Tigecycline Absorption Improved by Selected Excipients
Abstract
1. Introduction
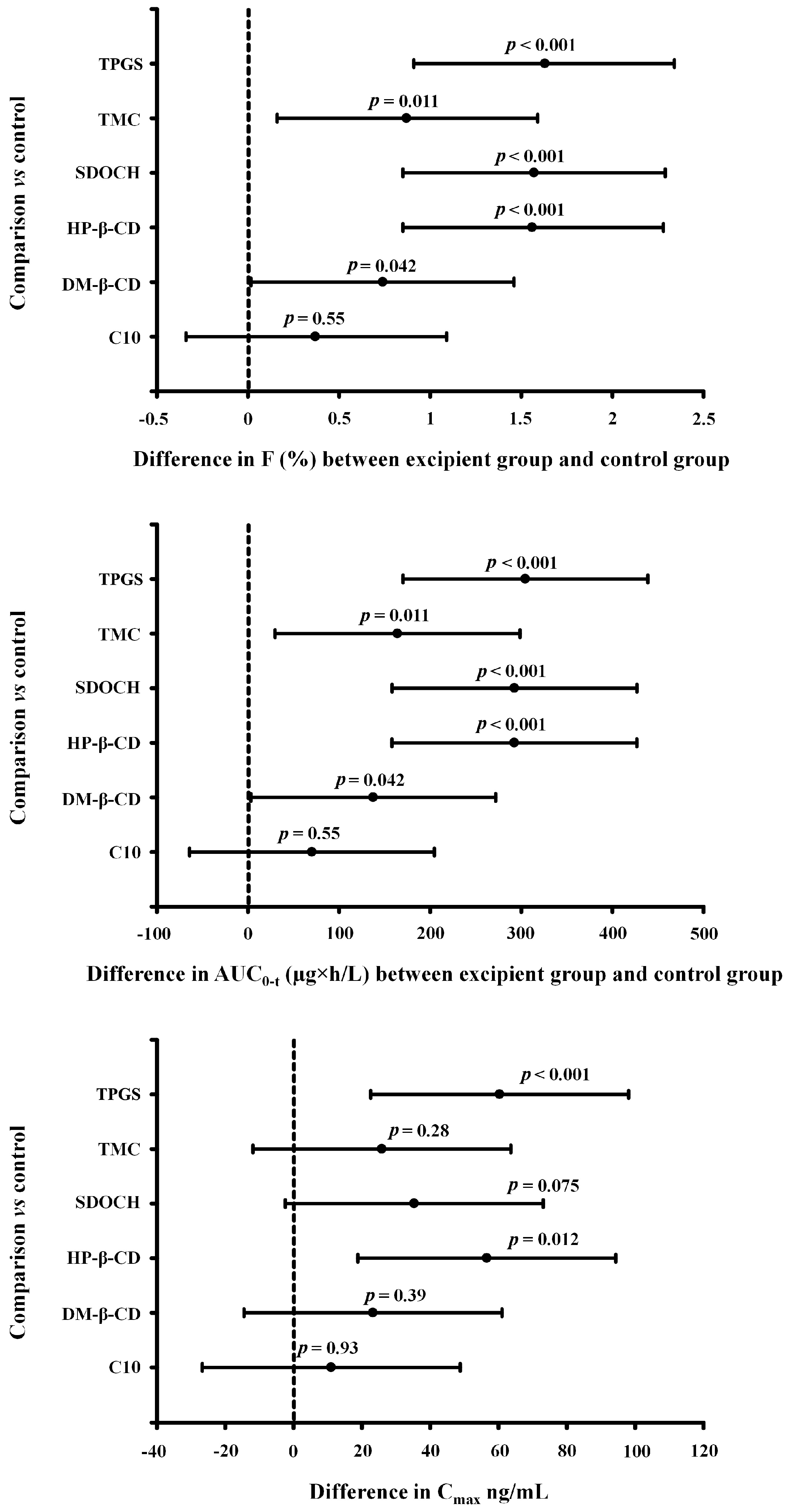
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Experimental Design
4.4. Chromatography and Sample Preparation
4.5. Pharmacokinetic Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tariq, S.; Rizvi, S.F.A.; Anwar, U. Tetracycline: Classification, Structure Activity Relationship and Mechanism of Action as a Theranostic Agent for Infectious Lesions—A Mini Review. Biomed. J. Sci. Tech. Res. 2018, 7, 5787–5796. [Google Scholar]
- Riviere, J.; Lees, P. USP veterinary pharmaceutical information monographs—Antibiotics: Tetracyclines. J. Vet. Pharmacol. Ther. 2003, 26 (Suppl. 2), 225–252. [Google Scholar]
- Diorio, C.R.; Shah, S.M.; Ali, K.A. Oral Formulation Comprising Tigecycline. PCT Patent WO2007075794A2, 5 July 2008. [Google Scholar]
- Chin, T.F.; Lach, J.L. Drug diffusion and bioavailability: Tetracycline metallic chelation. Am. J. Hosp. Pharm. 1975, 32, 625–629. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, H.; Jasiecka-Mikołajczyk, A.; Madej-Śmiechowska, H.; Janiuk, J.; Zygmuntowicz, A.; Dąbrowski, M. Comparative pharmacokinetics of chlortetracycline, tetracycline, minocycline, and tigecycline in broiler chickens. Poult. Sci. 2020, 99, 4750–4757. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, H. Bioavailability of tetracyclines is substantially increased by administration of cyclosporine A, a non-specific efflux-pump blocker. Drug Metab. Pharmacokinet. 2023, 50, 100493. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Lum, B.L.; Gosland, M.P. MDR expression in normal tissues. Pharmacologic implications for the clinical use of P-glycoprotein inhibitors. Hematol. Oncol. Clin. N. Am. 1995, 9, 319–336. [Google Scholar] [CrossRef]
- FDA. Drug Development and Drug Interactions. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactionstable-substrates-inhibitors-and-inducers (accessed on 10 March 2020).
- NIH. Available online: https://clinicaltrials.gov/ct2/show/NCT01042379?term=Encequidar&draw=2&rank=1 (accessed on 22 November 2022).
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Kalász, H.; Antal, I. Drug excipients. Curr. Med. Chem. 2006, 13, 2535–2563. [Google Scholar] [CrossRef]
- Jackson, K.; Young, D.; Pant, S. Drug-excipient interactions and their affect on absorption. Pharm. Sci. Technol. Today 2000, 3, 336–345. [Google Scholar] [CrossRef]
- Buggins, T.R.; Dickinson, P.A.; Taylor, G. The effects of pharmaceutical excipients on drug disposition. Adv. Drug Deliv. Rev. 2007, 59, 1482–1503. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.D.; Tippin, T.K.; Thakker, D.R. Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm. Sci. Technol. Today 2000, 3, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Feldman, S. Mechanisms of surfactant effects on drug absorption. J. Pharm. Sci. 1970, 59, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Goole, J.; Lindley, D.J.; Roth, W.; Carl, S.M.; Amighi, K.; Kauffmann, J.M.; Knipp, G.T. The effects of excipients on transporter mediated absorption. Int. J. Pharm. 2010, 393, 17–31. [Google Scholar] [CrossRef]
- Lu, R.; Zhou, Y.; Ma, J.; Wang, Y.; Miao, X. Strategies and Mechanism in Reversing Intestinal Drug Efflux in Oral Drug Delivery. Pharmaceutics 2022, 26, 1131. [Google Scholar] [CrossRef]
- Sharma, P.; Varma, M.V.S.; Chawla, H.P.S.; Panchagnula, R. Absorption enhancement, mechanistic and toxicity studies of medium chain fatty acids, cyclodextrins and bile salts as peroral absorption enhancers. Il Farmaco 2005, 60, 884–893. [Google Scholar] [CrossRef]
- Jitkova, Y.; Gronda, M.; Hurren, R.; Wang, X.; Goard, C.A.; Jhas, B.; Schimmer, A.D. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS ONE 2014, 9, e95281. [Google Scholar] [CrossRef]
- Pankey, G.A. Tigecycline. J. Antimicrob. Chemother. 2005, 56, 470–480. [Google Scholar] [CrossRef]
- Peterson, L.R. A review of tigecycline-the first glycylcycline. Int. J. Antimicrob. Agents 2008, 32 (Suppl. 4), 215–222. [Google Scholar] [CrossRef]
- Papich, M.G. Selection of antibiotics for meticillin-resistant Staphylococcus pseudintermedius: Time to revisit some old drugs? Vet. Dermatol. 2012, 23, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef]
- Arima, H.; Yunomae, K.; Hirayama, F.; Uekama, K. Contribution of P-glycoprotein to the enhancing effects of dimethyl-beta-cyclodextrin on oral bioavailability of tacrolimus. J. Pharmacol. Exp. Ther. 2001, 297, 547–555. [Google Scholar] [PubMed]
- Nguyen, T.; Duong, V.; Maeng, H. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Huang, J.D. Effects of sodium deoxycholate and sodium caprate on the transport of epirubicin in human intestinal epithelial Caco-2 cell layers and everted gut sacs of rats. Biochem. Pharmacol. 2000, 59, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Samani, S.M.; Sobhani, Z. Enhanced intestinal permeation of doxorubicin using chitosan nanoparticles. Adv. Pharm. Bull. 2018, 8, 411–417. [Google Scholar] [CrossRef]
- Tilloy, S.; Monnaert, V.; Fenart, L.; Bricout, H.; Cecchelli, R.; Monflier, E. Methylated beta-cyclodextrin as P-gp modulators for deliverance of doxorubicin across an in vitro model of blood-brain barrier. Bioorg. Med. Chem. Lett. 2006, 16, 2154–2157. [Google Scholar] [CrossRef]
- Eljaaly, K.; Helal, A.; Almandeel, T.; Algarni, R.; Alshehri, S. Multivalent cations interactions with fluoroquinolones or tetracyclines: A cross-sectional study. Saudi J. Biol. Sci. 2021, 28, 6929–6932. [Google Scholar] [CrossRef]
- Tao, R.E.; Prajapati, S.; Pixley, J.N.; Grada, A.; Feldman, S.R. Oral Tetracycline-Class Drugs in Dermatology: Impact of Food Intake on Absorption and Efficacy. Antibiotics 2023, 12, 1152. [Google Scholar] [CrossRef]
- Ziółkowski, H.; Madej-Śmiechowska, H.; Jaroszewski, J.J. Influence of beta-cyclodextrins on oral bioavailability of tigecycline in broiler chickens-preliminary study. J. Vet. Pharmacol. Ther. 2018, 41 (Suppl. 1), 133. [Google Scholar]
- EMA. Available online: https://www.ema.europa.eu/en/medicines/veterinary/EPAR/fungitraxx (accessed on 27 March 2019).
- EMA. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/koselugo (accessed on 16 January 2023).
- López-Sánchez, A.; Pérez-Cantero, A.; Torrado-Salmerón, C.; Martin-Vicente, A.; García-Herrero, V.; González-Nicolás, M.A.; Lázaro, A.; Tejedor, A.; Torrado-Santiago, S.; García-Rodríguez, J.J.; et al. Efficacy, Biodistribution, and Nephrotoxicity of Experimental Amphotericin B-Deoxycholate Formulations for Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2018, 62, e00489-18. [Google Scholar] [CrossRef]
- FDA. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/inactive-ingredients-database-download (accessed on 27 January 2023).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of sodium, potassium and calcium salts of fatty acids (E 470a) and magnesium salts of fatty acids (E 470b) as food additives. EFSA J. 2018, 16, e05180. [Google Scholar] [PubMed]
- EMA. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-cyclodextrins-used-excipients-medicinal-products-human-use_en.pdf (accessed on 9 October 2017).
- Yang, C.; Qin, Y.; Tu, K.; Xu, C.; Li, Z.; Zhang, Z. Star-shaped polymer of β-cyclodextrin-g-vitamin E TPGS for doxorubicin delivery and multidrug resistance inhibition. Colloids Surf. B Biointerfaces 2018, 169, 10–19. [Google Scholar] [CrossRef]
- Guo, Y.; Chu, M.; Tan, S.; Zhao, S.; Liu, H.; Otieno, B.O.; Yang, X.; Xu, C.; Zhang, Z. Chitosan-g-TPGS nanoparticles for anticancer drug delivery and overcoming multidrug resistance. Mol. Pharm. 2014, 11, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Hsiung, E.; Celebioglu, A.; Chowdhury, R.; Kilic, M.E.; Durgun, E.; Altier, C.; Uyar, T. Antibacterial nanofibers of pullulan/tetracycline-cyclodextrin inclusion complexes for Fast-Disintegrating oral drug delivery. J. Colloids Interface Sci. 2022, 610, 321–333. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotze, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Petkova, T.; Milanova, A.; Poźniak, B. The effects of cyclosporine A or activated charcoal co-administration on the pharmacokinetics of enrofloxacin in chickens. Poult. Sci. 2023, 102, 102225. [Google Scholar] [CrossRef]
- Haritova, A.M.; Schrickx, J.; Fink-Gremmels, J. Expression of drug efflux transporters in poultry tissues. Res. Vet. Sci. 2010, 89, 104–107. [Google Scholar] [CrossRef]
- Schrickx, J.; Fink-Gremmels, J. P-glycoprotein-mediated transport of oxytetracycline in the Caco-2 cell model. J. Vet. Pharmacol. Ther. 2007, 30, 25–31. [Google Scholar] [CrossRef]
- van der Merwe, S.M.; Verhoef, J.C.; Verheijden, J.H.; Kotzé, A.F.; Junginger, H.E. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur. J. Pharm. Biopharm. 2004, 58, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Evrard, B.; Chiap, P.; DeTullio, P.; Ghalmi, F.; Pielv, G.; Van Hees, T.; Crommen, J.; Losson, B.; Delattre, L. Oral bioavailability in sheep of albendazole from a suspension and from a solution containing hydroxypropyl-beta-cyclodextrin. J. Control Release 2002, 85, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Panchagnula, R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: Effect on solubility and permeability in vitro, in situ and in vivo. Eur. J. Pharm. Sci. 2005, 25, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Guarini, S.; Ferrari, W. Sodium deoxycholate promotes the absorption of heparin administered orally, probably by acting on gastrointestinal mucosa, in rats. Experientia 1985, 41, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Raoof, A.A.; Ramtoola, Z.; McKenna, B.; Yu, R.Z.; Hardee, G.; Geary, R.S. Effect of sodium caprate on the intestinal absorption of two modified antisense oligonucleotides in pigs. Eur. J. Pharm. Sci. 2002, 17, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Jasiecka-Mikołajczyk, A.; Jaroszewski, J.J. Determination of tigecycline in turkey plasma by LC-MS/MS: Validation and application in a pharmacokinetic study. Pol. J. Vet. Sci. 2017, 20, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Informa Healthcare: New York, NY, USA, 1982; pp. 409–417. [Google Scholar]

| Pharmacokinetic Parameters | Tigecycline (Control) | Tigecycline + Trimethyl Chitosan | Tigecycline + Sodium Caprate | Tigecycline + Sodium Desoxycholate | Tigecycline + Tocopherol Polyethylene Glycol 1000 Succinate | Tigecycline + (2,6-di-O-methyl)-β-Cyclodextrin | Tigecycline + (2-Hydroxy propyl)-β-Cyclodextrin | Tigecycline Intravenous | |
|---|---|---|---|---|---|---|---|---|---|
| AUC(0-t) (µg×h/L) | 312.28 ±52.29 | 476.40 * ±104.53 | 382.49 ±42.32 | 604.84 *** ±131.16 | 616.79 *** ±86.13 | 449.82 * ±81.52 | 604.70 *** ±157.46 | 18,682.07 ±3219.19 | |
| AUC(0-∞) (µg×h/L) | 849.92 ±280.50 | 1158.50 ±372.069 | 1065.04 ±533.03 | 1492.16 ±407.07 | 1316.84 ±318.41 | 1254.72 ±407.82 | 1261.21 ±408.61 | 20,069.14 ±3089.97 | |
| β (h−1) | 0.029 ±0.033 | 0.022 ±0.015 | 0.022 ±0.014 | 0.014 ±0.003 | 0.018 ±0.009 | 0.017 ±0.006 | 0.018 ±0.01 | 0.015 ±0.006 | |
| t1/2β (h) | 39.44 ±16.56 | 42.81 ±16.17 | 46.3 ±32.12 | 53.13 ±10.13 | 46.92 ±20.77 | 48.31 ±24.06 | 45.18 ±23.39 | 53.88 ±25.31 | |
| Cmax (µg/mL) | 0.035 ±0.006 | 0.061 ±0.033 | 0.046 ±0.018 | 0.071 ±0.029 | 0.096 * ±0.028 | 0.058 ±0.027 | 0.091 * ±0.043 | 28.61 ±9.84 | |
| tmax(h) | 2.50 ±0.80 | 2.06 ±0.56 | 2.63 ±1.89 | 1.50 ±0.27 | 2.50 ±0.53 | 2.57 ±0.77 | 2.44 ±0.82 | 0.083 | |
| Clast (µg/mL) | 0.010 ±0.001 | 0.011 ±0.002 | 0.010 ±0.002 | 0.011 ±0.003 | 0.011 ±0.001 | 0.010 ±0.003 | 0.010 ±0.001 | 0.019 ±0.003 | |
| tlast(h) | 22.25 ±0.49 | 27.0 ±8.49 | 24.0 | 39.0 ±15.38 | 30.0 * ±6.42 | 24 | 28.50 ±6.21 | 96 | |
| AUMC(0-t) (mg×h×h/L) | 2897.21 ±832.68 | 5239.9 ±2466.15 | 3483.17 ±312.98 | 8516.48 ±3295.12 | 6175.31 ±2115.48 | 3907.29 ±483.96 | 5663.75 ±2426.45 | 220,673.25 ±48,869.82 | |
| AUMC(0-∞) (mg×h×h/L) | 51,143.95 ±28,612.95 | 73,317.77 ±44,132.72 | 86,998.69 ±107,239.9 | 115,013.7 *** ±51,553.9 | 83,919.44 * ±59,131.27 | 88,961.75 ±93,875.95 | 78,914.06 * ±62,095.32 | 477,072.60 ±119,564.68 | |
| MRT(0-t) (h) | 9.04 ±1.85 | 10.57 ±3.54 | 9.16 ±0.87 | 14.63 * ±4.33 | 9.89 ±2.72 | 8.81 ±1.07 | 9.11 ±1.84 | 11.93 ±2.77 | |
| MRT(0-∞) (h) | 53.82 ±22.32 | 56.93 ±24.09 | 62.03 ±25.88 | 70.37 ±13.51 | 57.85 ±29 | 62.83 ±35.84 | 54.71 ±33.07 | 24.36 ±7.85 | |
| kab (h) | 0.97 ±0.54 | 1.21 ±1.25 | 0.85 ±0.91 | 1.29 ±0.68 | 0.74 ±0.54 | 1.17 ±0.67 | 0.78 ±0.76 | Cl (L/h*kg) | 0.41 ±0.08 |
| t1/2kab (h) | 0.91 ±0.77 | 1.73 ±1.90 | 1.50 ±0.97 | 0.76 ±0.52 | 1.81 ±2.07 | 1.02 ±1.03 | 1.54 ±0.97 | Vdarea (L/kg) | 32.22 ±17.20 |
| MAT (h) | 1.32 ±1.12 | 2.50 ±2.74 | 2.46 ±1.40 | 1.10 ±0.76 | 2.61 ±2.99 | 1.43 ±1.51 | 2.23 ±1.40 | Vdss (L/kg) | 4.98 ±1.98 |
| F (%) | 1.67 ±0.27 | 2.55 ** ±0.56 | 2.04 ±0.23 | 3.24 *** ±0.70 | 3.30 *** ±0.46 | 2.41 * ±0.43 | 3.24 *** ±0.84 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkowski, H.; Szteyn, K.; Jędrzkiewicz, D.; Rasiński, B.; Jaroszewski, J. Tigecycline Absorption Improved by Selected Excipients. Pharmaceuticals 2023, 16, 1111. https://doi.org/10.3390/ph16081111
Ziółkowski H, Szteyn K, Jędrzkiewicz D, Rasiński B, Jaroszewski J. Tigecycline Absorption Improved by Selected Excipients. Pharmaceuticals. 2023; 16(8):1111. https://doi.org/10.3390/ph16081111
Chicago/Turabian StyleZiółkowski, Hubert, Kalina Szteyn, Dawid Jędrzkiewicz, Bartosz Rasiński, and Jerzy Jaroszewski. 2023. "Tigecycline Absorption Improved by Selected Excipients" Pharmaceuticals 16, no. 8: 1111. https://doi.org/10.3390/ph16081111
APA StyleZiółkowski, H., Szteyn, K., Jędrzkiewicz, D., Rasiński, B., & Jaroszewski, J. (2023). Tigecycline Absorption Improved by Selected Excipients. Pharmaceuticals, 16(8), 1111. https://doi.org/10.3390/ph16081111








