Evaluation of Cisplatin-Induced Acute Renal Failure Amelioration Using Fondaparinux and Alteplase
Abstract
1. Introduction
2. Results
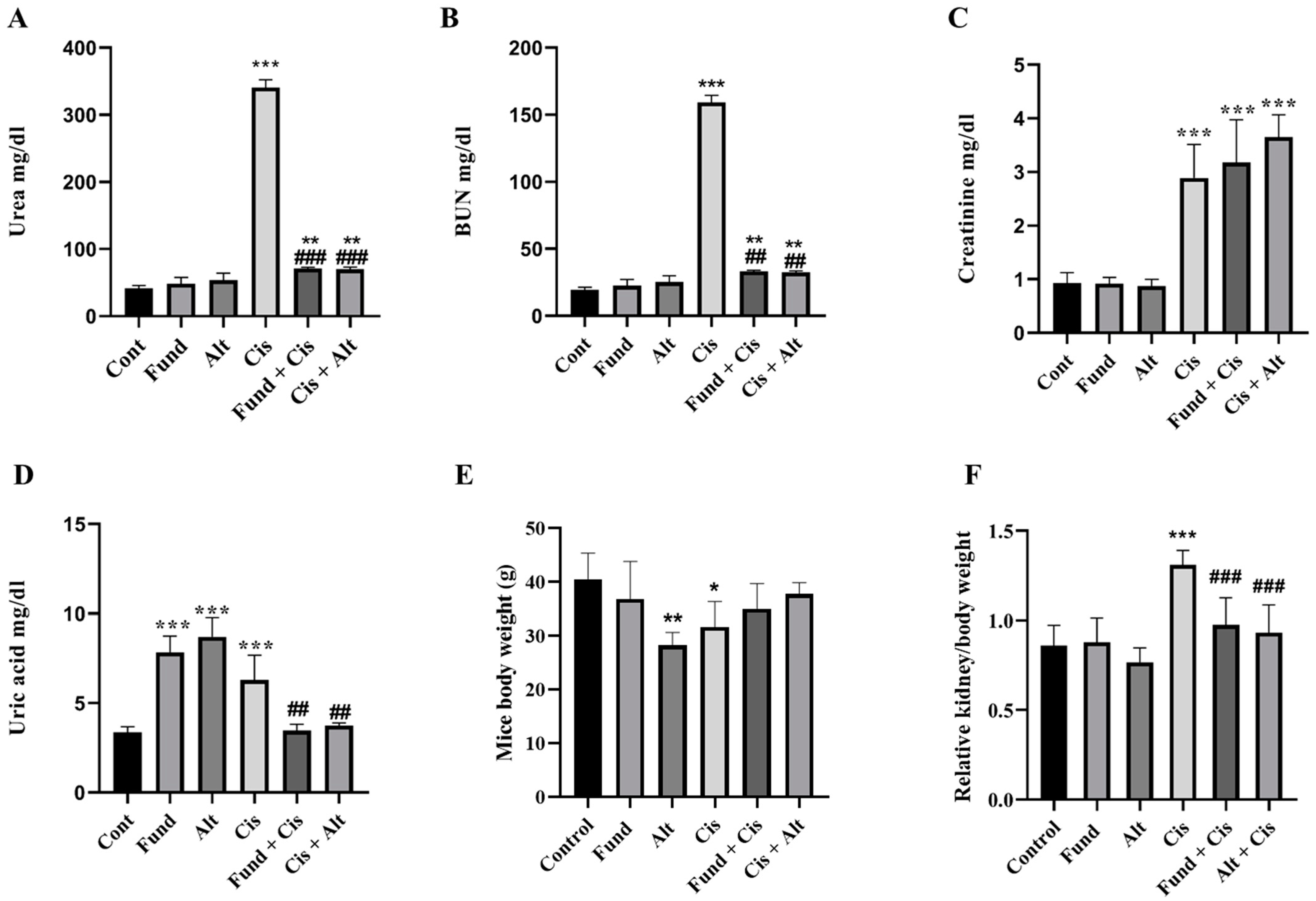
2.1. Effect of Fondaparinux (Fund) or Alteplase (Alt) with/without Cisplatin on the Kidney
2.2. Effect of Cisplatin Time Course on White Blood Cells (WBCs), Red Blood Cells (RBCs), Platelets (PLT), Prothrombin Time and Glucose Level
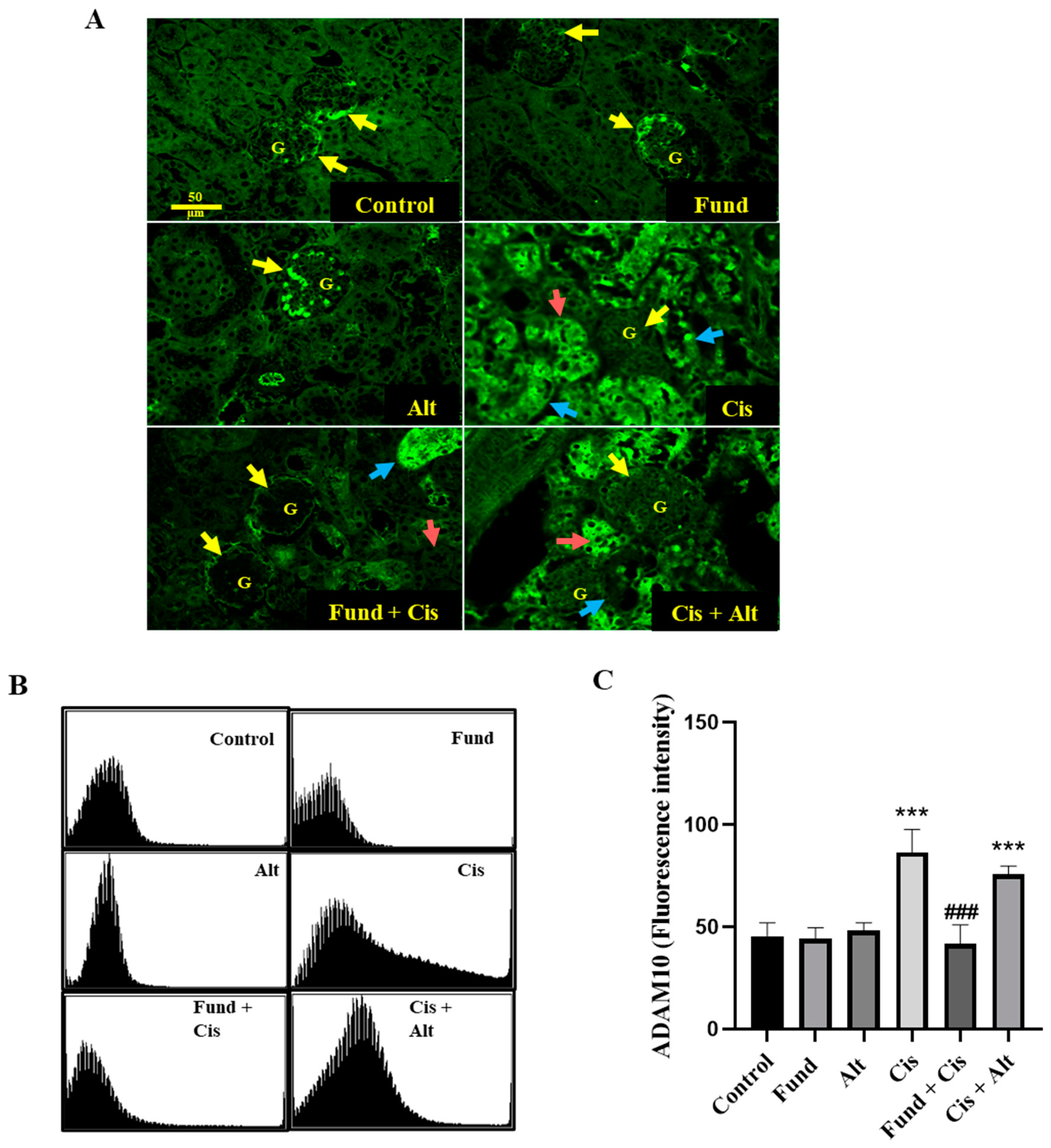
2.3. Effect of Cis with or without Fund or Alt on ADAM10 Protein Expression in Renal Mice Tissues
2.4. Effect of Cis with/without Fund or Alt on RXR-α Protein Expression in Renal Mice Tissues
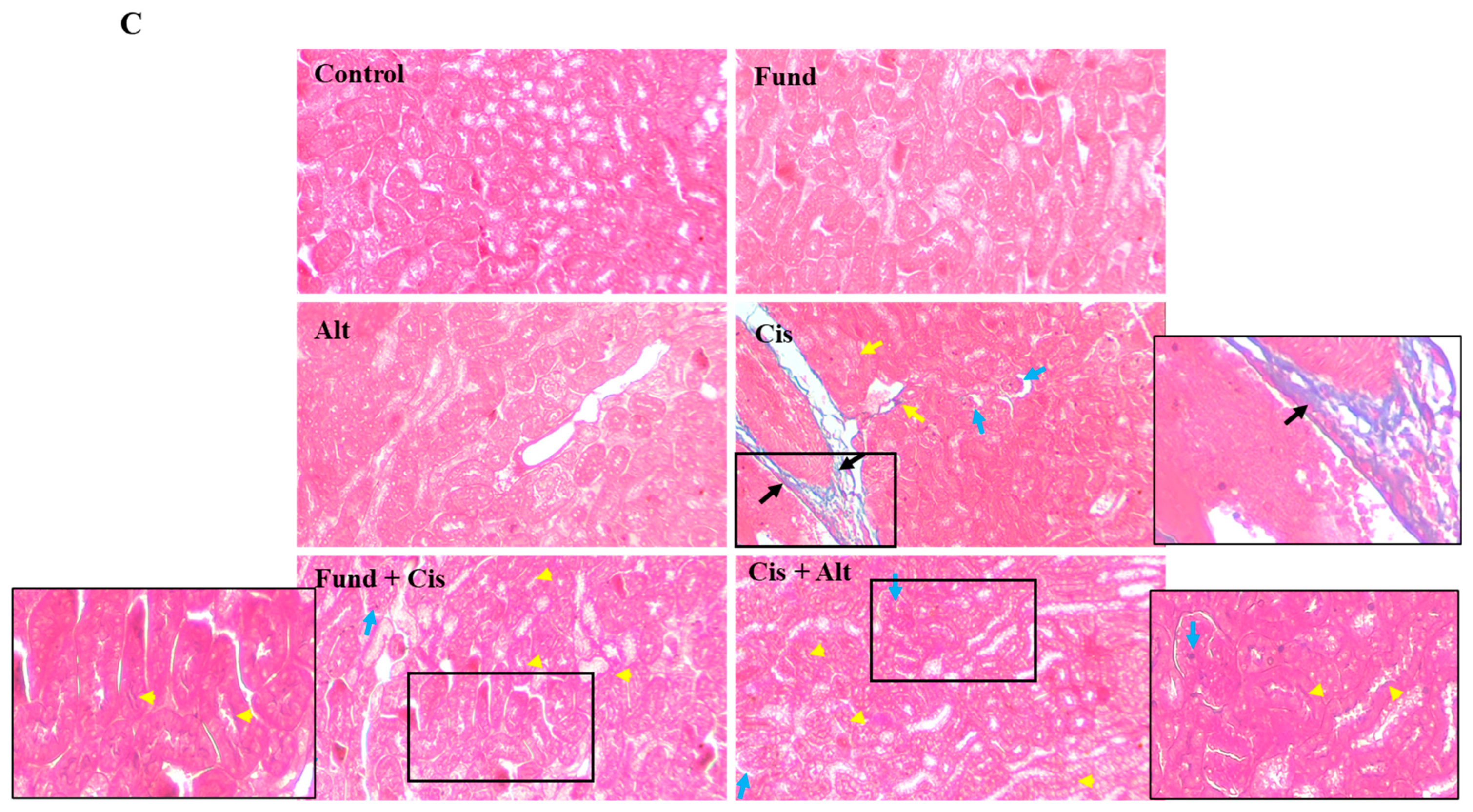
2.5. Effect of Cis with/without Fund and Alt on the Structural Architecture
2.6. Effect of Cis with/without Fund and Alt on p-AKT and Caspase-3 Proteins Expression in Mice Renal Tissues
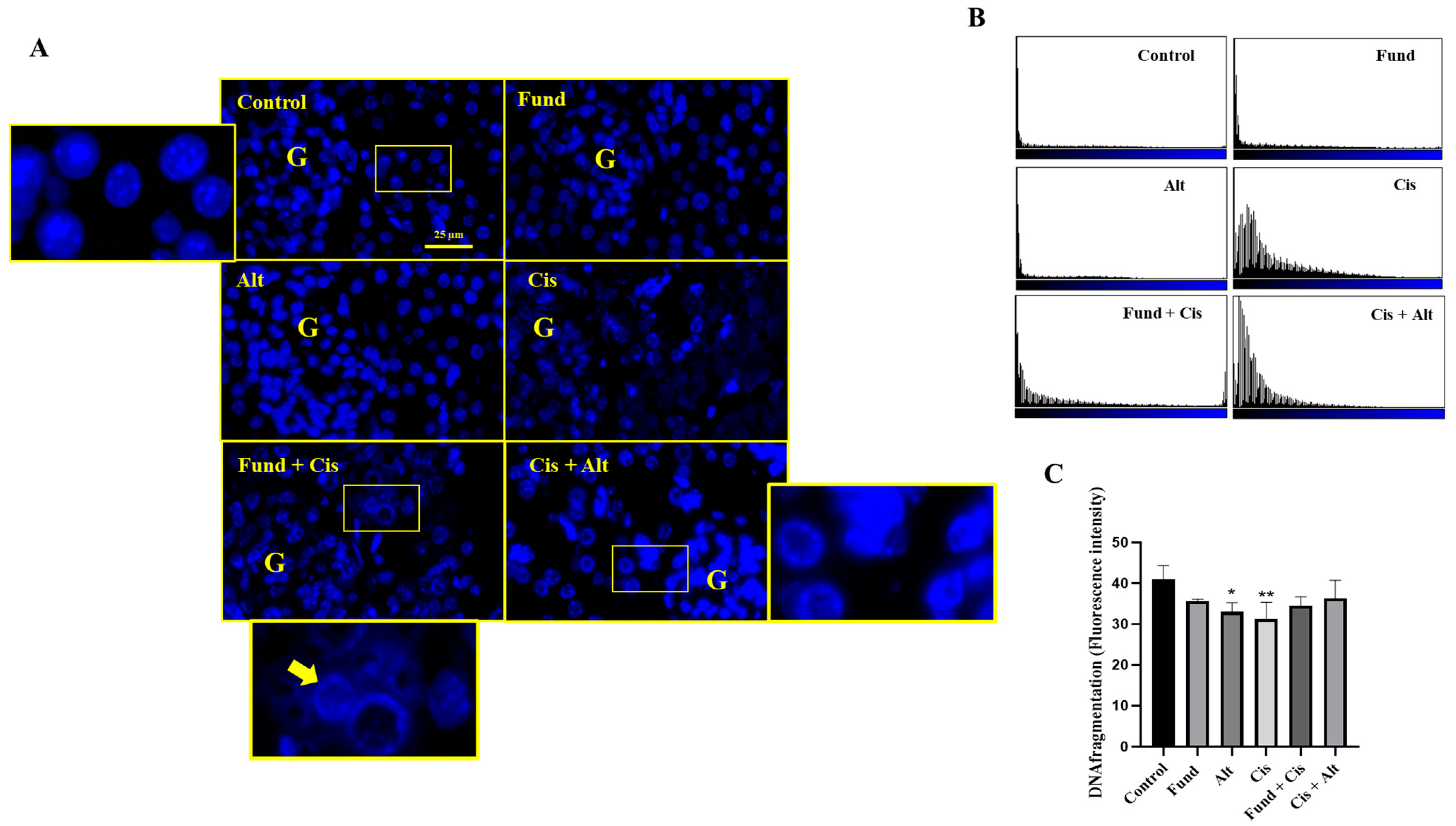
2.7. Nuclear Morphology after Treatment with Cis with/without Fund or Alt Using DAPI Staining
2.8. Effect of Cis with/without Fund or Alt on PAR-2 Protein Expression in Mice Renal Tissues
2.9. Effect of Cis with/without Fund or Alt on Fibrinogen Protein Expression in Mice Renal Tissues
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Experimental Design
4.4. Blood Sampling and Preparation
4.5. Tissue Sampling and Calculation of Relative Kidney Weight
4.6. Determination of Creatinine Level
4.7. Determination of Complete Blood Count (CBC)
4.8. Histopathological Analysis
4.9. Single Immunofluorescence Analysis of ADAM10, Fibrinogen, RXR-α and PAR-2 in Renal Tissue Sections
4.10. Double Immunofluorescence Analysis of Caspase-3 and p-Akt in Renal Tissue Sections
4.11. Trichrome Stain
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.P.; Muthuraman, A.; Jaggi, A.S.; Singh, N.; Grover, K.; Dhawan, R. Animal models of acute renal failure. Pharmacol. Reports 2012, 64, 31–44. [Google Scholar] [CrossRef]
- Patschan, D.; Müller, G.A. Acute Kidney Injury. J. Inj. Violence Res. 2014, 7, 19–26. [Google Scholar]
- Koziolek, M.J. Role of CX3C-chemokine CX3C-L/fractalkine expression in a model of slowly progressive renal failure. Nephrol. Dial. Transplant. 2009, 25, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Mendolicchio, G.L. Adhesion Mechanisms in Platelet Function. Circ. Res. 2007, 100, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Yang, H.N.; Kim, H.W.; Jo, S.K.; Cho, W.Y.; Kim, H.K. IL-10 mediates rosiglitazone-induced kidney protection in cisplatin nephrotoxicity. J. Korean Med. Sci. 2010, 25, 557–563. [Google Scholar] [CrossRef]
- Hammer, G.D.; McPhee, S.J. Notice. In Pathophysiology of Disease: An Introduction to Clinical Medicine; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Livingston, R.B. Cisplatin in the Treatment of Solid Tumors: Effect of Dose and Schedule. JNCI J. Natl. Cancer Inst. 1989, 81, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, Z.; Khosravi, A.; Nekuei, M.; Khoshnood, S.; Zandi, E.; Eslamian, M.; Nematbakhsh, M. Time course of cisplatin-induced nephrotoxicity and hepatotoxicity. J. Nephropathol. 2017, 6, 163–167. [Google Scholar] [CrossRef]
- Yang, F.; Long, W.; Xuechuan, H.; Xueqin, L.; Hongyun, M.; Yonghui, D. Upregulation of Fas in epithelial ovarian cancer reverses the development of resistance to cisplatin. BMB Rep. 2015, 48, 30–35. [Google Scholar]
- Matthews, A.L.; Noy, P.J.; Reyat, J.S.; Tomlinson, M.G. Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids. Platelets 2016, 28, 333–341. [Google Scholar] [CrossRef]
- Shahid, S.; Ikeda, A.; Layana, M.C.; Bartlett, J.D. ADAM10: Possible functions in enamel development. Front. Physiol. 2022, 13, 2502. [Google Scholar] [CrossRef]
- Schramme, A.; Abdel-Bakky, M.S.; Kämpfer-Kolb, N.; Pfeilschifter, J.; Gutwein, P. The role of CXCL16 and its processing metalloproteinases ADAM10 and ADAM17 in the proliferation and migration of human mesangial cells. Biochem. Biophys. Res. Commun. 2008, 370, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Du, Y.; Zhao, C.; Wu, Y. PAX2 may induce ADAM10 expression in renal tubular epithelial cells and contribute to epithelial-to-mesenchymal transition. Int. Urol. Nephrol. 2018, 50, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhu, C.; Dong, L.; Qin, J.; Xiang, W.; Davidson, A.J.; Jiang, H. ADAM10 mediates ectopic proximal tubule development and renal fibrosis through Notch signalling. J. Pathol. 2020, 252, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Schramme, A.; Abdel-Bakky, M.S.; Gutwein, P.; Obermüller, N.; Baer, P.C.; Hauser, I.A.; Pfeilschifter, J. Characterization of CXCL16 and ADAM10 in the normal and transplanted kidney. Kidney Int. 2008, 74, 328–338. [Google Scholar] [CrossRef]
- Aboyoussef, A.M.; Abdel-Sattar, A.R.; Abdel-Bakky, M.S.; Messiha, B.A.S. Enoxaparin prevents CXCL16/ADAM10-mediated cisplatin renal toxicity: Role of the coagulation system and the transcriptional factor NF-kappaB. Life Sci. 2021, 270, 119120. [Google Scholar] [CrossRef]
- Penniston, K.L.; Tanumihardjo, S.A. The acute and chronic toxic effects of vitamin A14. Am. J. Clin. Nutr. 2006, 83, 191–201. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Evans, R.M. The RXR heterodimers and orphan receptors. Cell 1995, 83, 841–850. [Google Scholar] [CrossRef]
- Avet, C.; Sturino, C.; Grastilleur, S.; Le Gouill, C.; Semache, M.; Gross, F.; Gendron, L.; Bennani, Y.; Mancini, J.A.; Sayegh, C.E.; et al. The PAR2 inhibitor I-287 selectively targets G-alpha and G-alpha12/13 signaling and has anti-inflammatory effects. Commun. Biol. 2020, 3, 719. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, C.; Zhou, M.; Zhou, H.; Dong, W.; Wu, X.; Feng, Q. RXR-enriched cancer stem cell-like properties triggered by CDDP in head and neck squamous cell carcinoma (HNSCC). Carcinogenesis 2017, 39, 252–262. [Google Scholar] [CrossRef]
- Portilla, D.; Dai, G.; McClure, T.; Bates, L.; Kurten, R.; Megyesi, J.; Li, S. Alterations of PPAR and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002, 62, 1208–1218. [Google Scholar] [CrossRef]
- Coelho, A.-M.; Ossovskaya, V.; Bunnett, N. Proteinase-Activated Receptor-2: Physiological and Pathophysiological Roles. Curr. Med. Chem. Hematol. Agents 2003, 1, 61–72. [Google Scholar] [CrossRef]
- Iyer, A.; Humphries, T.L.; Owens, E.P.; Zhao, K.N.; Masci, P.P.; Johnson, D.W.; Vesey, D.A. PAR2 Activation on Human Kidney Tubular Epithelial Cells Induces Tissue Factor Synthesis, That Enhances Blood Clotting. Front. Physiol. 2021, 12, 615428. [Google Scholar] [CrossRef]
- Palygin, O.; Ilatovskaya, D.V.; Staruschenko, A. Protease-activated receptors in kidney disease progression. Am. J. Physiol. Physiol. 2016, 311, F1140–F1144. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Margina, D. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Endres, K.; Deller, T. Regulation of Alpha-Secretase ADAM10 In vitro and In vivo: Genetic, Epigenetic, and Protein-Based Mechanisms. Front. Mol. Neurosci. 2017, 10, 56. [Google Scholar] [CrossRef]
- Bauersachs, R.M. Fondaparinux: An update on new study results. Eur. J. Clin. Investig. 2005, 35, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.A.; Hawkins, D.W.; Peters, P.C.; Petitou, M.; Herbert, J.M.; van Boeckel, C.A.; Meuleman, D.G. Fondaparinux, a Synthetic Pentasaccharide: The First in a New Class of Antithrombotic Agents–The Selective Factor Xa Inhibitors. Cardiovasc. Drug Rev. 2006, 20, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Tan, L.; Pan, N.; Zhang, L. The clinical use of Fondaparinux: A synthetic heparin pentasaccharide. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–53. [Google Scholar] [CrossRef]
- Mosimah, C.I.; Murray, P.J.; Simpkins, J.W. Not all clots are created equal: A review of deficient thrombolysis with tissue plasminogen activator (tPA) in patients with metabolic syndrome. Int. J. Neurosci. 2018, 129, 612–618. [Google Scholar] [CrossRef]
- Katsanos, A.H.; Tsivgoulis, G. Is intravenous thrombolysis still necessary in patients who undergo mechanical thrombectomy? Curr. Opin. Neurol. 2019, 32, 3–12. [Google Scholar] [CrossRef]
- Thuwaini, M. Ameliorating effect of Alteplase in bleomycin-induced pulmonary fibrosis in adult rats. In Proceedings of the 2nd International Multi-Disciplinary Conference Theme: Integrated Sciences and Technologies, IMDC-IST 2021, Sakarya, Turkey, 7−9 September 2021. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Qiu, H.Y.; Tong, X.X.; Guo, Y.; Han, M.F.; Yang, C.S.; Yang, Y. Evaluation of efficacy and safety of Reteplase and Alteplase in the treatment of hyper-acute cerebral infarction. Biosci. Rep. 2018, 38, BSR20170730. [Google Scholar] [CrossRef]
- Meyer-Schwesinger, C.; Seipold, L.; Saftig, P. Ectodomain shedding by ADAM proteases as a central regulator in kidney physiology and disease. Biochim. Biophys. Acta–Mol. Cell Res. 2022, 1869, 119165. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Obach, V.; Cervera, A.; Revilla, M.; Deulofeu, R.; Aponte, J.H. Prognostic Significance of Uric Acid Serum Concentration in Patients With Acute Ischemic Stroke. Stroke 2002, 33, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Amaro, S.; Planas, A.M.; Chamorro, Á. Uric acid administration in patients with acute stroke: A novel approach to neuroprotection. Expert Rev. Neurother. 2008, 8, 259–270. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Splenser, A.E.; Valavanidis, A.; Zhang, H.; Uppu, R.M.; Pryor, W.A. Reaction of Uric Acid with Peroxynitrite and Implications for the Mechanism of Neuroprotection by Uric Acid. Arch. Biochem. Biophys. 2000, 376, 333–337. [Google Scholar] [CrossRef]
- Giebeler, N.; Zigrino, P. A Disintegrin and Metalloprotease (ADAM): Historical Overview of Their Functions. Toxins 2016, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Mullooly, M.; McGowan, P.M.; Crown, J.; Duffy, M.J. The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol. Ther. 2016, 17, 870–880. [Google Scholar] [CrossRef]
- Seegar, T.C.M.; Blacklow, S.C. Domain integration of ADAM family proteins: Emerging themes from structural studies. Exp. Biol. Med. 2019, 244, 1510–1519. [Google Scholar] [CrossRef]
- Wetzel, S.; Seipold, L.; Saftig, P. The metalloproteinase ADAM10: A useful therapeutic target? Biochim. Biophys. Acta–Mol. Cell Res. 2017, 1864, 2071–2081. [Google Scholar] [CrossRef]
- Sachs, M.; Wetzel, S.; Reichelt, J.; Sachs, W.; Schebsdat, L.; Zielinski, S.; Meyer-Schwesinger, C. ADAM10-Mediated Ectodomain Shedding Is an Essential Driver of Podocyte Damage. J. Am. Soc. Nephrol. 2021, 32, 1389–1408. [Google Scholar] [CrossRef]
- Gutwein, P.; Abdel-Bakky, M.S.; Doberstein, K.; Schramme, A.; Beckmann, J.; Schaefer, L.; Pfeilschifter, J. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J. Cell. Mol. Med. 2009, 13, 3809–3825. [Google Scholar] [CrossRef]
- Fu, L.; Liu, N.; Han, Y.; Xie, C.; Li, Q.; Wang, E. ADAM10 regulates proliferation, invasion, and chemoresistance of bladder cancer cells. Tumor Biol. 2014, 35, 9263–9268. [Google Scholar] [CrossRef]
- Musumeci, G.; Coleman, R.; Imbesi, R.; Magro, G.; Parenti, R.; Szychlinska, M.A.; Castrogiovanni, P. ADAM-10 could mediate cleavage of N-cadherin promoting apoptosis in human atherosclerotic lesions leading to vulnerable plaque: A morphological and immunohistochemical study. Acta Histochem. 2014, 116, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Germain, P.; Chambon, P.; Eichele, G.; Evans, R.M.; Lazar, M.A.; Leid, M.; Gronemeyer, H. International Union of Pharmacology. LXIII. Retinoid X Receptors. Pharmacol. Rev. 2006, 58, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Almeida, N.R.; Conda-Sheridan, M. A review of the molecular design and biological activities of RXR agonists. Med. Res. Rev. 2019, 39, 1372–1397. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre-Pégorier, M.; Vilar, J.; Ferrier, M.L.; Moreau, E.; Freund, N.; Gilbert, T.; Merlet-Bénichou, C. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998, 54, 1455–1462. [Google Scholar] [CrossRef]
- Schaier, M.; Schaier, M.; Lehrke, I.; Schade, K.; Morath, C.; Shimizu, F.; Kawachi, H.; Wagner, J. Isotretinoin alleviates renal damage in rat chronic glomerulonephritis. Kidney Int. 2001, 60, 2222–2234. [Google Scholar] [CrossRef] [PubMed]
- Lucio-Cazana, J.; Nakayama, K.; Xu, Q.; Konta, T.; Moreno-Manzano, V.; Furusu, A.; Kitamura, M. Suppression of Constitutive but Not IL-1beta-Inducible Expression of Monocyte Chemoattractant Protein-1 in Mesangial Cells by Retinoic Acids: Intervention in the Activator Protein-1 Pathway. J. Am. Soc. Nephrol. 2001, 12, 688–694. [Google Scholar] [CrossRef]
- Otoyama, Y.; Arai, J.; Goto, K.; Nozawa, H.; Nakagawa, R.; Muroyama, R.; Yoshida, H. Retinoids Decrease Soluble MICA Concentration by Inhibiting the Enzymatic Activity of ADAM9 and ADAM10. Anticancer Res. 2021, 41, 2307–2320. [Google Scholar] [CrossRef]
- Tippmann, F.; Hundt, J.; Schneider, A.; Endres, K.; Fahrenholz, F. Up-regulation of the-secretase ADAM10 by retinoic acid receptors and acitretin. FASEB J. 2009, 23, 1643–1654. [Google Scholar] [CrossRef]
- Lerner, A.J.; Gustaw-Rothenberg, K.; Smyth, S.; Casadesus, G. Retinoids for treatment of Alzheimer’s disease. BioFactors 2012, 38, 84–89. [Google Scholar] [CrossRef]
- Noubouossie, D.; Key, N.S.; Ataga, K.I. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev. 2016, 30, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Vesey, D.A.; Suen, J.Y.; Seow, V.; Lohman, R.J.; Liu, L.; Gobe, G.C.; Fairlie, D.P. {PAR}2-induced inflammatory responses in human kidney tubular epithelial cells. Am. J. Physiol. Physiol. 2013, 304, F737–F750. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, T.; Kerlin, B.A.; Isermann, B. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 2015, 12, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Ding, Y.; Ricks, T.K.; Gullapalli, A.; Wolfe, B.L.; Trejo, J. Protease-Activated Receptor-2 Is Essential for Factor VIIa and Xa-Induced Signaling, Migration, and Invasion of Breast Cancer Cells. Cancer Res. 2006, 66, 307–314. [Google Scholar] [CrossRef]
- Rothmeier, A.S.; Liu, E.; Chakrabarty, S.; Disse, J.; Mueller, B.M.; Østergaard, H.; Ruf, W. Identification of the integrin-binding site on coagulation factor VIIa required for proangiogenic PAR2 signaling. Blood 2018, 131, 674–685. [Google Scholar] [CrossRef]
- Samad, F.; Ruf, W. Inflammation, obesity, and thrombosis. Blood 2013, 122, 3415–3422. [Google Scholar] [CrossRef]
- Krupiczojc, M.; Scotton, C.; Chambers, R. Coagulation signalling following tissue injury: Focus on the role of factor Xa. Int. J. Biochem. Cell Biol. 2008, 40, 1228–1237. [Google Scholar] [CrossRef]
- Chambers, R.C. Procoagulant signalling mechanisms in lung inflammation and fibrosis: Novel opportunities for pharmacological intervention? Br. J. Pharmacol. 2008, 153, S367–S378. [Google Scholar] [CrossRef]
- Ritchie, E.; Saka, M.; Mackenzie, C.; Drummond, R.; Wheeler-Jones, C.; Kanke, T.; Plevin, R. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br. J. Pharmacol. 2007, 150, 1044–1054. [Google Scholar] [CrossRef]
- Suen, J.Y.; Gardiner, B.; Grimmond, S.; Fairlie, D.P. Profiling Gene Expression Induced by Protease-Activated Receptor 2 (PAR2) Activation in Human Kidney Cells. PLoS ONE 2010, 5, e13809. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Ruf, W.; Pryzdial, E.L.G. Tissue factor and glycoprotein C on herpes simplex virus type 1 are protease-activated receptor 2 cofactors that enhance infection. Blood 2012, 119, 3638–3645. [Google Scholar] [CrossRef]
- Li, Y.; Feng, C.; Wei, X.; Zhang, J.; Zan, R.; Zheng, G.; Zhai, J. Activation of protease-activated receptor-2 disrupts vaginal epithelial barrier function. Cell Biol. Int. 2014, 38, 1247–1251. [Google Scholar] [CrossRef]
- Montague, S.J.; Hicks, S.M.; Lee, C.S.M.; Coupland, L.A.; Parish, C.R.; Lee, W.M.; Gardiner, E.E. Fibrin exposure triggers alpha-IIb-beta3-independent platelet aggregate formation, ADAM10 activity and glycoprotein VI shedding in a charge-dependent manner. J. Thromb. Haemost. 2020, 18, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Večerić-Haler, Ž. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. Biomed Res. Int. 2018, 2018, 1–29. [Google Scholar] [CrossRef]
- Summaria, L.; Caprini, J.A.; McMillan, R.; Sandesara, J.Y.O.T.I.; Axelrod, C.A.; Mueller, M.E.; Fareed, J. Relationship between postsurgical fibrinolytic parameters and deep vein thrombosis in surgical patients treated with compression devices. Am. Surg. 1988, 54, 156–160. [Google Scholar] [PubMed]
- Zuo, P.; Zhou, Q.; Zuo, Z.; Wang, X.; Chen, L.; Ma, G. Effects of the Factor Xa Inhibitor, Fondaparinux, on the Stability of Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice. Circ. J. 2015, 79, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wan, J.; Bian, Y.; Li, F.; Lan, J. Baicalein and its underlying mechanism as a protector against liver injury induced by cisplatin in mice. Biotechnol. Biotechnol. Equip. 2016, 31, 193–199. [Google Scholar] [CrossRef]
- Orset, C.; Haelewyn, B.; Allan, S.M.; Ansar, S.; Campos, F.; Cho, T.H.; Vivien, D. Efficacy of Alteplase in a Mouse Model of Acute Ischemic Stroke: A Retrospective Pooled Analysis. Stroke 2016, 47, 1312–1318. [Google Scholar] [CrossRef]
- Frank, R.D.; Schabbauer, G.; Holscher, T.; Sato, Y.; Tencati, M.; Pawlinski, R.; Mackman, N. The synthetic pentasaccharide fondaparinux reduces coagulation, inflammation and neutrophil accumulation in kidney ischemia–reperfusion injury. J. Thromb. Haemost. 2005, 3, 531–540. [Google Scholar] [CrossRef]
- Todd, J.C.; Sanford, A.H.; Davidsohn, I.; Henry, J.B. Clinical Diagnosis and Management by Laboratory Methods; Saunders: London, UK, 1979. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef]
- Abdel-Bakky, M.S.; Hammad, M.A.; Walker, L.A.; Ashfaq, M.K. Silencing of tissue factor by antisense deoxyoligonucleotide prevents monocrotaline/LPS renal injury in mice. Arch. Toxicol. 2011, 85, 1245–1256. [Google Scholar] [CrossRef] [PubMed]








| Control | Fund | Alt | Cis | Fund + Cis | Cis + Alt | |
|---|---|---|---|---|---|---|
| WBCs (109/L) | 5.613 ± 0.12 | 5.047 ± 0.04 *** | 5.147 ± 0.35 *** | 4.253 ± 0.14 ** | 2.973 ± 0.21 ***, ### | 1.727 ± 0.13 ***, ### |
| RBCs (106 cell/L) | 5.467 ± 0.05 | 5.353 ± 0.08 | 5.893 ± 0.06 | 6.283 ± 0.09 *** | 5.433 ± 0.11 ### | 5.5 ± 0.1 ### |
| PLT (109/L) | 795 ± 14.47 | 737 ± 9.29 ** | 288 ± 10.6 *** | 463.3 ± 13.54 *** | 730 ± 8.88 **, ### | 334.3 ± 5.78 ***, ### |
| Prothrombin time (s) | 8.6 ± 0.31 | 10.96 ± 0.26 *** | 10.60 ± 0.24 *** | 14.98 ± 0.09 *** | 11.54 ± 0.31 ***, ### | 11.38 ± 0.23 ***, ### |
| Glucose (mg/dL) | 115 ± 9.640 | 117.7 ± 10.97 | 113.5 ± 9.926 | 142.9 ± 13.54 | 77 ± 10.38 ### | 62.33 ± 3.930 *,### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Bakky, M.S.; Aldakhili, A.S.A.; Ali, H.M.; Babiker, A.Y.; Alhowail, A.H.; Mohammed, S.A.A. Evaluation of Cisplatin-Induced Acute Renal Failure Amelioration Using Fondaparinux and Alteplase. Pharmaceuticals 2023, 16, 910. https://doi.org/10.3390/ph16070910
Abdel-Bakky MS, Aldakhili ASA, Ali HM, Babiker AY, Alhowail AH, Mohammed SAA. Evaluation of Cisplatin-Induced Acute Renal Failure Amelioration Using Fondaparinux and Alteplase. Pharmaceuticals. 2023; 16(7):910. https://doi.org/10.3390/ph16070910
Chicago/Turabian StyleAbdel-Bakky, Mohamed S., Anas S. A. Aldakhili, Hussein M. Ali, Ali Y. Babiker, Ahmad H. Alhowail, and Salman A. A. Mohammed. 2023. "Evaluation of Cisplatin-Induced Acute Renal Failure Amelioration Using Fondaparinux and Alteplase" Pharmaceuticals 16, no. 7: 910. https://doi.org/10.3390/ph16070910
APA StyleAbdel-Bakky, M. S., Aldakhili, A. S. A., Ali, H. M., Babiker, A. Y., Alhowail, A. H., & Mohammed, S. A. A. (2023). Evaluation of Cisplatin-Induced Acute Renal Failure Amelioration Using Fondaparinux and Alteplase. Pharmaceuticals, 16(7), 910. https://doi.org/10.3390/ph16070910







