Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models
Abstract
1. Introduction
2. Results
2.1. The Performance of DeepBiomarker in AD + P Patients
2.2. Risk Factors Identified by the DeepBiomarker Model with Significant Contributions
3. Discussion
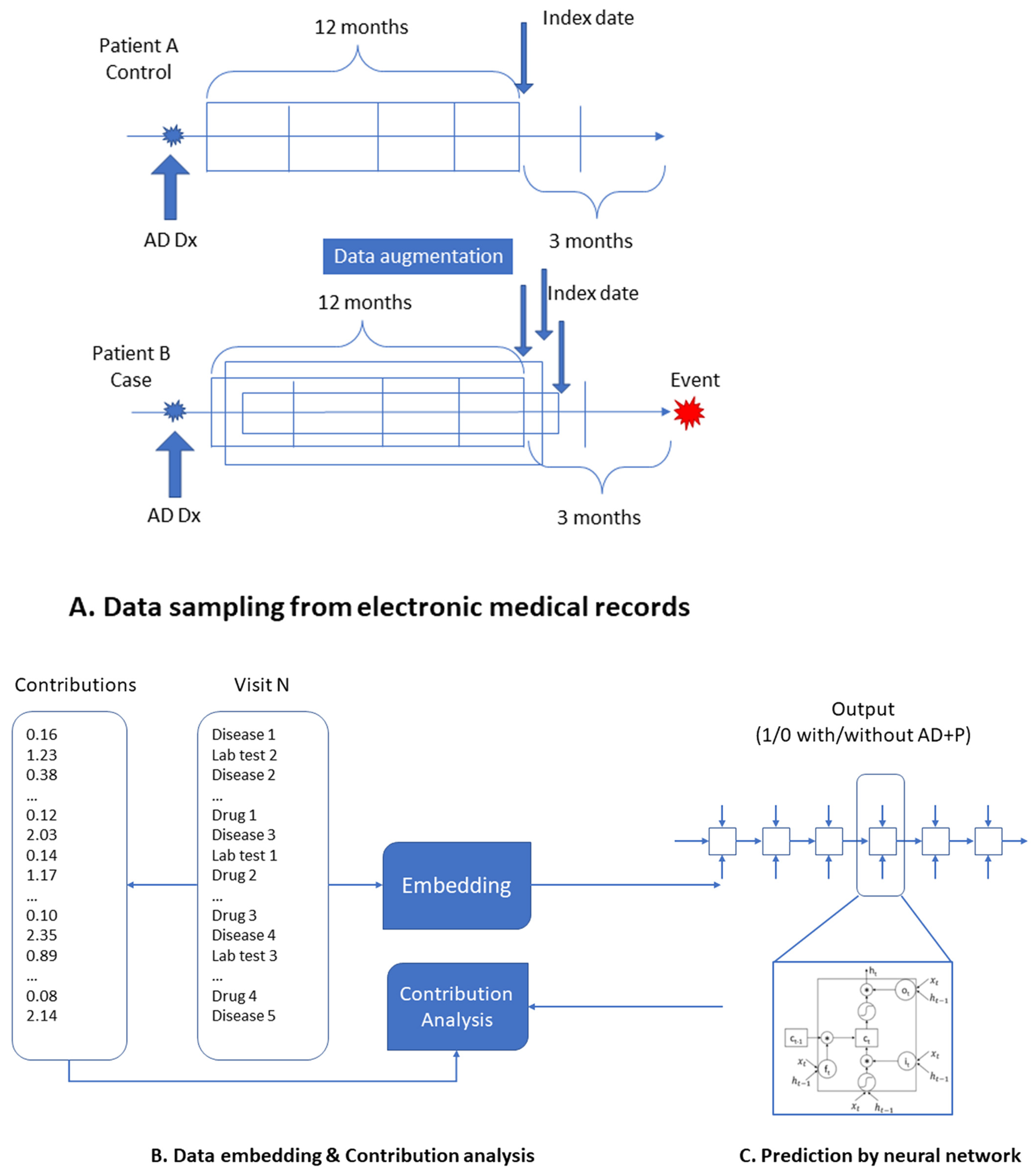
4. Materials and Methods
4.1. Data Source
4.2. Inclusion/Exclusion Criteria and Data Preparation
4.3. Data Augementation
4.4. Model Construction and Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar] [CrossRef]
- Thorgrimsen, L.; Selwood, A.; Spector, A.; Royan, L.; de Madariaga Lopez, M.; Woods, R.; Orrell, M. Whose quality of life is it anyway?: The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis. Assoc. Disord. 2003, 17, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Prince, M. World Alzheimer Report. The Global Economic Impact of Dementia; Alzheimer’s Disease International: London, UK, 2010. [Google Scholar]
- Murray, P.S.; Kumar, S.; Demichele-Sweet, M.A.; Sweet, R.A. Psychosis in Alzheimer’s disease. Biol. Psychiatry 2014, 75, 542–552. [Google Scholar] [CrossRef]
- Ropacki, S.A.; Jeste, D.V. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: A review of 55 studies published from 1990 to 2003. Am. J. Psychiatry 2005, 162, 2022–2030. [Google Scholar] [CrossRef]
- Battaglia, S.; Nazzi, C.; Thayer, J.F. Fear-induced bradycardia in mental disorders: Foundations, current advances, future perspectives. Neurosci. Biobehav. Rev. 2023, 149, 105163. [Google Scholar] [CrossRef]
- Lanctôt, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Ismail, Z.; Lyketsos, C.; Miller, D.S.; Musiek, E.; et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement. 2017, 3, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.A.; Bennett, D.A.; Graff-Radford, N.R.; Mayeux, R. Assessment and familial aggregation of psychosis in Alzheimer’s disease from the National Institute on Aging Late Onset Alzheimer’s Disease Family Study. Brain J. Neurol. 2010, 133, 1155–1162. [Google Scholar] [CrossRef]
- Krivinko, J.M.; Erickson, S.L.; Ding, Y.; Sun, Z.; Penzes, P.; MacDonald, M.L.; Yates, N.A.; Ikonomovic, M.D.; Lopez, O.L.; Sweet, R.A.; et al. Synaptic Proteome Compensation and Resilience to Psychosis in Alzheimer’s Disease. Am. J. Psychiatry 2018, 175, 999–1009. [Google Scholar] [CrossRef]
- DeChellis-Marks, M.R.; Wei, Y.; Ding, Y.; Wolfe, C.M.; Krivinko, J.M.; MacDonald, M.L.; Lopez, O.L.; Sweet, R.A.; Kofler, J. Psychosis in Alzheimer’s Disease Is Associated With Increased Excitatory Neuron Vulnerability and Post-transcriptional Mechanisms Altering Synaptic Protein Levels. Front. Neurol. 2022, 13, 778419. [Google Scholar] [CrossRef] [PubMed]
- Krivinko, J.; DeChellis-Marks, M.; Zeng, L.; Fan, P.; Lopez, O.; Ding, Y.; Wang, L.; Kofler, J.; MacDonald, M.; Sweet, R. Targeting the Post-Synaptic Proteome in Alzheimer Disease with Psychosis. Commun. Biol. 2023, 6, 538. [Google Scholar] [CrossRef] [PubMed]
- Madhusoodanan, S.; Shah, P. Management of psychosis in patients with Alzheimer’s disease: Focus on aripiprazole. Clin. Interv. Aging 2008, 3, 491–501. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Streim, J.; Carpenter, D.; Docherty, J.P. Expert consensus guidelines for using antipsychotic agents in older patients. J. Clin. Psychiatry 2004, 65, 5–99. [Google Scholar] [PubMed]
- Burke, A.D.; Burke, W.J. Antipsychotics FOR patients WITH dementia: The road less traveled: Second-generation agents have an important but limited role in treating behavioral and psychological symptoms. Curr. Psychiatry 2018, 17, 26–36. [Google Scholar]
- Dorsey, E.R.; Rabbani, A.; Gallagher, S.A.; Conti, R.M.; Alexander, G.C. Impact of FDA black box advisory on antipsychotic medication use. Arch. Intern. Med. 2010, 170, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, J.E.; Lopez, O.L.; Houck, P.R.; Becker, J.T.; Weamer, E.A.; Demichele-Sweet, M.A.; Kuller, L.; Sweet, R.A. Trajectory of cognitive decline as a predictor of psychosis in early Alzheimer disease in the cardiovascular health study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2011, 19, 160–168. [Google Scholar] [CrossRef]
- Tampi, R.R.; Tampi, D.J.; Balachandran, S.; Srinivasan, S. Antipsychotic use in dementia: A systematic review of benefits and risks from meta-analyses. Ther. Adv. Chronic Dis. 2016, 7, 229–245. [Google Scholar] [CrossRef]
- Fan, P.; Kofler, J.; Ding, Y.; Marks, M.; Sweet, R.A.; Wang, L. Efficacy difference of antipsychotics in Alzheimer’s disease and schizophrenia: Explained with network efficiency and pathway analysis methods. Brief. Bioinform. 2022, 23, bbac394. [Google Scholar] [CrossRef]
- DeMichele-Sweet, M.A.A.; Klei, L.; Creese, B.; Harwood, J.C.; Weamer, E.A.; McClain, L.; Sims, R.; Hernandez, I.; Moreno-Grau, S.; Tarraga, L.; et al. Genome-wide association identifies the first risk loci for psychosis in Alzheimer disease. Mol. Psychiatry 2021, 26, 5797–5811. [Google Scholar] [CrossRef]
- Bote-Curiel, L.; Muñoz-Romero, S.; Gerrero-Curieses, A.; Rojo-Álvarez, J.L. Deep Learning and Big Data in Healthcare: A Double Review for Critical Beginners. Appl. Sci. 2019, 9, 2331. [Google Scholar] [CrossRef]
- Salehinejad, H.; Sankar, S.; Barfett, J.; Colak, E.; Valaee, S.J. Recent advances in recurrent neural networks. arXiv 2017, arXiv:1801.01078. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 30. [Google Scholar]
- Supriya, M.; Deepa, A.J. Machine learning approach on healthcare big data: A review. Big Data Inf. Anal. 2020, 5, 58–75. [Google Scholar] [CrossRef]
- Pham, T.; Tran, T.; Phung, D.; Venkatesh, S. Deepcare: A deep dynamic memory model for predictive medicine. In Proceedings of the Pacific-Asia Conference on Knowledge Discovery and Data Mining, Auckland, New Zealand, 19–22 April 2016; pp. 30–41. [Google Scholar]
- Dernoncourt, F.; Lee, J.Y.; Uzuner, O.; Szolovits, P. De-identification of patient notes with recurrent neural networks. J. Am. Med. Inform. Assoc. 2017, 24, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Purushotham, S.; Khemani, R.; Liu, Y. Distilling knowledge from deep networks with applications to healthcare domain. arXiv 2015, arXiv:1512.03542. [Google Scholar]
- Cheng, Y.; Wang, F.; Zhang, P.; Hu, J. Risk prediction with electronic health records: A deep learning approach. In Proceedings of the SIAM International Conference on Data Mining, Miami, FL, USA, 5–7 May 2016; pp. 432–440. [Google Scholar]
- Choi, Y.; Chiu, C.Y.; Sontag, D. Learning Low-Dimensional Representations of Medical Concepts. AMIA Summits Transl. Sci. Proc. 2016, 2016, 41–50. [Google Scholar]
- Shickel, B.; Tighe, P.J.; Bihorac, A.; Rashidi, P. Deep EHR: A Survey of Recent Advances in Deep Learning Techniques for Electronic Health Record (EHR) Analysis. IEEE J. Biomed. Health Inform. 2018, 22, 1589–1604. [Google Scholar] [CrossRef]
- Wiemken, T.L.; Kelley, R.R. Machine Learning in Epidemiology and Health Outcomes Research. Annu. Rev. Public. Health 2020, 41, 21–36. [Google Scholar] [CrossRef]
- Rasmy, L.; Zhu, J.; Li, Z.; Hao, X.; Tran, H.T.; Zhou, Y.; Tiryaki, F.; Xiang, Y.; Xu, H.; Zhi, D. Simple Recurrent Neural Networks is all we need for clinical events predictions using EHR data. arXiv 2021, arXiv:.00998. [Google Scholar]
- Miranda, O.; Fan, P.; Qi, X.; Yu, Z.; Ying, J.; Wang, H.; Brent, D.A.; Silverstein, J.C.; Chen, Y.; Wang, L. DeepBiomarker: Identifying Important Lab Tests from Electronic Medical Records for the Prediction of Suicide-Related Events among PTSD Patients. J. Pers. Med. 2022, 12, 524. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Wang, X.; Zhang, Q.; Chen, R.; He, D.; Xie, X. Towards a deep and unified understanding of deep neural models in nlp. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019; pp. 2454–2463. [Google Scholar]
- Wang, L.; Ying, J.; Fan, P.; Weamer, E.A.; DeMichele-Sweet, M.A.A.; Lopez, O.L.; Kofler, J.K.; Sweet, R.A. Effects of Vitamin D Use on Outcomes of Psychotic Symptoms in Alzheimer Disease Patients. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2019, 27, 908–917. [Google Scholar] [CrossRef]
- Fan, P.; Qi, X.; Sweet, R.A.; Wang, L. Network systems pharmacology-based mechanism study on the beneficial effects of vitamin d against psychosis in Alzheimer’s disease. Sci. Rep. 2020, 10, 6136. [Google Scholar] [CrossRef] [PubMed]
- Calsolaro, V.; Antognoli, R.; Okoye, C.; Monzani, F. The Use of Antipsychotic Drugs for Treating Behavioral Symptoms in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1465. [Google Scholar] [CrossRef]
- Vigen, C.L.; Mack, W.J.; Keefe, R.S.; Sano, M.; Sultzer, D.L.; Stroup, T.S.; Dagerman, K.S.; Hsiao, J.K.; Lebowitz, B.D.; Lyketsos, C.G.; et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: Outcomes from CATIE-AD. Am. J. Psychiatry 2011, 168, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Raskin, J.; Wiltse, C.G.; Siegal, A.; Sheikh, J.; Xu, J.; Dinkel, J.J.; Rotz, B.T.; Mohs, R.C. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: An 8-week, double-blind, placebo-controlled trial. Am. J. Psychiatry 2007, 164, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Lee, S.W.; Lee, B.J.; Park, S.W.; Kim, G.M.; Kim, Y.H. Adjunctive memantine therapy for cognitive impairment in chronic schizophrenia: A placebo-controlled pilot study. Psychiatry Investig. 2012, 9, 166–173. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Papadakis, K.; Csernansky, J.; Litman, R.; Volavka, J.; Jia, X.D.; Gage, A.; Group, M.-M.-S. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 1322–1329. [Google Scholar] [CrossRef]
- Veerman, S.R.; Schulte, P.F.; Smith, J.D.; de Haan, L. Memantine augmentation in clozapine-refractory schizophrenia: A randomized, double-blind, placebo-controlled crossover study. Psychol. Med. 2016, 46, 1909–1921. [Google Scholar] [CrossRef]
- Krivoy, A.; Weizman, A.; Laor, L.; Hellinger, N.; Zemishlany, Z.; Fischel, T. Addition of memantine to antipsychotic treatment in schizophrenia inpatients with residual symptoms: A preliminary study. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2008, 18, 117–121. [Google Scholar] [CrossRef]
- Omranifard, V.; Rajabi, F.; Mohammadian-Sichani, M.; Maracy, M. The effect of add-on memantine on global function and quality of life in schizophrenia: A randomized, double-blind, controlled, clinical trial. Adv. Biomed. Res. 2015, 4, 211. [Google Scholar]
- Kotsiantis, S.B.; Zaharakis, I.; Pintelas, P.J. Supervised machine learning: A review of classification techniques. Emerg. Artif. Intell. Appl. Comput. Eng. 2007, 160, 3–24. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Lou, N.; Takano, T.; Pei, Y.; Xavier, A.L.; Goldman, S.A.; Nedergaard, M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. USA 2016, 113, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y. Mechanisms linking apolipoprotein E isoforms with cardiovascular and neurological diseases. Curr. Opin. Lipidol. 2010, 21, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modeling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan—Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, Z.; Shen, Y.; Zhu, H. A novel perspective linkage between kidney function and alzheimer’s disease. Front. Cell. Neurosci. 2018, 12, 384. [Google Scholar] [CrossRef]
- Han, S.W.; Park, Y.H.; Jang, E.S.; Nho, K.; Kim, S. Implications of liver enzymes in the pathogenesis of alzheimer’s disease. J. Alzheimers Dis. 2022, 88, 1371–1376. [Google Scholar] [CrossRef]
- Harciarek, M.; Williamson, J.B.; Biedunkiewicz, B.; Lichodziejewska-Niemierko, M.; Dębska-Ślizień, A.; Rutkowski, B. Risk factors for selective cognitive decline in dialyzed patients with end-stage renal disease: Evidence from verbal fluency analysis. J. Int. Neuropsychol. Soc. 2012, 18, 162–167. [Google Scholar] [CrossRef]
- Liao, W.; Xu, J.; Li, B.; Ruan, Y.; Li, T.; Liu, J. Deciphering the Roles of Metformin in Alzheimer’s Disease: A Snapshot. Front. Pharmacol. 2021, 12, 728315. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Prusty, S.K.; Sahu, P.K.; Das, D.J. Irbesartan protects against aluminium chloride induced amyloidogenesis and cognitive impairment. J. Krishna Inst. Med. Sci. 2022, 11, 18–30. [Google Scholar]
- Sushko, V.V.; Sushko, V.V. Use Acetazolamide in the Complex Therapy of Alzheimer’s Disease. Alzheimer’s Dement. 2022, 18, e059982. [Google Scholar] [CrossRef]
- Sberna, G.; Saez-Valero, J.; Beyreuther, K.; Masters, C.L.; Small, D.H. The amyloid beta-protein of Alzheimer’s disease increases acetylcholinesterase expression by increasing intracellular calcium in embryonal carcinoma P19 cells. J. Neurochem. 1997, 69, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Umukoro, S.; Bakre, T.O.; Onwuchekwa, C. Anti-psychotic and sedative effect of calcium channel blockers in mice. Afr. J. Med. Med. Sci. 2010, 39, 61–66. [Google Scholar]
- Stuve, O.; Weideman, R.A.; McMahan, D.M.; Jacob, D.A.; Little, B.B. Diclofenac reduces the risk of Alzheimer’s disease: A pilot analysis of NSAIDs in two US veteran populations. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420935676. [Google Scholar] [CrossRef]
- Yin, C.; Zhao, R.; Qian, B.; Lv, X.; Zhang, P. Domain knowledge guided deep learning with electronic health records. In Proceedings of the IEEE International Conference on Data Mining (ICDM), Beijing, China, 8–11 November 2019; pp. 738–747. [Google Scholar]
- Su, C.; Xu, Z.; Pathak, J.; Wang, F. Deep learning in mental health outcome research: A scoping review. Transl. Psychiatry 2020, 10, 116. [Google Scholar] [CrossRef]
- Zehraoui, F.; Sendi, N.; Abchiche-Mimouni, N. MS-LSTMEA: Predicting clinical events for Hypertension using Multi-Sources LSTM Explainable Approach. SSRN 4123459 2022. [Google Scholar] [CrossRef]
- Zhang, J. Representation Learning of Longitudinal Electronic Health Record Data for Patient Characterization and Prediction of Health Outcomes. Ph.D. Thesis, University of Virginia, Charlottesville, VA, USA, 2017. [Google Scholar]
- Visweswaran, S.; McLay, B.; Cappella, N.; Morris, M.; Milnes, J.T.; Reis, S.E.; Silverstein, J.C.; Becich, M.J. An atomic approach to the design and implementation of a research data warehouse. J. Am. Med. Inform. Assoc. 2022, 29, 601–608. [Google Scholar] [CrossRef]
- Rao, S.; Li, Y.; Ramakrishnan, R.; Hassaine, A.; Canoy, D.; Zhu, Y.; Salimi-Khorshidi, G.; Rahimi, K. BEHRT-HF: An interpretable transformer-based, deep learning model for prediction of incident heart failure. Eur. Heart J. 2020, 41, ehaa946.3553. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to obtain the P value from a confidence interval. BMJ 2011, 343, d2304. [Google Scholar] [CrossRef] [PubMed]
- Bonferroni, C. Teoria Statistica delle Classi e Calcolo delle Probabilita; Pubblicazioni del R. Istituto Superiore di Scienze Economiche e Commericiali di Firenze: Florence, Italy, 1936; Volume 8, pp. 3–62. [Google Scholar]
| Validation AUC | Test AUC | Validation AUC Std. | Test AUC Std. | |
|---|---|---|---|---|
| T-LSTM | 0.921 | 0.903 | 0.006 | 0.005 |
| RETAIN | 0.935 | 0.907 | 0.004 | 0.002 |
| LR | 0.837 | 0.822 | 0.009 | 0.012 |
| Feature | RC | CI95up | CI95down | Q Value * |
|---|---|---|---|---|
| Hypoxemia | 0.718 | 0.85 | 0.606 | 0.002 |
| Arthropathy, unspecified, site unspecified 1 | 0.747 | 0.831 | 0.672 | <0.001 |
| Pain in joint, shoulder region | 0.764 | 0.898 | 0.651 | 0.01 |
| Activities involving walking, marching, and hiking | 0.782 | 0.876 | 0.699 | 0.001 |
| Acute kidney failure, unspecified 1 | 0.86 | 0.945 | 0.783 | 0.014 |
| Unspecified osteoarthritis, unspecified site 1 | 0.877 | 0.944 | 0.814 | 0.006 |
| Esophageal reflux | 1.112 | 1.174 | 1.054 | 0.002 |
| Depressive disorder, not elsewhere classified | 1.117 | 1.195 | 1.045 | 0.01 |
| Hypothyroidism, unspecified 1 | 1.136 | 1.234 | 1.045 | 0.02 |
| Disorientation, unspecified 1 | 1.148 | 1.268 | 1.039 | 0.039 |
| Atherosclerotic heart disease of native coronary artery without angina pectoris | 1.154 | 1.228 | 1.085 | <0.001 |
| Abnormality of gait | 1.171 | 1.292 | 1.061 | 0.014 |
| Type 2 diabetes mellitus without complications | 1.191 | 1.261 | 1.125 | <0.001 |
| Obstructive sleep apnea | 1.207 | 1.354 | 1.076 | 0.012 |
| Central pain syndrome | 1.221 | 1.397 | 1.067 | 0.025 |
| Diabetes mellitus without mention of complication, type II or unspecified type, not stated as uncontrolled 1 | 1.234 | 1.328 | 1.147 | <0.001 |
| Aortic valve disorders | 1.274 | 1.506 | 1.077 | 0.03 |
| Atrial fibrillation | 1.358 | 1.477 | 1.249 | <0.001 |
| Dependence on renal dialysis | 1.361 | 1.602 | 1.156 | 0.003 |
| Hypocalcemia | 1.417 | 1.689 | 1.189 | 0.002 |
| Long term (current) use of insulin | 1.582 | 1.722 | 1.454 | <0.001 |
| Primary hypercoagulable state | 1.582 | 1.722 | 1.454 | <0.001 |
| Acute venous embolism and thrombosis of unspecified deep vessels of lower extremity | 1.687 | 2.325 | 1.223 | 0.012 |
| Feature | Indication/Drug Class | RC | CI95up | CI95down | Q Value * |
|---|---|---|---|---|---|
| Glucosamine–Chondroitin | Osteoarthritis, reduce joint pain and inflammation | 0.359 | 0.7 | 0.184 | 0.02 |
| Dextromethorphan–Guaifenesin | Cough suppressant | 0.439 | 0.757 | 0.255 | 0.022 |
| Fish Oil | Dietary supplement | 0.456 | 0.73 | 0.285 | 0.01 |
| Sucralfate | Gastrointestinal ulcers | 0.472 | 0.716 | 0.311 | 0.005 |
| Midodrine | Alpha-adrenergic agonist for hypotension | 0.477 | 0.65 | 0.35 | <0.001 |
| Irbesartan | Angiotensin receptor blocker for hypertension | 0.509 | 0.769 | 0.338 | 0.012 |
| Esomeprazole Magnesium | Proton pump inhibitor | 0.538 | 0.698 | 0.414 | <0.001 |
| Cyclobenzaprine | Skeletal muscle relaxant | 0.572 | 0.734 | 0.445 | <0.001 |
| Budesonide–Formoterol | Corticosteroid/beta2-adrenergic receptor agonist | 0.578 | 0.763 | 0.437 | 0.002 |
| Lactulose | Constipation and portal systemic encephalopathy | 0.599 | 0.835 | 0.429 | 0.019 |
| Duloxetine | Antidepressant | 0.606 | 0.757 | 0.485 | <0.001 |
| Ezetimibe | Hyperlipidemia | 0.624 | 0.854 | 0.457 | 0.022 |
| Magnesium Hydroxide | Laxative and antacid | 0.66 | 0.823 | 0.529 | 0.003 |
| Famotidine | Histamine H2 receptor antagonist | 0.672 | 0.823 | 0.548 | 0.002 |
| Nitroglycerin | Nitrate vasodilator | 0.679 | 0.84 | 0.549 | 0.005 |
| Alprazolam | Anxiety disorders and panic disorders | 0.692 | 0.857 | 0.56 | 0.008 |
| Isosorbide Mononitrate | Prevent and treat angina in coronary artery disease | 0.719 | 0.89 | 0.582 | 0.018 |
| Quetiapine | Antipsychotics | 0.726 | 0.875 | 0.603 | 0.008 |
| Glipizide | Type 2 diabetes | 0.732 | 0.923 | 0.581 | 0.046 |
| Memantine | Alzheimer’s disease | 0.749 | 0.83 | 0.676 | <0.001 |
| Triamcinolone Acetonide | Corticosteroid | 0.751 | 0.923 | 0.612 | 0.038 |
| Losartan | Angiotensin receptor blocker for hypertension | 0.766 | 0.869 | 0.676 | 0.001 |
| Clopidogrel | Antiplatelet | 0.777 | 0.918 | 0.658 | 0.022 |
| Docusate Sodium | Stool softener | 0.78 | 0.907 | 0.671 | 0.011 |
| Calcium Carbonate-Vitamin D3 | Calcium supplement/osteoporosis | 0.781 | 0.902 | 0.676 | 0.008 |
| Cephalexin | Antibiotics | 0.793 | 0.917 | 0.686 | 0.014 |
| Tramadol | Opioid agonist and serotonin/norepinephrine reuptake inhibitor | 0.795 | 0.937 | 0.674 | 0.037 |
| Aspirin | Antiplatelet/Non-steroidal anti-inflammatory drugs | 0.808 | 0.904 | 0.721 | 0.003 |
| Pantoprazole | Proton pump inhibitor | 0.835 | 0.95 | 0.734 | 0.036 |
| Warfarin | Anticoagulated/vitamin K antagonist | 1.289 | 1.478 | 1.124 | 0.004 |
| Fluconazole | Antifungal medication | 1.58 | 2.175 | 1.147 | 0.032 |
| Allopurinol | Xanthine oxidase inhibitor/reduce uric acid concentrations | 1.639 | 2.157 | 1.245 | 0.005 |
| Cholestyramine–Aspartame | Lower cholesterol levels | 1.642 | 2.233 | 1.207 | 0.013 |
| Terazosin | Alpha-1 adrenergic antagonist/hypertension | 1.842 | 2.633 | 1.288 | 0.009 |
| Metoclopramide | Antiemetic agent and dopamine D2 antagonist | 1.879 | 2.697 | 1.309 | 0.007 |
| Clobetasol | High potency corticosteroid topical medication | 2.054 | 3.043 | 1.387 | 0.004 |
| Feature | RC | CI95up | CI95down | Q Value * |
|---|---|---|---|---|
| Aspartate Aminotransferase (AST) Test | 0.704 | 0.864 | 0.574 | 0.008 |
| Alkaline Phosphatase (ALP) Test | 0.826 | 0.924 | 0.739 | 0.009 |
| Urea Nitrogen | 0.84 | 0.909 | 0.776 | 0.001 |
| Anion Gap | 0.863 | 0.944 | 0.789 | 0.012 |
| Glucose | 0.871 | 0.941 | 0.806 | 0.006 |
| Chloride (Cl) | 0.886 | 0.952 | 0.825 | 0.01 |
| Drugs | Mechanism of Action | Targets * | Ability to Penetrate Blood–Brain Barrier | Predicted Effects against AD + P |
|---|---|---|---|---|
| Glucosamine–Chondroitin | Maintain healthy cartilage by providing the building blocks for its synthesis and supporting its repair | UDP-glucose 2-epimerase/ManNAc kinase (GNE) gene | No | Beneficial |
| Dextromethorphan–Guaifenesin | Cough suppressant that works by acting on the cough center in the brain/thinning and loosening mucus in the airways | GRIN1 GRIN2A GRIN2B SIGMAR1 HTR3A HTR3B | Yes | Beneficial |
| Fish Oil | Incorporating into cell membranes and modulating the production of eicosanoids | Yes | Beneficial | |
| Sucralfate | Forming a protective barrier over the ulcer or damaged area, which helps to prevent further damage and promote healing | No | Beneficial | |
| Midodrine | Selective alpha-1 adrenergic agonist, which increases peripheral vascular resistance and blood pressure | ADRA1A | No | Beneficial |
| Irbesartan | Selectively blocking the angiotensin II receptor type 1 (AT1) in the renin–angiotensin–aldosterone system, which leads to vasodilation and a decrease in blood pressure | AT1 AGTR1 | No | Beneficial |
| Esomeprazole Magnesium | Inhibiting the proton pump (H+/K+ ATPase) in the stomach | the proton pump (H+/K+ ATPase) | Yes | Beneficial |
| Cyclobenzaprine | A centrally-acting muscle relaxant, which reduces muscle tone and spasm by blocking the activity of alpha motor neurons in the spinal cord | alpha motor neurons in the spinal cord | Yes | Beneficial |
| Budesonide–Formoterol | Binding to glucocorticoid receptors in the lungs, leading to the suppression of inflammation and immune responses | Glucocorticoid receptors Beta-2 adrenergic receptors | Yes | Beneficial |
| Lactulose | Beneficial | |||
| Duloxetine | Inhibition of the reuptake of two neurotransmitters in the brain, serotonin and norepinephrine | SLC6A2 SLC6A4 | Yes | Beneficial |
| Ezetimibe | Increasing the osmotic pressure in the colon, which draws water into the colon and softens the stool | NPC1L1 SOAT1 | No | Beneficial |
| Magnesium Oxide | Providing magnesium ions to the body, which are essential for many biological processes | Yes | Beneficial | |
| Famotidine | Inhibiting the activity of histamine h2 receptors in the stomach | histamine H2 receptor | Yes | Beneficial |
| Nitroglycerin | A potent vasodilator by releasing nitric oxide in the smooth muscle of blood vessels, leading to relaxation of vascular smooth muscle and vasodilation | NPR1 | Yes | Beneficial |
| Alprazolam | Enhancing the activity of gamma-aminobutyric acid (GABA) in the brain | GABA-A receptor benzodiazepine receptor | Yes | Beneficial |
| Isosorbide Mononitrate | It acts as a vasodilator by releasing nitric oxide in the smooth muscle of blood vessels, leading to relaxation of vascular smooth muscle and vasodilation | NPR1 | No | Beneficial |
| Quetiapine | Antagonist of several neurotransmitter receptors in the brain, including dopamine, serotonin, and histamine receptors | DRD2 HTR1A HTR2A HRH1 | Yes | Beneficial |
| Glipizide | Stimulating the release of insulin from the beta cells of the pancreas. | ATP-sensitive potassium channels in pancreatic beta cells SUR1 | No | Beneficial |
| Memantine | Blocking of the activity of the NMDA (n-methyl-d-aspartate) subtype of glutamate receptors in the brain. | NMDA subtype of glutamate receptors | Yes | Beneficial |
| Triamcinolone Acetonide | A synthetic glucocorticoid, which reduces inflammation and swelling by inhibiting the production and release of inflammatory mediators | Inflammatory mediators and their signaling pathways. NR3C1 | No | Beneficial |
| Losartan | Angiotensin II receptor antagonist, blocking the binding of angiotensin II to specific receptors in the body, which inhibits its vasoconstrictive and pro-inflammatory effects | angiotensin II receptor | Yes | Beneficial |
| Clopidogrel | Irreversibly inhibits the P2Y12 receptor, which is found on the surface of platelets. Reduces the activation and aggregation of platelets. | P2Y12 receptor | Yes | Beneficial |
| Docusate Sodium | Increasing the amount of water and fat in the stool | No | Beneficial | |
| Vitamin D | Binding to vitamin d receptors (VDR) in cells, leading to changes in gene expression and protein synthesis | Vitamin D receptor (VDR) | Yes | Beneficial |
| Cephalexin | Inhibiting bacterial cell wall synthesis by binding to penicillin-binding proteins (PBPS) | bacterial PBPs | No | Beneficial |
| Tramadol | An opioid agonist, which means it binds to and activates opioid receptors in the brain, inhibits the reuptake of serotonin and norepinephrine, which are neurotransmitters involved in pain processing, further enhancing its analgesic effect | OPRM1 SLC6A2 SLC6A4 SCN2A NMDA receptors ADORA1 | Yes | Beneficial |
| Aspirin | Irreversibly inhibit the cyclooxygenase (COX) enzyme | PTGS1 PTGS2 AKR1C1 EDNRA TP53 HSPA5 RPS6KA3 NFKBIA | Yes | Beneficial |
| Pantoprazole | Irreversibly blocking the H+/K+-atpase enzyme in the parietal cells of the stomach | ATP4A ATP4B | No | Beneficial |
| Warfarin | Inhibiting the synthesis of vitamin K-dependent clotting factors in the liver, specifically factors II, VII, IX, and X | VKORC1 NR1I2 | Yes | Hazardous |
| Fluconazole | Inhibiting fungal cytochrome P450-dependent enzymes | fungal cytochrome P450-dependent enzymes | Yes | Hazardous |
| Allopurinol | Inhibiting the xanthine oxidase enzyme, which is involved in the metabolism of purines | xanthine oxidase enzyme | Yes | Hazardous |
| Cholestyramine–Aspartame | Binding to bile acids in the intestine and preventing their reabsorption | bile acids | No | Hazardous |
| Terazosin | Blocking the alpha-1 adrenergic receptors in smooth muscle tissue, including the prostate and blood vessels | ADRA1A ADRA1B ADRA1D | No | Hazardous |
| Metoclopramide | Blocking dopamine receptors and stimulating 5-HT4 serotonin receptors in the gastrointestinal tract | DRD1 DRD2 DRD3 DRD4 DRD5 HTR4 | Yes | Hazardous |
| Clobetasol | Binding to and activating glucocorticoid receptors in skin cells | NR3C1 | No | Hazardous |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Miranda, O.; Qi, X.; Kofler, J.; Sweet, R.A.; Wang, L. Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals 2023, 16, 911. https://doi.org/10.3390/ph16070911
Fan P, Miranda O, Qi X, Kofler J, Sweet RA, Wang L. Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals. 2023; 16(7):911. https://doi.org/10.3390/ph16070911
Chicago/Turabian StyleFan, Peihao, Oshin Miranda, Xiguang Qi, Julia Kofler, Robert A. Sweet, and Lirong Wang. 2023. "Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models" Pharmaceuticals 16, no. 7: 911. https://doi.org/10.3390/ph16070911
APA StyleFan, P., Miranda, O., Qi, X., Kofler, J., Sweet, R. A., & Wang, L. (2023). Unveiling the Enigma: Exploring Risk Factors and Mechanisms for Psychotic Symptoms in Alzheimer’s Disease through Electronic Medical Records with Deep Learning Models. Pharmaceuticals, 16(7), 911. https://doi.org/10.3390/ph16070911










