar] [CrossRef]
- Amin, I.; Cheah, S. Determination of vitamin C, β-carotene and riboflavin contents in five green vegetables organically and conventionally grown. Malays. J. Nutr. 2003, 9, 31–39. [Google Scholar]
- Dawilai, S.; Muangnoi, C.; Praengamthanachoti, P.; Tuntipopipat, S. Anti-inflammatory activity of bioaccessible fraction from Eryngium foetidum leaves. BioMed Res. Int. 2013, 2013, 958567. [Google Scholar] [CrossRef]

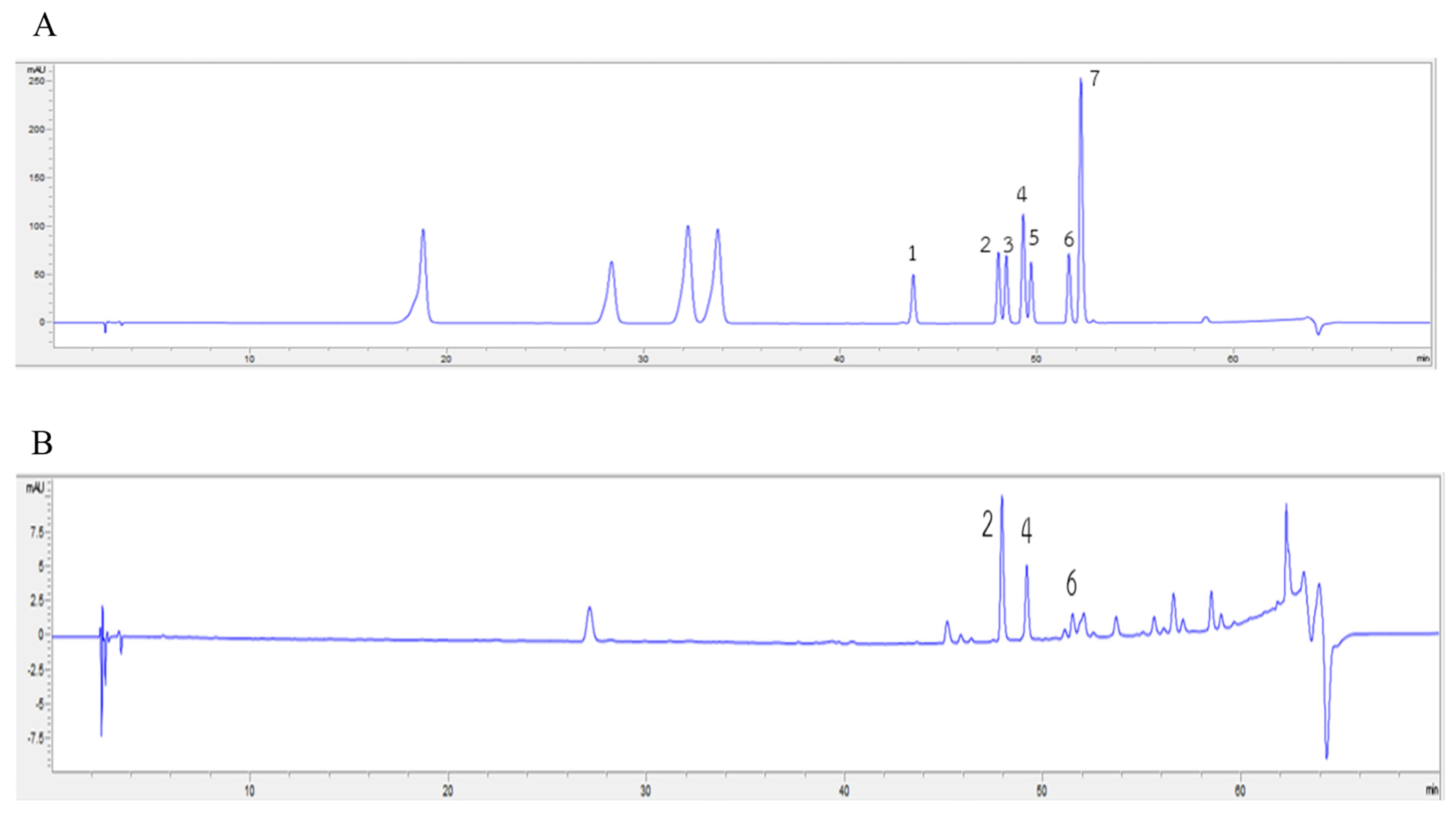
| Carotenoids | Content (µg/100 g FW) |
|---|---|
| α-carotene | 4706.57 ± 37.05 |
| β-carotene | 819.32 ± 24.85 |
| lutein | 4137.28 ± 162.20 |
| zeaxanthin | ND |
| β-cryptoxanthin | ND |
| Total carotenoids | 9663.17 ± 224.10 |
| Flavonoids | Content (µg/g FW) |
| quercetin | 989.42 ± 53.40 |
| luteolin | 254.67 ± 9.83 |
| kaempferol | 103.41 ± 9.05 |
| myricetin | ND |
| apigenin | ND |
| naringenin | ND |
| hesperidin | ND |
| Total flavonoids | 1347.50 ± 72.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoraksa, O.; Chimkerd, C.; Thiyajai, P.; Judprasong, K.; Tuntipopipat, S.; Tencomnao, T.; Charoenkiatkul, S.; Muangnoi, C.; Sukprasansap, M. Neuroprotective Effects of Albizia lebbeck (L.) Benth. Leaf Extract against Glutamate-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Microglial Cells. Pharmaceuticals 2023, 16, 989. https://doi.org/10.3390/ph16070989
Phoraksa O, Chimkerd C, Thiyajai P, Judprasong K, Tuntipopipat S, Tencomnao T, Charoenkiatkul S, Muangnoi C, Sukprasansap M. Neuroprotective Effects of Albizia lebbeck (L.) Benth. Leaf Extract against Glutamate-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Microglial Cells. Pharmaceuticals. 2023; 16(7):989. https://doi.org/10.3390/ph16070989
Chicago/Turabian StylePhoraksa, Onuma, Chanika Chimkerd, Parunya Thiyajai, Kunchit Judprasong, Siriporn Tuntipopipat, Tewin Tencomnao, Somsri Charoenkiatkul, Chawanphat Muangnoi, and Monruedee Sukprasansap. 2023. "Neuroprotective Effects of Albizia lebbeck (L.) Benth. Leaf Extract against Glutamate-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Microglial Cells" Pharmaceuticals 16, no. 7: 989. https://doi.org/10.3390/ph16070989
APA StylePhoraksa, O., Chimkerd, C., Thiyajai, P., Judprasong, K., Tuntipopipat, S., Tencomnao, T., Charoenkiatkul, S., Muangnoi, C., & Sukprasansap, M. (2023). Neuroprotective Effects of Albizia lebbeck (L.) Benth. Leaf Extract against Glutamate-Induced Endoplasmic Reticulum Stress and Apoptosis in Human Microglial Cells. Pharmaceuticals, 16(7), 989. https://doi.org/10.3390/ph16070989










