The Carnitine Palmitoyltransferase 1A Inhibitor Teglicar Shows Promising Antitumour Activity against Canine Mammary Cancer Cells by Inducing Apoptosis
Abstract
1. Introduction
2. Results
2.1. Teglicar Inhibits the Viability of Canine Mammary Tumour Cells
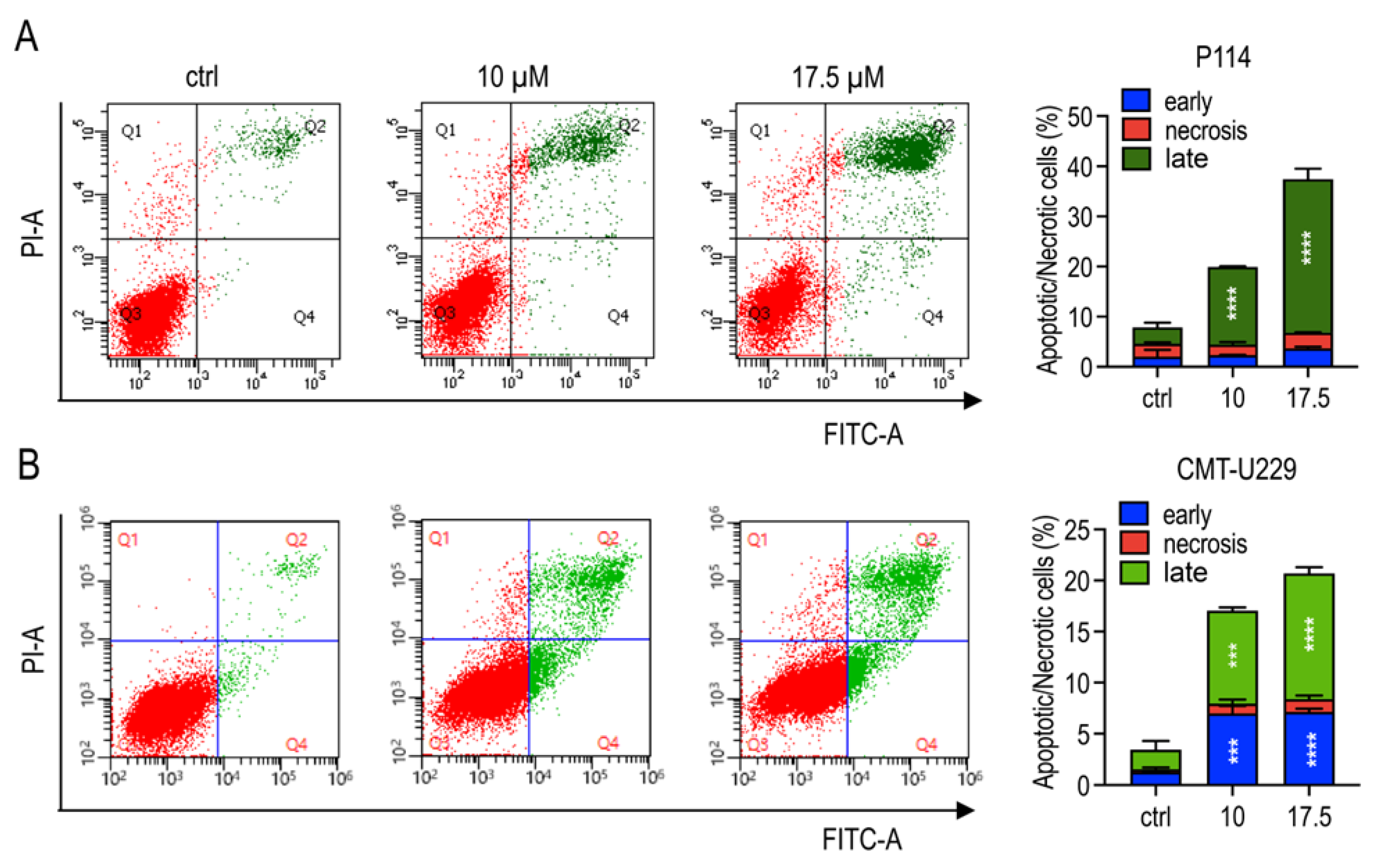
2.2. Teglicar Indues Cell Death in Canine Mammary Tumour Cells
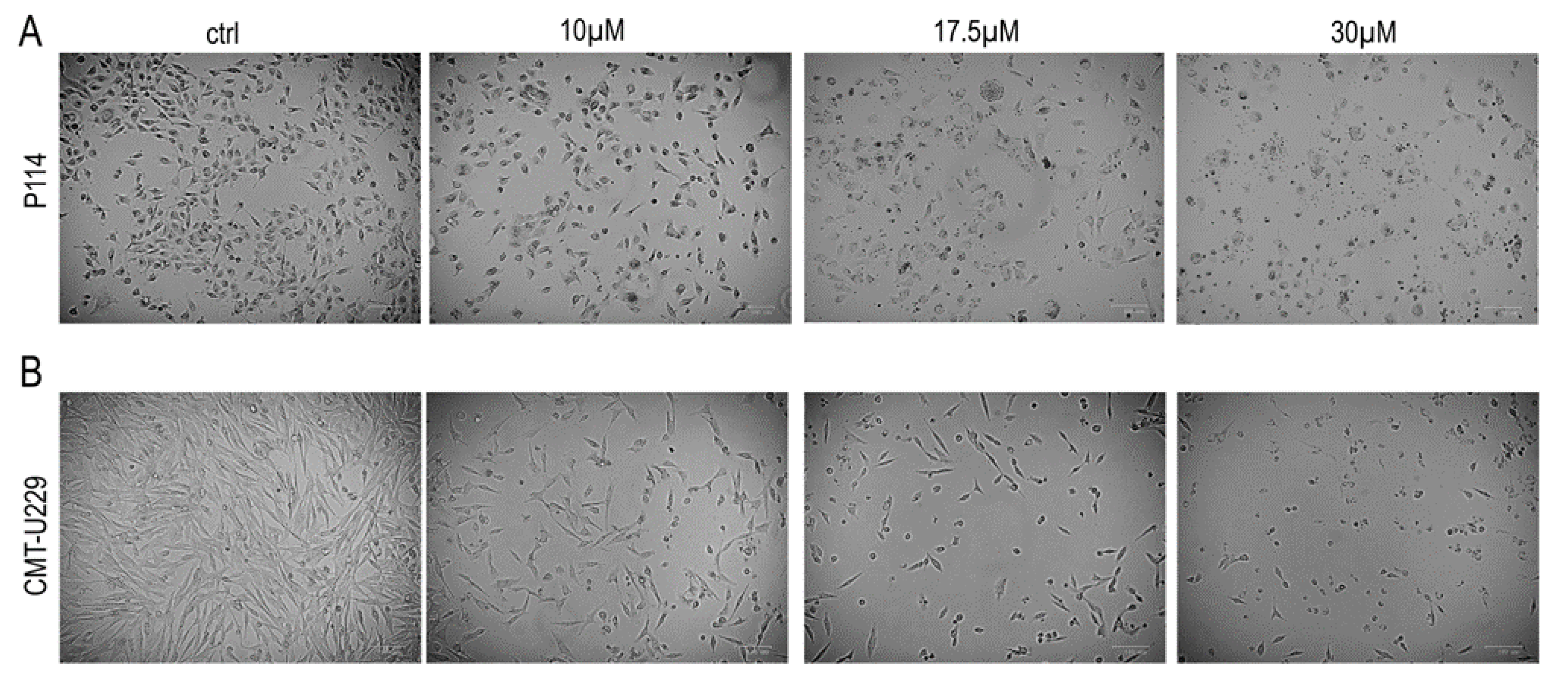
2.3. Teglicar Induces Morphological Changes in CMT Cells
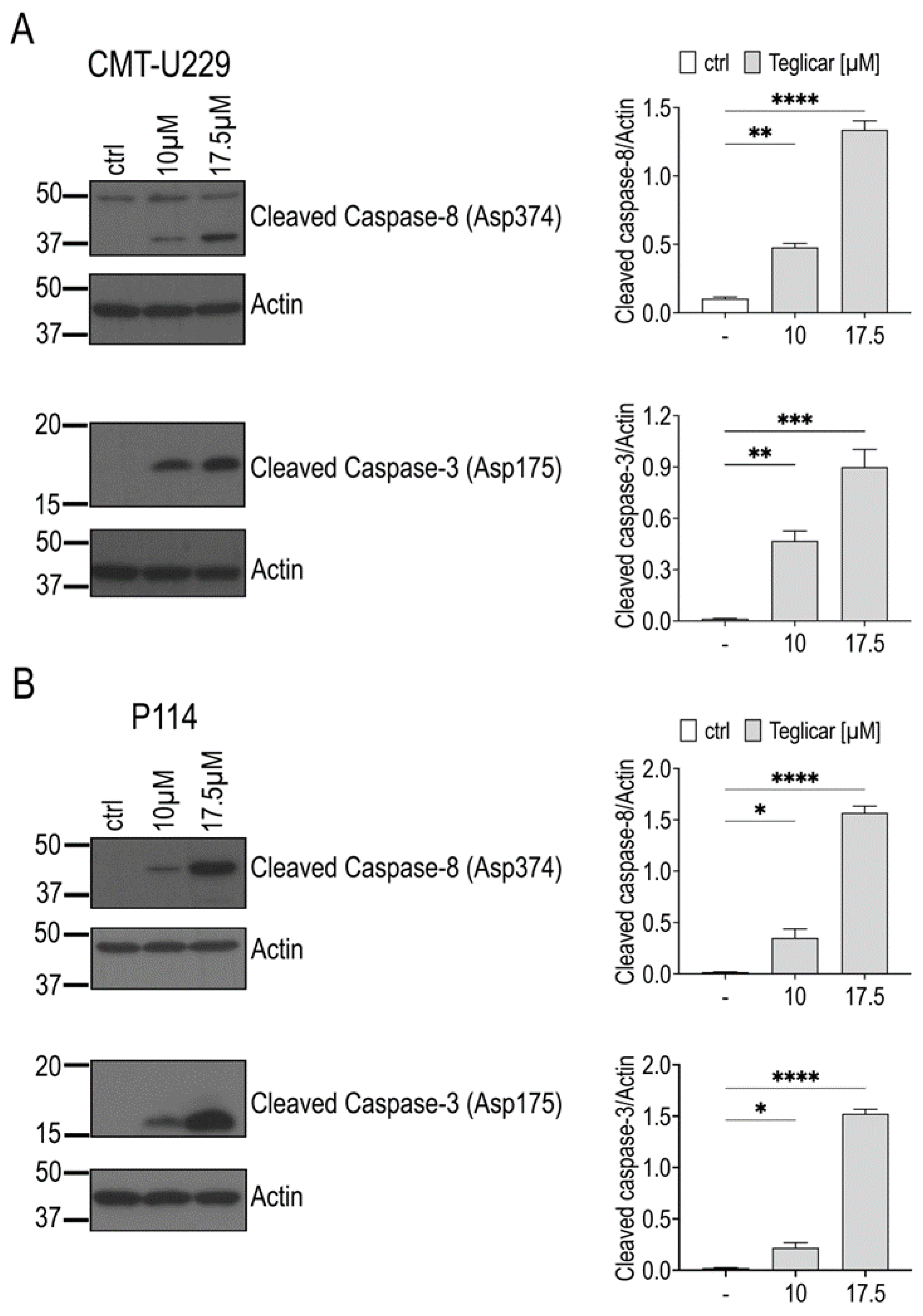
2.4. Teglicar Triggers Apoptosis in CMT Cells
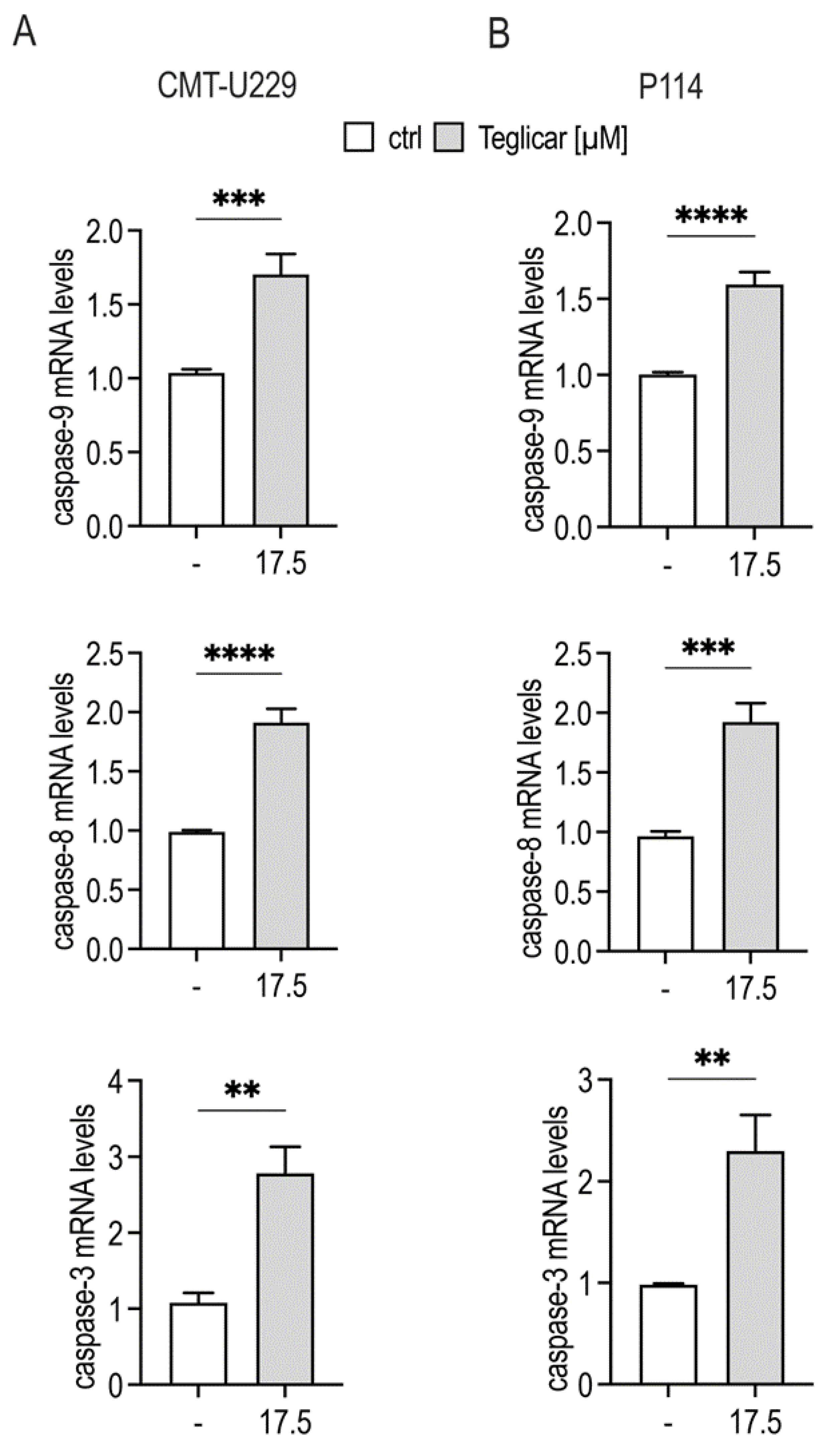
2.5. Teglicar-Induced Apoptosis Is Mediated by Caspases Activation
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Crystal Violet Assay
4.4. Trypan Blue Assay
4.5. Annexin V-FITC Apoptosis Detection
4.6. RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR (qRT-PCR)
4.7. Preparation of Cell Lysates and Western Blotting Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, H.; Chen, W.; Guan, Q.; He, J.; Guo, Z.; Li, J. A Qualitative Transcriptional Signature for Predicting Extreme Resistance of ER-Negative Breast Cancer to Paclitaxel, Doxorubicin, and Cyclophosphamide Neoadjuvant Chemotherapy. Front. Mol. Biosci. 2020, 7, 34. [Google Scholar] [CrossRef]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Domrazek, K.; Jurka, P. The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Vet. Sci. 2022, 9, 526. [Google Scholar] [CrossRef]
- Valdivia, G.; Alonso-Diez, A.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Valentino, A.; Margarucci, S.; Galderisi, U.; Giordano, A.; Peluso, G. The carnitine system and cancer metabolic plasticity. Cell Death Dis. 2018, 9, 228. [Google Scholar] [CrossRef]
- Wang, M.; Wang, K.; Liao, X.; Hu, H.; Chen, L.; Meng, L.; Gao, W.; Li, Q. Carnitine Palmitoyltransferase System: A New Target for Anti-Inflammatory and Anticancer Therapy? Front. Pharmacol. 2021, 12, 760581. [Google Scholar] [CrossRef]
- Tang, M.; Dong, X.; Xiao, L.; Tan, Z.; Luo, X.; Yang, L.; Li, W.; Shi, F.; Li, Y.; Zhao, L.; et al. CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma. Cell Death Dis. 2022, 13, 331. [Google Scholar] [CrossRef]
- Linher-Melville, K.; Zantinge, S.; Sanli, T.; Gerstein, H.; Tsakiridis, T.; Singh, G. Establishing a relationship between prolactin and altered fatty acid β-Oxidation via carnitine palmitoyl transferase 1 in breast cancer cells. BMC Cancer 2011, 11, 56. [Google Scholar] [CrossRef]
- Mozihim, A.K.; Chung, I.; Said, N.A.B.M.; Jamil, A.H.A. Reprogramming of Fatty Acid Metabolism in Gynaecological Cancers: Is There a Role for Oestradiol? Metabolites 2022, 12, 350. [Google Scholar] [CrossRef]
- Park, J.H.; Vithayathil, S.; Kumar, S.; Sung, P.-L.; Dobrolecki, L.E.; Putluri, V.; Bhat, V.B.; Bhowmik, S.K.; Gupta, V.; Arora, K.; et al. Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell Rep. 2016, 14, 2154–2165. [Google Scholar] [CrossRef]
- Gugiatti, E.; Tenca, C.; Ravera, S.; Fabbi, M.; Ghiotto, F.; Mazzarello, A.N.; Bagnara, D.; Reverberi, D.; Zarcone, D.; Cutrona, G.; et al. A reversible carnitine palmitoyltransferase (CPT1) inhibitor offsets the proliferation of chronic lymphocytic leukemia cells. Haematologica 2018, 103, e531–e536. [Google Scholar] [CrossRef] [PubMed]
- Mozolewska, P.; Duzowska, K.; Pakiet, A.; Mika, A.; Śledziński, T. Inhibitors of Fatty Acid Synthesis and Oxidation as Potential Anticancer Agents in Colorectal Cancer Treatment. Anticancer Res. 2020, 40, 4843–4856. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.-H.; Liu, G.-Y.; Wang, R.; Moon, S.H.; Gross, R.W.; Patti, G.J. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of β-oxidation. PLoS Biol. 2018, 16, e2003782. [Google Scholar] [CrossRef] [PubMed]
- Pacilli, A.; Calienni, M.; Margarucci, S.; D’apolito, M.; Petillo, O.; Rocchi, L.; Pasquinelli, G.; Nicolai, R.; Koverech, A.; Calvani, M.; et al. Carnitine-Acyltransferase System Inhibition, Cancer Cell Death, and Prevention of Myc-Induced Lymphomagenesis. J. Natl. Cancer Inst. 2013, 105, 489–498. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Mirabilii, S.; Allegretti, M.; Licchetta, R.; Calarco, A.; Torrisi, M.R.; Foà, R.; Nicolai, R.; Peluso, G.; Tafuri, A. Targeting the leukemia cell metabolism by the CPT1a inhibition: Functional preclinical effects in leukemias. Blood 2015, 126, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, N.A.; Sgadari, M.; Petillo, O.; Margarucci, S.; Martano, M.; Cocchia, N.; Maiolino, P.; Restucci, B. Carnitine palmitoyltransferase 1 A expression profile in canine mammary tumors. Vet. J. 2020, 257, 105453. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Worley, D.R.; Zappulli, V. Tumors of the mammary gland. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D., Thamm, D., Liptack, J., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 604–625. [Google Scholar] [CrossRef]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Martinez-Perez, C.; Kay, C.; Turnbull, A.K.; Morrison, L.R.; Pang, L.Y.; Argyle, D. Naturally-Occurring Canine Mammary Tumors as a Translational Model for Human Breast Cancer. Front. Oncol. 2020, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.; Jiang, X.; Gao, A.; Li, X.; Lin, Z.; Pei, S. Celastrol Inhibits Canine Mammary Tumor Cells by Inducing Apoptosis via the Caspase Pathway. Front. Vet.-Sci. 2021, 8, 801407. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Kohn, B.; Gruber, A.D. Mechanisms of tumour resistance against chemotherapeutic agents in veterinary oncology. Vet. J. 2016, 207, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, N.; Failing, K.; Richter, A.; Wehrend, A. Mammary Tumor Recurrence in Bitches After Regional Mastectomy. Vet. Surg. 2008, 37, 82–86. [Google Scholar] [CrossRef]
- Schoos, A.; Knab, V.M.; Gabriel, C.; Tripolt, S.; Wagner, D.A.; Bauder, B.; Url, A.; Fux, D.A. In vitro study to assess the efficacy of CDK4/6 inhibitor Palbociclib (PD-0332991) for treating canine mammary tumours. Vet.-Comp. Oncol. 2019, 17, 507–521. [Google Scholar] [CrossRef]
- Cirillo, A.; Di Salle, A.; Petillo, O.; Melone, M.A.B.; Grimaldi, G.; Bellotti, A.; Torelli, G.; De Santi, M.S.; Cantatore, G.; Marinelli, A.; et al. High grade glioblastoma is associated with aberrant expression of ZFP57, a protein involved in gene imprinting, and of CPT1A and CPT1C that regulate fatty acid metabolism. Cancer Biol. Ther. 2014, 15, 735–741. [Google Scholar] [CrossRef]
- Pucci, S.; Zonetti, M.J.; Fisco, T.; Polidoro, C.; Bocchinfuso, G.; Palleschi, A.; Novelli, G.; Spagnoli, L.G.; Mazzarelli, P. Carnitine palmitoyl transferase-1A (CPT1A): A new tumor specific target in human breast cancer. Oncotarget 2016, 7, 19982–19996. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Rider, L.; Rodrigues, L.U.; Gijón, M.A.; Pac, C.T.; Romero, L.; Cimic, A.; Sirintrapun, S.J.; Glodé, L.M.; Eckel, R.H.; et al. Lipid Catabolism via CPT1 as a Therapeutic Target for Prostate Cancer. Mol. Cancer Ther. 2014, 13, 2361–2371. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Mohamed, E.M.; Xu, G.G.; Waters, M.; Jing, K.; Ma, Y.; Zhang, Y.; Spiegel, S.; Idowu, M.O.; Fang, X. Carnitine palmitoyltransferase 1A functions to repress FoxO transcription factors to allow cell cycle progression in ovarian cancer. Oncotarget 2016, 7, 3832–3846. [Google Scholar] [CrossRef]
- Giannessi, F.; Pessotto, P.; Tassoni, E.; Chiodi, P.; Conti, R.; De Angelis, F.; Dell’Uomo, N.; Catini, R.; Deias, R.; Tinti, M.O.; et al. Discovery of a Long-Chain Carbamoyl Aminocarnitine Derivative, a Reversible Carnitine Palmitoyltransferase Inhibitor with Antiketotic and Antidiabetic Activity. J. Med. Chem. 2003, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Conti, R.; Mannucci, E.; Pessotto, P.; Tassoni, E.; Carminati, P.; Giannessi, F.; Arduini, A. Selective Reversible Inhibition of Liver Carnitine Palmitoyl-Transferase 1 by Teglicar Reduces Gluconeogenesis and Improves Glucose Homeostasis. Diabetes 2011, 60, 644–651. [Google Scholar] [CrossRef]
- Zhang, R.; Nie, D.L.; Chen, Y.; Zeng, Y.L.; Huang, Y.; Xiao, N. Teglicar Induces Apoptosis in HeLa Cervical Cancer Cells via Endoplasmic Reticulum Stress-Mediated Pathway. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 973–979. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Orning, P.; Lien, E. Multiple roles of caspase-8 in cell death, inflammation, and innate immunity. J. Leukoc. Biol. 2020, 109, 121–141. [Google Scholar] [CrossRef]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef]
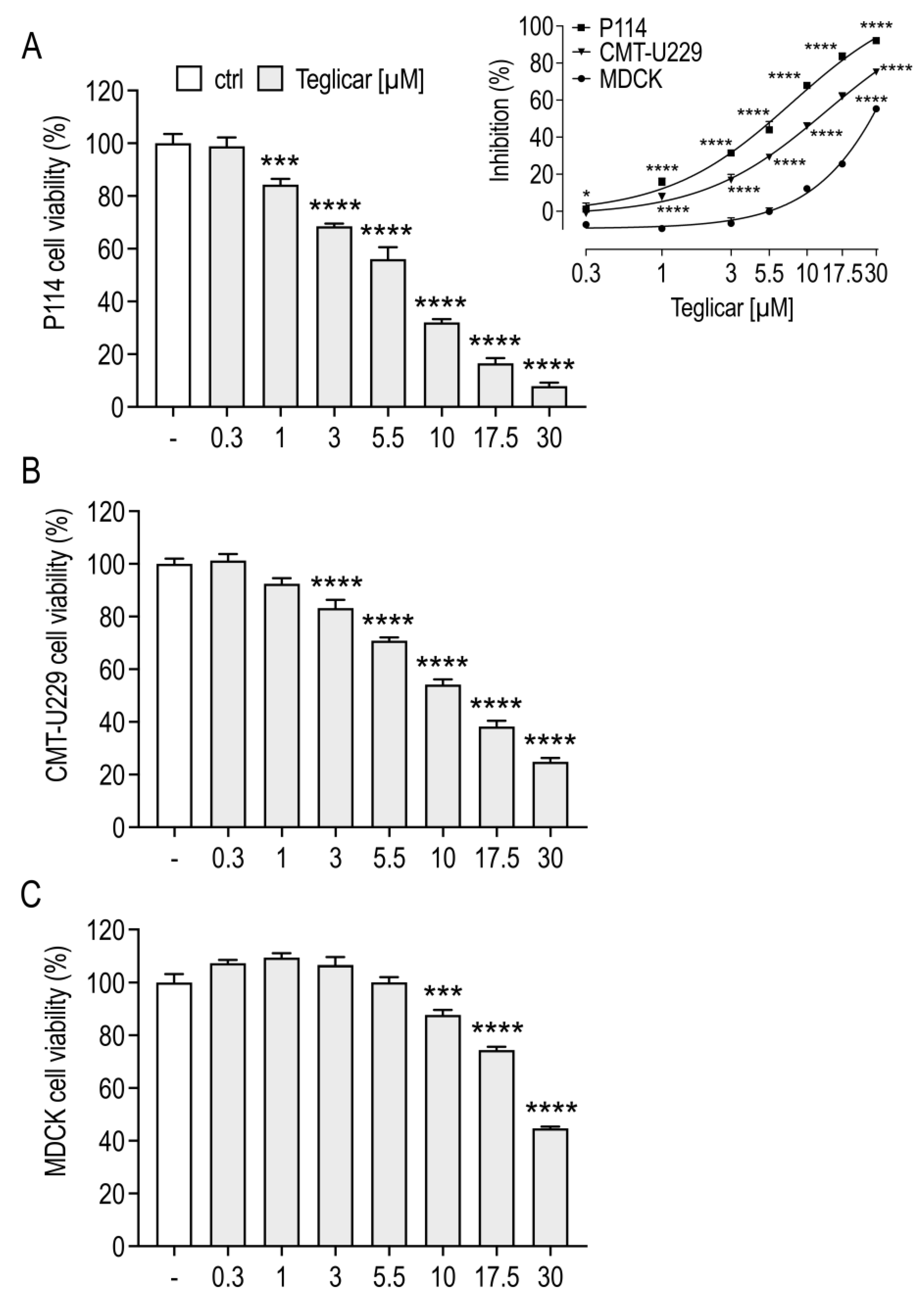

| Cell Line | Teglicar (μM) IC50 ± SEM |
|---|---|
| P114 | 7.9 ± 1.280 |
| CMT-U229 | 13.5 ± 1.483 |
| MDCK | 90.9 ± 7.096 |
| Gene | Primers |
|---|---|
| Rps-5 | Fw: 5′-TCACTGGTGAGAACCCCCT-3′ Rv: 5′-CCTGATTCACACGGCGTAG-3′ |
| Caspase-3 | Fw: 5′-GCAAACCTCAGGGAAACATT-3′ Rv: 5′-CTTAGAAGCACGCAAACAAAAC-3′ |
| Caspase-8 | Fw: 5′-GATGCAGATGCGTTGAGTAA-3′ Rv: 5′-AGCCATAGATGATGCCCTTGT-3′ |
| Caspase-9 | Fw: 5′-GGGAAGCCCAACGTCTTCTT-3′ Rv: 5′-AGTGGGCAAACTAGACACGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciola, N.A.; Sepe, F.; Fioriniello, S.; Petillo, O.; Margarucci, S.; Scivicco, M.; Peluso, G.; Balestrieri, A.; Bifulco, G.; Restucci, B.; et al. The Carnitine Palmitoyltransferase 1A Inhibitor Teglicar Shows Promising Antitumour Activity against Canine Mammary Cancer Cells by Inducing Apoptosis. Pharmaceuticals 2023, 16, 987. https://doi.org/10.3390/ph16070987
Cacciola NA, Sepe F, Fioriniello S, Petillo O, Margarucci S, Scivicco M, Peluso G, Balestrieri A, Bifulco G, Restucci B, et al. The Carnitine Palmitoyltransferase 1A Inhibitor Teglicar Shows Promising Antitumour Activity against Canine Mammary Cancer Cells by Inducing Apoptosis. Pharmaceuticals. 2023; 16(7):987. https://doi.org/10.3390/ph16070987
Chicago/Turabian StyleCacciola, Nunzio Antonio, Fabrizia Sepe, Salvatore Fioriniello, Orsolina Petillo, Sabrina Margarucci, Marcello Scivicco, Gianfranco Peluso, Anna Balestrieri, Giovanna Bifulco, Brunella Restucci, and et al. 2023. "The Carnitine Palmitoyltransferase 1A Inhibitor Teglicar Shows Promising Antitumour Activity against Canine Mammary Cancer Cells by Inducing Apoptosis" Pharmaceuticals 16, no. 7: 987. https://doi.org/10.3390/ph16070987
APA StyleCacciola, N. A., Sepe, F., Fioriniello, S., Petillo, O., Margarucci, S., Scivicco, M., Peluso, G., Balestrieri, A., Bifulco, G., Restucci, B., & Severino, L. (2023). The Carnitine Palmitoyltransferase 1A Inhibitor Teglicar Shows Promising Antitumour Activity against Canine Mammary Cancer Cells by Inducing Apoptosis. Pharmaceuticals, 16(7), 987. https://doi.org/10.3390/ph16070987







