Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage
Abstract
1. Introduction
2. Results
2.1. Effect of SDF on Weight Loss, DAI, and Colon Length
2.1.1. Female Mice
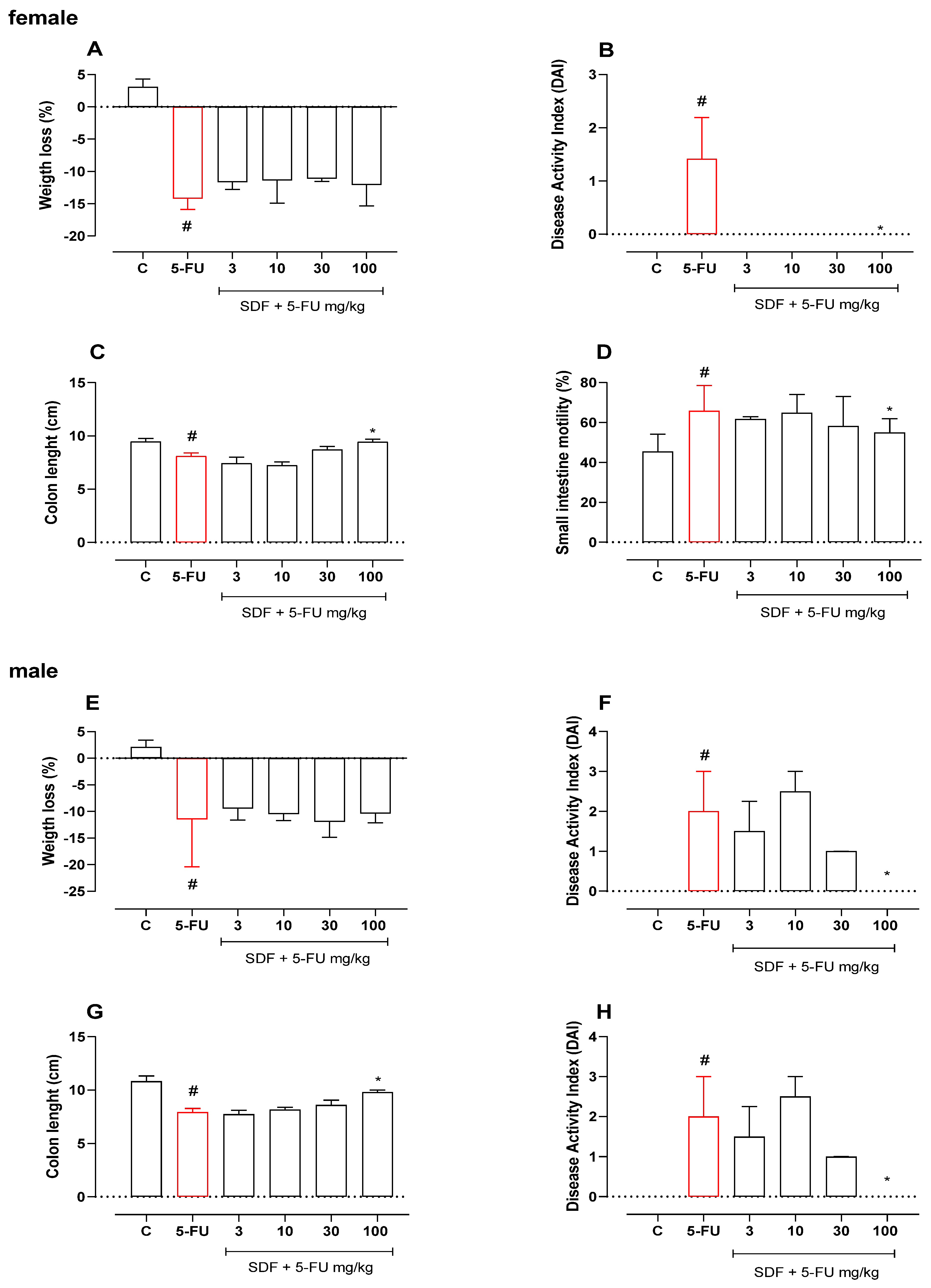
2.1.2. Male Mice
2.2. Effect of SDF on Small Intestinal Motility
2.2.1. Female Mice
2.2.2. Male Mice
2.3. Effect of SDF on the Weight of Spleen and Liver
2.3.1. Female Mice
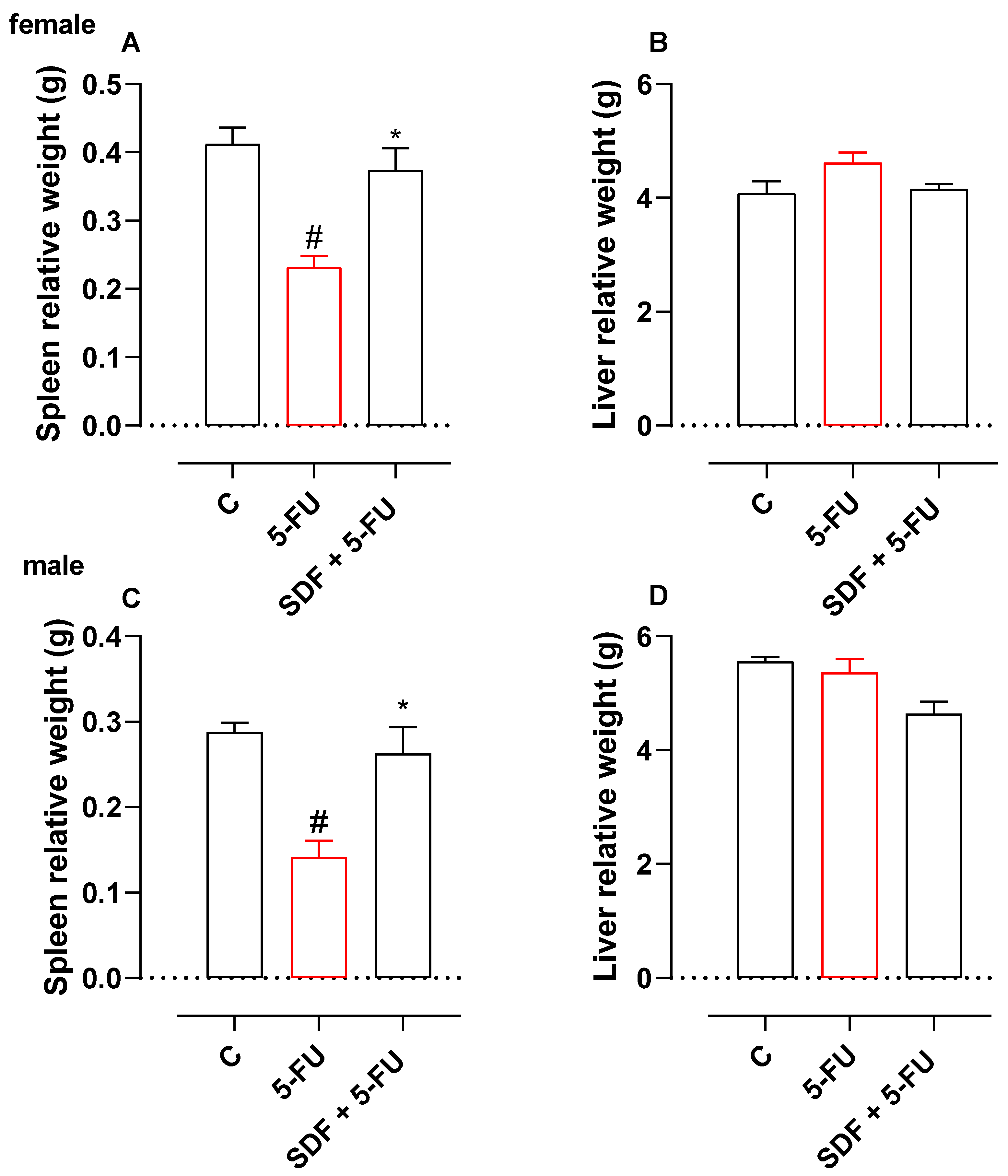
2.3.2. Male Mice
2.4. Effect of SDF on Oxidative Stress Parameters
2.4.1. Female Mice
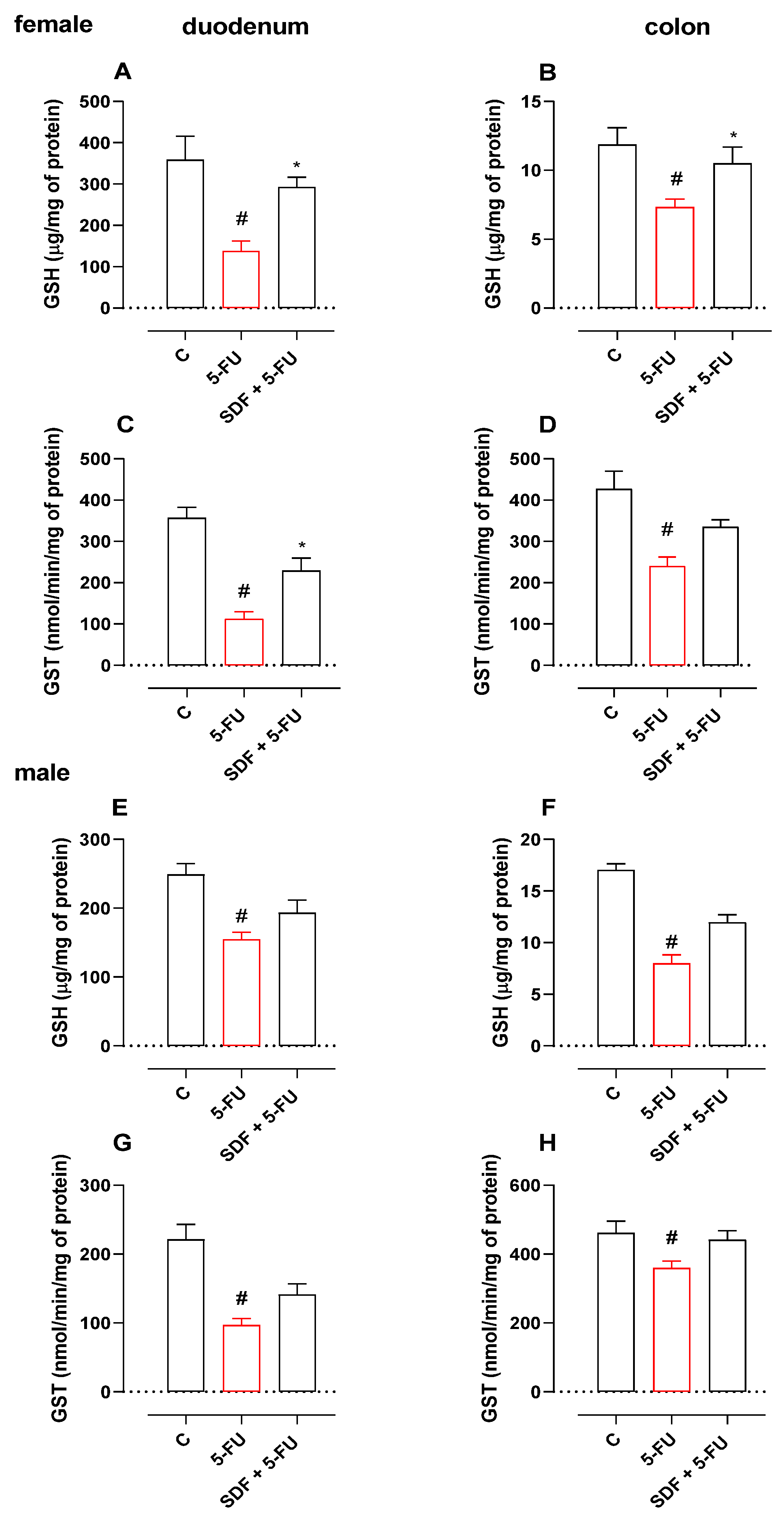
2.4.2. Male Mice
2.5. Effect of SDF on Inflammatory Parameters
2.5.1. Female Mice
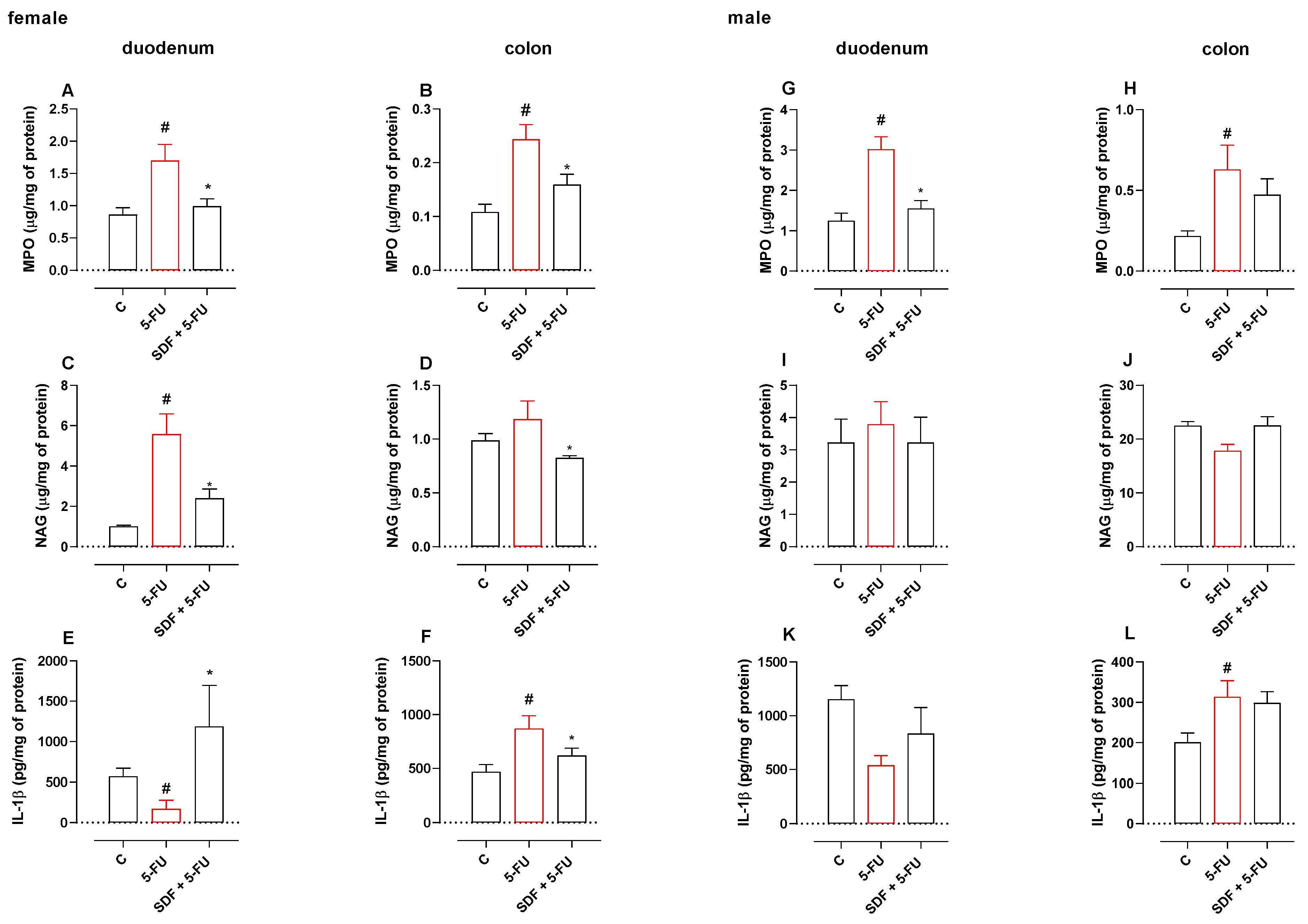
2.5.2. Male mice
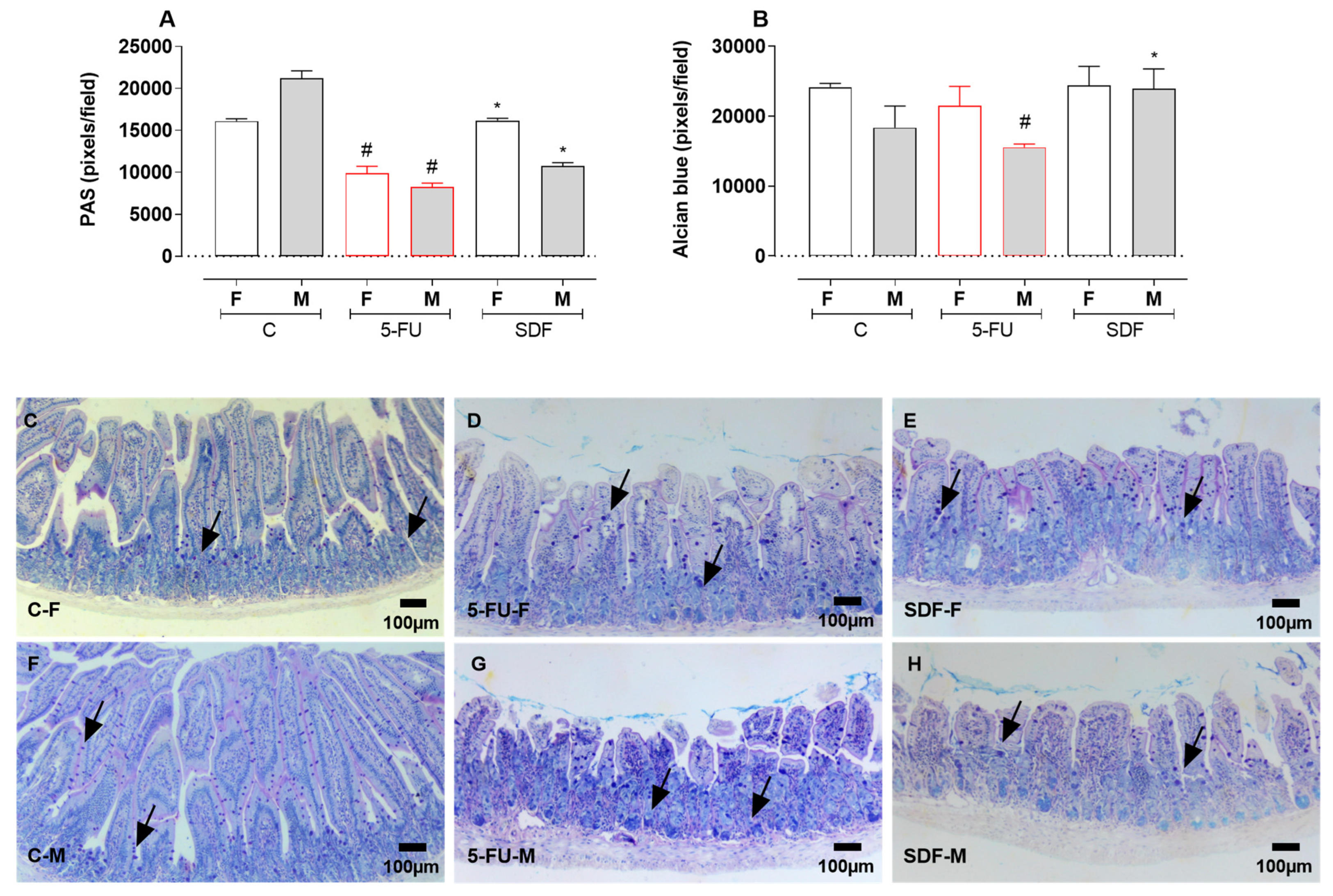
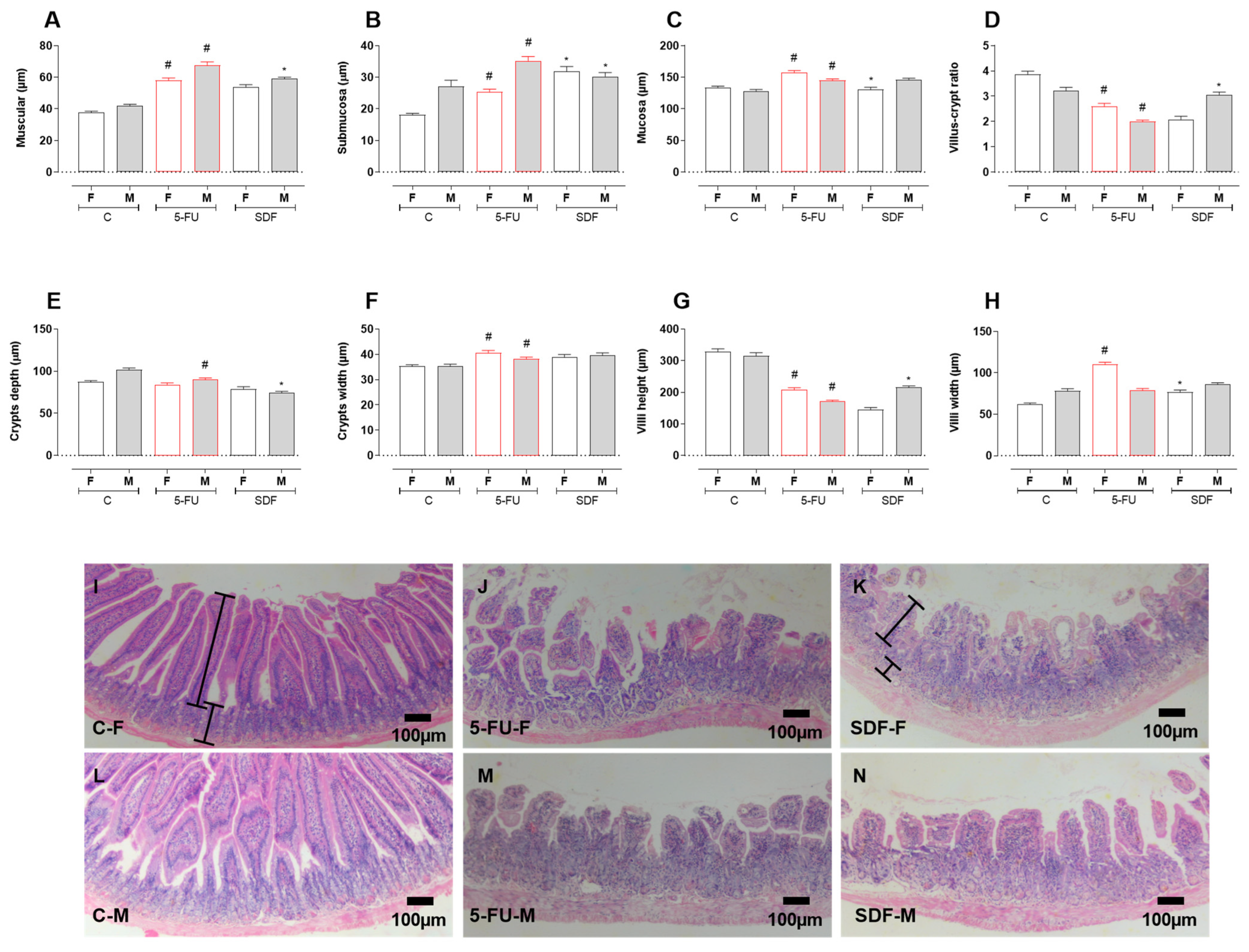
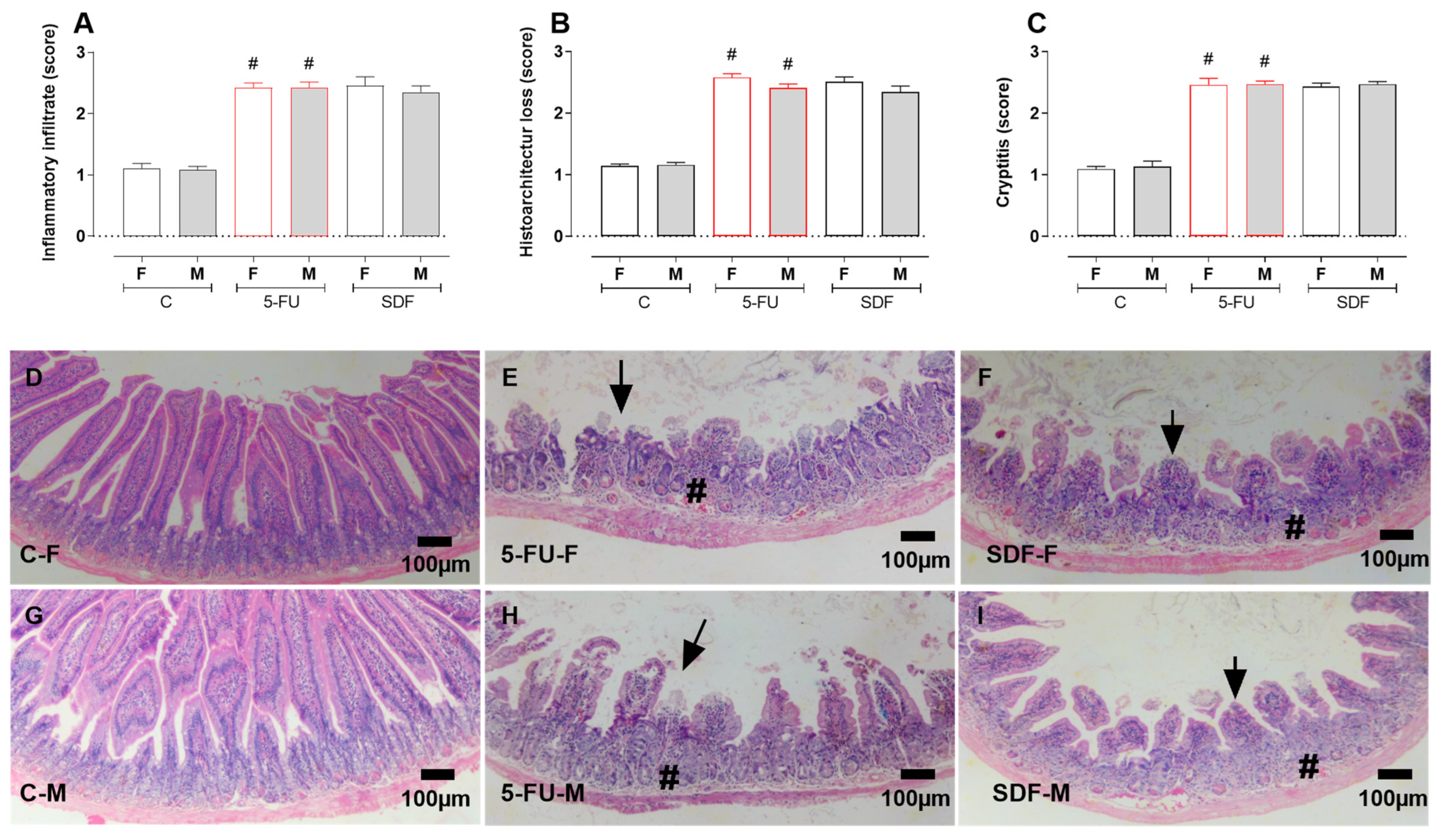
2.6. Effect of SDF on Duodenum Histological Damage of Female and Male Mice
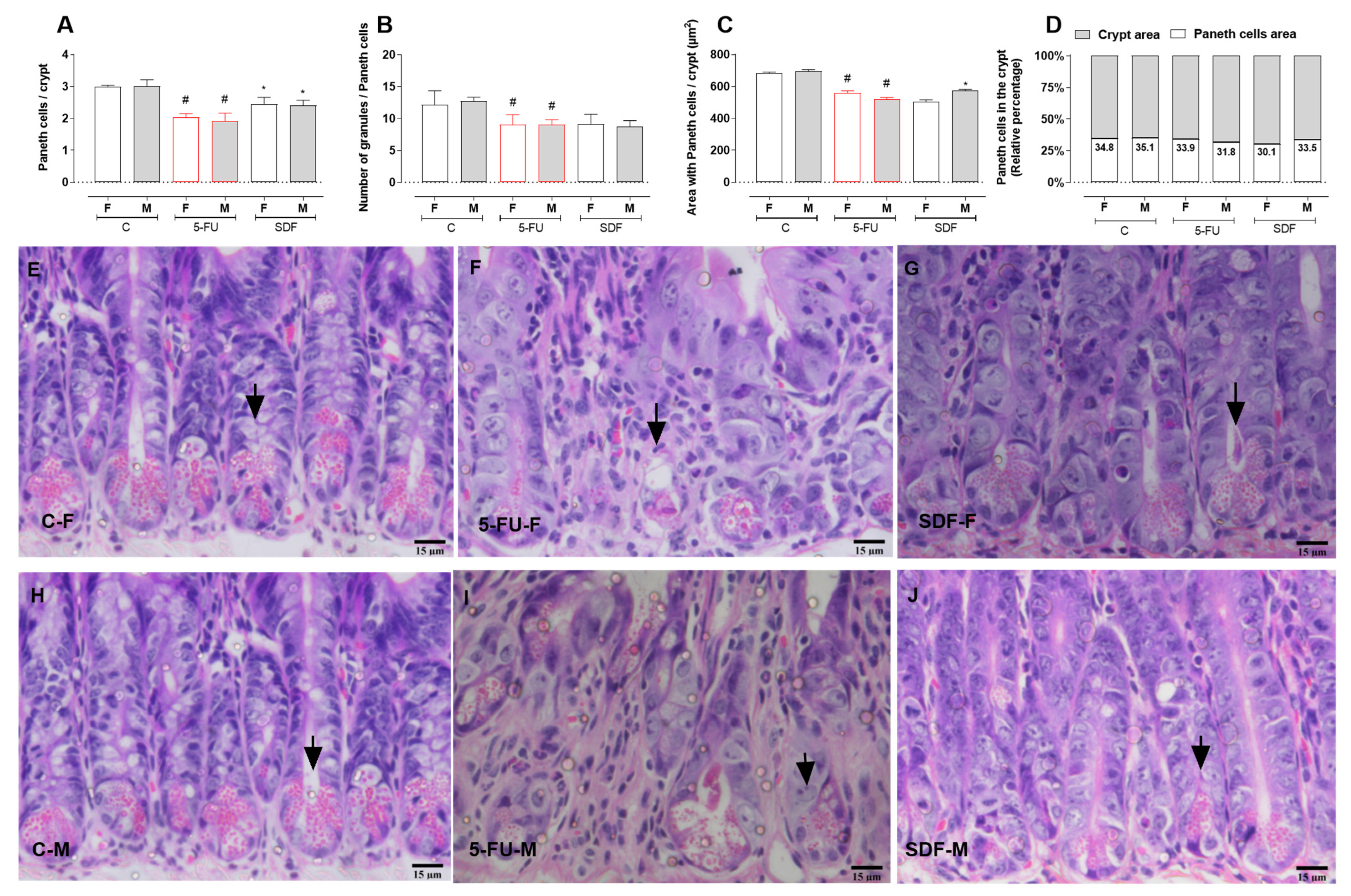
2.7. Effect of SDF on Colon Histological Damage of Female and Male Mice
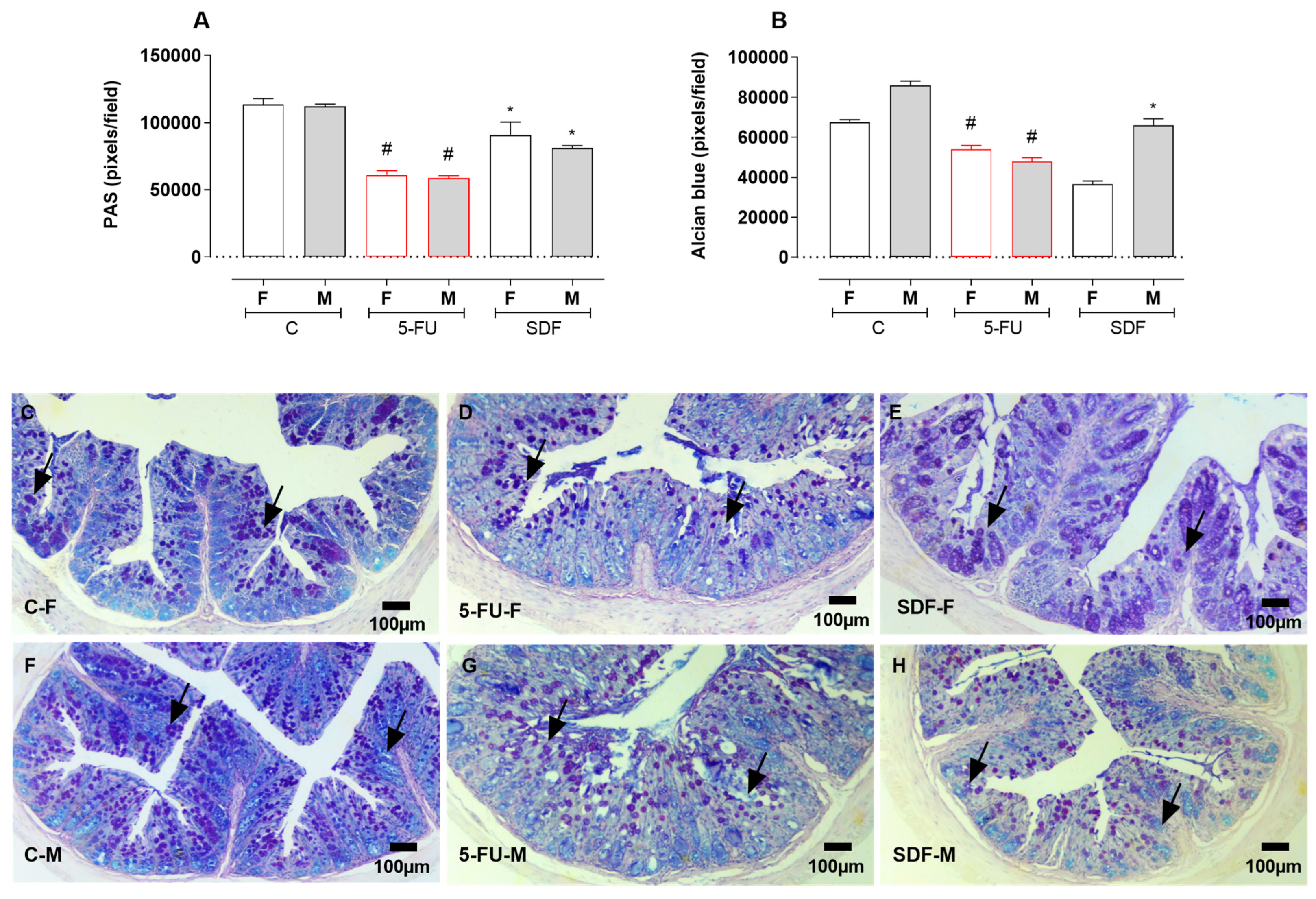
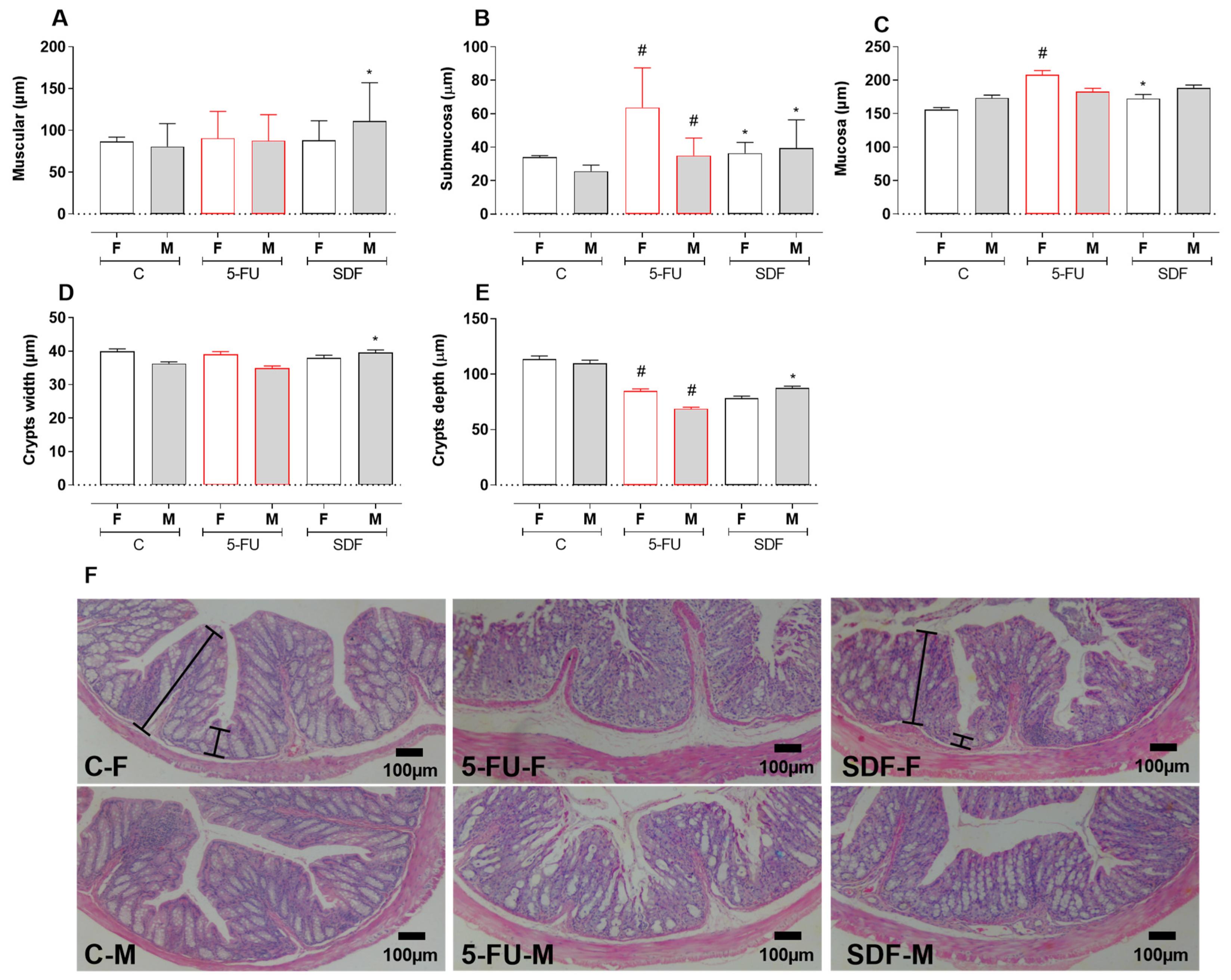
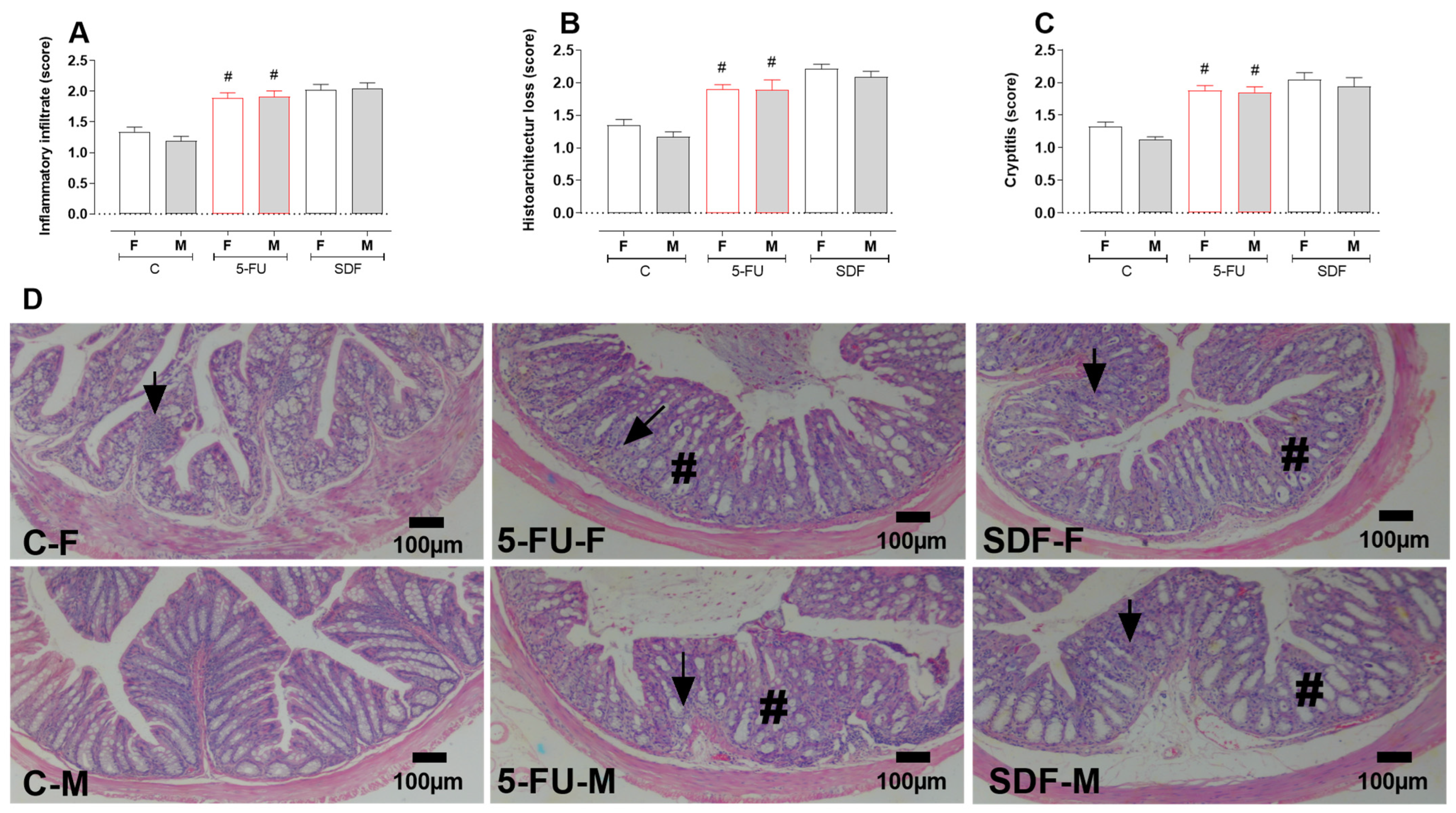
3. Discussion
4. Materials and Methods
4.1. Soluble Dietary Fiber Extraction and Characterization
4.2. Animals and Ethics Statement
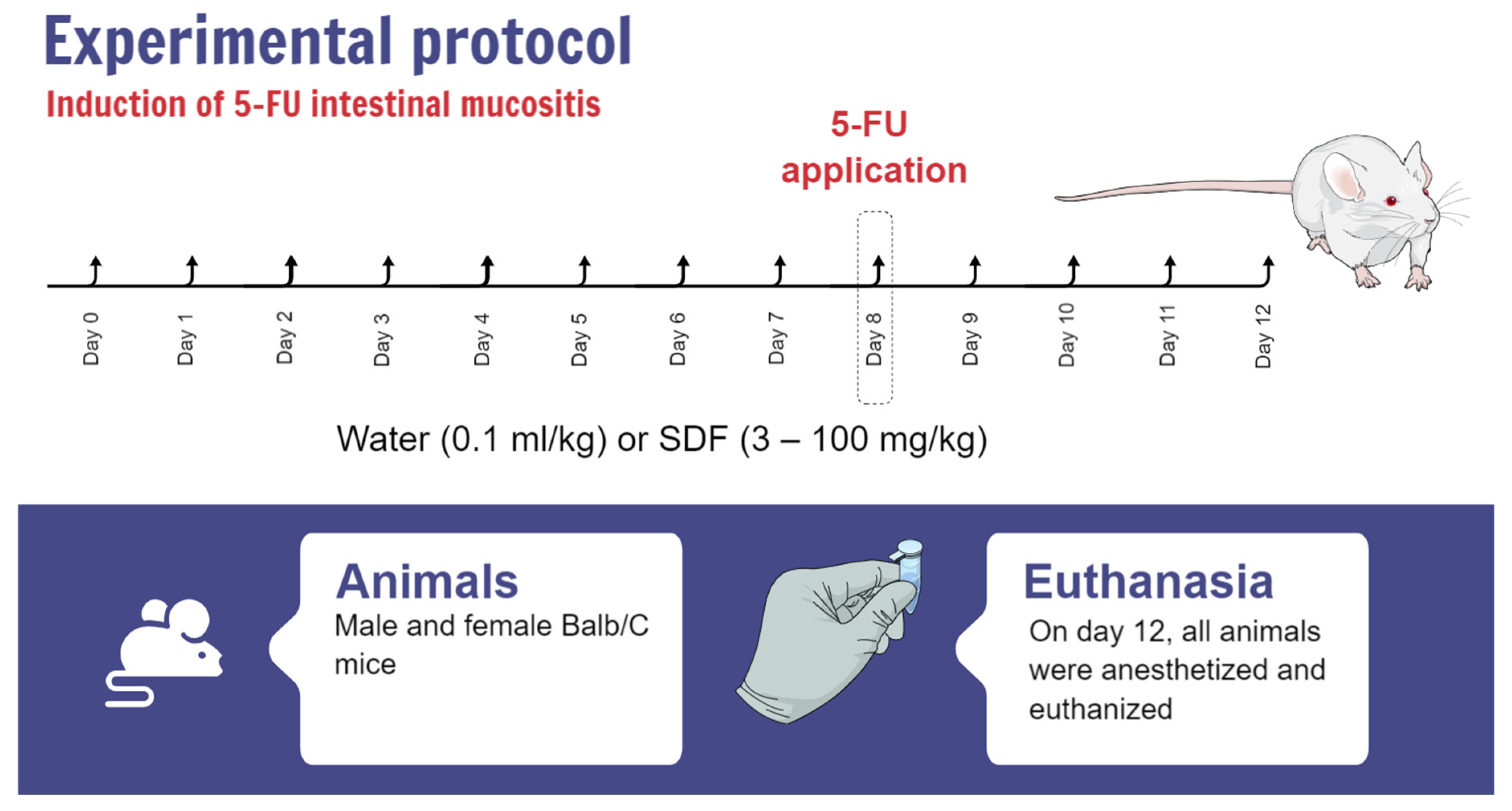
4.3. 5-FU Intestinal Mucositis Induction
4.4. Assessment of Intestinal Motility
4.5. Tissue Preparation for the Determination of Oxidative Stress and Inflammation Parameters
4.6. Oxidative Stress
4.6.1. GSH Determination
4.6.2. GST Determination
4.7. Inflammatory Parameters
4.7.1. Determination of MPO and NAG Activities
4.7.2. Evaluation of IL-1β
4.8. Histopathological Analysis
4.9. Histomorphometric Analysis
4.10. Quantification of Goblet Cells
4.11. Quantitative and Histomorphometric Analysis of Paneth Cells
4.12. Histopathological Evaluation
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Artwork
References
- Chen, X.; Eslamfam, S.; Fang, L.; Qiao, S.; Ma, X. Maintenance of Gastrointestinal Glucose Homeostasis by the Gut-Brain Axis. Curr. Protein Pept. Sci. 2017, 18, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Včev, A.; Meštrović, T.; Pustijanac, E.; Jukić, M.; Škrlec, I. Homeostasis and Dysbiosis of the Intestinal Microbiota: Comparing Hallmarks of a Healthy State with Changes in Inflammatory Bowel Disease. Microorganisms 2022, 10, 2405. [Google Scholar] [CrossRef] [PubMed]
- Thoo, L.; Noti, M.; Krebs, P. Keep Calm: The Intestinal Barrier at the Interface of Peace and War. Cell Death Dis. 2019, 10, 849. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Lo, Y.; Mah, A.; Kuo, C. The Intestinal Stem Cell Niche: Homeostasis and Adaptations. Trends Cell Biol. 2018, 28, 1062–1078. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and Inflammation in the Intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, I.; Fantini, M.C.; Monteleone, G. Regulation of Homeostasis and Inflammation in the Intestine. Gastroenterology 2011, 140, 1768–1775. [Google Scholar] [CrossRef]
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley Des Varannes, S.; Le Vacon, F.; De La Cochetière, M.F. Systematic Review: The Role of the Gut Microbiota in Chemotherapy- or Radiation-Induced Gastrointestinal Mucositis—Current Evidence and Potential Clinical Applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, Y.; Zhang, H.; Tu, W.; Zhang, S. Radiotherapy-Induced Digestive Injury: Diagnosis, Treatment and Mechanisms. Front. Oncol. 2021, 11, 757973. [Google Scholar] [CrossRef]
- Loman, B.R.; Jordan, K.R.; Haynes, B.; Bailey, M.T.; Pyter, L.M. Chemotherapy-Induced Neuroinflammation Is Associated with Disrupted Colonic and Bacterial Homeostasis in Female Mice. Sci. Rep. 2019, 9, 16490. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Sohda, M.; Saito, H.; Kuriyama, K.; Yoshida, T.; Kumakura, Y.; Hara, K.; Yokobori, T.; Miyazaki, T.; Murata, K.; et al. Docetaxel, Cisplatin, and 5-Fluorouracil Combination Chemoradiotherapy for Patients with Cervical Esophageal Cancer: A Single-Center Retrospective Study. Cancer Chemother. Pharmacol. 2019, 83, 1121–1126. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Putri, A.R. Metode Kemoterapi Terkini Berbasis Circadian Chronoteraphy Dalam Menurunkan Resistensi SeL SiHa Terhadap 5-Fluorourasil Pada Kanker Serviks. J. Agromed. 2019, 6, 370–378. [Google Scholar] [CrossRef]
- Negarandeh, R.; Salehifar, E.; Saghafi, F.; Jalali, H.; Janbabaei, G.; Abdhaghighi, M.J.; Nosrati, A. Evaluation of Adverse Effects of Chemotherapy Regimens of 5-Fluoropyrimidines Derivatives and Their Association with DPYD Polymorphisms in Colorectal Cancer Patients. BMC Cancer 2020, 20, 560. [Google Scholar] [CrossRef] [PubMed]
- Kadoyama, K.; Miki, I.; Tamura, T.; Brown, J.B.; Sakaeda, T.; Okuno, Y. Adverse Event Profiles of 5-Fluorouracil and Capecitabine: Data Mining of the Public Version of the FDA Adverse Event Reporting System, AERS, and Reproducibility of Clinical Observations. Int. J. Med. Sci. 2012, 9, 33–39. [Google Scholar] [CrossRef]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-Induced Intestinal Mucositis: Pathophysiology and Potential Treatment Strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef]
- Kuiken, N.S.S.; Rings, E.H.H.M.; Tissing, W.J.E. Risk Analysis, Diagnosis and Management of Gastrointestinal Mucositis in Pediatric Cancer Patients. Crit. Rev. Oncol. Hematol. 2015, 94, 87–97. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Keefe, D.M.K.; Gibson, R.J.; Hauer-Jensen, M. Gastrointestinal Mucositis. Semin. Oncol. Nurs. 2004, 20, 38–47. [Google Scholar] [CrossRef]
- Delgado, M.E.; Grabinger, T.; Brunner, T. Cell Death at the Intestinal Epithelial Front Line. FEBS J. 2016, 283, 2701–2719. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, H.; Li, X.; Liu, J.; Li, Z.; Xu, R.; Ma, Y.; Liu, C.; Xue, B. Activation of G Protein Coupled Estrogen Receptor Prevents Chemotherapy-Induced Intestinal Mucositis by Inhibiting the DNA Damage in Crypt Cell in an Extracellular Signal-Regulated Kinase 1- and 2- Dependent Manner. Cell Death Dis. 2021, 12, 1034. [Google Scholar] [CrossRef]
- Johnston, P.G.; Kaye, S. Capecitabine: A Novel Agent for the Treatment of Solid Tumors. Anticancer Drugs 2001, 12, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M.; et al. Effects of 5-Fluorouracil on Morphology, Cell Cycle, Proliferation, Apoptosis, Autophagy and ROS Production in Endothelial Cells and Cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef] [PubMed]
- Mayo, B.J.; Stringer, A.M.; Bowen, J.M.; Bateman, E.H.; Keefe, D.M. Irinotecan-Induced Mucositis: The Interactions and Potential Role of GLP-2 Analogues. Cancer Chemother. Pharmacol. 2017, 79, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding Chemotherapy-Induced Intestinal Mucositis and Strategies to Improve Gut Resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New Frontiers in the Pathobiology and Treatment of Cancer Regimen-Related Mucosal Injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef]
- Yeung, C.-Y.; Chan, W.-T.; Jiang, C.-B.; Cheng, M.-L.; Liu, C.-Y.; Chang, S.-W.; Chiang Chiau, J.-S.; Lee, H.-C. Amelioration of Chemotherapy-Induced Intestinal Mucositis by Orally Administered Probiotics in a Mouse Model. PLoS ONE 2015, 10, e0138746. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The Broadening Scope of Oral Mucositis and Oral Ulcerative Mucosal Toxicities of Anticancer Therapies. CA. Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Pereira, J.R.R.; Galante, F. Peel flour of the Passiflora edulis f. flavicarpa Deg (yellow passion fruit): From therapeutic potentials to side effects. Rev. Bras. Plantas Med. 2016, 18, 563–569. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan-Hyaluronic Acid/Nano Silver Composite Sponges for Drug Resistant Bacteria Infected Diabetic Wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- da Barud, H.S.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; de Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-Natural Composite Wound Dressing Films of Essential Oils Encapsulated in Sodium Alginate with Antimicrobial Properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Tahmouzi, S.; Ghodsi, M. Optimum Extraction of Polysaccharides from Motherwort Leaf and Its Antioxidant and Antimicrobial Activities. Carbohydr. Polym. 2014, 112, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.-L.; Xu, J.; Shi, M.-J.; Wang, X.-L.; Li, Y.-T.; Kong, L.-M.; Hider, R.C.; Zhou, T. Preparation, Antioxidant and Antimicrobial Evaluation of Hydroxamated Degraded Polysaccharides from Enteromorpha Prolifera. Food Chem. 2017, 237, 481–487. [Google Scholar] [CrossRef] [PubMed]
- El-Batal, A.I.; Mosalam, F.M.; Ghorab, M.M.; Hanora, A.; Elbarbary, A.M. Antimicrobial, Antioxidant and Anticancer Activities of Zinc Nanoparticles Prepared by Natural Polysaccharides and Gamma Radiation. Int. J. Biol. Macromol. 2018, 107, 2298–2311. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Thakur, K.; Liao, B.-Y.; Zhang, J.-G.; Wei, Z.-J. Antioxidant and Antimicrobial Potential of Polysaccharides Sequentially Extracted from Polygonatum Cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasab, F.S.; Hashemi, S.M.; Dehghan, A.; Sharififar, F.; Setayesh, M.; Sasanpour, P.; Tasbandi, M.; Raeiszadeh, M. Effects of a Plantago Ovata-Based Herbal Compound in Prevention and Treatment of Oral Mucositis in Patients with Breast Cancer Receiving Chemotherapy: A Double-Blind, Randomized, Controlled Crossover Trial. J. Integr. Med. 2020, 18, 214–221. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, W.; Tan, W.; Chen, Z.; Lu, H.; You, Y.; Tian, C.; Zhou, X.; Zhou, L.; Luo, R.; et al. Protective Effect of Polysaccharides Isolated from the Seeds of Cuscuta Chinensis Lam. on 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Acta Cir. Bras. 2022, 37, e370204. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Macedo, C.; Silva, A.M.; Delerue-Matos, C.; Costa, P.; Rodrigues, F. Natural Products for the Prevention and Treatment of Oral Mucositis-A Review. Int. J. Mol. Sci. 2022, 23, 4385. [Google Scholar] [CrossRef]
- da Silva, K.S.; da Silveira, B.C.; Bueno, L.R.; da Silva, L.C.M.; Fonseca, L.d.S.; Fernandes, E.S.; Maria-Ferreira, D. Beneficial Effects of Polysaccharides on the Epithelial Barrier Function in Intestinal Mucositis. Front. Physiol. 2021, 12, 714846. [Google Scholar] [CrossRef] [PubMed]
- Bueno, L.R.; da Silva Soley, B.; Abboud, K.Y.; França, I.W.; da Silva, K.S.; de Oliveira, N.M.T.; Barros, J.S.; Gois, M.B.; Cordeiro, L.M.C.; Maria-Ferreira, D. Protective Effect of Dietary Polysaccharides from Yellow Passion Fruit Peel on DSS-Induced Colitis in Mice. Oxid. Med. Cell. Longev. 2022, 2022, 6298662. [Google Scholar] [CrossRef]
- Paiva, A.A.d.O.; Castro, A.J.G.; Nascimento, M.S.; Will, L.S.E.P.; Santos, N.D.; Araújo, R.M.; Xavier, C.A.C.; Rocha, F.A.; Leite, E.L. Antioxidant and Anti-Inflammatory Effect of Polysaccharides from Lobophora Variegata on Zymosan-Induced Arthritis in Rats. Int. Immunopharmacol. 2011, 11, 1241–1250. [Google Scholar] [CrossRef]
- Dore, C.M.P.G.; das C Faustino Alves, M.G.; Will, L.S.E.P.; Costa, T.G.; Sabry, D.A.; de Souza Rêgo, L.A.R.; Accardo, C.M.; Rocha, H.A.O.; Filgueira, L.G.A.; Leite, E.L. A Sulfated Polysaccharide, Fucans, Isolated from Brown Algae Sargassum Vulgare with Anticoagulant, Antithrombotic, Antioxidant and Anti-Inflammatory Effects. Carbohydr. Polym. 2013, 91, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.M.; Liang, Y.Q.; Tang, L.J.; Ding, Y.; Wang, X.H. Antioxidant and Anti-Inflammatory Effects of Astragalus Polysaccharide on EA.Hy926 Cells. Exp. Ther. Med. 2013, 6, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Dore, C.M.P.G.; Alves, M.G.d.C.F.; Santos, M.D.G.L.; De Souza, L.A.R.; Baseia, I.G.; Leite, E.L. Antioxidant and Anti-Inflammatory Properties of an Extract Rich in Polysaccharides of the Mushroom Polyporus Dermoporus. Antioxidants 2014, 3, 730–744. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Huang, W.; Suo, J.; Chen, X.; Ding, K.; Sun, Q.; Zhang, H. Antioxidant and Anti-Inflammatory Activities of an Anti-Diabetic Polysaccharide Extracted from Gynostemma Pentaphyllum Herb. Int. J. Biol. Macromol. 2020, 145, 484–491. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, J.; Chen, H.; Liu, E. Trametes Orientalis Polysaccharide Alleviates PM2.5-Induced Lung Injury in Mice through Its Antioxidant and Anti-Inflammatory Activities. Food Funct. 2019, 10, 8005–8015. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An Insight into Anti-Inflammatory Effects of Natural Polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Li, Y.-T.; Chen, B.-J.; Wu, W.-D.; Ge, K.; Wei, X.-Y.; Kong, L.-M.; Xie, Y.-Y.; Gu, J.-P.; Zhang, J.-C.; Zhou, T. Antioxidant and Antimicrobial Evaluation of Carboxymethylated and Hydroxamated Degraded Polysaccharides from Sargassum Fusiforme. Int. J. Biol. Macromol. 2018, 118, 1550–1557. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Duan, W.; Li, D.; Tang, X.; Duan, Z. Effects of Polysaccharides From Auricularia Auricula on the Immuno-Stimulatory Activity and Gut Microbiota in Immunosuppressed Mice Induced by Cyclophosphamide. Front. Immunol. 2020, 11, 595700. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Cao, L.; Li, X.; Zhang, Q.; Xue, C.; Tang, Q. The Squid Ink Polysaccharides Protect Tight Junctions and Adherens Junctions from Chemotherapeutic Injury in the Small Intestinal Epithelium of Mice. Nutr. Cancer 2015, 67, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Cao, L.; Xue, C.; Tang, Q.-J. Dietary Squid Ink Polysaccharide Induces Goblet Cells to Protect Small Intestine from Chemotherapy Induced Injury. Food Funct. 2015, 6, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, O.W.; Yakubov, G.E.; Gartaula, G.; McGuckin, M.A.; Gidley, M.J. Mucoadhesive Functionality of Cell Wall Structures from Fruits and Grains: Electrostatic and Polymer Network Interactions Mediated by Soluble Dietary Polysaccharides. Sci. Rep. 2017, 7, 15794. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The Impacts of Natural Polysaccharides on Intestinal Microbiota and Immune Responses—A Review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Qian, C.; Hussain, M.; Liu, S.; Zhang, A.; He, R.; Sun, P. The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review. Biology 2023, 12, 122. [Google Scholar] [CrossRef]
- Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods 2020, 9, 843. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res. Int. 2013, 51, 756–763. [Google Scholar] [CrossRef]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.; Garcia, P.S.; Petkowicz, C.L.D.O.; Jagadevan, S.; Gimenes, M.L. Extraction of pectin from passion fruit peel (Passiflora edulis f. flavicarpa) by microwave-induced heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.T.; Cunha, M.A.L.; Sabaa-Srur, A.U.O.; Pires, V.C.F.; Cardoso, A.A.; Diniz, M.F.F.M.; Medeiros, C.C.M. Use of Passiflora edulis f. flavicarpa on cholesterol reduction. Braz. J. Pharmacogn. 2007, 17, 592–597. [Google Scholar] [CrossRef]
- Macagnan, F.T.; Santos, L.R.d.; Roberto, B.S.; de Moura, F.A.; Bizzani, M.; da Silva, L.P. Biological Properties of Apple Pomace, Orange Bagasse and Passion Fruit Peel as Alternative Sources of Dietary Fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Lima, G.C.; Vuolo, M.M.; Batista, Â.G.; Dragano, N.R.; Solon, C.; Maróstica Junior, M.R. Passiflora edulis peel intake improves insulin sensitivity, increasing incretins and hypothalamic satietogenic neuropeptide in rats on a high-fat diet. Nutrition 2016, 32, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Bussolo de Souza, C.; Jonathan, M.; Isay Saad, S.M.; Schols, H.A.; Venema, K. Characterization and in Vitro Digestibility of By-Products from Brazilian Food Industry: Cassava Bagasse, Orange Bagasse and Passion Fruit Peel. Bioact. Carbohydr. Diet. Fibre 2018, 16, 90–99. [Google Scholar] [CrossRef]
- Cazarin, C.B.; da Silva, J.K.; Colomeu, T.C.; Batista, Â.G.; Vilella, C.A.; Ferreira, A.L.; Junior, S.B.; Fukuda, K.; Augusto, F.; de Meirelles, L.R.; et al. Passiflora Edulis Peel Intake and Ulcerative Colitis: Approaches for Prevention and Treatment. Exp. Biol. Med. 2014, 239, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, J.; Guerra-Hernández, E.; Braga, P.A.C.; Reyes, F.G.R.; Maróstica, M.R.; et al. Intestinal anti-inflammatory effects of Passiflora edulis peel in the dextran sodium sulphate model of mouse colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Abboud, K.Y.; da Luz, B.B.; Dallazen, J.L.; Werner, M.F.d.P.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Iacomini, M.; Cordeiro, L.M.C. Gastroprotective Effect of Soluble Dietary Fibres from Yellow Passion Fruit (Passiflora Edulis f. Flavicarpa) Peel against Ethanol-Induced Ulcer in Rats. J. Funct. Foods 2019, 54, 552–558. [Google Scholar] [CrossRef]
- Keefe, D.M.; Schubert, M.M.; Elting, L.S.; Sonis, S.T.; Epstein, J.B.; Raber-Durlacher, J.E.; Migliorati, C.A.; McGuire, D.B.; Hutchins, R.D.; Peterson, D.E.; et al. Updated Clinical Practice Guidelines for the Prevention and Treatment of Mucositis. Cancer 2007, 109, 820–831. [Google Scholar] [CrossRef]
- Thomsen, M.; Vitetta, L. Adjunctive Treatments for the Prevention of Chemotherapy- and Radiotherapy-Induced Mucositis. Integr. Cancer Ther. 2018, 17, 1027–1047. [Google Scholar] [CrossRef]
- Liu, J.-H.; Hsieh, C.-H.; Liu, C.-Y.; Chang, C.-W.; Chen, Y.-J.; Tsai, T.-H. Anti-Inflammatory Effects of Radix Aucklandiae Herbal Preparation Ameliorate Intestinal Mucositis Induced by 5-Fluorouracil in Mice. J. Ethnopharmacol. 2021, 271, 113912. [Google Scholar] [CrossRef]
- Graham, J.S.; Cassidy, J. Adjuvant Therapy in Colon Cancer. Expert Rev. Anticancer Ther. 2012, 12, 99–109. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Mafi, A.; Rezaee, M.; Hedayati, N.; Hogan, S.D.; Reiter, R.J.; Aarabi, M.-H.; Asemi, Z. Melatonin and 5-Fluorouracil Combination Chemotherapy: Opportunities and Efficacy in Cancer Therapy. Cell Commun. Signal. 2023, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Ciaffaglione, V.; Modica, M.N.; Pittalà, V.; Romeo, G.; Salerno, L.; Intagliata, S. Mutual Prodrugs of 5-Fluorouracil: From a Classic Chemotherapeutic Agent to Novel Potential Anticancer Drugs. ChemMedChem 2021, 16, 3496–3512. [Google Scholar] [CrossRef]
- Kim, S.H.; Chun, H.J.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; Um, S.H.; Kim, C.D. Ursodeoxycholic Acid Attenuates 5-Fluorouracil-Induced Mucositis in a Rat Model. Oncol. Lett. 2018, 16, 2585–2590. [Google Scholar] [CrossRef]
- Huang, J.; Hwang, A.Y.M.; Jia, Y.; Kim, B.; Iskandar, M.; Mohammed, A.I.; Cirillo, N. Experimental Chemotherapy-Induced Mucositis: A Scoping Review Guiding the Design of Suitable Preclinical Models. Int. J. Mol. Sci. 2022, 23, 15434. [Google Scholar] [CrossRef]
- Smith, P.; Lavery, A.; Turkington, R.C. An Overview of Acute Gastrointestinal Side Effects of Systemic Anti-Cancer Therapy and Their Management. Best Pract. Res. Clin. Gastroenterol. 2020, 48–49, 101691. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef]
- Van Vliet, M.J.; Harmsen, H.J.M.; De Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.V.; Lazzarotto, C.R.; Aquino, C.C.; Figueiredo, I.L.; Costa, T.B.; Alves, L.A.d.O.; Ribeiro, R.A.; Bertolini, L.R.; Lima, A.a.M.; Brito, G.a.C.; et al. Alanyl-Glutamine Attenuates 5-Fluorouracil-Induced Intestinal Mucositis in Apolipoprotein E-Deficient Mice. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. E Biol. 2015, 48, 493–501. [Google Scholar] [CrossRef]
- Pereira, V.B.M.; Melo, A.T.; Assis-Júnior, E.M.; Wong, D.V.T.; Brito, G.A.C.; Almeida, P.R.C.; Ribeiro, R.A.; Lima-Júnior, R.C.P. A New Animal Model of Intestinal Mucositis Induced by the Combination of Irinotecan and 5-Fluorouracil in Mice. Cancer Chemother. Pharmacol. 2016, 77, 323–332. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef]
- Sangild, P.T.; Shen, R.L.; Pontoppidan, P.; Rathe, M. Animal Models of Chemotherapy-Induced Mucositis: Translational Relevance and Challenges. Am. J. Physiol.-Gastrointest. Liver Physiol. 2018, 314, G231–G246. [Google Scholar] [CrossRef]
- Galdino, F.M.P.; Andrade, M.E.R.; Barros, P.A.V.d.; Generoso, S.d.V.; Alvarez-Leite, J.I.; Almeida-Leite, C.M.d.; Peluzio, M.d.C.G.; Fernandes, S.O.A.; Cardoso, V.N. Pretreatment and Treatment with Fructo-Oligosaccharides Attenuate Intestinal Mucositis Induced by 5-FU in Mice. J. Funct. Foods 2018, 49, 485–492. [Google Scholar] [CrossRef]
- Lee, C.S.; Ryan, E.J.; Doherty, G.A. Gastro-Intestinal Toxicity of Chemotherapeutics in Colorectal Cancer: The Role of Inflammation. World J. Gastroenterol. 2014, 20, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Justino, P.F.C.; Melo, L.F.M.; Nogueira, A.F.; Costa, J.V.G.; Silva, L.M.N.; Santos, C.M.; Mendes, W.O.; Costa, M.R.; Franco, A.X.; Lima, A.A.; et al. Treatment with Saccharomyces Boulardii Reduces the Inflammation and Dysfunction of the Gastrointestinal Tract in 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Br. J. Nutr. 2014, 111, 1611–1621. [Google Scholar] [CrossRef]
- De Miranda, J.A.L.; Martins, C.d.S.; Fideles, L.d.S.; Barbosa, M.L.L.; Barreto, J.E.F.; Pimenta, H.B.; Freitas, F.O.R.; Pimentel, P.V.d.S.; Teixeira, C.S.; Scafuri, A.G.; et al. Troxerutin Prevents 5-Fluorouracil Induced Morphological Changes in the Intestinal Mucosa: Role of Cyclooxygenase-2 Pathway. Pharmaceuticals 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Li, X.; Chang, Y.; Duan, G.; Yu, L.; Zheng, R.; Xue, C.; Tang, Q. Dietary Fucoidan of Acaudina Molpadioides and Its Enzymatically Degraded Fragments Could Prevent Intestinal Mucositis Induced by Chemotherapy in Mice. Food Funct. 2015, 6, 415–422. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, F.; Zhang, R.; Dong, L.; Jia, X.; Liu, L.; Yi, Y.; Zhang, M. Longan Pulp Polysaccharides Relieve Intestinal Injury In Vivo and In Vitro by Promoting Tight Junction Expression. Carbohydr. Polym. 2020, 229, 115475. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus Barrier, Mucins and Gut Microbiota: The Expected Slimy Partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction With the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M.; et al. Homeostasis of the Gut Barrier and Potential Biomarkers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, Y.; Ichikawa, T.; Iwai, T.; Goso, Y.; Okayasu, I.; Ikezawa, T.; Shikama, N.; Saigenji, K.; Ishihara, K. Changes in the Mucus Barrier of the Rat during 5-Fluorouracil-Induced Gastrointestinal Mucositis. Scand. J. Gastroenterol. 2008, 43, 59–65. [Google Scholar] [CrossRef]
- Song, M.-K.; Park, M.-Y.; Sung, M.-K. 5-Fluorouracil-Induced Changes of Intestinal Integrity Biomarkers in BALB/c Mice. J. Cancer Prev. 2013, 18, 322. [Google Scholar] [CrossRef]
- Stringer, A.; Gibson, R.; Logan, R.; Bowen, J.; Yeoh, A.; Hamilton, J.; Keefe, D. Gastrointestinal Microflora and Mucins May Play a Critical Role in the Development of 5-Fluorouracil-Induced Gastrointestinal Mucositis. Exp. Biol. Med. 2009, 234, 430–441. [Google Scholar] [CrossRef]
- Rakha, E.A.; Boyce, R.W.G.; Abd El-Rehim, D.; Kurien, T.; Green, A.R.; Paish, E.C.; Robertson, J.F.R.; Ellis, I.O. Expression of Mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and Their Prognostic Significance in Human Breast Cancer. Mod. Pathol. 2005, 18, 1295–1304. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H.; Wen, Y.; Jiang, D.; Zhu, S.; He, X.; Xiong, Q.; Gao, J.; Hou, S.; Huang, S.; et al. Tremella Fuciformis Polysaccharides Ameliorated Ulcerative Colitis via Inhibiting Inflammation and Enhancing Intestinal Epithelial Barrier Function. Int. J. Biol. Macromol. 2021, 180, 633–642. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; Santana-Filho, A.P.; Watanabe, P.d.S.; Sant’Ana, D.d.M.G.; Luciano, F.B.; Bocate, K.C.P.; van den Wijngaard, R.M.; Werner, M.F.d.P.; et al. Rhamnogalacturonan, a Chemically-Defined Polysaccharide, Improves Intestinal Barrier Function in DSS-Induced Colitis in Mice and Human Caco-2 Cells. Sci. Rep. 2018, 8, 12261. [Google Scholar] [CrossRef]
- Yoshino, F.; Yoshida, A.; Nakajima, A.; Wada-Takahashi, S.; Takahashi, S.; Lee, M.C. Alteration of the Redox State with Reactive Oxygen Species for 5-Fluorouracil-Induced Oral Mucositis in Hamsters. PLoS ONE 2013, 8, e82834. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Kim, E.-K.; Jang, M.; Song, M.-J.; Kim, D.; Kim, Y.; Jang, H.H. Redox-Mediated Mechanism of Chemoresistance in Cancer Cells. Antioxidants 2019, 8, 471. [Google Scholar] [CrossRef]
- Wen, L.; Yang, S.; Li, P.; Chen, R.; Wang, Q.; Kaspo, B.; Fan, H.; Hu, J. IASPP-Mediated ROS Inhibition Drives 5-Fu Resistance Dependent on Nrf2 Antioxidative Signaling Pathway in Gastric Adenocarcinoma. Dig. Dis. Sci. 2020, 65, 2873–2883. [Google Scholar] [CrossRef]
- Li, D.; Song, C.; Zhang, J.; Zhao, X. ROS and Iron Homeostasis Dependent Ferroptosis Play a Vital Role in 5-Fluorouracil Induced Cardiotoxicity in Vitro and in Vivo. Toxicology 2022, 468, 153113. [Google Scholar] [CrossRef]
- Bowen, J.M.; Gibson, R.J.; Cummins, A.G.; Keefe, D.M.K. Intestinal Mucositis: The Role of the Bcl-2 Family, P53 and Caspases in Chemotherapy-Induced Damage. Support. Care Cancer 2006, 14, 713–731. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Besnard, A.G.; Menezes, G.B.; Secher, T.; Jabir, M.S.; Amaral, S.S.; Braun, H.; Lima-Junior, R.C.P.; Ribeiro, R.A.; Cunha, F.Q.; et al. IL-33 Targeting Attenuates Intestinal Mucositis and Enhances Effective Tumor Chemotherapy in Mice. Mucosal Immunol. 2014, 7, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.M.G.; Mota, J.M.S.C.; Souza, E.P.; Justino, P.F.C.; Franco, A.X.; Cunha, F.Q.; Ribeiro, R.A.; Souza, M.H.L.P. Inflammatory Intestinal Damage Induced by 5-Fluorouracil Requires IL-4. Cytokine 2013, 61, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Role of the Small Intestine, Colon and Microbiota in Determining the Metabolic Fate of Polyphenols. Biochem. Pharmacol. 2017, 139, 24–39. [Google Scholar] [CrossRef]
- Kurita, A.; Kado, S.; Kaneda, N.; Onoue, M.; Hashimoto, S.; Yokokura, T. Modified Irinotecan Hydrochloride (CPT-11) Administration Schedule Improves Induction of Delayed-Onset Diarrhea in Rats. Cancer Chemother. Pharmacol. 2000, 46, 211–220. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Cordeiro, G.S.; Góis, M.B.; Santos, L.S.; Espírito-Santo, D.A.; Silva, R.T.; Pereira, M.U.; Santos, J.N.; Conceição-Machado, M.E.P.; Deiró, T.C.B.J.; Barreto-Medeiros, J.M. Perinatal and Post-Weaning Exposure to a High-Fat Diet Causes Histomorphometric, Neuroplastic, and Histopathological Changes in the Rat Ileum. J. Dev. Orig. Health Dis. 2023, 14, 231–241. [Google Scholar] [CrossRef]
- Wzorek França Dos Santos, I.; Sauruk da Silva, K.; Regis Bueno, L.; Suzane Schneider, V.; Silva Schiebel, C.; Mulinari Turin de Oliveira, N.; Cristine Malaquias da Silva, L.; Soares Fernandes, E.; Biondaro Gois, M.; Mach Cortes Cordeiro, L.; et al. Polysaccharide Fraction from Campomanesia Adamantium and Campomanesia Pubescens Attenuates 5-Fluorouracil-Induced Intestinal Mucosal Inflammation in Mice. Nutr. Cancer 2023, 75, 1382–1398. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Lee, H.-Y.; Kim, T.K.; Kim, M.-S.; Park, Y.M.; Kim, J.; Park, K.; Kweon, M.-N.; Kim, S.-H.; Bae, J.-W.; et al. Obesogenic Diet-Induced Gut Barrier Dysfunction and Pathobiont Expansion Aggravate Experimental Colitis. PLoS ONE 2017, 12, e0187515. [Google Scholar] [CrossRef] [PubMed]
| Feces Appearance | Score |
|---|---|
| normal | 0 |
| slightly altered or damp | 1 |
| moist with little perianal dirt | 2 |
| moist with perianal dirt | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, K.S.; Abboud, K.Y.; Schiebel, C.S.; de Oliveira, N.M.T.; Bueno, L.R.; de Mello Braga, L.L.V.; da Silveira, B.C.; Santos, I.W.F.d.; Gomes, E.d.S.; Gois, M.B.; et al. Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage. Pharmaceuticals 2023, 16, 912. https://doi.org/10.3390/ph16070912
da Silva KS, Abboud KY, Schiebel CS, de Oliveira NMT, Bueno LR, de Mello Braga LLV, da Silveira BC, Santos IWFd, Gomes EdS, Gois MB, et al. Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage. Pharmaceuticals. 2023; 16(7):912. https://doi.org/10.3390/ph16070912
Chicago/Turabian Styleda Silva, Karien Sauruk, Kahlile Youssef Abboud, Carolina Silva Schiebel, Natalia Mulinari Turin de Oliveira, Laryssa Regis Bueno, Lara Luisa Valerio de Mello Braga, Bruna Carla da Silveira, Isabella Wzorek França dos Santos, Everton dos Santos Gomes, Marcelo Biondaro Gois, and et al. 2023. "Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage" Pharmaceuticals 16, no. 7: 912. https://doi.org/10.3390/ph16070912
APA Styleda Silva, K. S., Abboud, K. Y., Schiebel, C. S., de Oliveira, N. M. T., Bueno, L. R., de Mello Braga, L. L. V., da Silveira, B. C., Santos, I. W. F. d., Gomes, E. d. S., Gois, M. B., Cordeiro, L. M. C., & Maria Ferreira, D. (2023). Polysaccharides from Passion Fruit Peels: From an Agroindustrial By-Product to a Viable Option for 5-FU-Induced Intestinal Damage. Pharmaceuticals, 16(7), 912. https://doi.org/10.3390/ph16070912








