Reviving a Classic Antigen with a Cutting-Edge Approach: Nanobodies for HER2+ Breast Cancer
Abstract
1. Introduction
1.1. mAbs Limitations in Cancer-Target Therapy
1.2. NBs Discovery
1.3. NBs Mechanism of Production
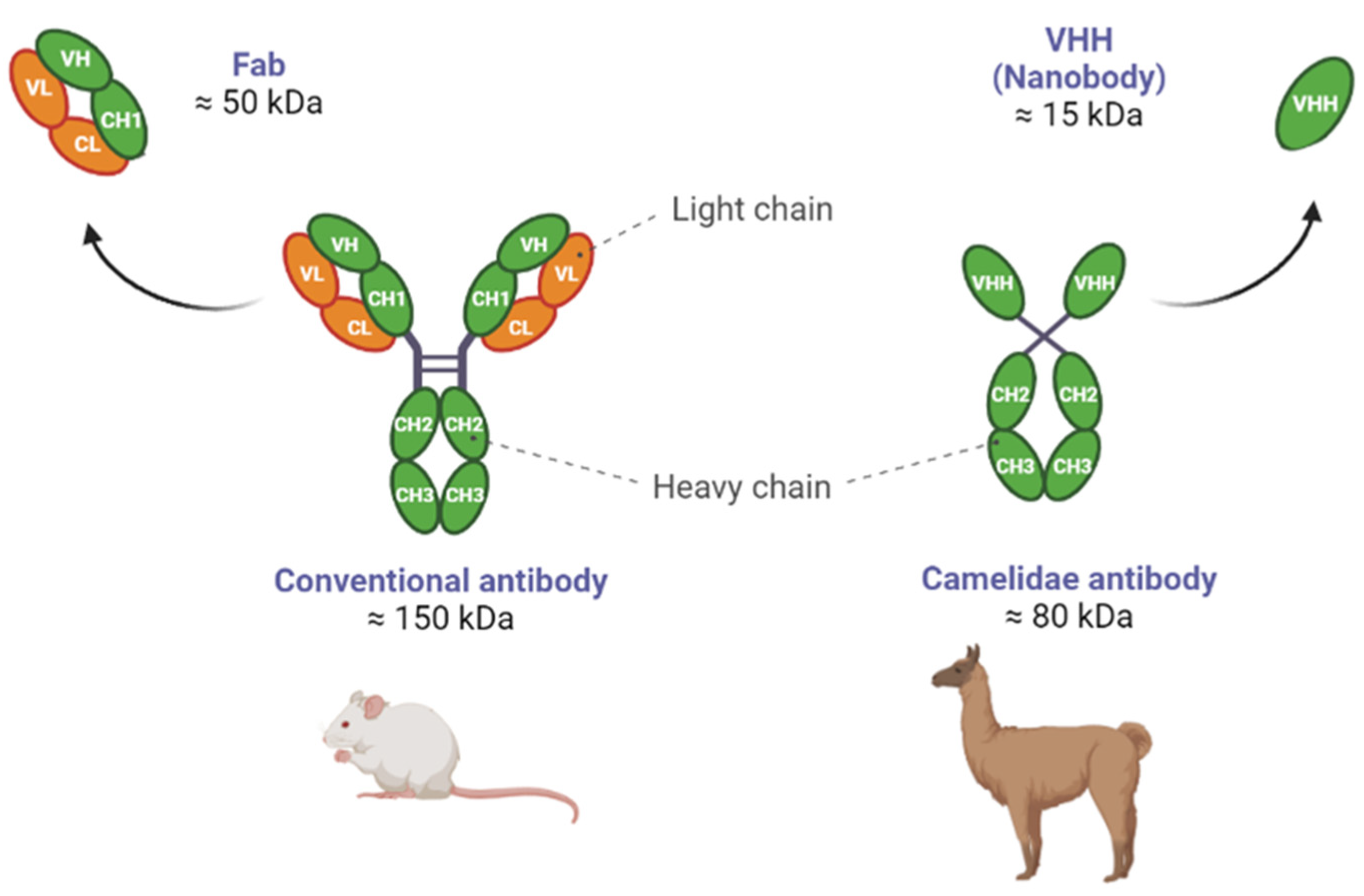
1.4. Differences between NBs and Abs
1.5. NBs as Enzymatic Modulators
1.6. NBs Extracellular Targets
2. NBs for the Diagnosis of HER2
2.1. Radioisotope-Based Diagnostic Techniques
2.2. Non-Radioisotope-Based Diagnostic Techniques
3. NBs against HER2 in Therapy
3.1. Identification of NBs against HER2 Extracellular Domain and Tyrosine-Kinase Domain
3.2. Engineered NBs
3.2.1. Bispecific NBs against HER2
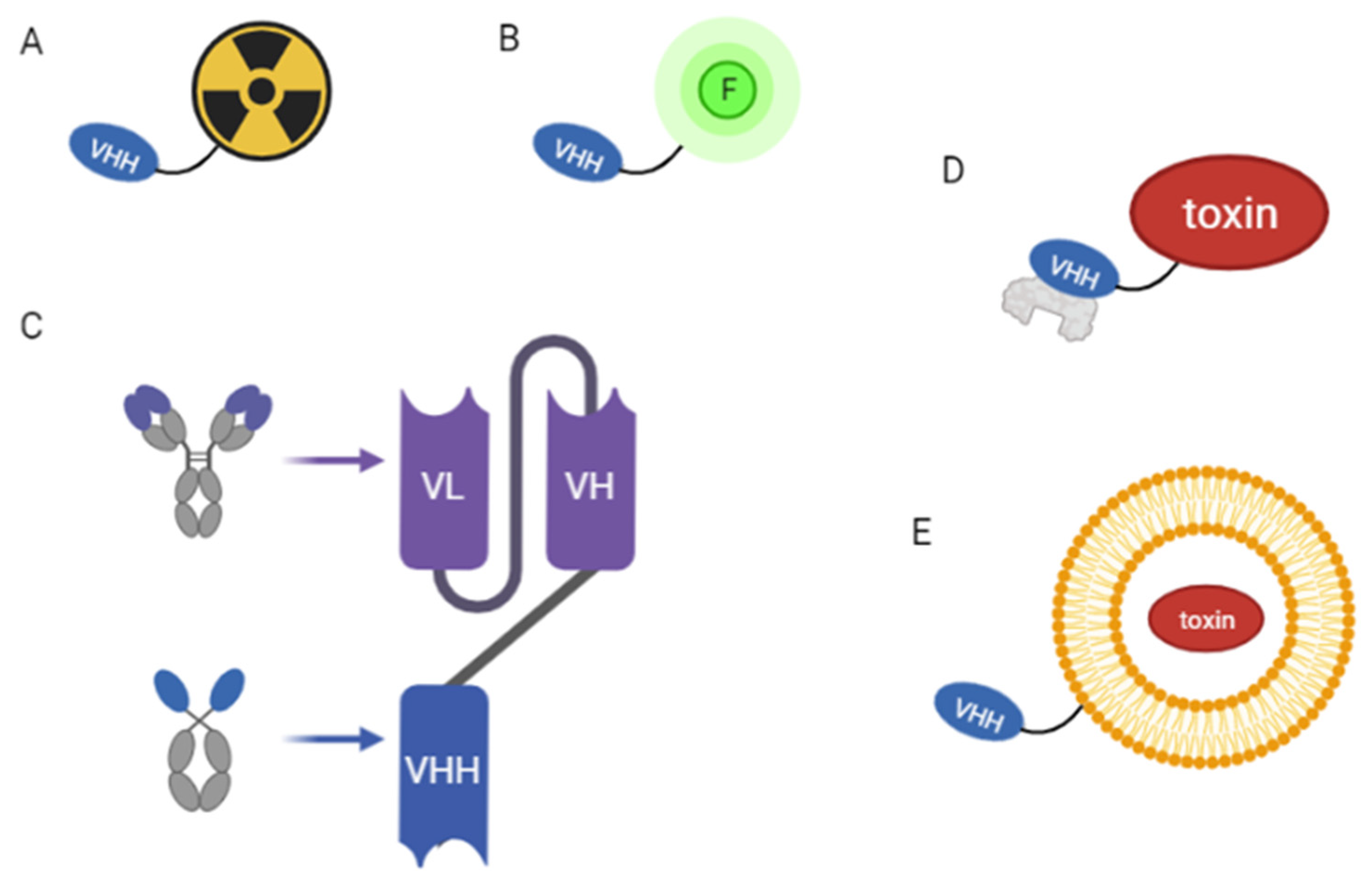
3.2.2. NBs as Drug Carriers
3.2.3. Improving Pharmacokinetic Properties
3.2.4. NBs in Radiopharmaceutical Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habeeb, N.W.-A.; Kulasingam, V.; Diamandis, E.P.; Yousef, G.M.; Tsongalis, G.J.; Vermeulen, L.; Zhu, Z.; Kamel-Reid, S. The Use of Targeted Therapies for Precision Medicine in Oncology. Clin. Chem. 2016, 62, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, G.M. Targeted Therapy: Attacking Cancer with Molecular and Immunological Targeted Agents. Asia. Pac. J. Oncol. Nurs. 2018, 5, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Yang, E.Y.; Shah, K. Nanobodies: Next Generation of Cancer Diagnostics and Therapeutics. Front. Oncol. 2020, 10, 1182. [Google Scholar] [CrossRef]
- Kolkman, J.A.; Law, D.A. Nanobodies—From Llamas to Therapeutic Proteins. Drug Discov. Today Technol. 2010, 7, e139–e146. [Google Scholar] [CrossRef]
- Weinstein, J.N.; van Osdol, W. Early Intervention in Cancer Using Monoclonal Antibodies and Other Biological Ligands: Micropharmacology and the “Binding Site Barrier”. Cancer Res. 1992, 52, 2747s–2751s. [Google Scholar]
- Adams, G.P.; Schier, R.; McCall, A.M.; Simmons, H.H.; Horak, E.M.; Alpaugh, R.K.; Marks, J.D.; Weiner, L.M. High Affinity Restricts the Localization and Tumor Penetration of Single-Chain Fv Antibody Molecules. Cancer Res. 2001, 61, 4750–4755. [Google Scholar]
- Vermeer, A.W.P.; Norde, W. The Thermal Stability of Immunoglobulin: Unfolding and Aggregation of a Multi-Domain Protein. Biophys. J. 2000, 78, 394–404. [Google Scholar] [CrossRef]
- Majidi, J.; Barar, J.; Baradaran, B.; Abdolalizadeh, J.; Omidi, Y. Target Therapy of Cancer: Implementation of Monoclonal Antibodies and Nanobodies. Hum. Antibodies 2009, 18, 81–100. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Avila, D.; Hughes, M.; Hughes, A.; McKinney, E.C.; Flajnik, M.F. A New Antigen Receptor Gene Family That Undergoes Rearrangement and Extensive Somatic Diversification in Sharks. Nature 1995, 374, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.B.; Gijsbers, R.; D’Huyvetter, M.; Devoogdt, N.; Lahoutte, T.; et al. Size and Affinity Kinetics of Nanobodies Influence Targeting and Penetration of Solid Tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Odongo, S.; Radwanska, M.; Magez, S. Nanobodies: A Review of Generation, Diagnostics and Therapeutics. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. A Guide to: Generation and Design of Nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef]
- Arbabi Ghahroudi, M.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and Identification of Single Domain Antibody Fragments from Camel Heavy-Chain Antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Rothbauer, U. Special Issue: Nanobody. Antibodies 2020, 9, 6. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Li, C.; Tang, Z.; Hu, Z.; Wang, Y.; Yang, X.; Mo, F.; Lu, X. Natural Single-Domain Antibody-Nanobody: A Novel Concept in the Antibody Field. J. Biomed. Nanotechnol. 2018, 14, 1–19. [Google Scholar] [CrossRef]
- Liu, M.; Li, L.; Jin, D.; Liu, Y. Nanobody—A Versatile Tool for Cancer Diagnosis and Therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1697. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Dougan, M.; Ingram, J.R.; Ho, C.C.M.; Kauke, M.J.; Almo, S.C.; Ploegh, H.L.; Garcia, K.C. Durable Antitumor Responses to CD47 Blockade Require Adaptive Immune Stimulation. Proc. Natl. Acad. Sci. USA 2016, 113, E2646–E2654. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Hamers, R.; Wyns, L.; Muyldermans, S. Camel Heavy-Chain Antibodies: Diverse Germline V(H)H and Specific Mechanisms Enlarge the Antigen-Binding Repertoire. EMBO J. 2000, 19, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Bannas, P.; Hambach, J.; Koch-Nolte, F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies as Antitumor Therapeutics. Front. Immunol. 2017, 8, 1603. [Google Scholar] [CrossRef]
- Muyldermans, S.; Cambillau, C.; Wyns, L. Recognition of Antigens by Single-Domain Antibody Fragments: The Superfluous Luxury of Paired Domains. Trends Biochem. Sci. 2001, 26, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wu, T.; Shi, H.; Wu, Y.; Yang, H.; Zhong, K.; Wang, Y.; Liu, Y. Modular Design of Nanobody–Drug Conjugates for Targeted-Delivery of Platinum Anticancer Drugs with an MRI Contrast Agent. Chem. Commun. 2019, 55, 5175–5178. [Google Scholar] [CrossRef]
- Sun, S.; Ding, Z.; Yang, X.; Zhao, X.; Zhao, M.; Gao, L.; Chen, Q.; Xie, S.; Liu, A.; Yin, S.; et al. Nanobody: A Small Antibody with Big Implications for Tumor Therapeutic Strategy. Int. J. Nanomed. 2021, 16, 2337–2356. [Google Scholar] [CrossRef]
- Könning, D.; Zielonka, S.; Grzeschik, J.; Empting, M.; Valldorf, B.; Krah, S.; Schröter, C.; Sellmann, C.; Hock, B.; Kolmar, H. Camelid and Shark Single Domain Antibodies: Structural Features and Therapeutic Potential. Curr. Opin. Struct. Biol. 2017, 45, 10–16. [Google Scholar] [CrossRef]
- Zavrtanik, U.; Lukan, J.; Loris, R.; Lah, J.; Hadži, S. Structural Basis of Epitope Recognition by Heavy-Chain Camelid Antibodies. J. Mol. Biol. 2018, 430, 4369–4386. [Google Scholar] [CrossRef]
- Menzel, S.; Schwarz, N.; Haag, F.; Koch-Nolte, F. Nanobody-Based Biologics for Modulating Purinergic Signaling in Inflammation and Immunity. Front. Pharm. 2018, 9, 266. [Google Scholar] [CrossRef]
- Fumey, W.; Koenigsdorf, J.; Kunick, V.; Menzel, S.; Schütze, K.; Unger, M.; Schriewer, L.; Haag, F.; Adam, G.; Oberle, A.; et al. Nanobodies Effectively Modulate the Enzymatic Activity of CD38 and Allow Specific Imaging of CD38+ Tumors in Mouse Models in Vivo. Sci. Rep. 2017, 7, 14289. [Google Scholar] [CrossRef]
- Barlow, J.N.; Conrath, K.; Steyaert, J. Substrate-Dependent Modulation of Enzyme Activity by Allosteric Effector Antibodies. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Oyen, D.; Wechselberger, R.; Srinivasan, V.; Steyaert, J.; Barlow, J.N. Mechanistic Analysis of Allosteric and Non-Allosteric Effects Arising from Nanobody Binding to Two Epitopes of the Dihydrofolate Reductase of Escherichia coli. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Cabalteja, C.C.; Sachdev, S.; Cheloha, R.W. Characterization of a Nanobody-Epitope Tag Interaction and Its Application for Receptor Engineering. ACS Chem. Biol. 2022, 17, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Nanobody-Based Cancer Therapy of Solid Tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine (NLM). Available online: https://clinicaltrials.gov (accessed on 29 March 2023).
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/Neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef]
- Seshadri, R.; Firgaira, F.A.; Horsfall, D.J.; McCaul, K.; Setlur, V.; Kitchen, P. Clinical Significance of HER-2/Neu Oncogene Amplification in Primary Breast Cancer. The South Australian Breast Cancer Study Group. J. Clin. Oncol. 1993, 11, 1936–1942. [Google Scholar] [CrossRef]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.-J.; Stickeler, E.; Mottaghy, F.M. HER2-Directed Antibodies, Affibodies and Nanobodies as Drug-Delivery Vehicles in Breast Cancer with a Specific Focus on Radioimmunotherapy and Radioimmunoimaging. Eur. J. Nucl. Med. 2020, 48, 1371–1389. [Google Scholar] [CrossRef]
- Grifone, T.J. Cell Polarity and Oncogenesis: Common Mutations Contribute to Altered Cellular Polarity and Promote Malignancy. Nucleus 2020, 63, 91–106. [Google Scholar] [CrossRef]
- Albanell, J.; Baselga, J. Trastuzumab, a Humanized Anti-HER2 Monoclonal Antibody, for the Treatment of Breast Cancer. Drugs Today 1999, 35, 931–946. [Google Scholar]
- Loibl, S.; Gianni, L. HER2-Positive Breast Cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NIH). Available online: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq#_1375 (accessed on 19 May 2023).
- Li, T.; Akinade, T.; Zhou, J.; Wang, H.; Tong, Q.; He, S.; Rinebold, E.; Valencia Salazar, L.E.; Bhansali, D.; Zhong, Y.; et al. Therapeutic Nanocarriers Inhibit Chemotherapy-Induced Breast Cancer Metastasis. Adv. Sci. 2022, 9, 2203949. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated with Electronic Circuits. Adv. Mater. 2022, 34, 2203224. [Google Scholar] [CrossRef]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef] [PubMed]
- Vi, C.; Mandarano, G.; Shigdar, S. Diagnostics and Therapeutics in Targeting Her2 Breast Cancer: A Novel Approach. Int. J. Mol. Sci. 2021, 22, 6163. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; O’Hanlon, S.; Latham, B. False-Negative Contrast-Enhanced Spectral Mammography: Use of More than One Imaging Modality and Application of the Triple Test Avoids Misdiagnosis. BMJ Case Rep. 2017, 2017, 218556. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular Imaging and Therapy of Cancer with Radiolabeled Nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68 Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II Trial of HER2-PET/CT Using 68Ga-Anti-HER2 VHH1 for Characterization of HER2 Presence in Brain Metastases of Breast Cancer Patients. Ann. Oncol. 2019, 30, iii25–iii26. [Google Scholar] [CrossRef]
- Xavier, C.; Blykers, A.; Laoui, D.; Bolli, E.; Vaneyken, I.; Bridoux, J.; Baudhuin, H.; Raes, G.; Everaert, H.; Movahedi, K.; et al. Clinical Translation of [68Ga]Ga-NOTA-Anti-MMR-SdAb for PET/CT Imaging of Protumorigenic Macrophages. Mol. Imaging Biol. 2019, 21, 898–906. [Google Scholar] [CrossRef]
- de Vos, J.; Devoogdt, N.; Lahoutte, T.; Muyldermans, S. Camelid Single-Domain Antibody-Fragment Engineering for (Pre)Clinical in Vivo Molecular Imaging Applications: Adjusting the Bullet to Its Target. Expert Opin. Biol. 2013, 13, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Rashidian, M.; Ploegh, H. Nanobodies as Non-Invasive Imaging Tools. Immuno-Oncol. Technol. 2020, 7, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “Magic Bullet” for Molecular Imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. (18)F-Nanobody for PET Imaging of HER2 Overexpressing Tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Z.; McDougald, D.; Meshaw, R.L.; Vaidyanathan, G.; Zalutsky, M.R. Site-Specific Radioiodination of an Anti-HER2 Single Domain Antibody Fragment with a Residualizing Prosthetic Agent. Nucl. Med. Biol. 2021, 92, 171–183. [Google Scholar] [CrossRef]
- Vaneycken, I.; Devoogdt, N.; van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical Screening of Anti-HER2 Nanobodies for Molecular Imaging of Breast Cancer. FASEB J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, C.; Xing, Y.; He, J.; O’doherty, J.; Huang, W.; Zhao, J. Development of a 99mTc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer. Mol. Pharm. 2021, 18, 3616–3622. [Google Scholar] [CrossRef]
- Verhaar, E.R.; Woodham, A.W.; Ploegh, H.L. Nanobodies in Cancer. Semin. Immunol. 2021, 52, 101425. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Dorrestijn, B.; Muns, J.A.; Adamzek, K.; Doulkeridou, S.; Houthoff, H.J.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Homogeneous Tumor Targeting with a Single Dose of HER2-Targeted Albumin-Binding Domain-Fused Nanobody-Drug Conjugates Results in Long-Lasting Tumor Remission in Mice. Theranostics 2021, 11, 5525–5538. [Google Scholar] [CrossRef]
- Kijanka, M.; Warnders, F.-J.; el Khattabi, M.; Lub-de Hooge, M.; van Dam, G.M.; Ntziachristos, V.; de Vries, L.; Oliveira, S.; van Bergen En Henegouwen, P.M.P. Rapid Optical Imaging of Human Breast Tumour Xenografts Using Anti-HER2 VHHs Site-Directly Conjugated to IRDye 800CW for Image-Guided Surgery. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1718–1729. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, X.; Li, L.; Zhang, R.; Zhu, Y.; Wu, Z.; Ding, K. A Novel Small Molecular Antibody, HER2-Nanobody, Inhibits Tumor Proliferation in HER2-Positive Breast Cancer Cells In Vitro and In Vivo. Front. Oncol. 2021, 11, 669393. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, F.; Rasaee, M.J.; Shokrgozar, M.A.; Dizaji, M.M.; Rahbarizadeh, F.; Ahmadvande, D. Isolation of a Novel Nanobody against HER-2/Neu Using Phage Displays Technology. Lab. Med. 2010, 41, 69–76. [Google Scholar] [CrossRef]
- Lamtha, T.; Tabtimmai, L.; Bangphoomi, K.; Kiriwan, D.; Malik, A.A.; Chaicumpa, W.; van Bergen En Henegouwen, P.M.P.; Choowongkomon, K. Generation of a Nanobody against HER2 Tyrosine Kinase Using Phage Display Library Screening for HER2-Positive Breast Cancer Therapy Development. Protein Eng. Des. Sel. 2021, 34, gzab030. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Rahbarizadeh, F.; Ahmadvand, D.; Parhamifar, L. Heavy Chain Only Antibodies: A New Paradigm in Personalized HER2+ Breast Cancer Therapy. BioImpacts 2013, 3, 1–4. [Google Scholar]
- Wu, X.; Chen, S.; Lin, L.; Liu, J.; Wang, Y.; Li, Y.; Li, Q.; Wang, Z. A Single Domain–Based Anti-Her2 Antibody Has Potent Antitumor Activities. Transl. Oncol. 2018, 11, 366–373. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Xiao, Z.; Li, W.; Dimitrov, D.S.; Chen, W. Human Domain Antibodies to Conserved Epitopes on HER2 Potently Inhibit Growth of HER2-Overexpressing Human Breast Cancer Cells In Vitro. Antibodies 2019, 8, 25. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, M.; Zhu, S.; Wang, H.; Wu, K. Recent Advances and Challenges of Bispecific Antibodies in Solid Tumors. Exp. Hematol. Oncol. 2021, 10, 56. [Google Scholar] [CrossRef]
- James, N.D.; Atherton, P.J.; Jones, J.; Howie, A.J.; Tchekmedyian, S.; Curnow, R.T. A Phase II Study of the Bispecific Antibody MDX-H210 (Anti-HER2 × CD64) with GM-CSF in HER2+ Advanced Prostate Cancer. Br. J. Cancer 2001, 85, 152–156. [Google Scholar] [CrossRef]
- Vaishampayan, U.; Thakur, A.; Rathore, R.; Kouttab, N.; Lum, L.G. Phase I Study of Anti-CD3 × Anti-Her2 Bispecific Antibody in Metastatic Castrate Resistant Prostate Cancer Patients. Prostate Cancer 2015, 2015, 285193. [Google Scholar] [CrossRef]
- Li, A.; Xing, J.; Li, L.; Zhou, C.; Dong, B.; He, P.; Li, Q.; Wang, Z. A Single-Domain Antibody-Linked Fab Bispecific Antibody Her2-S-Fab Has Potent Cytotoxicity against Her2-Expressing Tumor Cells. AMB Express 2016, 6, 32. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, W.W.; Huang, Q.; Zhang, P.; Zhang, L.-Z.; Jiang, G.; Tian, Y. Effects of Lapatinib or Trastuzumab, Alone and in Combination, in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Cancer Med. 2016, 5, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, L.; Zhou, C.; Li, J.; Liu, J.; Shu, R.; Dong, B.; Li, Q.; Wang, Z. A HER2 Bispecific Antibody Can Be Efficiently Expressed in Escherichia coli with Potent Cytotoxicity. Oncol. Lett. 2018, 16, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Helma, J.; Schneider, A.F.L.; Leonhardt, H.; Hackenberger, C.P.R. Nanobodies: Chemical Functionalization Strategies and Intracellular Applications. Angew. Chem. Int. Ed. Engl. 2018, 57, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.; Muyldermans, S. Nanobody-Based Delivery Systems for Diagnosis and Targeted Tumor Therapy. Front. Immunol. 2017, 8, 1442. [Google Scholar] [CrossRef]
- Farasat, A.; Rahbarizadeh, F.; Ahmadvand, D.; Ranjbar, S.; Khoshtinat Nikkhoi, S. Effective Suppression of Tumour Cells by Oligoclonal HER2-Targeted Delivery of Liposomal Doxorubicin. J. Liposome Res. 2019, 29, 53–65. [Google Scholar] [CrossRef]
- Martínez-Jothar, L.; Beztsinna, N.; van Nostrum, C.F.; Hennink, W.E.; Oliveira, S. Selective Cytotoxicity to HER2 Positive Breast Cancer Cells by Saporin-Loaded Nanobody-Targeted Polymeric Nanoparticles in Combination with Photochemical Internalization. Mol. Pharm. 2019, 16, 1633–1647. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent Conjugation of Extracellular Vesicles with Peptides and Nanobodies for Targeted Therapeutic Delivery. J. Extracell. Vesicles 2021, 10, 12057. [Google Scholar] [CrossRef]
- Artigas, C.; Mileva, M.; Flamen, P.; Karfis, I. Targeted Radionuclide Therapy: An Emerging Field in Solid Tumours. Curr. Opin. Oncol. 2021, 33, 493–499. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Targeted Radionuclide Therapy: A Historical and Personal Review. Semin. Nucl. Med. 2020, 50, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical Therapy in Cancer: Clinical Advances and Challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A Promising Toolkit for Molecular Imaging and Disease Therapy. EJNMMI Res. 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Meshaw, R.; McDougald, D.; Zhou, Z.; Zhao, X.G.; Jannetti, S.A.; Reiman, R.E.; Pippen, E.; Marjoram, R.; Schaal, J.L.; et al. Evaluation of an 131I-Labeled HER2-Specific Single Domain Antibody Fragment for the Radiopharmaceutical Therapy of HER2-Expressing Cancers. Sci. Rep. 2022, 12, 3020. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. 131I-Labeled Anti-HER2 Camelid SdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- Zhao, L.; Gong, J.; Qi, Q.; Liu, C.; Su, H.; Xing, Y.; Zhao, J. 131I-Labeled Anti-HER2 Nanobody for Targeted Radionuclide Therapy of HER2-Positive Breast Cancer. Int. J. Nanomed. 2023, 18, 1915–1925. [Google Scholar] [CrossRef]
- Silberstein, E.B. Radioiodine: The Classic Theranostic Agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Miladinova, D. Molecular Imaging of HER2 Receptor: Targeting HER2 for Imaging and Therapy in Nuclear Medicine. Front. Mol. Biosci. 2023, 10, 1144817. [Google Scholar] [CrossRef]
- Feng, Y.; Meshaw, R.; Zhao, X.G.; Jannetti, S.; Vaidyanathan, G.; Zalutsky, M.R. Effective Treatment of Human Breast Carcinoma Xenografts with Single-Dose 211At-Labeled Anti-HER2 Single-Domain Antibody Fragment. J. Nucl. Med. 2023, 64, 124–130. [Google Scholar] [CrossRef]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 Labeled Anti-HER2 5F7 Single Domain Antibody Fragment Conjugates: Radiolabeling and Preliminary Evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef]
- Tseu, G.Y.W.; Kamaruzaman, K.A. A Review of Different Types of Liposomes and Their Advancements as a Form of Gene Therapy Treatment for Breast Cancer. Molecules 2023, 28, 1498. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Dutra, J.A.P.; Tofani, L.B.; de Araújo, J.T.C.; Di Filippo, L.D.; Marchetti, J.M.; Chorilli, M. Targeted Liposomes: A Nonviral Gene Delivery System for Cancer Therapy. Pharmaceutics 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Gao, Y.-G.; Lin, X.; Li, Y.; Dang, K.; Tian, Y.; Zhang, W.-J.; Jiang, S.-F.; Qadir, A.; Qian, A.-R. The Development of Functional Non-Viral Vectors for Gene Delivery. Int. J. Mol. Sci. 2019, 20, 5491. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Bigham, A.; Taheriazam, A.; Saghari, Y.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Karimi-Maleh, H.; Nazarzadeh Zare, E.; et al. (Nano)Platforms in Breast Cancer Therapy: Drug/Gene Delivery, Advanced Nanocarriers and Immunotherapy. Med. Res. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Karn, V.; Sandhya, S.; Hsu, W.; Parashar, D.; Singh, H.N.; Jha, N.K.; Gupta, S.; Dubey, N.K.; Kumar, S. CRISPR/Cas9 System in Breast Cancer Therapy: Advancement, Limitations and Future Scope. Cancer Cell Int. 2022, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, W. CRISPR-Mediated Targeting of HER2 Inhibits Cell Proliferation through a Dominant Negative Mutation. Cancer Lett. 2017, 385, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ylä-Herttuala, S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol. Ther. 2012, 20, 1831–1832. [Google Scholar] [CrossRef]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Schimmer, J.; Breazzano, S. Investor Outlook: Rising from the Ashes; GSK’s European Approval of Strimvelis for ADA-SCID. Hum. Gene Clin. Dev. 2016, 27, 57–61. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Han, W.; Zhang, Y. Tisagenlecleucel, an Approved Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Leukemia. Drugs Today 2017, 53, 597. [Google Scholar] [CrossRef]
- Axicabtagene Ciloleucel (Yescarta) for B-Cell Lymphoma. Med. Lett. Drugs 2018, 60, e122–e123.
- Leroy, B.P.; Fischer, M.D.; Flannery, J.G.; MacLaren, R.E.; Dalkara, D.; Scholl, H.P.N.; Chung, D.C.; Spera, C.; Viriato, D.; Banhazi, J. Gene Therapy for Inherited Retinal Disease: Long-Term Durability of Effect. Ophthalmic Res. 2022, 66, 179–196. [Google Scholar] [CrossRef] [PubMed]
| Advantages | Disadvantages | |
|---|---|---|
| Abs |
|
|
| NBs |
|
|
| Condition/Disease | Target | NB Drug | Clinical Trial | Phase | Study Start |
|---|---|---|---|---|---|
| Hidradenitis Suppurativa 1 | IL17A, IL17F, IL17A/F | M1095 | NCT05322473 | II | April 2022 |
| Generalized Myasthenia Gravis 2 | AChR | ALXN1720 | NCT05556096 | III | November 2022 |
| Solid tumor PET/CT 3 | PD-L1 | 68Ga-THP-APN09 | NCT05156515 | NA | December 2021 |
| Solid Tumor PET/CT 4 | CLDN18.2 | 18F-FDG | NCT05436093 | NA | June 2022 |
| Non-small-cell lung cancer, mesothelioma, colorectal cancer 5 | MSLN | α-PD1-MSLN-CAR-T cells | NCT05373147 NCT05089266 | Early I I | October 2020 November 2021 |
| Malignant lymphoma 6 | IL21 | JS014 | NCT05296772 | I | February 2022 |
| Malignant Neoplasm of Digestive System 7 | CLDN18.2 | DR30303 | NCT05639153 | I | May 2022 |
| Cancer SPECT/CT 8 | HER2 | 99mTc-MIRC208 | NCT04591652 | II | April 2019 |
| Metastatic breast cancer PET/CT 9 | HER2 | 68GaNOTA-anti-HER2 VHH1 | NCT03331601 NCT03924466 | II II | October 2017 April 2019 |
| Malignant solid tumor PET/CT, Cardiovascular atherosclerosis, Lymphoma, Cardiac Sarcoidosis 10 | 68Ga-NOTA-anti-MMR VHH2 | NCT04168528 NCT04758650 | I–II II | November 2019 January 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrignano, C.; Di Scipio, F.; Franco, F.; Mognetti, B.; Berta, G.N. Reviving a Classic Antigen with a Cutting-Edge Approach: Nanobodies for HER2+ Breast Cancer. Pharmaceuticals 2023, 16, 794. https://doi.org/10.3390/ph16060794
Castrignano C, Di Scipio F, Franco F, Mognetti B, Berta GN. Reviving a Classic Antigen with a Cutting-Edge Approach: Nanobodies for HER2+ Breast Cancer. Pharmaceuticals. 2023; 16(6):794. https://doi.org/10.3390/ph16060794
Chicago/Turabian StyleCastrignano, Chiara, Federica Di Scipio, Francesco Franco, Barbara Mognetti, and Giovanni Nicolao Berta. 2023. "Reviving a Classic Antigen with a Cutting-Edge Approach: Nanobodies for HER2+ Breast Cancer" Pharmaceuticals 16, no. 6: 794. https://doi.org/10.3390/ph16060794
APA StyleCastrignano, C., Di Scipio, F., Franco, F., Mognetti, B., & Berta, G. N. (2023). Reviving a Classic Antigen with a Cutting-Edge Approach: Nanobodies for HER2+ Breast Cancer. Pharmaceuticals, 16(6), 794. https://doi.org/10.3390/ph16060794






