The Effect of Statins on Ocular Disorders: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Results
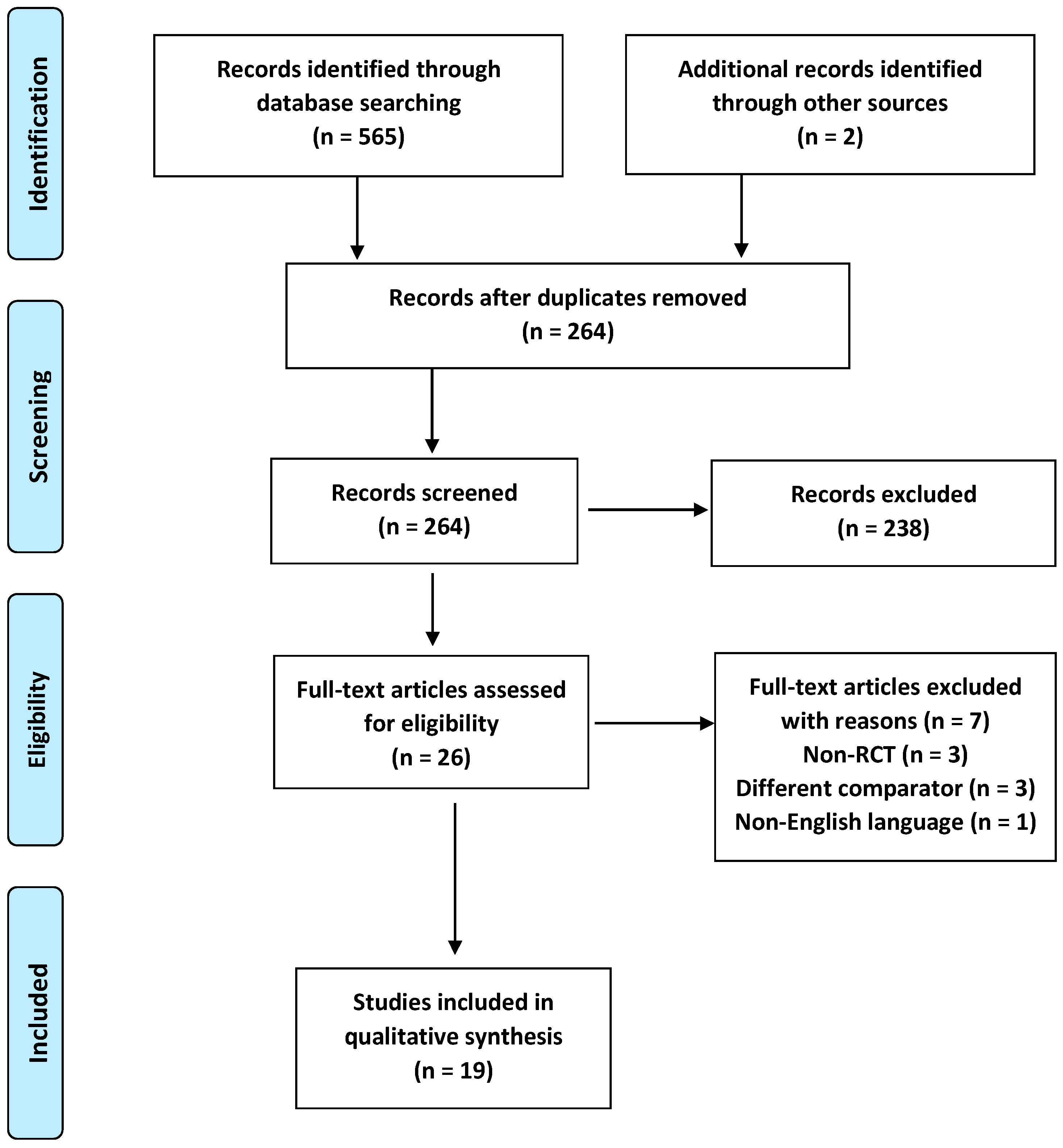
2.1. Study Selection
2.2. Study Characteristics
2.3. Outcomes of the Included Studies
2.4. Quality Appraisal
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design
4.2. Search Strategy
4.3. Eligibility Criteria
4.4. Data Extraction
4.5. Assessment of Risk of Bias
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toth, P.P.; Banach, M. Statins: Then and Now. Methodist Debakey Cardiovasc. J. 2019, 15, 23–31. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Elisaf, M.S.; Nair, D.R.; Mikhailidis, D.P. All for Statins and Statins for All; An Update. Curr. Pharm. Des. 2016, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L., 2nd; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cheung, B.M.; Tomlinson, B. Safety of statins: An update. Ther. Adv. Drug Saf. 2012, 3, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Bedi, O.; Dhawan, V.; Sharma, P.L.; Kumar, P. Pleiotropic effects of statins: New therapeutic targets in drug design. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 695–712. [Google Scholar] [CrossRef]
- Blanco-Colio, L.M.; Tunon, J.; Martin-Ventura, J.L.; Egido, J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003, 63, 12–23. [Google Scholar] [CrossRef]
- Ooi, K.G.; Khoo, P.; Vaclavik, V.; Watson, S.L. Statins in ophthalmology. Surv. Ophthalmol. 2019, 64, 401–432. [Google Scholar] [CrossRef]
- Olson, E.A.; Hainsworth, D.P.; Davis, G.; Hagan, J.C., 3rd. Eye on statins: A comprehensive review. MO Med. 2013, 110, 344–348. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Murakami, T.; Kato, S.; Shigeeda, T.; Itoh, H.; Komuro, I.; Takeuchi, M.; Yoshimura, N.; ophthalmology substudy of EMPATHY Investigators. Intensive treat-to-target statin therapy and severity of diabetic retinopathy complicated by hypercholesterolaemia. Eye 2021, 35, 2221–2228. [Google Scholar] [CrossRef]
- Maguire, M.G.; Ying, G.S.; McCannel, C.A.; Liu, C.; Dai, Y.; Complications of Age-related Macular Degeneration Prevention Trial (CAPT) Research Group. Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology 2009, 116, 2381–2385. [Google Scholar] [CrossRef] [PubMed]
- Al-Holou, S.N.; Tucker, W.R.; Agron, E.; Clemons, T.E.; Cukras, C.; Ferris, F.L., 3rd; Chew, E.Y.; Age-Related Eye Disease Study 2 Research, G. The Association of Statin Use with Age-Related Macular Degeneration Progression: The Age-Related Eye Disease Study 2 Report Number 9. Ophthalmology 2015, 122, 2490–2496. [Google Scholar] [CrossRef]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Lovato, J.F.; Perdue, L.H.; Greven, C.; Genuth, S.; Goff, D.C.; Leiter, L.A.; Ismail-Beigi, F.; et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014, 121, 2443–2451. [Google Scholar] [CrossRef]
- Gaede, P.; Lund-Andersen, H.; Parving, H.H.; Pedersen, O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008, 358, 580–591. [Google Scholar] [CrossRef]
- Tobert, J.A.; Shear, C.L.; Chremos, A.N.; Mantell, G.E. Clinical experience with lovastatin. Am. J. Cardiol. 1990, 65, 23F–26F. [Google Scholar] [CrossRef]
- Gehlbach, P.; Li, T.; Hatef, E. Statins for age-related macular degeneration. Cochrane Database Syst. Rev. 2016, 2016, CD006927. [Google Scholar] [CrossRef]
- Havel, R.J.; Hunninghake, D.B.; Illingworth, D.R.; Lees, R.S.; Stein, E.A.; Tobert, J.A.; Bacon, S.R.; Bolognese, J.A.; Frost, P.H.; Lamkin, G.E.; et al. Lovastatin (mevinolin) in the treatment of heterozygous familial hypercholesterolemia. A multicenter study. Ann. Intern. Med. 1987, 107, 609–615. [Google Scholar] [CrossRef]
- Bach, L.A.; Cooper, M.E.; O’Brien, R.C.; Jerums, G. The use of simvastatin, an HMG CoA reductase inhibitor, in older patients with hypercholesterolemia and atherosclerosis. J. Am. Geriatr. Soc. 1990, 38, 10–14. [Google Scholar] [CrossRef]
- Lundh, B.L.; Nilsson, S.E. Lens changes in matched normals and hyperlipidemic patients treated with simvastatin for 2 years. Acta Ophthalmol. 1990, 68, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Laties, A.M.; Shear, C.L.; Lippa, E.A.; Gould, A.L.; Taylor, H.R.; Hurley, D.P.; Stephenson, W.P.; Keates, E.U.; Tupy-Visich, M.A.; Chremos, A.N. Expanded clinical evaluation of lovastatin (EXCEL) study results. II. Assessment of the human lens after 48 weeks of treatment with lovastatin. Am. J. Cardiol. 1991, 67, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chylack, L.T., Jr.; Mantell, G.; Wolfe, J.K.; Friend, J.; Rosner, B. Lovastatin and the human lens; results of a two year study. The MSDRL Study Group. Optom. Vis. Sci. 1993, 70, 937–943. [Google Scholar] [CrossRef]
- Blankenhorn, D.H.; Azen, S.P.; Kramsch, D.M.; Mack, W.J.; Cashin-Hemphill, L.; Hodis, H.N.; DeBoer, L.W.; Mahrer, P.R.; Masteller, M.J.; Vailas, L.I.; et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS). Ann. Intern. Med. 1993, 119, 969–976. [Google Scholar] [CrossRef]
- Pedersen, T.R.; Berg, K.; Cook, T.J.; Faergeman, O.; Haghfelt, T.; Kjekshus, J.; Miettinen, T.; Musliner, T.A.; Olsson, A.G.; Pyorala, K.; et al. Safety and tolerability of cholesterol lowering with simvastatin during 5 years in the Scandinavian Simvastatin Survival Study. Arch. Intern. Med. 1996, 156, 2085–2092. [Google Scholar] [CrossRef]
- Harris, M.L.; Bron, A.J.; Brown, N.A.; Keech, A.C.; Wallendszus, K.R.; Armitage, J.M.; MacMahon, S.; Snibson, G.; Collins, R. Absence of effect of simvastatin on the progression of lens opacities in a randomised placebo controlled study. Oxford Cholesterol Study Group. Br. J. Ophthalmol. 1995, 79, 996–1002. [Google Scholar] [CrossRef]
- Fried, L.F.; Forrest, K.Y.; Ellis, D.; Chang, Y.; Silvers, N.; Orchard, T.J. Lipid modulation in insulin-dependent diabetes mellitus: Effect on microvascular outcomes. J. Diabetes Complicat. 2001, 15, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Misra, A.; Kumar, A.; Pandey, R.M. Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res. Clin. Pract. 2002, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, V.; Thapar, S.; Bhansali, A. Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am. J. Ophthalmol. 2004, 137, 675–682. [Google Scholar] [CrossRef]
- Ozkiris, A.; Erkilic, K.; Koc, A.; Mistik, S. Effect of atorvastatin on ocular blood flow velocities in patients with diabetic retinopathy. Br. J. Ophthalmol. 2007, 91, 69–73. [Google Scholar] [CrossRef]
- Narang, S.; Sood, S.; Kaur, B.; Singh, R.; Mallik, A.; Kaur, J. Atorvastatin in clinically-significant macular edema in diabetics with a normal lipid profile. Nepal. J. Ophthalmol. 2012, 4, 23–28. [Google Scholar] [CrossRef]
- Guymer, R.H.; Baird, P.N.; Varsamidis, M.; Busija, L.; Dimitrov, P.N.; Aung, K.Z.; Makeyeva, G.A.; Richardson, A.J.; Lim, L.; Robman, L.D. Proof of concept, randomized, placebo-controlled study of the effect of simvastatin on the course of age-related macular degeneration. PLoS ONE 2013, 8, e83759. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Gan, W.L.; Kawasaki, R.; Hodgson, L.; Lee, K.Y.; Wong, T.Y.; Lamoureux, E.; Robman, L.; Guymer, R. Effect of simvastatin on retinal vascular caliber: The Age-Related Maculopathy Statin Study. Acta Ophthalmol. 2013, 91, e418–e419. [Google Scholar] [CrossRef]
- Bang, C.N.; Greve, A.M.; La Cour, M.; Boman, K.; Gohlke-Barwolf, C.; Ray, S.; Pedersen, T.; Rossebo, A.; Okin, P.M.; Devereux, R.B.; et al. Effect of Randomized Lipid Lowering With Simvastatin and Ezetimibe on Cataract Development (from the Simvastatin and Ezetimibe in Aortic Stenosis Study). Am. J. Cardiol. 2015, 116, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Bosch, J.; Dagenais, G.; Zhu, J.; Xavier, D.; Liu, L.; Pais, P.; Lopez-Jaramillo, P.; Leiter, L.A.; Dans, A.; et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N. Engl. J. Med. 2016, 374, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Shirinsky, I.V.; Biryukova, A.A.; Shirinsky, V.S. Simvastatin as an Adjunct to Conventional Therapy of Non-infectious Uveitis: A Randomized, Open-Label Pilot Study. Curr. Eye Res. 2017, 42, 1713–1718. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Huang, H.; Cai, Y.; Xi, Y.; Bai, Y.; Ma, C. Effects of Rosuvastatin and Aspirin on Retinal Vascular Structures in Hypercholesterolemic Patients with Low-to-Moderate Risk of Coronary Artery Disease. Am. J. Cardiovasc. Drugs 2019, 19, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Albert, M.A.; Danielson, E.; Rifai, N.; Ridker, P.M. Effect of statin therapy on C-reactive protein levels: The pravastatin inflammation/CRP evaluation (PRINCE): A randomized trial and cohort study. JAMA 2001, 286, 64–70. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Gilbert, R.; Al-Janabi, A.; Tomkins-Netzer, O.; Lightman, S. Statins as anti-inflammatory agents: A potential therapeutic role in sight-threatening non-infectious uveitis. Porto Biomed. J. 2017, 2, 33–39. [Google Scholar] [CrossRef]
- Leuschen, J.; Mortensen, E.M.; Frei, C.R.; Mansi, E.A.; Panday, V.; Mansi, I. Association of statin use with cataracts: A propensity score-matched analysis. JAMA Ophthalmol. 2013, 131, 1427–1434. [Google Scholar] [CrossRef]
- Kostis, J.B.; Dobrzynski, J.M. Prevention of cataracts by statins: A meta-analysis. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 191–200. [Google Scholar] [CrossRef]
- Yu, S.; Chu, Y.; Li, G.; Ren, L.; Zhang, Q.; Wu, L. Statin Use and the Risk of Cataracts: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e004180. [Google Scholar] [CrossRef]
- Miyahara, S.; Kiryu, J.; Yamashiro, K.; Miyamoto, K.; Hirose, F.; Tamura, H.; Katsuta, H.; Nishijima, K.; Tsujikawa, A.; Honda, Y. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am. J. Pathol. 2004, 164, 1697–1706. [Google Scholar] [CrossRef]
- Weis, M.; Heeschen, C.; Glassford, A.J.; Cooke, J.P. Statins have biphasic effects on angiogenesis. Circulation 2002, 105, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Pranata, R.; Vania, R.; Victor, A.A. Statin reduces the incidence of diabetic retinopathy and its need for intervention: A systematic review and meta-analysis. Eur. J. Ophthalmol. 2021, 31, 1216–1224. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Du, J.; Wang, M.; Zhang, R.; Fu, Y. The association between statin use and risk of age-related macular degeneration. Sci. Rep. 2015, 5, 18280. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, E.; Heidari-Soureshjani, S. The Relationship between Statin and Risk of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2022, 2022, 8564818. [Google Scholar] [CrossRef] [PubMed]
- Borkar, D.S.; Tham, V.M.; Shen, E.; Parker, J.V.; Uchida, A.; Vinoya, A.C.; Acharya, N.R. Association between statin use and uveitis: Results from the Pacific Ocular Inflammation study. Am. J. Ophthalmol. 2015, 159, 707–713. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiong, R.; Wu, Y.; Ha, J.; Wang, W.; Han, X.; He, M. Associations of statin use with the onset and progression of open-angle glaucoma: A systematic review and meta-analysis. EClinicalMedicine 2022, 46, 101364. [Google Scholar] [CrossRef] [PubMed]


| First Author | Year | Country | Participants (No) | Study Duration | Comorbidities | Mean Age (Years) | Ocular disorder | Statin Therapy (Dosage) | Comparator | Outcomes | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Havel [17] | 1987 | US | 101 | 6 weeks | Heterozygous familial hypercholesterolemia | 44 | Cataract | Lovastatin (5–40 mg twice/day or 20 to 40 once/day) | Placebo | No change in the prevalence of lens opacities | N/A |
| Bach [18] | 1990 | Australia | 20 | 4 weeks | Hyperlipidemia, coronary artery disease | N/A | Cataract | Simvastatin (2.5, 5, 10, or 20 mg/day) | Placebo | No difference regarding lens opacities and visual acuity | N/A |
| Lundh [19] | 1990 | Sweden | 29 | 2 years | Hypercholesterolemia | N/A | Cataract | Simvastatin (10–20 mg twice/day) | Control | No harmful effect in the human lens | p = N/S |
| Laties [20] | 1991 | US | 8245 | 48 weeks | Hypercholesterolemia | 55 | Cataract | Lovastatin (20 or 40 mg once or twice/day) | Placebo | No effect on the human lens | p = N/S |
| Chylack [21] | 1993 | US | 192 | 2 years | Hypercholesterolemia | 53.5 | Cataract | Lovastatin (40 mg/day) | Placebo | Cataract progression showed no significant difference between the two groups | p = N/S (Only for nuclear cataract in right eyes, p < 0.02) |
| Blankenhorn [22] | 1993 | US | 270 | 4 years | Coronary artery disease | 58 | Cataract | Lovastatin (40 mg twice/day) | Placebo | No difference was found between groups in new onset or worsening of lens opacities | N/A |
| Pedersen [23] | 1994 | Scandinavian countries | 4444 | 5.4 years | Angina pectoralis or previous myocardial infarction, hypercholesterolemia | 58.9 | Cataract | Simvastatin (20–40 mg once/day) | Placebo | No difference between the two groups | p = 0.19 |
| Harris [24] | 1995 | UK | 474 | 18 months | High risk of coronary heart disease | 53.9 | Cataract | Simvastatin (20 or 40 mg/day) | Placebo | No differences between the two groups | N/A |
| Fried [25] | 2000 | US | 39 | 2 years | Insulin-dependent diabetes mellitus | 32.1 | Diabetic Retinopathy | Simvastatin | Diet | No difference in change in retinopathy status | N/A |
| Sen [26] | 2001 | India | 50 | 180 days | Diabetes mellitus, hypercholesterolemia | 53.9 | Diabetic Retinopathy | Simvastatin (20 mg/day) | Placebo | Delay the progression of diabetic retinopathy | p = 0.009 |
| Gupta [27] | 2004 | India | 30 | 18 weeks | Diabetes, Hyperlipidemia, macular edema with hard exudates | 54.1 | Diabetic macular edema | Atorvastatin (10 mg/day) | Control | Reduction of hard exudates and none of the patients suffered from subfoveal lipid migration | p = 0.07 |
| Ozkiris [28] | 2006 | Turkey | 45 | 10 weeks | Diabetes type 2 | 58.3 | Diabetic Retinopathy | Atorvastatin (10 mg/day) | Placebo | Vascular resistance improvement, decrease of mean peak systolic velocity of the ophthalmic artery and the central retinal artery | p < 0.05 |
| Narang [29] | 2012 | India | 30 | 6 months | Non-insulin-dependent diabetes, non-proliferative diabetic retinopathy with clinically significant macular edema (CSME) | 55.9 | CSME | Atorvastatin (20 mg/day) | Placebo | No significant difference in visual acuity, macular edema, and hard exudate among the two groups | p = 0.39 for visual acuity p = 0.62 for macular edema |
| Guymer [30] | 2013 | Australia | 114 | 6 years | Non-advanced AMD | 74.6 | AMD | Simvastatin (40 mg/day) | Placebo | Simvastatin retarded the AMD progression compared to the placebo group | p = 0.047 |
| Sasaki [31] | 2013 | Australia | 102 | 3 years | Non-advanced AMD | N/A | Retinal vascular diseases | Simvastatin (40 mg/day) | Placebo | Simvastatin group had a significantly larger retinal arteriolar caliber compared to the control group | p = 0.443 |
| Bang [32] | 2015 | Denmark | 1873 | 4.3 years | Asymptomatic aortic stenosis | 67.5 | Cataract | Simvastatin (40 mg/day) | Placebo | Simvastatin plus ezetimibe was associated with a 44% lower risk of cataract | p = 0.034 |
| Yusuf [33] | 2016 | N/A | 12,705 | 5.6 years | Cardiovascular risk factors | 65.7 | Cataract | Rosuvastatin (10 mg/day) | Placebo | More patients in the rosuvastatin group needed cataract surgery compared to the placebo group | p = 0.02 |
| Shrinsky [34] | 2017 | Russia | 50 | 2 months | Active non-infectious uveitis | 43.9 | Non-infectious uveitis | Simvastatin (40 mg/day) | Control | Patients in the simvastatin group received significantly less steroid treatment and showed an improvement in visual acuity and reduction in ocular inflammation. | p < 0.001 |
| Li [35] | 2019 | China | 127 | 12 months | Hypercholesterolemia | 53.7 | Retinal vascular diseases | Rosuvastatin (10 mg/day) | Control | A significant effect of rosuvastatin on retinal microvasculature was detected, including artery vein ratio increase, venular constriction, and arteriolar dilation | p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lymperopoulou, C.; Kandarakis, S.A.; Tzanaki, I.; Mylona, I.; Xanthos, T.; Agouridis, A.P. The Effect of Statins on Ocular Disorders: A Systematic Review of Randomized Controlled Trials. Pharmaceuticals 2023, 16, 711. https://doi.org/10.3390/ph16050711
Lymperopoulou C, Kandarakis SA, Tzanaki I, Mylona I, Xanthos T, Agouridis AP. The Effect of Statins on Ocular Disorders: A Systematic Review of Randomized Controlled Trials. Pharmaceuticals. 2023; 16(5):711. https://doi.org/10.3390/ph16050711
Chicago/Turabian StyleLymperopoulou, Charoula, Stylianos A. Kandarakis, Ismini Tzanaki, Ioanna Mylona, Theodoros Xanthos, and Aris P. Agouridis. 2023. "The Effect of Statins on Ocular Disorders: A Systematic Review of Randomized Controlled Trials" Pharmaceuticals 16, no. 5: 711. https://doi.org/10.3390/ph16050711
APA StyleLymperopoulou, C., Kandarakis, S. A., Tzanaki, I., Mylona, I., Xanthos, T., & Agouridis, A. P. (2023). The Effect of Statins on Ocular Disorders: A Systematic Review of Randomized Controlled Trials. Pharmaceuticals, 16(5), 711. https://doi.org/10.3390/ph16050711






