Compounded Effervescent Magnesium for Familial Hypomagnesemia: A Case Report
Abstract
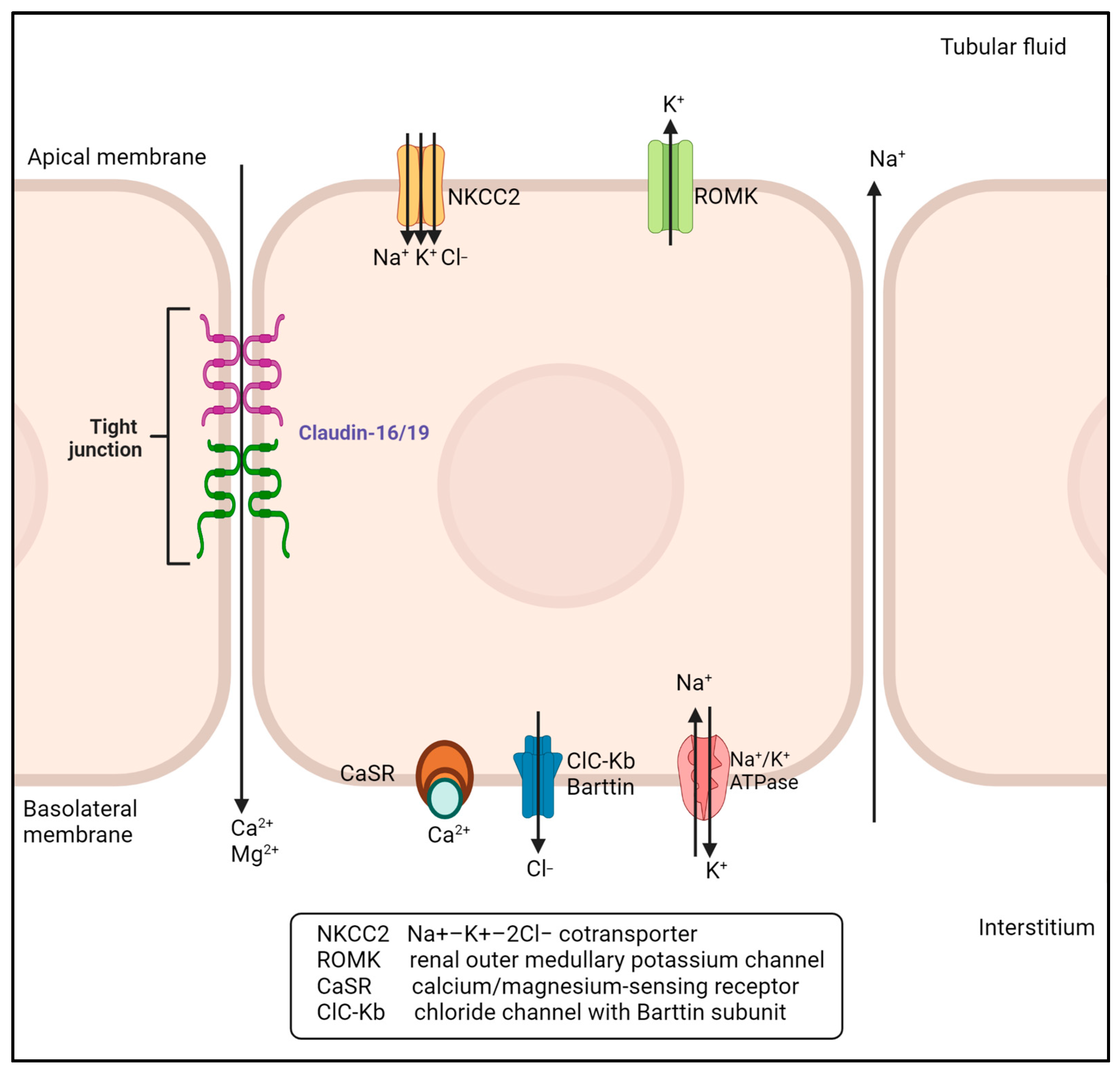
1. Introduction
2. Case and Methods
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vall-Palomar, M.; Madariaga, L.; Ariceta, G. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Pediatr. Nephrol. 2021, 36, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Godron, A.; Harambat, J.; Boccio, V.; Mensire, A.; May, A.; Rigothier, C.; Couzi, L.; Barrou, B.; Godin, M.; Chauveau, D.; et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: Phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin. J. Am. Soc. Nephrol. 2012, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Praga, M.; Vara, J.; González-Parra, E.; Andrés, A.; Alamo, C.; Araque, A.; Ortiz, A.; Rodicio, J.L. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis. Kidney Int. 1995, 47, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Prot-Bertoye, C.; Houillier, P. Claudins in Renal Physiology and Pathology. Genes 2020, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Schaller, A.; Seelow, D.; Pandey, A.V.; Waldegger, S.; Lesslauer, A.; Vitzthum, H.; Suzuki, Y.; Luk, J.M.; Becker, C.; et al. Mutations in the Tight-Junction Gene Claudin 19 (CLDN19) Are Associated with Renal Magnesium Wasting, Renal Failure, and Severe Ocular Involvement. Am. J. Hum. Genet. 2006, 79, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Claverie-Martín, F.; Vargas-Poussou, R.; Müller, D.; García-Nieto, V. Clinical utility gene card for: Familial hypomagnesemia with hypercalciuria and nephrocalcinosis with/without severe ocular involvement. Eur. J. Hum. Genet. 2014, 23, 889. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.; Bockenhauer, D.; Bolignano, D.; Calò, L.A.; Cosyns, E.; Devuyst, O.; Ellison, D.H.; Frankl, F.E.K.; Knoers, N.V.; Konrad, M.; et al. Gitelman syndrome: Consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2017, 91, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W. The PedsQL Measurement Model for the Pediatric Quality of Life Inventory. Available online: https://www.pedsql.org/index.html (accessed on 30 November 2022).
- Ates, M.; Kizildag, S.; Yuksel, O.; Hosgorler, F.; Yuce, Z.; Guvendi, G.; Kandis, S.; Karakilic, A.; Koc, B.; Uysal, N. Dose-Dependent Absorption Profile of Different Magnesium Compounds. Biol. Trace Element Res. 2019, 192, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V.V.; Somberg, J.C. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am. J. Ther. 2001, 8, 345–357. [Google Scholar]
- Schuette, S.A.; Lashner, B.A.; Janghorbani, M. Bioavailability of Magnesium Diglycinate vs. Magnesium Oxide in Patients with Ileal Resection. J. Parenter. Enter. Nutr. 1994, 18, 430–435. [Google Scholar] [CrossRef]
- Ruml, L.A.; Pak, C.Y. Effect of potassium magnesium citrate on thiazide-induced hypokalemia and magnesium loss. Am. J. Kidney Dis. 1999, 34, 107–113. [Google Scholar] [CrossRef]
- Rylander, R. Bioavailability of magnesium salts—A review. J. Pharm. Nutr. Sci. 2014, 4, 57–59. [Google Scholar] [CrossRef]
- Ettinger, B.; Pak, C.Y.; Citron, J.T.; Thomas, C.; Adams-Huet, B.; Vangessel, A. Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J. Urol. 1997, 158, 2069–2073. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. The Importance of Magnesium in Clinical Healthcare. Scientifica 2017, 2017, 4179326. [Google Scholar] [CrossRef] [PubMed]
- Kari, J.A.; Shalaby, M.A.; Qari, F.A.; Albanna, A.S.; Alhasan, K.A. Childhood nephrolithiasis and nephrocalcinosis caused by metabolic diseases and renal tubulopathy: A retrospective study from 2 tertiary centers. Saudi Med. J. 2022, 43, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Donowitz, M. Magnesium-Induced Diarrhea and New Insights into the Pathobiology of Diarrhea. N. Engl. J. Med. 1991, 324, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.M.; Kim, H.; Averch, T.D.; Kim, H.J. Effect of Magnesium on Calcium and Oxalate Ion Binding. J. Endourol. 2013, 27, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.C. Measurement of feelings using visual analogue scales. Proc. R. Soc. Med. 1969, 62, 989–993. [Google Scholar] [PubMed]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Psychol. Psychother. Theory Res. Pr. 1974, 47, 211–218. [Google Scholar] [CrossRef]
| Magnesium Salts | Carbonate | Chloride | Citrate | Fumarate | Gluconate | Glycinate | L-lactate | ||
|---|---|---|---|---|---|---|---|---|---|
| Elemental Mg2+/dose, mg (mEq) | 232 (19.0) | 64 (5.26) | - (25) | 530 (44.16) | Tablet 27 (2.2) | Liquid 54 (4.4) | 100 (8.33) | 84 (7) | |
| Solubility in water | Nearly insoluble | High | Very good | Good | Moderate | Good | Excellent | ||
| Bioavailability | Extremely low | Good | Good | Good | Good; similar to chloride | Good | Excellent | ||
| Oral absorption, % (mEq) | - | 19.68 (1.04) | 29.64 (ionic) | - | 19.25 (0.82–0.43) | 23.5 - | 42.3 (2.96) | ||
| Delivery system | Tablets | Enteric coated Tablets | Liquid, Tablets | Tablets | Tablets, liquid | Ingestion | Sustained-release caplets | ||
| Dosage | 70 mg elemental Mg (each Tablet) | 640 mg/d, 1–2 tabs TID | 25 mEq Mg, 2–5 Tablets | 1 Tablet | 648 mg/d, 2–4 Tablets TID | 100 mg | 1–2 caplets q 12 h | ||
| Side effects | GI distress, diarrhea | GI distress, diarrhea | Laxative, evacuant | GI distress, diarrhea | Minor GI disturbances | ||||
| Comments | Not very soluble at pH of GI tract; some GI side effects; laxative | Enteric coating could delay absorption; some GI side effects; cathartic | Therapy-limiting side effects; limited absorption; low citraturic response | Expensive formulation to achieve Recommended daily allowance requirements | Good alternative in patients with intestinal resection | Sustained release increases absorption, reduces side effects; cathartic | |||
| Magnesium salts | Oxide | K Mg citrate | DL-aspartate | L-aspartate | Hydroxide | Salicylate | Sulfate | Aminoate | |
| Elemental Mg2+/dose, mg (mEq) | 241 (19–8) | — (24.5) | 5 | 5 | 2 × 10.3 mmol | 600 | 56.5 mmol | 500 (41.6) | |
| Solubility in water | Extremely low, 8.6 mg/L | High solubility | Good | Good | Practically insoluble | Freely soluble | Moderately soluble | ||
| Bioavailability | Extremely Low | Good; similar to Mg citrate | 86–100% | ||||||
| Oral absorption, % (mEq) | 22.8 (0.39) (2% ionic) | 44.5 | 41.7 | 4 (oral dose), limited and variable extent | |||||
| Delivery system | Tablets, capsules | Tablet | Tablet | Tablet | Tablet (Maalox) | Tablet | IV solution | Tablet | |
| Dosage | 2–4 tabs TID | 7 tablets, 3–5 mEq Mg ea | 1 Tablet | 1 Tablet | 2 Tablets | 600 mg, 1 Tablet | Intravenous Mg 9.9–49.3 mg/mL | 1 Tablet, 3 Tablets (100 mg ea Mg) | |
| Side effects | Emesis, diarrhea | No GI side effects | Occasional regurgitation and mild diarrhea | ||||||
| Comments | Virtually insoluble at pH of GI tract; some GI side effects; antacid | Yielded a greater Citraturic response in addition to primary absorbable K & Mg | Antacid; cathartic | Internal, antiinfective | Parenteral use may lead to magnesium toxicity | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennati, G.; Cirino, M.; Benericetti, G.; Maximova, N.; Zanier, M.; Pigato, F.; Parzianello, A.; Maestro, A.; Barbi, E.; Zanon, D. Compounded Effervescent Magnesium for Familial Hypomagnesemia: A Case Report. Pharmaceuticals 2023, 16, 785. https://doi.org/10.3390/ph16060785
Bennati G, Cirino M, Benericetti G, Maximova N, Zanier M, Pigato F, Parzianello A, Maestro A, Barbi E, Zanon D. Compounded Effervescent Magnesium for Familial Hypomagnesemia: A Case Report. Pharmaceuticals. 2023; 16(6):785. https://doi.org/10.3390/ph16060785
Chicago/Turabian StyleBennati, Giada, Mario Cirino, Giulia Benericetti, Natalia Maximova, Monica Zanier, Federico Pigato, Anna Parzianello, Alessandra Maestro, Egidio Barbi, and Davide Zanon. 2023. "Compounded Effervescent Magnesium for Familial Hypomagnesemia: A Case Report" Pharmaceuticals 16, no. 6: 785. https://doi.org/10.3390/ph16060785
APA StyleBennati, G., Cirino, M., Benericetti, G., Maximova, N., Zanier, M., Pigato, F., Parzianello, A., Maestro, A., Barbi, E., & Zanon, D. (2023). Compounded Effervescent Magnesium for Familial Hypomagnesemia: A Case Report. Pharmaceuticals, 16(6), 785. https://doi.org/10.3390/ph16060785









