The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
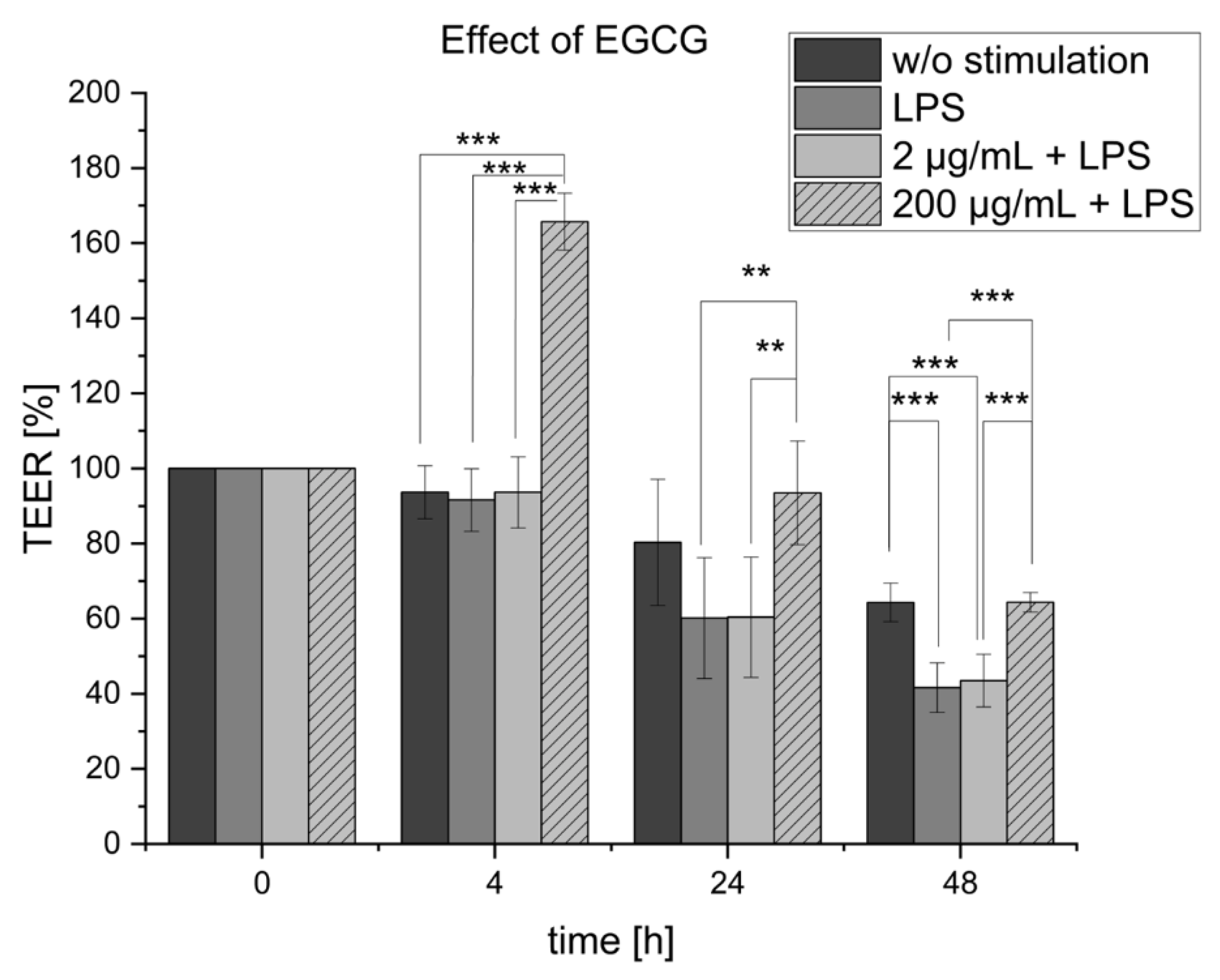
2.1. EGCG Treatment of the Inflamed Co-Culture
2.2. Effect of EGCG on Cytokine Release of MDM
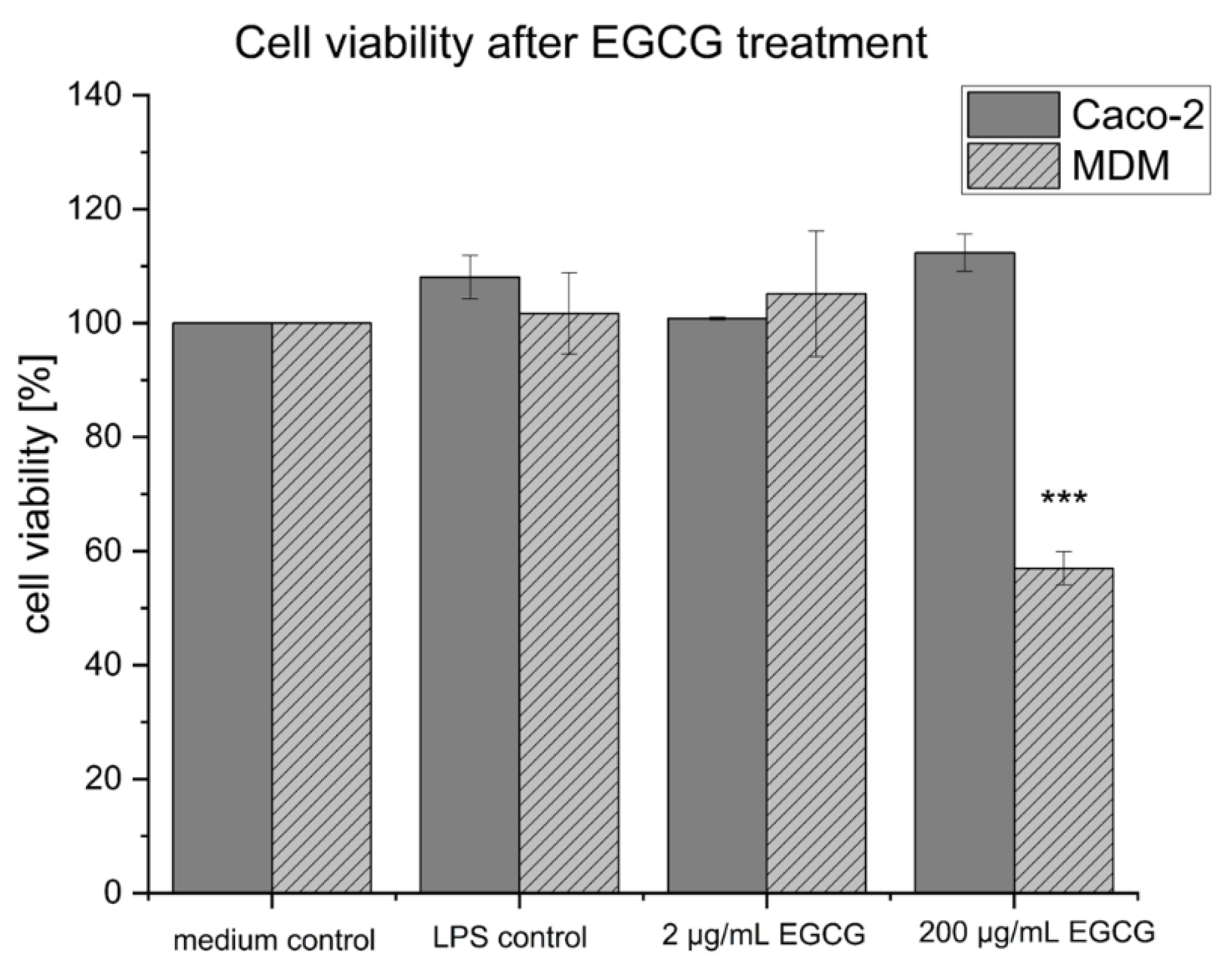
2.3. Cell Viability of the Co-Culture Setup after EGCG Treatment
2.4. Effect of EGCG on Caco-2 Monolayer
2.5. Comparison of EGCG’s Efficacy with IBD-Related Active Pharmaceutical Ingredients
3. Discussion
4. Materials and Methods
4.1. Cell Culture Experiments
4.1.1. Co-Culture Setup
4.1.2. Inflammation of Co-Culture
4.1.3. ECGC Treatment of Inflamed Co-Culture
4.1.4. Investigation of Cell Viability of the Co-Culture Setup after EGCG Treatment
4.1.5. Investigation of ECGC Effect on Caco-2 Monolayer
4.2. Analytical Methods
4.2.1. TEER Measurements
4.2.2. ELISA Measurements
4.2.3. MTT Assay
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Global Report on Traditional and Complementary Medicine; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?ua=1 (accessed on 13 January 2023).
- National Center for Complementary and Integrative Health. Complementary, Alternative, or Integrative Health: What’s in a Name? 2021. Available online: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name (accessed on 13 January 2023).
- Bodeker, G.; Kronenberg, F. A public health agenda for traditional, complementary, and alternative medicine. Am. J. Public Health 2002, 92, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- National Center for Complementary and Integrative Health. NCCIH Strategic Plan FY 2021–2025: Mapping the Pathway to Research on Whole Person Health. 2021. Available online: https://nccih-nih-gov.eur.idm.oclc.org/about/nccih-strategic-plan-2021–2025 (accessed on 13 January 2023).
- World Health Organization (WHO). WHO Traditional Medicine Strategy 2014–2023; World Health Organization: Geneva, Switzerland, 2013; pp. 1–76. [Google Scholar]
- Hilsden, R.J.; Verhoef, M.J.; Rasmussen, H.; Porcino, A.; Debruyn, J.C.C. Use of complementary and alternative medicine by patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Wulfert, H.; Lauche, R.; Klose, P.; Cramer, H.; Dobos, G.J.; Korzenik, J. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2023, 24, 95. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Mazzon, E.; Muià, C.; Di Paola, R.; Genovese, T.; Menegazzi, M.; De Sarro, A.; Suzuki, H.; Cuzzocrea, S. Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Radic. Res. 2005, 39, 1017–1025. [Google Scholar] [CrossRef]
- Ran, Z.H.; Chen, C.; Xiao, S.D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed. Pharmacother. 2008, 62, 189–196. [Google Scholar] [CrossRef]
- Oz, H.S.; Chen, T.; de Villiers, W.J.S. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef]
- Diwan, B.; Sharma, R. Green tea EGCG effectively alleviates experimental colitis in middle-aged male mice by attenuating multiple aspects of oxi-inflammatory stress and cell cycle deregulation. Biogerontology 2022, 23, 789–807. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Shi, F.; Zhang, Z.; Lv, H. Study on the preparation of EGCG-γ-Cyclodextrin inclusion complex and its drug-excipient combined therapeutic effects on the treatment of DSS-induced acute ulcerative colitis in mice. Int. J. Pharm. 2023, 630, 122419. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises ? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, P.; Ling, T.; Wang, Y.; Dong, R.; Zhang, C.; Zhang, L.; Han, M.; Wang, D.; Wan, X.; et al. Certain (-)-epigallocatechin-3-gallate ( EGCG ) auto-oxidation products ( EAOPs ) retain the cytotoxic activities of EGCG. Food Chem. 2016, 204, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (-)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef]
- Moridani, M.Y.; Scobie, H.; Salehi, P.; O’Brien, P.J. Catechin metabolism: Glutathione conjugate formation catalyzed by tyrosinase, peroxidase, and cytochrome P450. Chem. Res. Toxicol. 2001, 14, 841–848. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Li, D.; Chen, Y.; Qiao, X.; Fardous, R.; Lewandowski, A.; Liu, J.; Chan, T.; Dou, Q.P.; et al. Perspectives on the recent developments with green tea polyphenols in drug discovery. Expert Opin Drug Discov 2019, 13, 643–660. [Google Scholar]
- Schnur, S.; Wahl, V.; Metz, J.K.; Gillmann, J.; Hans, F.; Rotermund, K.; Zäh, R.-K.; Brück, D.A.; Schneider, M.; Hittinger, M. Inflammatory bowel disease addressed by Caco-2 and monocyte-derived macrophages: An opportunity for an in vitro drug screening assay. Vitr. Model. 2022, 1, 365–383. [Google Scholar] [CrossRef]
- Watson, J.L.; Ansari, S.; Cameron, H.; Wang, A.; Akhtar, M.; McKay, D.M. Green tea polyphenol (-)-epigallocatechin gallate blocks epithelial barrier dysfunction provoked by IFN-γ but not by IL-4. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, 954–961. [Google Scholar] [CrossRef]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Schlichter, S.; Amasheh, S.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 2008, 138, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Liu, X.; Wang, Y.; Dong, J.; Wu, F.; Mackenzie, G.G.; Su, Z. (-)-Epigallocatechin-3-gallate mitigates cyclophosphamide-induced intestinal injury by modulating the tight junctions, inflammation and dysbiosis in mice. Food Funct. 2021, 12, 11671–11685. [Google Scholar] [CrossRef] [PubMed]
- Abboud, P.A.; Hake, P.W.; Burroughs, T.J.; Odoms, K.; O’Connor, M.; Mangeshkar, P.; Wong, H.R.; Zingarelli, B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur. J. Pharmacol. 2008, 579, 411–417. [Google Scholar] [CrossRef]
- Du, Y.; Ding, H.; Vanarsa, K.; Soomro, S.; Baig, S.; Hicks, J.; Mohan, C. Low dose epigallocatechin gallate alleviates experimental colitis by subduing inflammatory cells and cytokines, and improving intestinal permeability. Nutrients 2019, 11, 1743. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-Epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Amoroso, M.; Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2012, 31, 379–446. [Google Scholar]
- Diamond, M.I.; Miner, J.N.; Yoshinaga, S.K.; Yamamoto, K.R. Transcription factor interactions: Selectors of positive or negative regulation from a single DNA element. Science 1990, 249, 1266–1272. [Google Scholar] [CrossRef]
- Ayroldi, E.; Migliorati, G.; Bruscoli, S.; Marchetti, C.; Zollo, O.; Cannarile, L.; D’Adamio, F.; Riccardi, C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor κB. Blood 2001, 98, 743–753. [Google Scholar] [CrossRef]
- Boivin, M.A.; Ye, D.; Kennedy, J.C.; Al-Sadi, R.; Shepela, C.; Ma, T.Y. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, 590–598. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-κB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory effects of green tea polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; De Villiers, W.J.S.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Kim, S.H.; Jeong, H.J.; Kim, S.Y.; Shin, T.Y.; Um, J.Y.; Hong, S.H.; Kim, H.M. Epigallocatechin-3-gallate inhibits secretion of TNF-α, IL-6 and IL-8 through the attenuation of ERK and NF-κB in HMC-1 cells. Int. Arch. Allergy Immunol. 2007, 142, 335–344. [Google Scholar] [CrossRef]
- Dryden, G.W.; Fernandez-Botran, G.R.; Hussien Qazzaz, H.M. EGCG Reduces Pro-Inflammatory Cytokine Production and Induces Apoptosis in Activated CD14+Macrophages, CD4+Cd45+RO T Cells, and Mixed Macrophage/T Cell Populations, but Not CD4+Cd45+RA T Cells From IBD Patients and Controls. Gastroenterology 2011, 140, S-838. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Parra, A.; Silva, A.M.; Egea, M.A.; Souto, E.B.; Garcia, M.L.; Calpena, A.C. Validation of a high performance liquid chromatography method for the stabilization of epigallocatechin gallate. Int. J. Pharm. 2014, 475, 181–190. [Google Scholar] [CrossRef]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Tachibana, H. Green tea polyphenol sensing. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 66–80. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Rehman, M.; Mir, M.; Farooq, A.; Rahid, S.M.; Ahmad, B.; Ahmad, S.B.; Ali, R.; Hussain, I.; Masoodi, M.; Muzamil, S.; et al. Naringenin (4,5,7-trihydroxyflavanone) suppresses the development of precancerous lesions via controlling hyperproliferation and inflammation in the colon of Wistar rats. Environ. Toxicol. 2018, 33, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.C.; Shaker, A.; Levin, M.S.; Louis, S.; Administration, V.; Louis, S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Tabish, M.; Ali, S.; Arafah, A.; Wahab, S.; Almarshad, F.M.; Rashid, S.; Rehman, M.U. Dietary Phytochemicals in Cancer Signalling Pathways: Role of miRNA Targeting. Curr. Med. Chem. 2021, 28, 8036–8067. [Google Scholar] [CrossRef] [PubMed]
- Menegazzi, M.; Campagnari, R.; Bertoldi, M.; Crupi, R.; Di Paola, R.; Cuzzocrea, S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: Could such a scenario be helpful to counteract COVID-19? Int. J. Mol. Sci. 2020, 21, 5171. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Scherließ, R. The MTT assay as tool to evaluate and compare excipient toxicity in vitro on respiratory epithelial cells. Int. J. Pharm. 2011, 411, 98–105. [Google Scholar] [CrossRef]


| API | TEER in % after 24 h | TNF-α | IL-6 | IL-8 |
|---|---|---|---|---|
| 5-ASA (level 1) | 68.23 ± 7.2% | - | - | ↓ |
| Prednisolone (level 2) | 81.29 ± 13.1% | - | ↓↓ | ↓↓↓ |
| 6-MP (level 3) | 106.73 ± 6.7% maximal effect after 48 h (134.33 ± 10.3%) | - | - | - |
| Infliximab (level 4) | 92.2 ± 6.2% | ↓ | - | ↓↓ |
| EGCG | 93.47 ± 14.8% maximal effect after 4 h (165.72 ± 4.6%) | - | ↓↓ | ↓↓↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnur, S.; Hans, F.; Dehne, A.; Osti, J.; Schneemann, M.-O.; Schneider, M.; Hittinger, M. The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2023, 16, 748. https://doi.org/10.3390/ph16050748
Schnur S, Hans F, Dehne A, Osti J, Schneemann M-O, Schneider M, Hittinger M. The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals. 2023; 16(5):748. https://doi.org/10.3390/ph16050748
Chicago/Turabian StyleSchnur, Sabrina, Fabian Hans, Annika Dehne, Janina Osti, Malte-Ole Schneemann, Marc Schneider, and Marius Hittinger. 2023. "The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease" Pharmaceuticals 16, no. 5: 748. https://doi.org/10.3390/ph16050748
APA StyleSchnur, S., Hans, F., Dehne, A., Osti, J., Schneemann, M.-O., Schneider, M., & Hittinger, M. (2023). The Potential of Epigallocatechin-3-gallate (EGCG) as Complementary Medicine for the Treatment of Inflammatory Bowel Disease. Pharmaceuticals, 16(5), 748. https://doi.org/10.3390/ph16050748








