Renoprotective Effect of Chrysanthemum coronarium L. Extract on Adenine-Induced Chronic Kidney Disease in Mice
Abstract
1. Introduction
2. Results
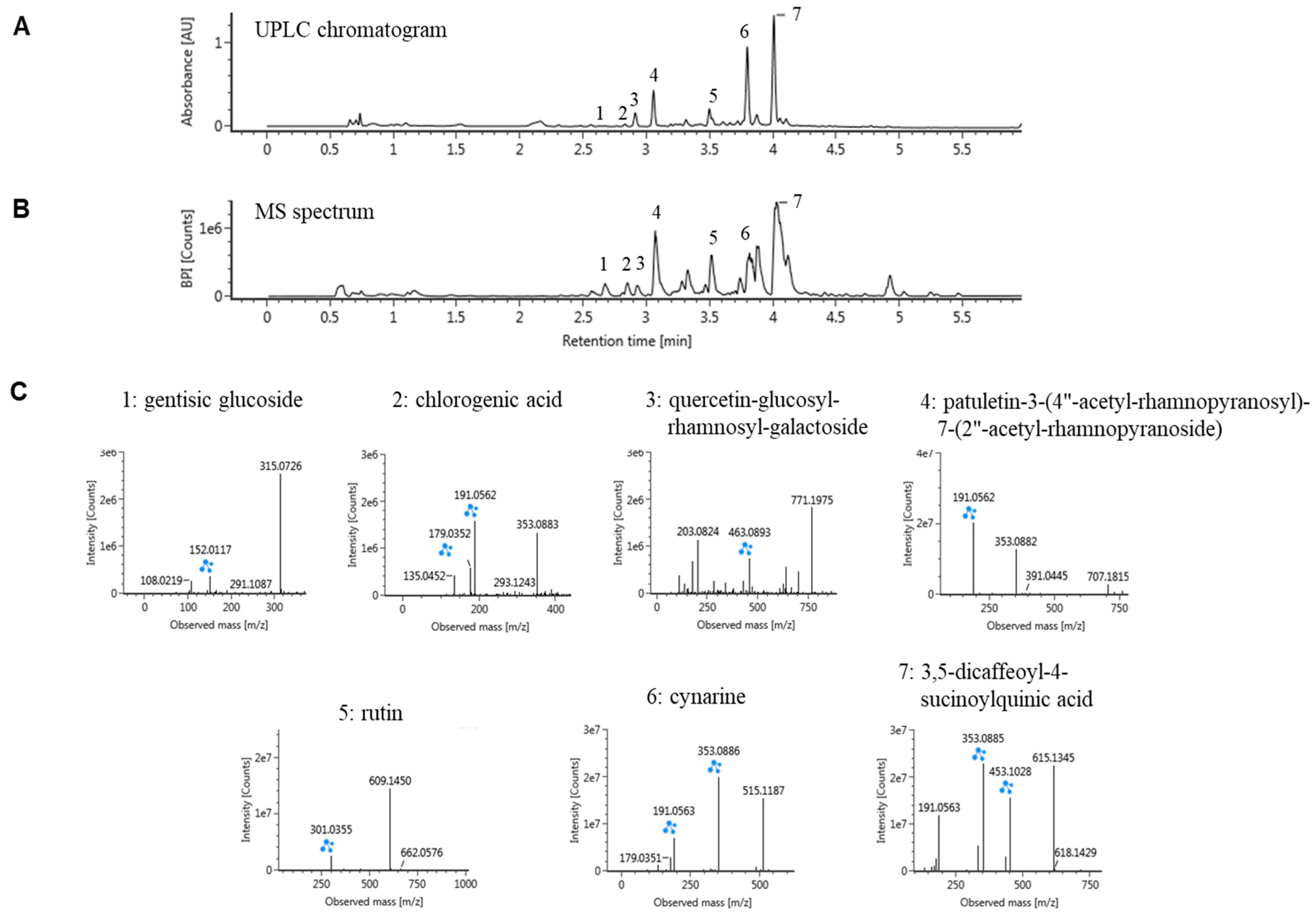
2.1. Ultraperformance Liquid Chromatography (UPLC)-Quadrupole Time-of-Flight Mass Spectrometry (QTOF/MS) Analysis of CC
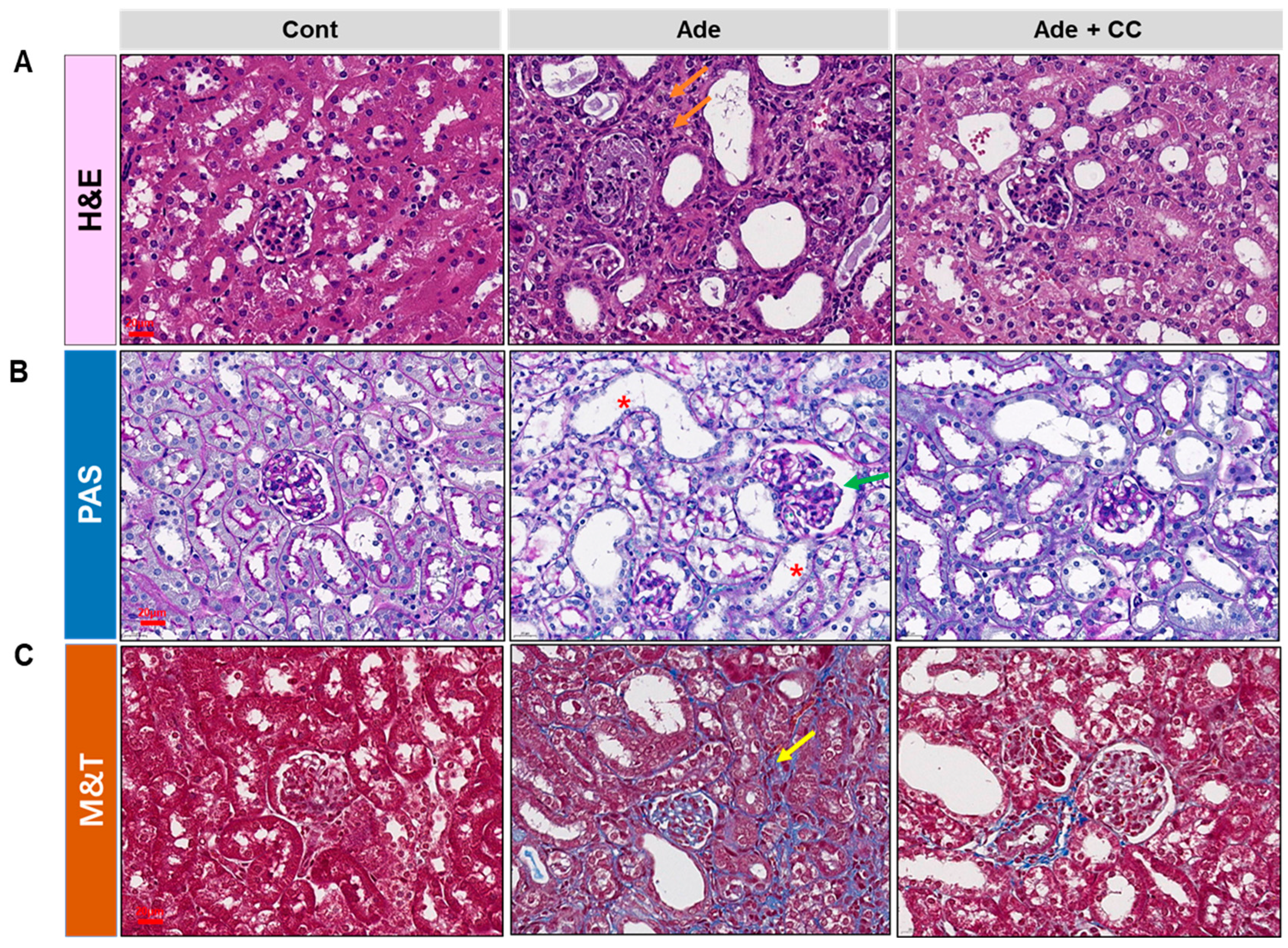
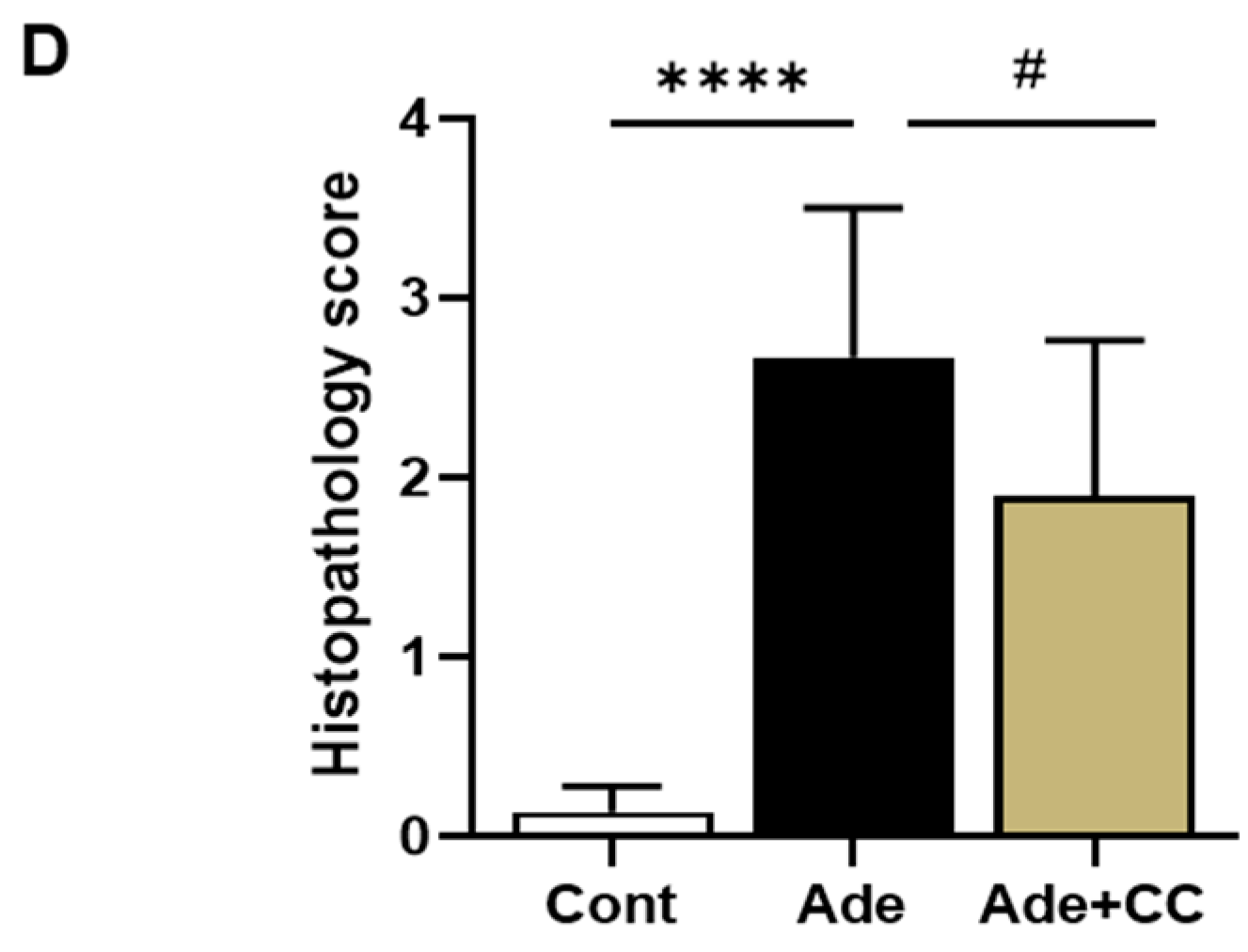
2.2. CC Attenuates AD-Induced Renal Injury
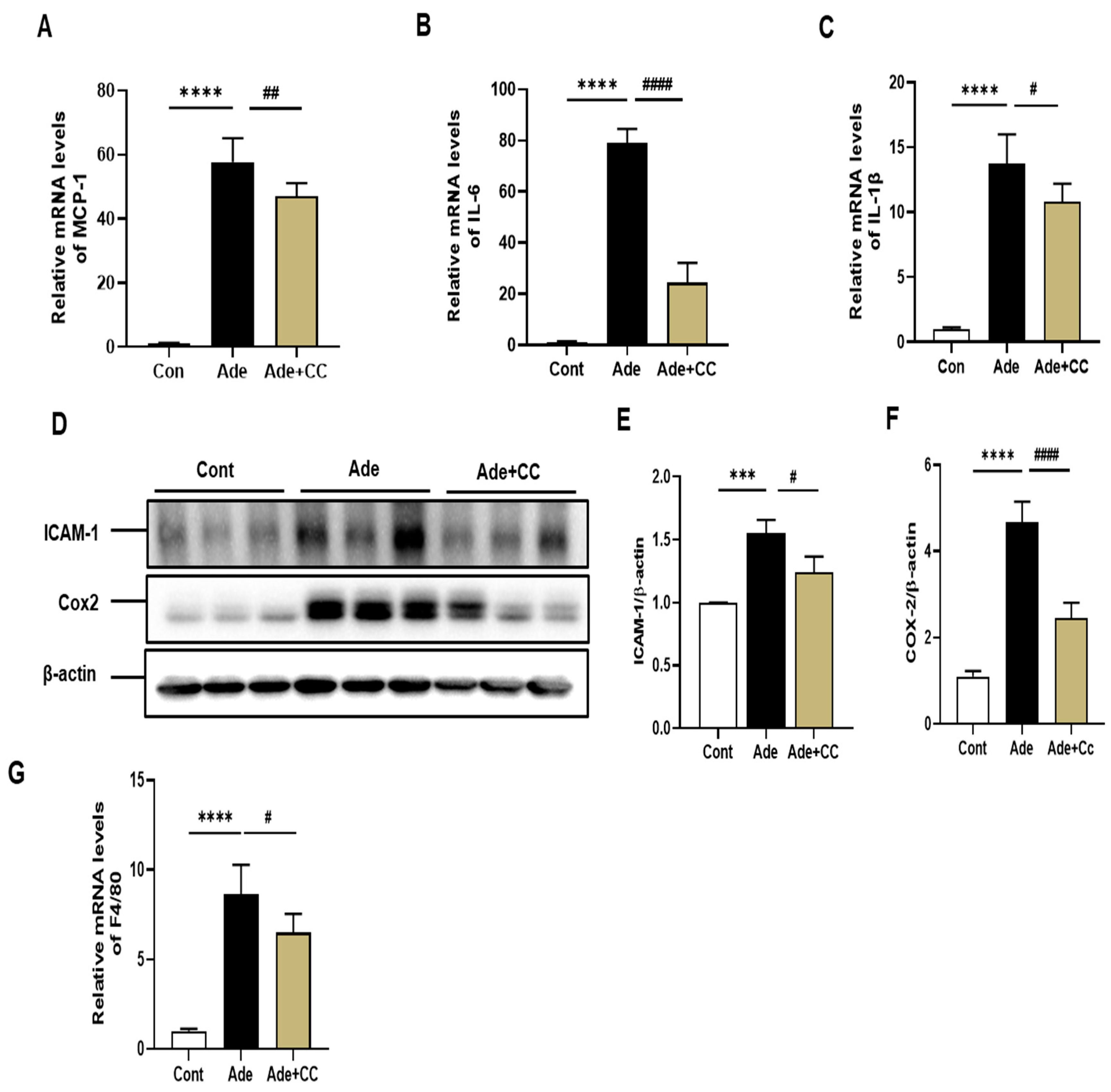
2.3. CC Suppresses Inflammatory Responses in AD-Induced Renal Injury Mice
2.4. CC Suppresses Extracellular Matrix (ECM) Deposition and Tubulointerstitial Fibrosis in AD-Induced Renal Injury Mice
2.5. CC Attenuates the Expression of Profibrotic Genes in AD-Induced Renal Injury Mice
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation and Analysis of CC
4.3. Animals
4.4. Experimental Procedure
4.5. Biochemical Parameters
4.6. Histopathological Analysis
4.7. IHC
4.8. RNA Isolation and qRT-PCR
4.9. Western Blotting Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Sohel, B.M.; Rumana, N.; Ohsawa, M.; Turin, T.C.; Kelly, M.A.; Al Mamun, M. Renal function trajectory over time and adverse clinical outcomes. Clin. Exp. Nephrol. 2016, 20, 379–393. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhu, J.-Y.; Ye, Y.; Zhang, M.; Zhang, L.-J.; Wang, S.-J.; Song, Y.-N.; Zhang, H. Erhuang Formula ameliorates renal damage in adenine–induced chronic renal failure rats via inhibiting inflammatory and fibrotic responses. Biomed. Pharmacother. 2017, 95, 520–528. [Google Scholar] [CrossRef]
- Sonfack, C.S.; Nguelefack-Mbuyo, E.P.; Kojom, J.J.; Lappa, E.L.; Peyembouo, F.P.; Fofié, C.K.; Nolé, T.; Nguelefack, T.B.; Dongmo, A.B. The aqueous extract from the stem bark of Garcinia lucida Vesque (Clusiaceae) exhibits cardioprotective and nephroprotective effects in adenine-induced chronic kidney disease in rats. Evid. Based Complement. Alternat. Med. 2021, 2021, 5581041. [Google Scholar] [CrossRef]
- Yi, H.; Huang, C.; Shi, Y.; Cao, Q.; Chen, J.; Chen, X.-M.; Pollock, C.A. Metformin attenuates renal fibrosis in a mouse model of adenine-induced renal injury through inhibiting TGF-β1 signaling pathways. Fron. Cell Dev. Biol. 2021, 9, 603802. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pennathur, S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol. 2015, 6, 495–504. [Google Scholar] [CrossRef]
- Gori, P.; Patel, A.; Solanki, N.; Shah, U.; Patel, V.; Patel, S. Protective effects of lycopene against adenine-induced chronic renal failure in rats. Indian J. Physiol. Pharmacol. 2021, 65, 74–85. [Google Scholar] [CrossRef]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, C.; Zhu, L.; Song, Z.; Dai, L.; Hu, Q.; Wang, L.; Chen, Y.; Xiong, J. Contribution of TFEB-mediated autophagy to tubulointerstitial fibrosis in mice with adenine-induced chronic kidney disease. Biomed. Pharmacother. 2021, 133, 110949. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Jin, W.; Niu, X.; Zhang, H. Effects and mechanism of low molecular weight fucoidan in mitigating the peroxidative and renal damage induced by adenine. Carbohydr. Polym. 2011, 84, 417–423. [Google Scholar] [CrossRef]
- Khan, M.A.; Kassianos, A.J.; Hoy, W.E.; Alam, A.K.; Healy, H.G.; Gobe, G.C. Promoting plant-based therapies for chronic kidney disease. J. Evid. Based Integr. Med. 2022, 27, 2515690X221079688. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Guo, M.; Lian, X.; Sun, M.; Guo, L. The efficacy of shen shuaining capsule on chronic kidney disease: A systematic review and meta-analysis. Evid. Based Complement. Alternat. Med. 2016, 2016, 7515413. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Li, Q.-M.; Fang, Q.; Pan, L.-H.; Zha, X.-Q.; Luo, J.-P. Renoprotective effect of Laminaria japonica polysaccharide in adenine-induced chronic renal failure. Molecules 2019, 24, 1491. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, J.; Luo, Y.; Wang, F.; He, G.; Zhou, G.; Peng, X. Lindera aggregata intervents adenine-induced chronic kidney disease by mediating metabolism and TGF-β/Smad signaling pathway. Biomed. Pharmacother. 2021, 134, 111098. [Google Scholar] [CrossRef]
- Cai, H.; Su, S.; Li, Y.; Zeng, H.; Zhu, Z.; Guo, J.; Zhu, Y.; Guo, S.; Yu, L.; Qian, D. Protective effects of Salvia miltiorrhiza on adenine-induced chronic renal failure by regulating the metabolic profiling and modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad signaling pathways. J. Ethnopharmacol. 2018, 212, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, A.S.; Hur, H.J.; Lee, S.H.; Sung, M.J. Preventive effects of chrysanthemum coronarium l. Extract on bone metabolism in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2020, 2020, 6975646. [Google Scholar] [CrossRef]
- Sung, M.J.; Lee, A.S. Chrysanthemum coronarium L. Protects against Premature Senescence in Human Endothelial Cells. Curr. Issues Mol. Biol. 2022, 44, 5839–5847. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, Y.-M.; Kim, J.-B.; Park, D.-S.; Go, J.-S.; Kim, H.-R. Comparison of physicochemical properties and antioxidant activity between raw and heat-treated vegetables. Korean J. Community Living Sci. 2014, 25, 5–18. [Google Scholar] [CrossRef][Green Version]
- Abd El Raheim, M.D. Biological activity of Chrysanthemum coronarium L. extracts. Annu. Res. Rev. Biol. 2014, 4, 2617–2627. [Google Scholar]
- Abd-Alla, H.I.; Albalawy, M.A.; Aly, H.F.; Shalaby, N.M.M.; Shaker, K.H. Flavone composition and antihypercholesterolemic and antihyperglycemic activities of Chrysanthemum coronarium L. Z. Nat. C 2014, 69, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.C.; Degaspari, S.; Catanozi, S.; Dellê, H.; de Sá Lima, L.; Silva, C.; Blanco, P.; Solez, K.; Scavone, C.; Noronha, I.L. Thalidomide suppresses inflammation in adenine-induced CKD with uraemia in mice. Nephrol. Dial. Transpl. 2013, 28, 1140–1149. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J. Nephrol. 2013, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, M.M.; Abdel-Rahman, M.S.; Badary, D.M.; Sabra, M.S. Effects of furosemide and tadalafil in both conventional and nanoforms against adenine-induced chronic renal failure in rats. Eur. J. Med. Res. 2022, 27, 117. [Google Scholar] [CrossRef]
- Gong, Q.; He, L.-L.; Wang, M.-L.; Ouyang, H.; Gao, H.-W.; Feng, Y.-L.; Yang, S.-L.; Du, L.-J.; Li, J.; Luo, Y.-Y. Anemoside B4 protects rat kidney from adenine-induced injury by attenuating inflammation and fibrosis and enhancing podocin and nephrin expression. Evid. Based Complement. Alternat. Med. 2019, 2019, 8031039. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.P.; Sharma, C.; Kang, S.C. Morin hydrate attenuates adenine-induced renal fibrosis via targeting cathepsin D signaling. Int. Immunopharmacol. 2021, 90, 107234. [Google Scholar] [CrossRef]
- Kondo, M.; Tahara, A.; Hayashi, K.; Abe, M.; Inami, H.; Ishikawa, T.; Ito, H.; Tomura, Y. Renoprotective effects of novel interleukin-1 receptor-associated kinase 4 inhibitor AS2444697 through anti-inflammatory action in 5/6 nephrectomized rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 909–919. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Jiang, H.; Pan, J.; Huang, X.-R.; Wang, Y.-C.; Huang, H.-F.; To, K.-F.; Nikolic-Paterson, D.J.; Lan, H.-Y.; Chen, J.-H. Macrophage-to-myofibroblast transition contributes to interstitial fibrosis in chronic renal allograft injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Booz, G.W.; Wang, Y.; Fan, F.; Roman, R.J. Inflammation and renal fibrosis: Recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 2018, 820, 65–76. [Google Scholar] [CrossRef]
- Jung, G.-S.; Hwang, Y.J.; Choi, J.-H.; Lee, K.-M. Lin28a attenuates TGF-β-induced renal fibrosis. BMB Rep. 2020, 53, 594–599. [Google Scholar] [CrossRef]
- Fan, J.-M.; Ng, Y.-Y.; Hill, P.A.; Nikolic-Paterson, D.J.; Mu, W.; Atkins, R.C.; Lan, H.Y. Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999, 56, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Manabe, E.; Dai, Y.; Ishihara, M.; Tsujino, T. Juzentaihoto improves adenine-induced chronic renal failure in BALB/c mice via suppression of renal fibrosis and inflammation. J. Pharmacol. Sci. 2022, 148, 172–178. [Google Scholar] [CrossRef]
- Mulsow, J.J.W.; Watson, R.W.G.; Fitzpatrick, J.M.; O’Connell, P.R. Transforming growth factor-β promotes pro-fibrotic behavior by serosal fibroblasts via PKC and ERK1/2 mitogen activated protein kinase cell signaling. Annal. Surg. 2005, 242, 880–889. [Google Scholar] [CrossRef]
- Han, Y.; Lu, J.-S.; Xu, Y.; Zhang, L.; Hong, B.-F. Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 4725. [Google Scholar] [PubMed]
- Deshmukh, R.; Kaundal, M.; Bansal, V. Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef]
- Veeren, B.; Bringart, M.; Turpin, C.; Rondeau, P.; Planesse, C.; Ait-Arsa, I.; Gimié, F.; Marodon, C.; Meilhac, O.; Gonthier, M.-P. Caffeic Acid, One of the Major Phenolic Acids of the Medicinal Plant Antirhea borbonica, Reduces Renal Tubulointerstitial Fibrosis. Biomedicines 2021, 9, 358. [Google Scholar] [CrossRef]
- Junaedy Yunus, M.S.; Lintin, G.B.R.; Muchtar, M.; Ratna, D.C.; Sari, N.A.; Romi, M.M. Chlorogenic acid attenuates kidney fibrosis via antifibrotic action of BMP-7 and HGF. Med. J. Malaysia 2020, 75, 5. [Google Scholar]
- Han, E.H.; Kim, J.Y.; Kim, H.G.; Chun, H.K.; Chung, Y.C.; Jeong, H.G. Inhibitory effect of 3-caffeoyl-4-dicaffeoylquinic acid from Salicornia herbacea against phorbol ester-induced cyclooxygenase-2 expression in macrophages. Chem. Biol. Interact. 2010, 183, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Adham, S.A.; Al Za’abi, M.; Waly, M.I.; Yasin, J.; Nemmar, A.; Schupp, N. Ameliorative effect of chrysin on adenine-induced chronic kidney disease in rats. PLoS ONE 2015, 10, e0125285. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci. Total Environ. 2023, 892, 164808. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, A.S.; Lee, H.B.; Hur, H.J.; Lee, S.H.; Sung, M.J. Soluble epoxide hydrolase inhibitor, TPPU, attenuates progression of atherosclerotic lesions and vascular smooth muscle cell phenotypic switching. Vascul. Pharmacol. 2022, 145, 107086. [Google Scholar] [CrossRef]



| Gene | Primer Sequence (5′-3′) | Tm (°C) |
|---|---|---|
| MCP-1 | F: GCCTGCTGTTCACAGTTGC | 59.1 |
| R: CAGGTGAGTGGGGCGTTA | 58.0 | |
| IL-6 | F: TGAGAGTAGTGAGGAACAAG | 52.8 |
| R: CGCAGAATGAGATGAGTTG | 53.0 | |
| IL-1β | F: TGAGCTCGCCAGTGAAATGAT | 59.1 |
| R: TCCATGGCCACAACAACTGA | 58.8 | |
| F4/80 | F: CCTGGACGAATCCTGTGAAG | 57.0 |
| R: GGTGGGACCACAGAGAGTTG | 59.0 | |
| Type IV collagen | F: TTAAAGGACTCCAGGGACCAC | 58.0 |
| R: CCCACTGAGCCTGTCACAC | 59.3 | |
| Fibronectin | F: CCCTATCTCTGATACCGTTGTCC | 58.8 |
| R: TGCCGCAACTACTGTGATTCGG | 62.4 | |
| TGFβ-1 | F: TCAGACATTCGGGAAGCAGT | 58.0 |
| R: ACGCCAGGAATTGTTGCTAT | 56.9 | |
| α-SMA | F: GCCCAGAGCAAGAGAGG | 55.6 |
| R: TGTCAGCAGTGTCGGATG | 56.1 | |
| GAPDH | F: AAATGGTGAAGGTCGGTGTG | 60.0 |
| R: TGAAGGGGTCGTTGATGG | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Lee, A.-S.; Hur, H.-J.; Lee, S.-H.; Na, H.-J.; Sung, M.-J. Renoprotective Effect of Chrysanthemum coronarium L. Extract on Adenine-Induced Chronic Kidney Disease in Mice. Pharmaceuticals 2023, 16, 1048. https://doi.org/10.3390/ph16071048
Kim Y-S, Lee A-S, Hur H-J, Lee S-H, Na H-J, Sung M-J. Renoprotective Effect of Chrysanthemum coronarium L. Extract on Adenine-Induced Chronic Kidney Disease in Mice. Pharmaceuticals. 2023; 16(7):1048. https://doi.org/10.3390/ph16071048
Chicago/Turabian StyleKim, Yi-Seul, Ae-Sin Lee, Haeng-Jeon Hur, Sang-Hee Lee, Hyun-Jin Na, and Mi-Jeong Sung. 2023. "Renoprotective Effect of Chrysanthemum coronarium L. Extract on Adenine-Induced Chronic Kidney Disease in Mice" Pharmaceuticals 16, no. 7: 1048. https://doi.org/10.3390/ph16071048
APA StyleKim, Y.-S., Lee, A.-S., Hur, H.-J., Lee, S.-H., Na, H.-J., & Sung, M.-J. (2023). Renoprotective Effect of Chrysanthemum coronarium L. Extract on Adenine-Induced Chronic Kidney Disease in Mice. Pharmaceuticals, 16(7), 1048. https://doi.org/10.3390/ph16071048






