Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities from the Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin
Abstract
1. Introduction
2. Results and Discussion
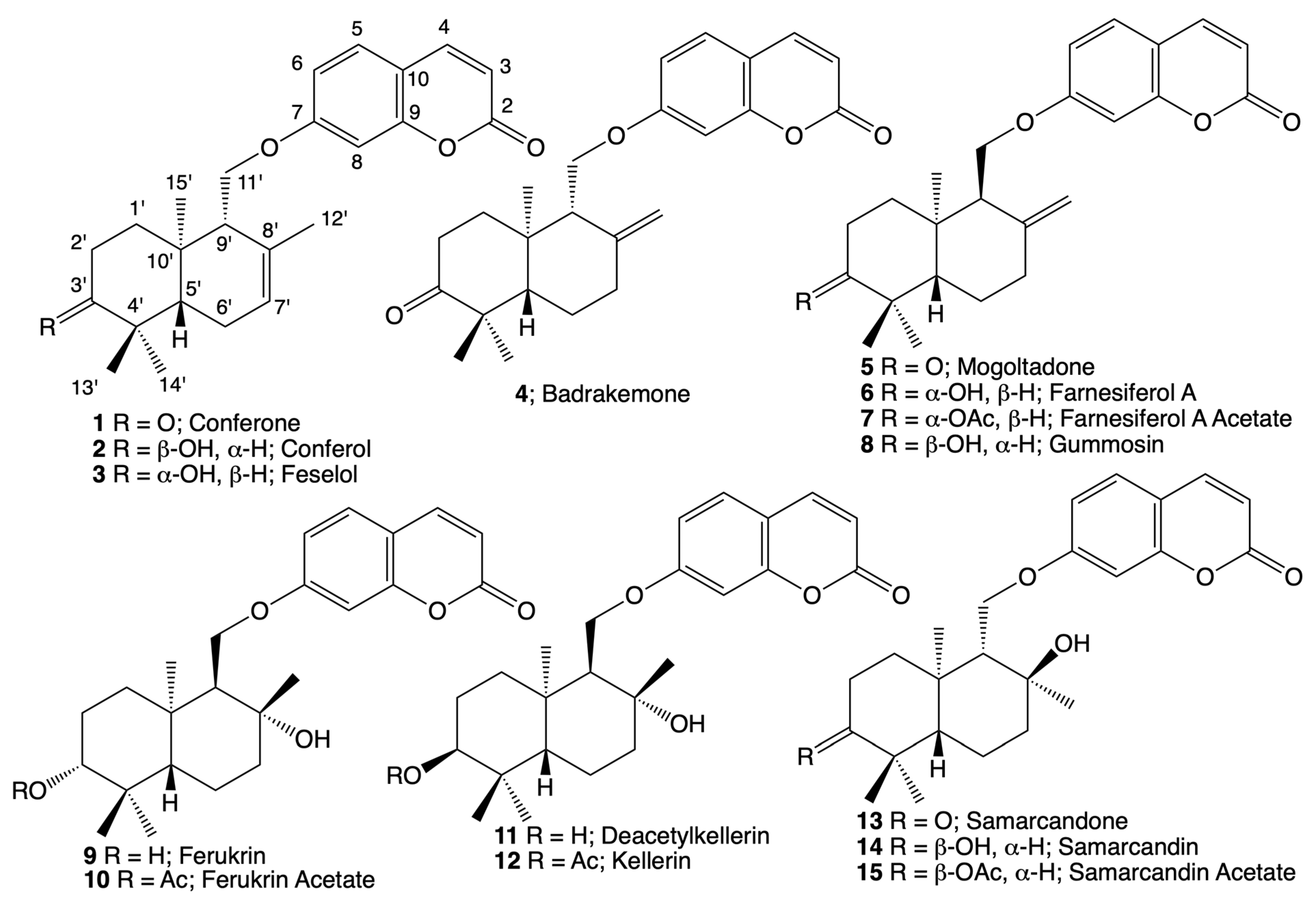
2.1. Bioactivity-Directed Isolation and Structure Elucidation of Cytotoxic Sesquiterpene Coumarin Ethers
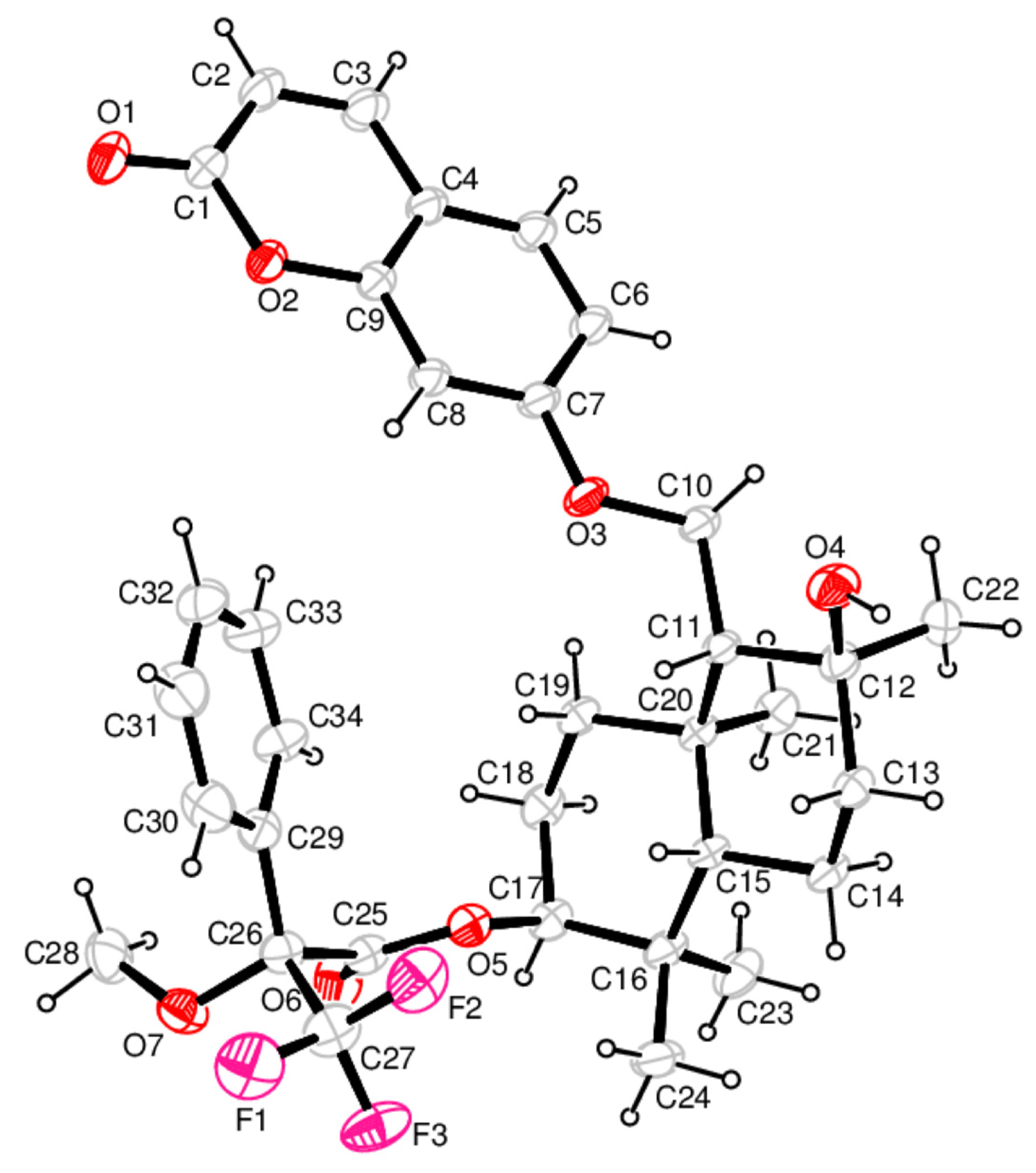
2.2. Determination of the Absolute Configuration of Samarcandin 14
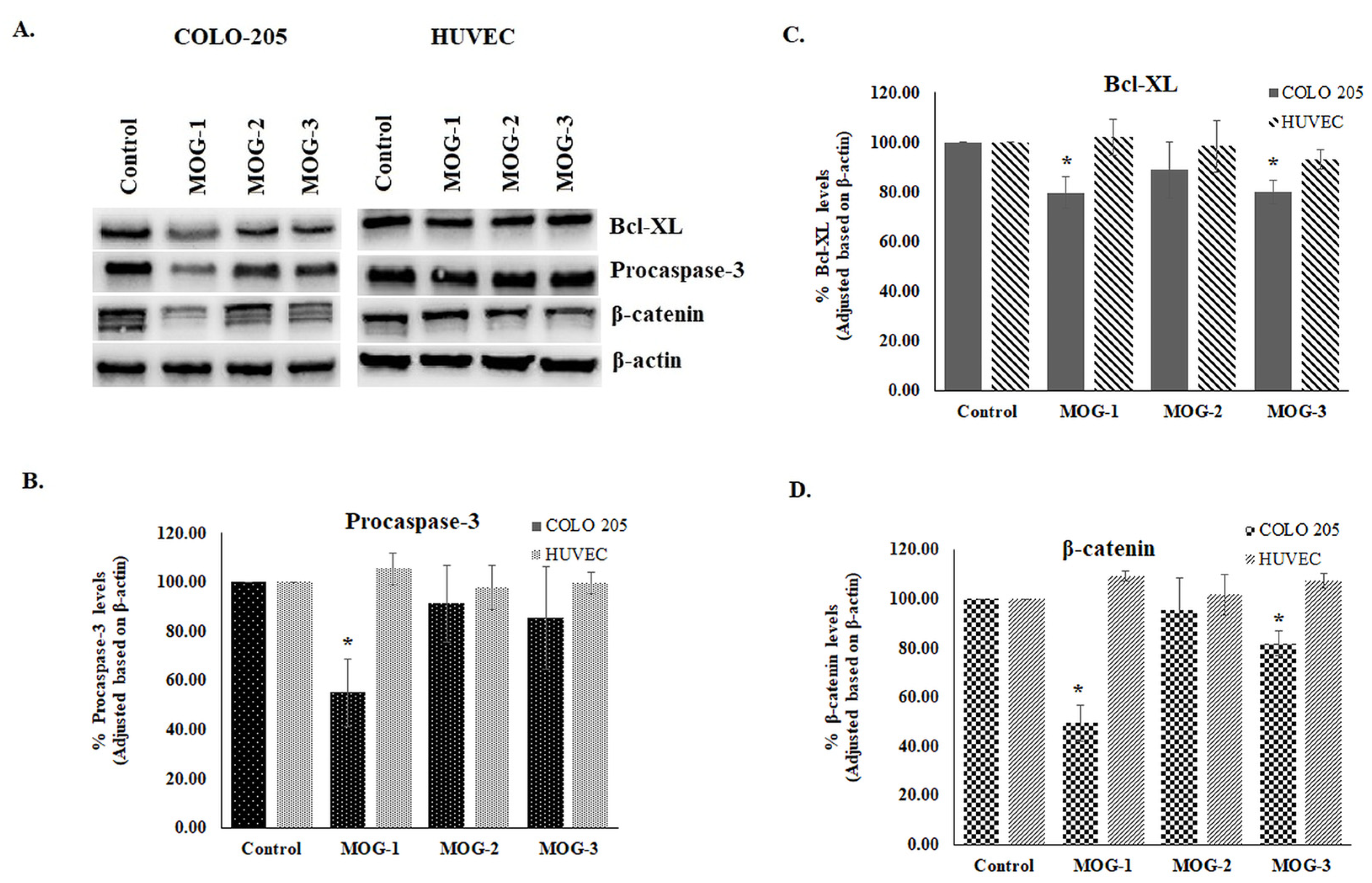
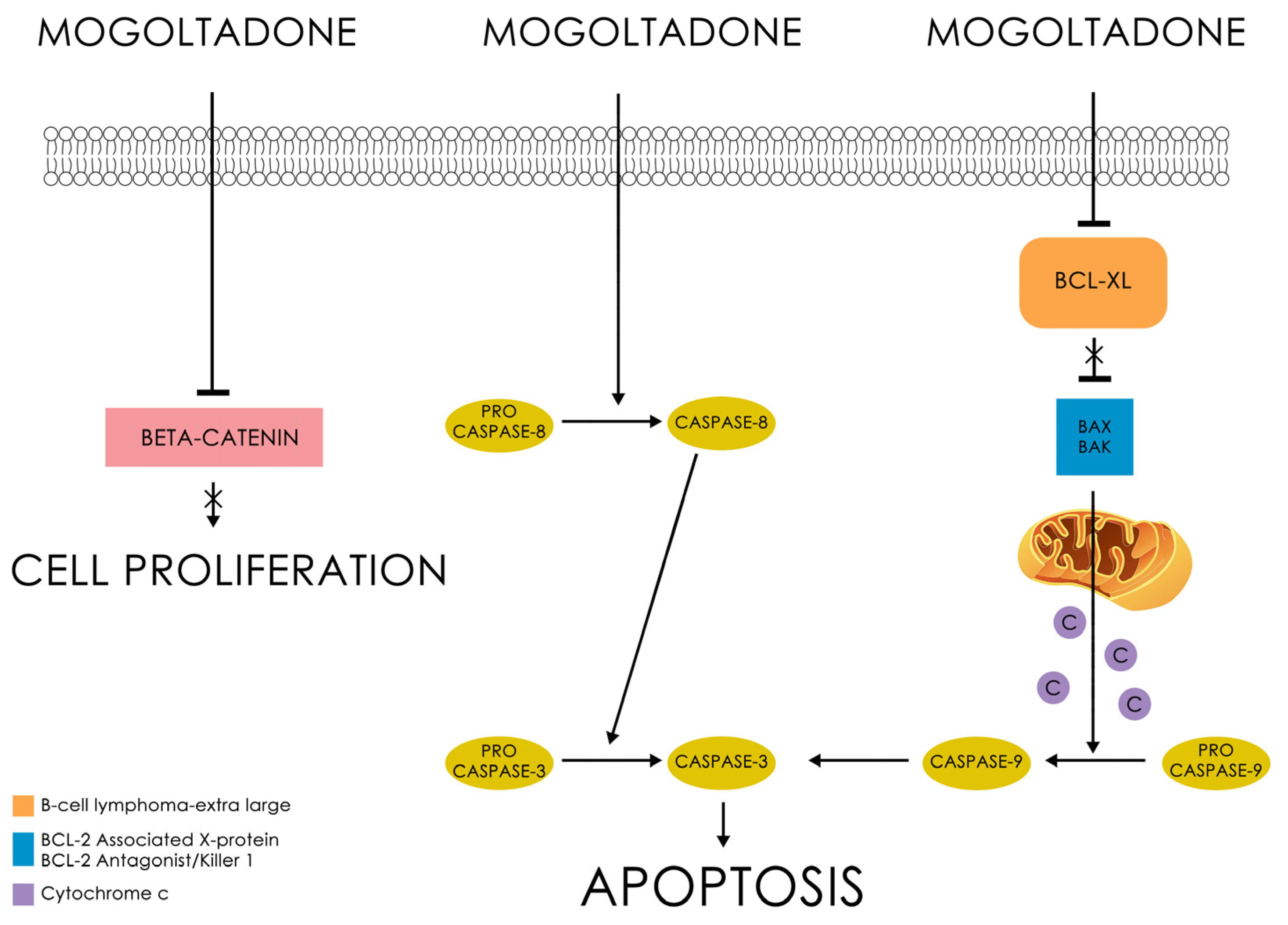
2.3. Cytotoxic Activities of the Sesquiterpene Coumarin Ethers Isolated from the Dichloromethane Extract of the Roots of Ferula huber-morathii
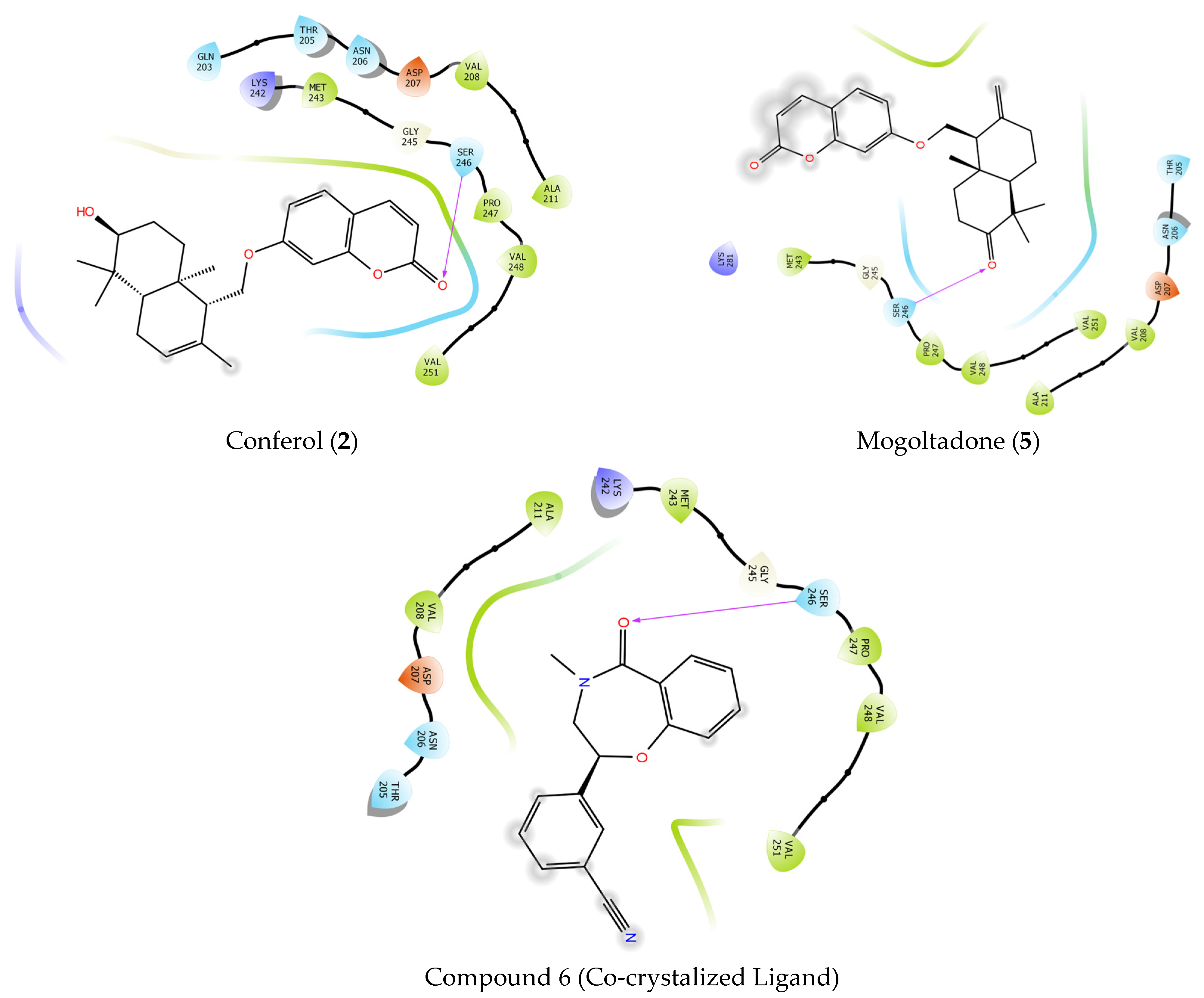
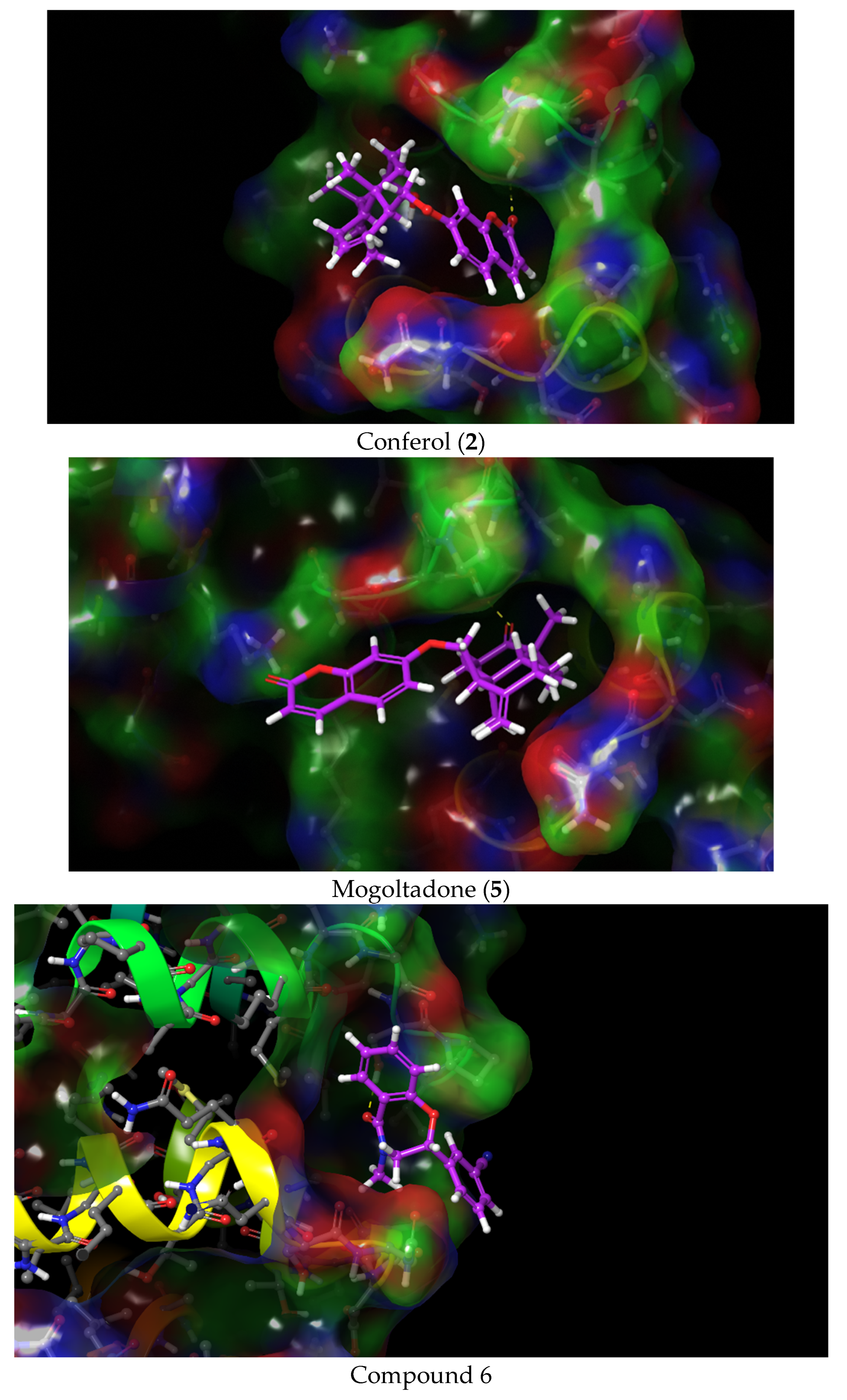
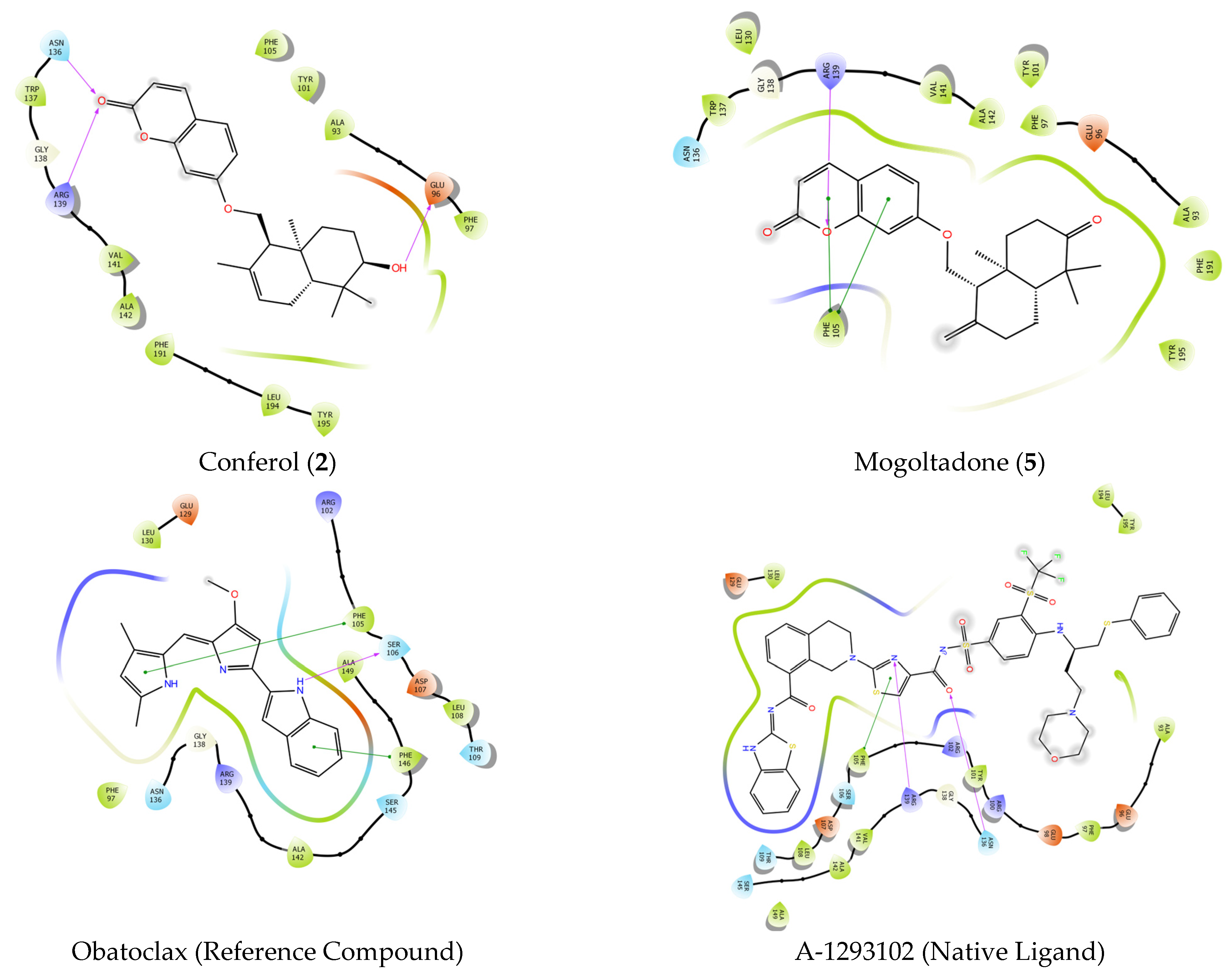
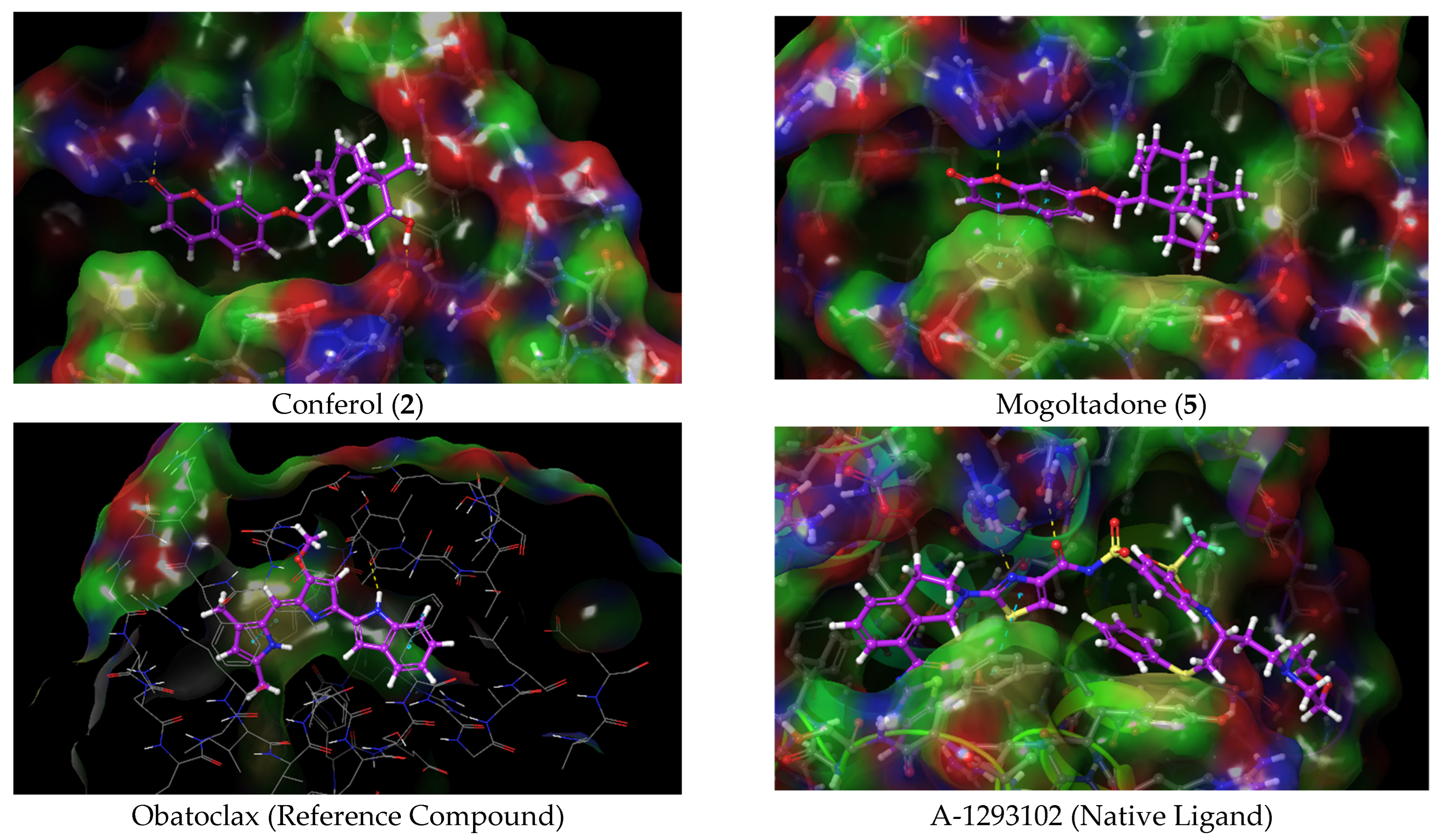
2.4. Molecular Docking Studies
2.4.1. Molecular Docking of Conferol (2), Mogoltadone (5), and Ligands with β-Catenin
2.4.2. Molecular Docking of Conferol (2), Mogoltadone (5), and Ligands with Bcl-XL
2.4.3. Calculated ADME Properties of Conferol (2) and Mogoltadone (5)
2.4.4. Evaluation of the Molecular Docking Study Results of Conferol (2) and Mogoltadone (5)
3. Materials and Methods
3.1. General Experimental Procedures
3.1.1. Chemical Reagents, Solvents and Chromatographic Adsorbents
3.1.2. Spectroscopic Analyses
3.1.3. Column and Thin Layer Chromatography (TLC)
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Chemical Transformations of Cytotoxic Sesquiterpene Coumarins
3.4.1. (R)-MTPA Ester of Samarcandin 24
3.4.2. X-ray Crystal Structure Analysis of the (R)-MTPA Ester of the Samarcandin 24
3.5. Cell Culture Conditions
3.6. Cytotoxic Activity
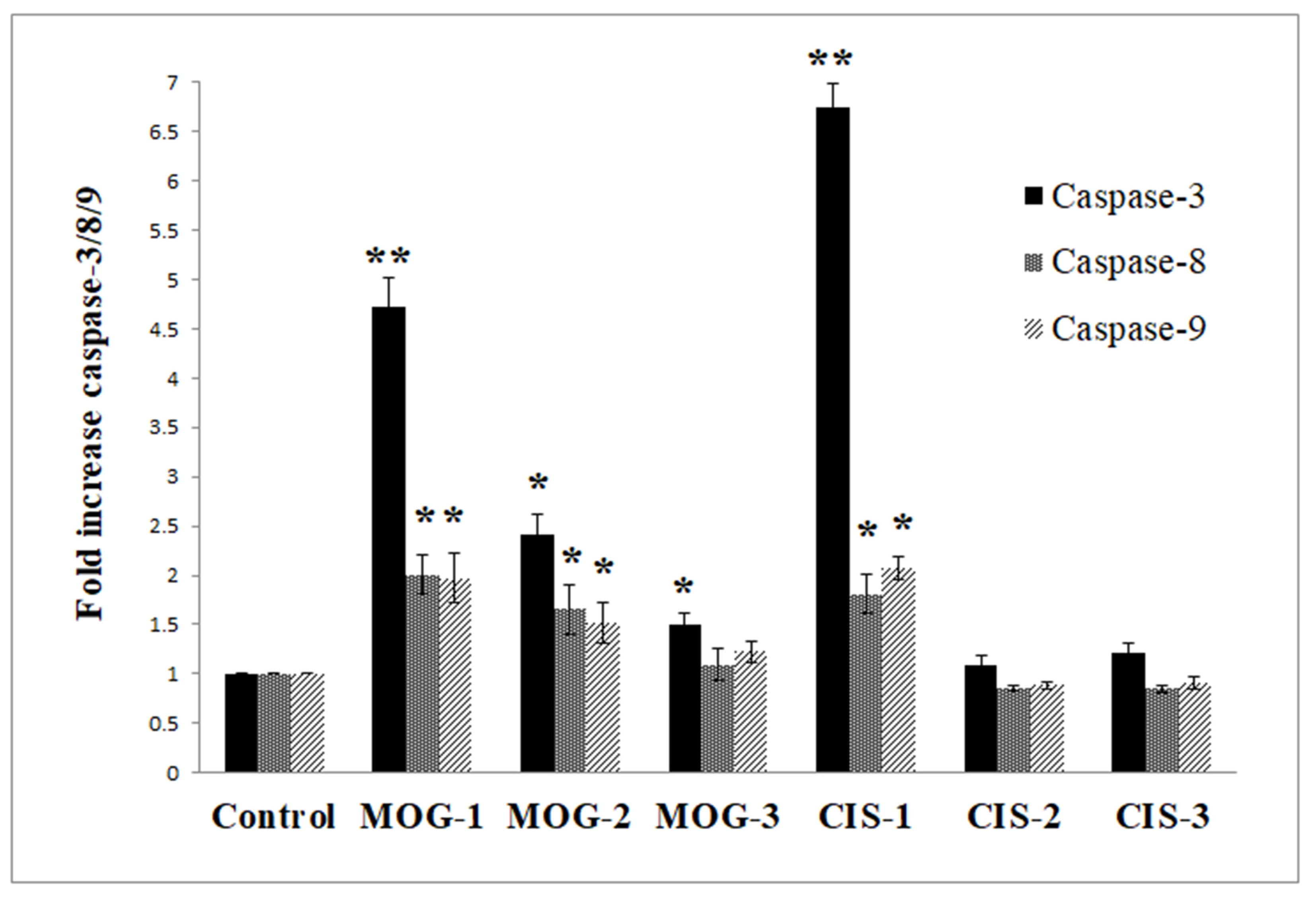
3.6.1. Caspase Activities
3.6.2. Western Blot Analysis
3.6.3. Statistical Analysis
3.7. Molecular Docking
3.8. In Silico ADME Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Gunther, R.T. The Greek Herbal of Dioscorides, 3rd ed.; Hafner Publishing Company: London, UK; New York, NY, USA, 1968; pp. 323, 328–332. [Google Scholar]
- Dioscorides, P. De Materia Medica: Being an Herbal with Many Other Medicinal Materials: Written in Greek in the First Century of the Common Era: A New Indexed Version in Modern English; Osbaldeston, T.A., Wood, R.P.A., Eds.; IBIDIS: Johannesburg, South Africa, 2000; pp. 72, 468, 479–480. [Google Scholar]
- Eisenman, S.W.; Zaurov, D.E.; Struwe, L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Springer: New York, NY, USA; Heidelberg/Berlin, Germany; Dordrecht, The Netherlands; London, UK, 2013; p. 10. [Google Scholar]
- Yapasert, R.; Sripanidkulchai, B.; Teerachaisakul, M.; Banchuen, K.; Banjerdpongchai, R. Anticancer Effects of a Traditional Thai Herbal Recipe Benja Amarit Extracts Against Human Hepatocellular Carcinoma and Colon Cancer Cell By Targeting Apoptosis Pathways. J. Ethnopharmacol. 2020, 254, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Jamous, R.M.; Salameh, N.M.; Jamous, R.M.; Hamadeh, A.M. Complementary and Alternative Medicine Use Among Cancer Patients in Palestine with Special Reference To Safety-Related Concerns. J. Ethnopharmacol. 2016, 187, 104–122. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I. Sesquiterpene Derivatives of the Genus Ferula. Chem. Nat. Compd. 1980, 4, 379–404. [Google Scholar]
- Miski, M.; Mabry, T.J. Daucane Esters from Ferula communis subsp. communis. Phytochemistry 1985, 24, 1735–1741. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Bermejo, J.; Diaz, J.G.; Arancibia, L.; de Paz, P.P. Humulenes and Other Constituents of Ferula latipinna. J. Nat. Prod. 1988, 51, 1140–1147. [Google Scholar] [CrossRef]
- Oughlissi-Dehak, K.; Lawton, P.; Michalet, S.; Bayet, C.; Darbour, N.; Hadj-Mahammed, M.; Badjah-Hadj-Ahmed, Y.A.; Dijoux-Franca, M.G.; Guilet, D. Sesquiterpenes from Aerial Parts of Ferula vesceritensis. Phytochemistry 2008, 69, 1933–1938. [Google Scholar] [CrossRef]
- Alkhatib, R.; Hennebelle, T.; Joha, S.; Idziorek, T.; Preudhomme, C.; Quesnel, B.; Sahpaz, S.; Bailleul, F. Activity of Elaeochytrin a from Ferula elaeochytris on Leukemia Cell Lines. Phytochemistry 2008, 69, 2979–2983. [Google Scholar] [CrossRef]
- Barthomeuf, C.; Demeule, M.; Grassi, J.; Saidkhodjaev, A.; Beliveau, R. Conferone from Ferula schtschurowskiana Enhances Vinblastine Cytotoxicity in MDCK-MDR1 Cells by Competitively Inhibiting P-glycoprotein Transport. Planta. Med. 2006, 72, 634–639. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated Multidrug Resistance in MCF-7/Adr Cancer Cells by Sesquiterpene Coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Miski, M.; Ulubelen, A.; Mabry, T.J. Six Sesquiterpene Alcohol Esters from Ferula elaeochytris. Phytochemistry 1983, 22, 2231–2233. [Google Scholar] [CrossRef]
- Miski, M.; Ulubelen, A.; Mabry, T.J.; Watson, W.H.; Vickovic, I.; Holub, M. A New Sesquiterpene Ester from Ferula tingitana. Tetrahedron 1984, 40, 5197–5201. [Google Scholar] [CrossRef]
- Miski, M.; Ulubelen, A. Sesquiterpene-coumarin Ethers of Ferula tingitana. J. Nat. Prod. 1985, 48, 326–327. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J. Fercolide, A Type of Sesquiterpene Lactone from Ferula communis subsp. communis and the Correct Structure of Vaginatin. Phytochemistry 1986, 25, 1673–1675. [Google Scholar]
- Miski, M.; Mabry, T.J. New Daucane Esters from Ferula tingitana. J. Nat. Prod. 1986, 49, 657–660. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J.; Saya, O. New Daucane and Germacrane Esters from Ferula orientalis var. orientalis. J. Nat. Prod. 1987, 50, 829–834. [Google Scholar] [CrossRef]
- Miski, M.; Mabry, T.J.; Saya, Ö. Apiene Esters from Ferula hausknechtii. Phytochemistry 1987, 26, 1733–1737. [Google Scholar] [CrossRef]
- Miski, M.; Jakupovic, J. Cyclic Farnesyl-Coumarin and Farnesyl-Chromone Derivatives from Ferula communis subsp. communis. Phytochemistry 1990, 29, 1995–1998. [Google Scholar] [CrossRef]
- Miski, M.; Jakupovic, J. Daucane Esters from Ferula rigidula. Phytochemistry 1990, 29, 173–178. [Google Scholar] [CrossRef]
- Akalin, E.; Tuncay, O.; Olcay, B.; Miski, M. A New Ferula (Apiaceae) Species from Southwest Anatolia: Ferula pisidica Akalın & Miski. Plants 2020, 9, 1–11. [Google Scholar]
- Peşmen, H. Ferula L. In Flora of Turkey and East Aegean Islands; Davis, P.H., Ed.; Edinburg University Press: Edinburgh, UK, 1972; pp. 440–453. [Google Scholar]
- Asghari, J.; Atabaki, V.; Baher, E.; Mazaheritehrani, M. Identification of Sesquiterpene Coumarins of Oleo-gum Resin of Ferula assa-foetida L. from the Yasuj Region. Nat. Prod. Res. 2016, 30, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Iranshahi, M.; Rezaee, R.; Sahebkar, A.; Bassarello, C.; Piacente, S.; Pizza, C. Sesquiterpene Coumarins from the Fruits of Ferula badrakema. Pharm. Biol. 2009, 47, 344–347. [Google Scholar] [CrossRef]
- Tosun, F.; Beutler, J.A.; Ransom, T.T.; Miski, M. Anatolicin, a Highly Potent And Selective Cytotoxic Sesquiterpene Coumarin from the Root Extract of Heptaptera anatolica. Molecules 2019, 24, 1153. [Google Scholar] [CrossRef] [PubMed]
- Valiahdi, S.M.; Iranshahi, M.; Sahebkar, A. Cytotoxic Activities of Phytochemicals from Ferula Species. Daru 2013, 21, 1–7. [Google Scholar] [CrossRef]
- Adhami, H.R.; Scherer, U.; Kaehlig, H.; Hettich, T.; Schlotterbeck, G.; Reich, E.; Krenn, L. Combination of Bioautography with HPTLC-MS/NMR: A Fast Identification of Acetylcholinesterase Inhibitors from Galbanum(†). Phytochem. Anal. 2013, 24, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Khasanov, T.K.; Saidkhodzhaeva, A.I.; Nikonov, G.K. Structure and Configuration of Polyanthin and Polyanthinin—New Coumarins from the Roots of Ferula polyantha. Chem. Nat. Compd. 1974, 10, 523–524. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz, J.F.; Yuste, A.; Rustaiyan, A. New Umbelliferone Sesquiterpene Ethers from Roots of Ligularia persica. Liebigs. Ann. Chem. 1991, 1991, 929–931. [Google Scholar] [CrossRef]
- Nabiev, A.A.; Khasanov, T.K.; Malikov, V.M. A Chemical Study of the Roots of Ferula kopetdagensis. Chem. Nat. Compd. 1979, 15, 14–16. [Google Scholar] [CrossRef]
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene Coumarins from Ferula sinkiangensis Act as Neuroinflammation Inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Steindl, T.M.; Schuster, D.; Kirchmair, J.; Anrain, K.; Ellmerer, E.P.; Langer, T.; Stuppner, H.; Wutzler, P.; Schmidtke, M. Structure-based Virtual Screening for the Discovery of Natural Inhibitors for Human Rhinovirus Coat Protein. J. Med. Chem. 2008, 51, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Kir’yalov, N.P.; Movchan, S.D. The Structure of Samarcandin and Samarcandone, Coumarin Compounds from Ferula samarcandica. Chem. Nat. Compd. 1968, 4, 63–65. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Atabaki, V.; Babaei, E.; Olfatkhah, S.R.; Dusek, M.; Eigner, V.; Soltani, A.; Khalaji, A.D. Isolation, Spectroscopic Characterization, X-ray, Theoretical Studies as well as In Vitro Cytotoxicity of Samarcandin. Bioorg. Chem. 2016, 66, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, F.; Baykan, S.; Soliman, G.A.; Yusufoglu, H.; Bedir, E. Evaluation of the Potential Aphrodisiac Activity of Sesquiterpenoids from Roots of Ferula huber-morathii Peşmen In Male Rats. J. Ethnopharmacol. 2020, 257, 1–8. [Google Scholar] [CrossRef]
- Baykan, S.; Aydogan, F.; Ozturk, M.B.; Debelec-Butuner, B.; Yengin, Ç.; Ozturk, B. Ferutinin Content and Cytotoxic Effects of Various Ferula L. Species On Prostate Cancer (PC-3) Cell Line. J. Res. Pharm. 2020, 24, 142–149. [Google Scholar] [CrossRef]
- Meng, H.; Guoyu Li, G.; Huang, J.; Zhang, K.; Wang, H.; Wang, J. Sesquiterpene Coumarin and Sesquiterpene Chromone Derivatives from Ferula ferulaeoides (Steud.) Korov. Fitoterapia 2013, 86, 70–77. [Google Scholar] [CrossRef]
- Pavlovic, I.; Petrovic, S.; Radenkovic, M.; Milenkovic, M.; Couladis, M.; Brankovic, S.; Drobac, M.P.; Niketic, M. Composition, Antimicrobial, Antiradical and Spasmolytic Activity of Ferula heuffelii Griseb. ex Heuffel (Apiaceae) Essential Oil. Food Chem. 2012, 130, 310–315. [Google Scholar] [CrossRef]
- Serkerov, S.V.; Rasulov, F.A.; Pimenov, M.G.; Belyi, M.G. 3,4-Methylenedioxy-5-methoxypropiophenone and Terpenoid Coumarins of Ferula caucasica. Chem. Nat. Comp. 1985, 21, 527–528. [Google Scholar] [CrossRef]
- Liu, T.; Osman, K.; Kaatz, W.G.; Gibbons, S.; Mu, Q. Antibacterial Sesquiterpenoid Derivatives from Ferula ferulaeoides. Planta Med. 2013, 79, 701–706. [Google Scholar] [CrossRef]
- Pavlovic, I.; Petrovic, S.; Milenkovic, M.; Stanojkovic, T.; Nikolic, D.; Krunic, A.; Niketic, M. Antimicrobial and Cytotoxic Activity of Extracts of Ferula heuffelii Griseb. Ex Heuff. and Its Metabolites. Chem. Biodivers. 2015, 12, 1585–1594. [Google Scholar] [CrossRef]
- Pavlovic, I.; Krunic, A.; Nikolic, D.; Radenkovic, M.; Brankovic, S.; Niketic, M.; Petrovic, S. Chloroform Extract of Underground Parts of Ferula heuffelii: Secondary Metabolites and Spasmolytic Activity. Chem. Biodivers. 2014, 11, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Neshati, V.; Matin, M.M.; Iranshahi, M.; Bahrami, A.R.; Behravan, J.; Mollazadeh, S.; Rassouli, F.B. Cytotoxicity of Vincristine on the 5637 Cell Line Is Enhanced By Combination With Conferone. Z. Naturforsch. C. J. Biosci. 2009, 64, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tong, X.; Zhang, J.; Huang, J.; Wang, J. DAW22, A Natural Sesquiterpene Coumarin Isolated from Ferula ferulaeoides (Steud.) Korov. That Induces C6 Glioma Cell Apoptosis and Endoplasmic Reticulum (ER) Stress. Fitoterapia 2015, 103, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, C.J.; Xie, H.Q.; Mu, Q. Guaiol-A Naturally Occurring Insecticidal Sesquiterpene. Nat. Prod. Commun. 2013, 8, 1353–1354. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.L.; Liu, T.; Xie, H.Q.; Xie, Y.H.; Mu, Q. Inhibition Effects on Hepatitis B Virus Replication by Hydrophobic Extracts from Ferula ferulaeoides (Steud.) Korov. J. Med. Plant Res. 2012, 6, 6581–6583. [Google Scholar]
- Nagatsu, A.; Isaka, K.; Kojima, K.; Ondognii, P.; Zevgeegiin, O.; Gombosurengyin, P.; Davgiin, K.; Irfan, B.; Iqubal, C.M.; Ogihara, Y. New Sesquiterpenes from Ferula ferulaeoides (Steud.) Korovin. VI. Isolation and Identification of Three New Dihydrofuro[2,3-b]Chromones. Chem. Pharm. Bull. 2002, 50, 675–677. [Google Scholar] [CrossRef]
- Kahraman, C.; Topcu, G.; Bedir, E.; Tatli, I.I.; Ekizoglu, M.; Akdemir, Z.S. Phytochemical Screening and Evaluation of the Antimicrobial and Antioxidant Activities of Ferula caspica M. Bieb. Extracts. Saudi Pharm. J. 2019, 27, 525–531. [Google Scholar] [CrossRef]
- Meng, H.; Guoyu Li, G.; Huang, J.; Zhang, K.; Wei, X.; Ma, Y.; Zhang, C.; Wang, J. Sesquiterpenoid Derivatives from Ferula ferulaeoides (Steud.) Korov. Phytochemistry 2013, 86, 151–158. [Google Scholar] [CrossRef]
- Eshbakova, K.A.; Saidkhodzhaev, A.I.; Vdovin, A.D.; Abdullaev, N.D. Terpenoid Coumarins from Ferula feruloides. Chem. Nat. Compd. 2009, 45, 708–709. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.-D.; Li, G.-Y.; Li, N.; Zuo, W.-J.; Zeng, Y.-M.; Meng, H.; Li, X.; Wang, J.-H. Two Novel Sesquiterpenoids from the Roots of Ferula ferulaeoides (Steud.) Korov. Helv. Chim. Acta. 2010, 93, 1019–1024. [Google Scholar] [CrossRef]
- NCI Samarcandin Yeast Assay Results. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5459231#section=Biological-Test-Results (accessed on 17 May 2023).
- Setlur, A.S.; Naik, S.Y.; Skariyachan, S. Herbal Lead as Ideal Bioactive Compounds Against Probable Drug Targets of Ebola Virus in Comparison with Known Chemical Analogue: A Computational Drug Discovery Perspective. Interdiscip. Sci. Comput. Life. Sci. 2017, 9, 254–277. [Google Scholar] [CrossRef] [PubMed]
- Dappiagi, F.; Pieraccini, S.; Potenza, D.; Vasile, F.; Podlipnik, C. Designing Antiviral Substances Targeting the Ebola Virus Viral Protein 24. In Emerging and Reemerging Viral Pathogens; Ennaji, M.M., Ed.; Academic Press: London, UK, 2020; pp. 147–177. [Google Scholar]
- Alqarni, M.H.; Soliman, G.A.; Salkini, M.A.A.; Alam, P.; Yusufoglu, H.S.; Baykan, S.; Ozturk, B.; Abdel-Kader, M.S. The Potential Aphrodisiac Effect of Ferula drudeana Korovin Extracts and Isolated Sesquiterpene Coumarins in Male Rats. Phcog. Mag. 2020, 16, 404–409. [Google Scholar]
- Bagirov, V.Y.; Kir’yalov, N.P.; Sheichenko, V.I. Structure of Samarcandin. Chem. Nat. Compd. 1970, 6, 475–476. [Google Scholar] [CrossRef]
- Saidkhodzhaev, A.I.; Malikov, V.M. The Stereochemistry of Terpenoid Coumarins. Chem. Nat. Compd. 1978, 14, 601–605. [Google Scholar] [CrossRef]
- Nasirov, S.M.; Saidhkhodzhaev, A.I.; Khasanov, T.K.; Yagudaev, M.R.; Malikov, V.M. Stereochemistry of Terpenoid Coumarins. Crystal and Molecular Structure of Samarcandin. Chem. Nat. Compd. 1985, 21, 171–177. [Google Scholar] [CrossRef]
- Li, N.; Guo, T.Y.; Zhou, D. Bioactive Sesquiterpene Coumarins From Plants. Stud. Nat. Prod. Chem. 2018, 59, 251–282. [Google Scholar]
- Malikov, V.M.; Saidkhodzhaev, A.I. Coumarins. Plants, Structure, Properties. Chem. Nat. Compd. 1998, 34, 345–409. [Google Scholar] [CrossRef]
- Gliszczynska, A.; Brodelius, P.E. Sesquiterpene Coumarins. Phytochem. Rev. 2012, 11, 77–96. [Google Scholar] [CrossRef]
- Abd El-Razek, M.H.; Ohta, S.; Hirata, T. Terpenoid Coumarins of the Genus Ferula. Heterocycles 2003, 60, 689–716. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Modulation of Multidrug Resistance Protein 2 Efflux in the Cisplatin Resistance Human Ovarian Carcinoma Cells A2780/RCIS By Sesquiterpene Coumarins. Phytother. Res. 2016, 30, 84–89. [Google Scholar] [CrossRef]
- Soltani, S.; Amin, G.; Salehi-Sourmaghi, M.H.; Iranshahi, M. Histone Deacetylase Inhibitory and Cytotoxic Activities of the Constituents from the Roots of Three Species of Ferula. Iran. J. Basic. Med. Sci. 2019, 22, 93–98. [Google Scholar] [PubMed]
- Cheraghi, O.; Dehghan, G.; Mahdavi, M.; Rahbarghazi, R.; Rezabakhsh, A.; Charoudeh, H.N.; Iranshahi, M.; Montazersaheb, S. Potent Anti-angiogenic and Cytotoxic Effect of Conferone on Human Colorectal Adenocarcinoma HT-29 Cells. Phytomedicine 2016, 23, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Khalili, L.; Dehghan, G.; Hosseinpour Feizi, M.A.; Sheibani, N.; Hamishekar, H. Development of an Albumin Decorated Lipid-polymer Hybrid Nanoparticle for Simultaneous Delivery of Methotrexate and Conferone to Cancer Cells. Int. J. Pharm. 2021, 599, 1–18. [Google Scholar] [CrossRef]
- Rahmani, A.; Rahimi, F.; Iranshahi, M.; Kahroba, H.; Zarebkohan, A.; Talebi, M.; Salehi, R.; Mousavi, H.Z. Co-delivery of Doxorubicin and Conferone by Novel pH-Responsive β-Cyclodextrin Grafted Micelles Triggers Apoptosis of Metastatic Human Breast Cancer Cells. Sci. Rep. 2021, 11, 1–21. [Google Scholar]
- Iranshahi, M.; Masullo, M.; Asili, A.; Hamedzadeh, A.; Jahanbin, B.; Festa, M.; Capasso, A.; Piacente, S. Sesquiterpene Coumarins from Ferula gumosa. J. Nat. Prod. 2010, 73, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Matin, M.M.; Iranshahi, M.; Bahrami, A.R.; Neshati, V.; Behnam-Rassouli, F. The Enhancement of Vincristine Cytotoxicity by Combination with Feselol. J. Asian Nat. Prod. Res. 2010, 12, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, S.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Behnam-Rassouli, M.; Rassouli, F.B.; Neshati, V. Feselol Enhances the Cytotoxicity and DNA Damage Induced by Cisplatin in 5637 Cells. Z. Naturforsch. C. J. Biosci. 2011, 66, 555–561. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Cao, L.; Zhang, L.; Shen, L.; Zhu, J.; Wang, J.; Si, J. Sesquiterpene Coumarins from Seeds of Ferula sinkiangensis. Fitoterapia 2015, 103, 222–226. [Google Scholar] [CrossRef]
- Rassouli, F.B.; Matin, M.M.; Iranshahi, M.; Bahrami, A.R.; Behravan, J.; Mollazadeh, S.; Neshati, V.; Kalalinia, F. Investigating the Enhancement of Cisplatin Cytotoxicity on 5637 Cells by Combination with Mogoltacin. Toxicol. In Vitro 2011, 25, 469–474. [Google Scholar] [CrossRef]
- Thakur, R.; Mishra, D.P. Pharmacological Modulation of Beta-Catenin and Its Applications in Cancer Therapy. J. Cell Mol. Med. 2013, 17, 449–456. [Google Scholar] [CrossRef]
- Schrödinger Program Suite. Available online: https://www.schrodinger.com/platform/drug-discovery (accessed on 22 May 2023).
- Joudeh, J.; Claxton, D. Obatoclax Mesylate: Pharmacology and Potential for Therapy of Hematological Neoplasms. Expert Opin. Investig. Drugs 2012, 21, 363–373. [Google Scholar] [CrossRef]
- Cournoyer, S.; Addioui, A.; Belounis, A.; Beaunoyer, M.; Nyalendo, C.; Le Gall, R.; Teira, P.; Haddad, E.; Vassal, G.; Sartelet, H. GX15-070 (Obatoclax), A Bcl-2 Family Proteins Inhibitor Engenders Apoptosis and Pro-survival Autophagy and Increases Chemosensitivity in Neuroblastoma. BMC Cancer 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Or, C.R.; Huang, C.W.; Chang, C.C.; Lai, Y.C.; Chen, Y.J.; Chang, C.C.; Obatoclax, A. Pan-BCL-2 Inhibitor, Downregulates Survivin to Induce Apoptosis in Human Colorectal Carcinoma Cells via Suppressing WNT/β-catenin Signaling. Int. J. Mol. Sci. 2020, 21, 1773. [Google Scholar] [CrossRef]
- Tao, Z.-F.; Wang, X.; Chen, J.; Ingram, J.P.; Jin, S.; Judge, R.A.; Kovar, P.J.; Park, C.; Sun, C.; Wakefield, B.D.; et al. Structure-based Design of A-1293102, a Potent and Selective BCL-XL Inhibitor. ACS Med. Chem. Lett. 2021, 12, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.; Mayer, M.; Zahn, S.K.; Zeeb, M.; Wöhrle, S.; Bergner, A.; Bruchhaus, J.; Ciftci, T.; Dahmann, G.; Dettling, M.; et al. Getting a Grip on the Undrugged: Targeting β-Catenin with Fragment-Based Methods. Chem. Med. Chem. 2021, 16, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Yazici Bektas, N.; Altiparmak Ulbegi, G.; Aksoy Sagirli, P.; Miski, M. Novel Cytotoxic Sesquiterpene Ester Derivatives from the Roots of Ferula mervynii. Chem. Biodivers. 2023, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.C.; Garg, C.P.; Kwag-Ting, L. The Oxidation of Secondary Alcohols In Diethyl Ether With Aqueous Chromic Acid. A Convenient Procedure for the Preparation of Ketones in High Epimeric Purity. J. Org. Chem. 1971, 36, 387–390. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS97. Program for the Solution of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL97. Program for the Refinement of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON. An Integrated Tool for the Analysis of A Single Crystal Structure Determination. Acta. Cryst. 1990, A46, C34. [Google Scholar]
- Buttke, T.M.; McCubrey, J.A.; Owen, T.C. Use of an Aqueous Soluble Tetrazolium/formazan Assay to Measure Viability and Proliferation of Lymphokine-dependent Cell Lines. J. Immunol. Methods 1993, 157, 233–240. [Google Scholar] [CrossRef]
- Li, J.; Abel, R.; Zhu, K.; Cao, Y.; Zhao, S.; Friesner, R.A. The VSGB 2.0 Model: A Next Generation Energy Model for High Resolution Protein Structure Modeling. Proteins 2011, 79, 2794–2812. [Google Scholar] [CrossRef]
- Ban’kovskii, A.I.; Ermatov, N.E.; Perel’son, M.E.; Bubeva-Ivanova, L.; Pavlova, N.S. Structure of the coumarins colladin and colladonin. II. Chem. Nat. Compd. 1970, 6, 170–176. [Google Scholar] [CrossRef]
| Extracts | IC50 (µg/mL) | |||
|---|---|---|---|---|
| COLO 205 | K-562 | MCF-7 | HUVEC | |
| Dichloromethane Extract | 29.52 ± 1.32 | 24.22 ± 1.33 | 63.09 ± 3.47 | 53.56 ± 0.74 |
| Methanol Extract | >100 | >100 | >100 | >100 |
| Cisplatin * | 12.21 ± 0.34 | 9.73 ± 0.30 | 35.52 ± 0.76 | 19.27 ± 1.57 |
| Position | Conferone (1) | Conferol (2) | Feselol (3) | Badrakemone (4) | ||||
|---|---|---|---|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 2 | - | 161.1 | - | 161.3 | - | 161.5 | - | 161.5 |
| 3 | 6.25; d; 9.5; 1H | 113.1 | 6.23; d; 9.5; 1H | 113.1 | 6.25; d; 9.4; 1H | 113.2 | 6.25; d; 9,5; 1H | 113.2 |
| 4 | 7.64; d; 9.5; 1H | 143.4 | 7.63; d; 9.5; 1H | 143.5 | 7.63; d; 9.4; 1H | 143.5 | 7.62; d; 9.5; 1H | 143.6 |
| 5 | 7.37; d; 8.6; 1H | 128.7 | 7.35; d; 8.5; 1H | 128.9 | 7.36; d; 8.5; 1H | 128.9 | 7.36; d; 8.7; 1H | 129 |
| 6 | 6.83; dd; 2.4; 8.6; 1H | 113 | 6.83; dd; 2.4, 8.5; 1H | 113.3 | 6.82; dd; 2.3; 8.5; 1H | 113.3 | 6.82; dd; 2.4; 8.7; 1H | 113.3 |
| 7 | - | 161.7 | - | 162.3 | - | 162.2 | - | 162.2 |
| 8 | 6.8; d; 2.4; 1H | 101.2 | 6.81; d; 2.5; 1H | 101.5 | 6.8; d; 2.3; 1H | 101.4 | 6.81; d; 2.4; 1H | 101.4 |
| 9 | - | 156 | - | 156.1 | - | 156.2 | - | 156.1 |
| 10 | - | 112.7 | - | 112.6 | - | 112.6 | - | 112.7 |
| 1′α | 2.28; m; 1H * | 38.5 | 1.68; m; 2H * | 31.9 | 2.01; dt; 3.6, 9.8; 1H | 37.9 | 2.09; ddd; 3.5; 6.3; 13.3; 1H | 37.8 |
| 1′β | 1.62; td; 4.2; 13.3; 1H | 1.33; td; 4.3;13.2; 1H | 1.81; td; 5.4; 13.3; 1H | |||||
| 2′ α | 2.75; td; 5.4; 14.6; 1H | 34.4 | 1.96; m; 1H ** | 25.3 | 1.65; m; 2H | 27.4 | 2.68; tdd; 6.2; 13.2; 15.3; 1H | 34.7 |
| 2′β | 2.28; m; 1H * | 1.65; m; 1H * | 2.41; ddd; 3.4; 5.3; 15.3; 1H | |||||
| 3′ | - | 216.1 | 3.47; bt; 2.4; 1H | 75.9 | 3.28; dd; 4.3; 11.4; 1H | 79.2 | - | 216.2 |
| 4′ | - | 47.6 | - | 35.7 | - | 38.8 | - | 48.1 |
| 5′ | 1.68; dd; 4.4; 12.2; 1H | 51.1 | 1.70; m; 1H * | 43.5 | 1.28; dd; 5.2; 11.7; 1H | 49.5 | 1.66; dd; 2.7; 12.5; 1H | 55.2 |
| 6′α | 2.13; dddd; 2.6; 4.8; 9.5; 19.4; 1H | 23.9 | 1.96; m; 2H ** | 23.3 | 2.05; m; 2H * | 23.4 | 1.57; dt; 6.8; 12.5; 1H | 24.6 |
| 6′β | 1.99; dt; 5.6; 17.3; 2H | 1.68; dp; 2.5; 12.4; 1H | ||||||
| 7′α | 5.6; dq; 1.9; 5.8; 1H | 123.9 | 5.54; bs; 1H | 124 | 5.56; brs; 1H | 123.9 | 2.15; td; 3.4; 13.3; 1H | 37.2 |
| 7′β | - | - | - | 2.5; ddd; 2.5; 4.2; 13.3; 1H | ||||
| 8′ | - | 132.5 | - | 132.6 | - | 132.4 | - | 145.8 |
| 9′ | 2.28; m; 1H * | 53.1 | 2.32; bs; 1H | 53.7 | 2.23; brs; 1H | 53.9 | 2.3; t; 5.9; 1H | 54.2 |
| 10′ | - | 35.9 | - | 37.4 | - | 35.9 | - | 38.8 |
| 11′a | 4.07; dd; 5.3; 9.8; 1H | 66.7 | 4.02; dd; 5.8, 9.4; 1H | 67.2 | 4.01; dd; 5.9; 9.7; 1H | 67.2 | 4.22; m; 2H | 65.8 |
| 11′b | 4.17; dd; 3.4; 9.8; 1H | 4.17; dd; 3.3, 9.4; 1H | 4.17; dd; 3.4; 9.7; 1H | |||||
| 12′a | 1.7; brt; 3H | 21.5 | 1.69; bs; 3H * | 21.9 | 1.68; d; 3.8; 3H | 21.8 | 4.59; brs; 1H | 108.9 |
| 12′b | 4.98; brs; 1H | |||||||
| 13′ | 1.08, s; 3H | 25.2 | 0.93; s; 3H | 22.5 | 1.02; s; 3H | 28.2 | 1.13; s; 3H | 26 |
| 14′ | 1.12; s; 3H | 22.3 | 0.96; s; 3H | 28.2 | 0.9; s; 3H | 15.4 | 1.06; s; 3H | 22.1 |
| 15′ | 1.13; s; 3H | 14.5 | 0.91; s; 3H | 14.9 | 0.88; s; 3H | 15 | 1.04; s; 3H | 15 |
| Position | Mogoltadone (5) | Farnesiferol A (6) | Farnesiferol A acetate (7) | Gummosin (8) | ||||
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 2 | - | 161.2 | - | 161.9 | - | 161.3 | - | 161.5 |
| 3 | 6.24; d; 9.5; 1H | 113 | 6.25; d; 9.5; 1H | 113.1 | 6.25; d; 9.4 | 113.1 | 6.22; d; 9.5; 1H | 112.9 |
| 4 | 7.62; d; 9.5; 1H | 143.4 | 7.63; d; 9.5; 1H | 143.5 | 7.63; d; 9.4; 1H | 143.6 | 7.6; d; 9.5; 1H | 143.6 |
| 5 | 7.35; d; 8.5; 1H | 128.7 | 7.35; d; 8.6; 1H | 128.7 | 7.36; d; 8.3; 1H | 128.9 | 7.32; d; 9.3; 1H | 128.7 |
| 6 | 6.78; dd; 2.5; 8.5; 1H | 113 | 6.81; dd; 2.4; 8.6; 1H | 113.4 | 6.8; dd; 2.1; 8.3; 1H | 113.2 | 6;81; dd; 2.4; 9.3; 1H | 113.3 |
| 7 | - | 161.7 | - | 161.9 | - | 161.9 | - | 162.3 |
| 8 | 6.77; d; 2.5; 1H | 101.4 | 6.8; d; 2.4; 1H | 101.7 | 6.79; d; 2.1; 1H | 101.9 | 6.75; d; 2.4; 1H | 101.8 |
| 9 | - | 155.7 | - | 155.9 | - | 155.9 | - | 155.9 |
| 10 | - | 112.6 | - | 112.6 | - | 112.6 | - | 112.5 |
| 1′α | 1.91; td; 4.5; 13.6; 1H | 35.5 | 1.37; dt; 3.2; 13.0; 1H | 35 | 1.73; 2H ** | 24.1 | 1.99; tdd; 2.2; 3.3; 13.9; 1H | 25.7 |
| 1′β | 1.69; ddd; 2.8; 5.9; 13.6; 1H | 1.63; td; 5.6; 12.0; 1H | 1.62; dq; 3.1; 13.9; 1H | |||||
| 2′ α | 2.76; td; 5.7; 14.7; 1H | 35.1 | 1.7; qd; 2.5; 12.5; 2H | 27.7 | 1.39; 1H * | 34.5 | 1.03; dt; 3.1; 12.5; 1H | 29.3 |
| 2′β | 2.36; m; 1H * | 1.72; 1H ** | 2.05; dd; 2.1; 13.2; 1H | |||||
| 3′ | - | 216.2 | 3.25; dd; 5.2; 10.2; 1H | 79.2 | 4.5; dd; 6.3; 8.5; 1H | 80.9 | 3.45; dd; 2.1; 3.5; 1H | 76.2 |
| 4′ | - | 47.8 | - | 39.2 | - | 38.1 | - | 37.7 |
| 5′ | 1.54; qd; 4.4; 13.03; 1H | 47.6 | 1.31; dd; 2.8; 12.6; 1H | 46.6 | 1.41; m; 1H * | 46.7 | 1.77; dd; 3.1; 12.9; 1H | 40.8 |
| 6′ α | 1.78; dd; 3.15; 12.6; 1H | 23.8 | 1.73; dq; 2.8; 10.5; 1H | 23.1 | 1.7; m; 2H ** | 23 | 1.60; dq; 2.8; 13.8; 1H | 23.1 |
| 6′ β | 1.64; m; 1H * | 1.43; qd; 4.4; 12.9; 1H | 1.37; qd; 4.2; 13.0; 1H | |||||
| 7′α | 2.1; td; 4.8; 13.6; 1H | 32 | 2.04; td; 5.1; 13.5; 1H | 32.5 | 2.05; m; 1H *** | 32.4 | 2.09; tdt; 2.3; 6.6; 14.1; 1H | 32.7 |
| 7′β | 2.39; dt; 3.27; 13.6; 1H | 2.33; dt; 3.1; 12.4; 1H | 2.35; dd; 3.9; 14.1; 1H | 2.31; ddt; 1.8; 4.0; 14.1; 1H | ||||
| 8′ | - | 146 | - | 146.8 | - | 146.6 | - | 147.1 |
| 9′ | 2.35; m; 1H * | 56 | 2.21; t; 6.1; 1H | 56.7 | 2.21; t; 6.0; 1H | 56.7 | 2.18; brt; 6.2; 1H | 57.1 |
| 10′ | - | 37.3 | - | 37.8 | - | 37.7 | - | 37.7 |
| 11′a | 4.03; dd; 6.1; 9.9; 1H | 67.9 | 4.02; dd; 6.3; 9.8; 1H | 68.2 | 4.02; dd; 6.0; 9.9; 1H | 68.3 | 4.07; dd; 6.9; 9.9; 1H | 68 |
| 11′b | 4.25; dd; 6.1; 9.9; 1H | 4.29; dd; 5.8; 9.8; 1H | 4.29; dd; 6.0; 9.9; 1H | 4.39; dd; 5.4; 9.9; 1H | ||||
| 12′a | 4.80; t; 1.9; 1H | 112.1 | 4.72; t; 1.9; 1H | 111.5 | 4.73; brs; 1H | 111.5 | 4.69; t; 2.0; 1H | 111.2 |
| 12′b | 4.89; t; 2.2; 1H | 4.83; t; 2.1; 1H | 4.82; t; 2.2; 1H | 4.79; t; 2.2; 1H | ||||
| 13′ | 1.13; s; 3H | 25.8 | 1.04; s; 3H | 28.6 | 0.92; s; 3H | 16.9 | 0.84; s; 3H | 22.5 |
| 14′ | 1.05; s; 3H | 22.3 | 0.81; s; 3H | 15.4 | 0.89; s; 3H | 28.4 | 0.98; s; 3H | 28.5 |
| 15′ | 1.2; s; 3H | 21.1 | 0.99; s; 3H | 22.2 | 1.01; s; 3H | 22.2 | 0.98; s; 3H | 22.1 |
| CH3- (OAc) | - | - | - | - | 2.06; s; 3H | 21.5 | - | - |
| C=O (OAc) | - | - | - | - | - | 171 | - | |
| Position | Ferukrin (9) | Ferukrin acetate (10) | Deacetylkellerin (11) | Kellerin (12) | ||||
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 2 | - | 161.3 | - | 161.4 | - | 161.4 | - | 161.3 |
| 3 | 6.2; d; 9.5; 1H | 113.1 | 6.26; d; 9.5; 1H | 113.3 | 6.23; d; 9.5; 1H | 113.2 | 6.25; d; 9.4; 1H | 113.6 |
| 4 | 7.6; d; 9.5; 1H | 143.6 | 7.64; d; 9.5; 1H | 143.6 | 7.62; d; 9.5; 1H | 143.5 | 7.63; d; 9.4; 1H | 143.5 |
| 5 | 7.35; d; 8.6; 1H | 129.2 | 7.37; d; 8.5; 1H | 128.7 | 7.34; d; 8.4; 1H | 129.1 | 7.37; d; 8.6; 1H | 128.9 |
| 6 | 6.78; dd; 2.4; 8.6; 1H | 112.7 | 6.79; dd; 2.2; 8.5; 1H | 112.5 | 6.82; dd; 2.5; 8.4; 1H | 113.1 | 6.86; dd; 2.4; 8.6; 1H | 113.1 |
| 7 | - | 161.8 | - | 161.7 | - | 162.1 | - | 162.1 |
| 8 | 6.75; d; 2.4; 1H | 101.3 | 6.78; d; 2.2; 1H | 101.4 | 6.81; d; 2.5; 1H | 101.7 | 6.82; d; 2.4; 1H | 101.3 |
| 9 | - | 155.8 | - | 155.9 | - | 156 | - | 156.2 |
| 10 | - | 112.7 | - | 112.7 | - | 112.8 | - | 112.9 |
| 1′α | 1.46; td; 3.1; 12.7; 1H | 35.5 | 1.38; dt; 3.8; 12.2; 1H | 35.4 | 1.04; dt; 3.0; 12.7; 1H | 29.9 | 1.09; dt; 3.9; 12.9; 1H | 31 |
| 1′β | 1.32; dt; 3.1; 12.7; 1H | 1.59; td; 3.6; 13.2; 1H | 1.93; td; 3.7; 13.3; 1H | 1.76; d; 3.2; 1H | ||||
| 2′α | 1.69; m; 1H * | 27.2 | 1.75; m; 1H * | 23.5 | 1.55; m; 1H * | 25.4 | 2; tt; 3.1; 14.2; 1H | 23 |
| 2′β | 1.59; dq; 5.5; 13.3; 1H | 1.68; dd; 4.3; 8.7; 1H | 2.04; tt; 3.1; 13.7; 1H | 1.6; dq; 3.4; 14.9; 1H | ||||
| 3′ | 3.12; dd; 4.4, 11.5; 1H | 78.9 | 4.4; dd; 4.65; 11.6; 1H | 80.8 | 3.4; t; 2.9; 1H | 76.3 | 4.63; t; 2.8; 1H | 78.6 |
| 4′ | - | 37.5 | - | 37.8 | - | 37.8 | - | 37.1 |
| 5′ | 1.38; dd; 2.1; 8.8; 1H | 48.5 | 1.51; dd; 1.8; 7.3; 1H | 48.6 | 1.8; m; 1H *** | 42.5 | 1.93; dd; 2.5; 12.3; 1H | 43.8 |
| 6′α | 1.53; dt; 3.4; 10.5; 1H | 18.3 | 1.73; m; 1H * | 18.2 | 1.44; dd; 5.9; 1.7; 1H | 18.3 | 1.69; qd; 5.1; 12.3; 1H | 18.1 |
| 6′ β | 1.68; m; 1H * | 1.56; dd; 2.5; 6.4; 1H | 1.68; m; 1H ** | 1.49; dq; 2.9; 12.9; 1H | ||||
| 7′ α | 1.68; m; 1H * | 39.5 | 1.73; m; 1H * | 39.7 | 1.73; td; 2.4; 7.6; 1H | 39.5 | 1.77; d; 3.1; 2H | 40 |
| 7′ β | 1.71; m; 1H * | 1.73; m; 1H * | 1.68; m; 1H ** | |||||
| 8′ | - | 73.3 | - | 73.2 | - | 73.8 | - | 73.8 |
| 9′ | 1.5; t; 2.4; 1H | 57.8 | 1.53; t; 3.2; 1H | 57.4 | 1.53; m; 1H * | 58.4 | 1.53; t; 3; 1H | 58.1 |
| 10′ | - | 37.9 | - | 37.6 | - | 38 | - | 37.9 |
| 11′a | 4.05; dd; 3.2; 10.3; 1H | 67.7 | 4.07; dd; 3.3; 10.4; 1H | 67.5 | 4.06; dd; 3.1; 10.3; 1H | 68.1 | 4.17; t; 2.8; 2H | 67.8 |
| 11′b | 4.08; dd; 2.4; 10.3; 1H | 4.1; dd; 2.7; 10.4; 1H | 4.2; dd; 3.1; 10.3; 1H | |||||
| 12′a | 1.26; s; 3H | 31.6 | 1.29; s; 3H | 31.7 | 1.29; s; 3H | 31.7 | 1.32; s; 3H | 31.9 |
| 12’b | ||||||||
| 13′ | 0.78 s 3H | 15.6 | 0.9; s; 3H | 16.7 | 0.87; s; 3H | 22.1 | 0.89; s; 3H | 28.4 |
| 14′ | 0.99; s; 3H | 28.7 | 0.91; s; 3H | 28.5 | 0.98; s; 3H | 28.6 | 0.93; s; 3H | 21.8 |
| 15′ | 1.29; s; 3H | 24.3 | 1.36; s; 3H | 24.2 | 1.34; s; 3H | 24.3 | 1.36; s; 3H | 24.4 |
| CH3- (OAc) | - | - | 2.02; s; 3H | 21.3 | - | - | 1.78; s; 3H | 21.2 |
| C=O (OAc) | - | - | - | 171 | - | - | - | 170.6 |
| Position | Samarcandone (13) | Samarcandin (14) | Samarcandin acetate (15) | |||||
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |||
| 2 | - | 161.3 | - | 161.5 | - | 161.3 | ||
| 3 | 6.26; d; 9.5; 1H | 113.5 | 6.22; d; 9.6; 1H | 113.2 | 6.25; d; 9.3; 1H | 113.3 | ||
| 4 | 7.64; d; 9.5; 1H | 143.5 | 7.61; d; 9.6; 1H | 143.6 | 7.63; d; 9.3; 1H | 143.5 | ||
| 5 | 7.37; d; 8.6; 1H | 128.9 | 7.33; d; 8.5; 1H | 128.8 | 7.36; d; 8.5; 1H | 128.9 | ||
| 6 | 6.85; dd; 2.4; 8.6; 1H | 113.3 | 6.83; dd; 2.5; 8.5; 1H | 113.3 | 6.86; dd; 2.3; 8.5; 1H | 113.4 | ||
| 7 | - | 161.6 | - | 161.9 | - | 161.9 | ||
| 8 | 6.91; d; 2.4; 1H | 101.7 | 6.88; d; 2.5; 1H | 101.7 | 6.90; d; 2.3; 1H | 101.8 | ||
| 9 | - | 156 | - | 156 | - | 156 | ||
| 10 | - | 113.1 | - | 112.7 | - | 112.8 | ||
| 1′ α | 2.03; ddd; 3.9; 7.3; 13.4; 1H | 38.7 | 1.44; dt; 13.4; 3.4; 1H | 32.9 | 1.51; m; 2H * | 33.6 | ||
| 1′ β | 1.73; ddd; 7.0; 10.9; 13.4; 1H | 1.66; dt; 3.4; 13.4; 1H | ||||||
| 2′ α | 2.56; ddd; 7.3; 10.9; 15.9; 1H | 33.9 | 1.57; m; 1H ** | 25.2 | 1.66; m; 1H *** | 22.7 | ||
| 2′ β | 2.45; dt; 3.9; 7.0; 16.1; 1H | 1.92; m; 1H * | 1.88; m; 1H ** | |||||
| 3′ | - | 216.5 | 3.42; bt; 2.6; 1H | 75.7 | 4.66; bt; 2.6; 1H | 77.8 | ||
| 4′ | - | 47.4 | - | 37.5 | - | 36.7 | ||
| 5′ | 1.58; m; 1H * | 54.8 | 1.51; bd; 12.6; 1H ** | 48.5 | 1.50; m; 1H * | 49.7 | ||
| 6′ α | 1.67; dq; 3.1; 13.4; 1H | 21.4 | 1.34; bdq; 3.6, 12.6;1H | 20.1 | 1.35; bdq; 3.5; 12.7; 1H | 19.9 | ||
| 6′ β | 1.48; td; 2.9; 12.4; 1H | 1.58; m; 1H ** | 1.63; m; 1H *** | |||||
| 7′ α | 1.98; dt; 3.1; 12.4; 1H | 43.4 | 1.92; m; 1H * | 44.2 | 1.94; m; 1H ** | 44.1 | ||
| 7′ β | 1.58; m; 1H * | 1.55; m; 1H ** | 1.58; m; 1H *** | |||||
| 8′ | - | 72.5 | - | 72.7 | - | 72.8 | ||
| 9′ | 1.86; t; 5.3; 1H | 58.5 | 1.85; bt; 5.2; 1H | 59.4 | 1.87; bt; 5.1; 1H ** | 59.4 | ||
| 10′ | - | 37.6 | - | 38 | - | 37.8 | ||
| 11′a | 4.21; dd; 5.6; 10.0; 1H | 66.6 | 4.17; dd; 5.4; 9.7; 1H | 66.7 | 4.19; dd; 5.3; 9.7; 1H | 66.9 | ||
| 11′b | 4.42; dd; 5.1; 10.0; 1H | 4.36; dd; 4.6; 9.7; 1H | 4.36; dd; 5.3; 9.7; 1H | |||||
| 12′a | 1.29; s; 3H | 24.7 | 1.22; s; 3H | 24.7 | 1.25; s; 3H | 24.9 | ||
| 12′b | ||||||||
| 13′ | 1.13; s; 3H | 26.8 | 0.96; s; 3H | 28.5 | 0.88; s; 3H | 28.1 | ||
| 14′ | 1.06; s; 3H | 21.5 | 0.83; s; 3H | 22.2 | 0.90; s; 3H | 21.9 | ||
| 15′ | 1.07; s; 3H | 15.8 | 0.93; s; 3H | 16.1 | 0.96; s; 3H | 16 | ||
| CH3- (OAc) | - | - | - | - | 2.08; s; 3H | 21.4 | ||
| C=O (OAc) | - | - | - | - | - | 170.7 | ||
| Compounds | IC50 (µM) a | |||
|---|---|---|---|---|
| COLO 205 | K-562 | MCF-7 | HUVEC | |
| Conferone (1) | 27.63 ± 0.69 | 55.50 ± 0.94 | 34.02 ± 0.68 | 46.12 ± 0.99 |
| Conferol (2) | 11.19 ± 0.68 | 35.23 ± 0.89 | 15.95 ± 0.46 | 61.03±0.29 |
| Feselol (3) | 38.41 ± 0.80 | 72.48 ± 0.74 | 35.95 ± 1.29 | 38.75 ± 0.83 |
| Badrakemone (4) | >200 | >200 | >200 | >200 |
| Mogoltadone (5) | 31.71 ± 0.15 | 21.11 ± 0.85 | 30.45 ± 0.60 | >200 |
| Farnesiferol A (6) | >200 | >200 | >200 | 74.43 ± 1.19 |
| Farnesiferol A acetate (7) | 66.03 ± 1.89 | 52.71 ± 0.90 | 27.40 ± 0.96 | 64.53 ± 1.56 |
| Gummosin (8) | >200 | 95.53 ± 4.87 | >200 | 174.78 ± 0.28 |
| Ferukrin (9) | >200 | >200 | 81.69 ± 1.96 | >200 |
| Ferukrin acetate (10) | 105.72 ± 1.35 | 88.42 ± 0.85 | >200 | >200 |
| Deacetylkellerin (11) | >200 | >200 | 47.62 ± 0.40 | >200 |
| Kellerin (12) | 51.05 ± 1.57 | 78.14± 3.13 | 18.24 ± 0.12 | 99.39 ±1.63 |
| Samarcandone (13) | 125.14 ± 2.13 | >200 | >200 | 190.44 ± 5.20 |
| Samarcandin (14) | 170.03 ± 3.62 | 143.03 ± 1.67 | 83.27 ± 0.39 | 169.16 ± 2.68 |
| Samarcandin acetate (15) | 70.29 ± 0.65 | 78.67 ± 1.65 | >200 | >200 |
| Cisplatin b | 111.87 ± 3.11 | 8.10 ± 0.25 | 64.22 ± 5.25 | 40.68 ± 1.12 |
| Doxorubicin c | 0.08 ± 0.00 | 0.33 ± 0.02 | 2.83 ± 0.25 | 0.20 ± 0.02 |
| Ligands | Docking Score | H-Bond | Pi-Pi Stacking |
|---|---|---|---|
| Compound 6 | −5544 | SER 246 | - |
| Conferol (2) | −3881 | SER 246 | - |
| Mogoltadone (5) | −3526 | SER 246 | - |
| Ligands | Docking Score | H-Bond | Pi-Pi Stacking |
|---|---|---|---|
| A-1293102 | −14,792 | ARG139, ASN 136 | PHE 105 |
| Obatoclax | −7744 | SER 106 | PHE 105, PHE 146 |
| Conferol (2) | −4935 | ARG 139, ASN 136, GLU 96 | |
| Mogoltadone (5) | −4811 | ARG 139 | PHE 105 |
| Compound | MW a | CNS b | donorHB c | accptHB d | QPlogPo/w e | QPlogS f | QPlogBB g | QPlogKhsa h | PHOA i | Rule of Five j |
|---|---|---|---|---|---|---|---|---|---|---|
| Conferol (2) | 382,499 | 0 | 1 | 5 | 4,14 | −5456 | −0.665 | 0.697 | 100 | 0 |
| Mogoltadone (5) | 380,483 | 0 | 0 | 5 | 3,97 | −5309 | −0.691 | 0.539 | 100 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eruçar, F.M.; Kuran, F.K.; Altıparmak Ülbegi, G.; Özbey, S.; Karavuş, Ş.N.; Arcan, G.G.; Yazıcı Tütüniş, S.; Tan, N.; Aksoy Sağırlı, P.; Miski, M. Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities from the Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin. Pharmaceuticals 2023, 16, 792. https://doi.org/10.3390/ph16060792
Eruçar FM, Kuran FK, Altıparmak Ülbegi G, Özbey S, Karavuş ŞN, Arcan GG, Yazıcı Tütüniş S, Tan N, Aksoy Sağırlı P, Miski M. Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities from the Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin. Pharmaceuticals. 2023; 16(6):792. https://doi.org/10.3390/ph16060792
Chicago/Turabian StyleEruçar, Fatma Memnune, Fadıl Kaan Kuran, Gülsüm Altıparmak Ülbegi, Süheyla Özbey, Şule Nur Karavuş, Gülşah Gamze Arcan, Seçil Yazıcı Tütüniş, Nur Tan, Pınar Aksoy Sağırlı, and Mahmut Miski. 2023. "Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities from the Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin" Pharmaceuticals 16, no. 6: 792. https://doi.org/10.3390/ph16060792
APA StyleEruçar, F. M., Kuran, F. K., Altıparmak Ülbegi, G., Özbey, S., Karavuş, Ş. N., Arcan, G. G., Yazıcı Tütüniş, S., Tan, N., Aksoy Sağırlı, P., & Miski, M. (2023). Sesquiterpene Coumarin Ethers with Selective Cytotoxic Activities from the Roots of Ferula huber-morathii Peşmen (Apiaceae) and Unequivocal Determination of the Absolute Stereochemistry of Samarcandin. Pharmaceuticals, 16(6), 792. https://doi.org/10.3390/ph16060792