New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review
Abstract
1. Introduction
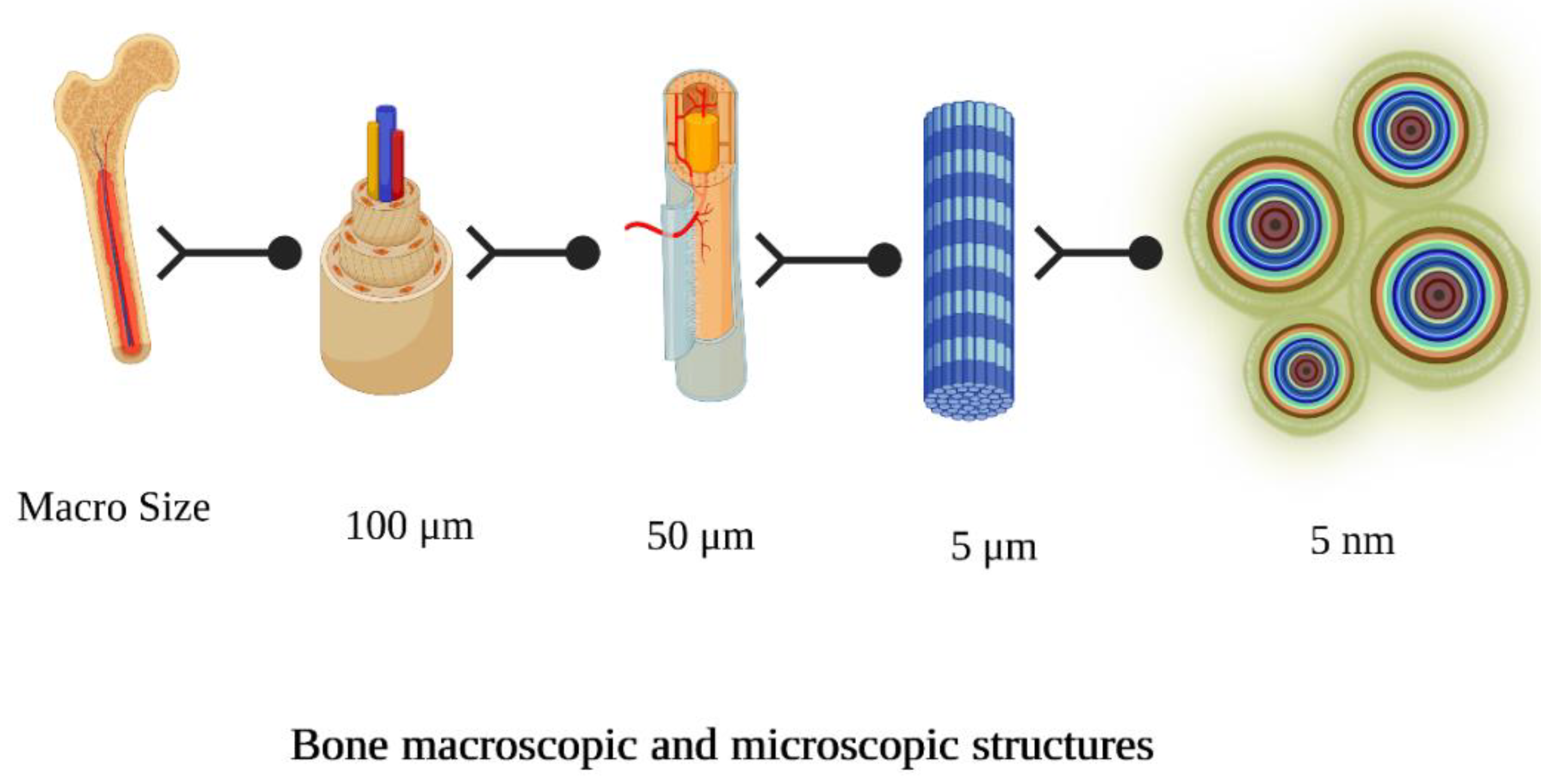
2. Bio Scaffolds for Dental and Osseous Tissue Engineering
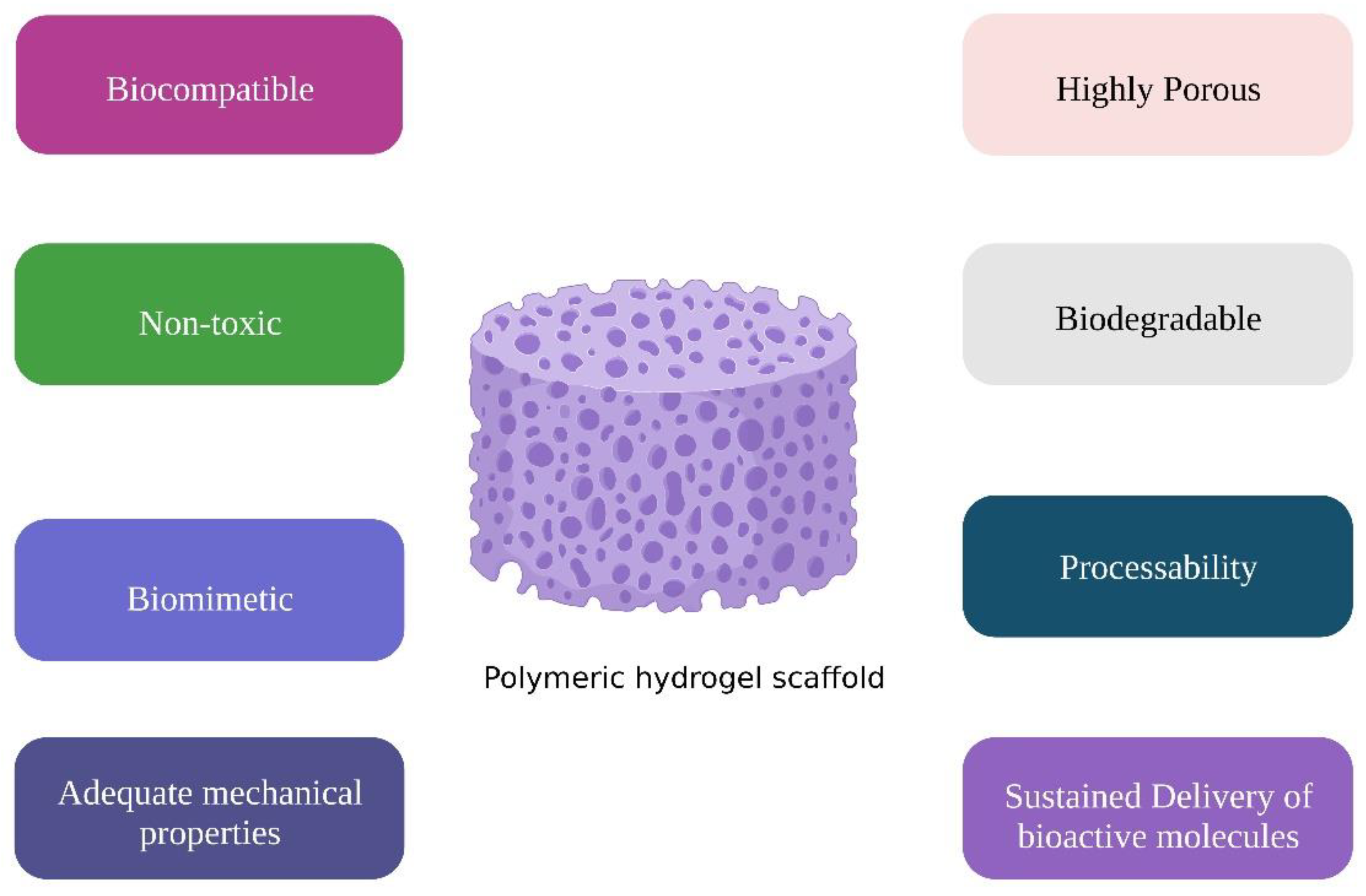
3. Pre-Requisites of Hydrogels for Dental and Osseous Regeneration
3.1. Cytotoxicity
3.2. Biological Responses
3.3. Mechanical Characteristics
3.4. Degradation
4. Classification of Hydrogels
5. Chemical Structure of Polymeric Hydrogels
5.1. Natural Polymers
5.1.1. Polysaccharides
Chitosan
Alginate
Hyaluronic Acid
Cellulose
Starch
Xyloglucan
Cyclodextrin
Dextran
Carrageenan
Gum
5.1.2. Proteins
Albumin
Collagen
Gelatin
Fibrinogen
Silk Fibroin
5.2. Synthetic Polymers
5.2.1. Polyethylene glycol (PEG)
5.2.2. Poly(a-Hydroxy Esters)
5.2.3. Poly (N-Isopropyl Acrylamide)
5.2.4. Pluronic Block Copolymers
5.3. Hybrid Polymeric Hydrogels
6. Composition of Polymeric Frameworks
7. Configuration of Polymeric Matrices
- (a)
- Amorphous.
- (b)
- Semicrystalline.
- (c)
- Crystalline.
8. Gelation Methods
8.1. Physically Crosslinked Hydrogels
8.1.1. Ionic Crosslinked Hydrogels
8.1.2. Hydrogen Bond Crosslinked Hydrogels
8.1.3. Thermally Triggered Hydrogels
8.2. Chemically Crosslinked Hydrogels
9. Classification of Hydrogels as Various Hydrogel Structure Used in Bone Regeneration
9.1. Hydrogel Microbeads
9.2. Hydrogel Nanoparticles
9.3. Hydrogel Fibers
9.4. Injectable Hydrogels
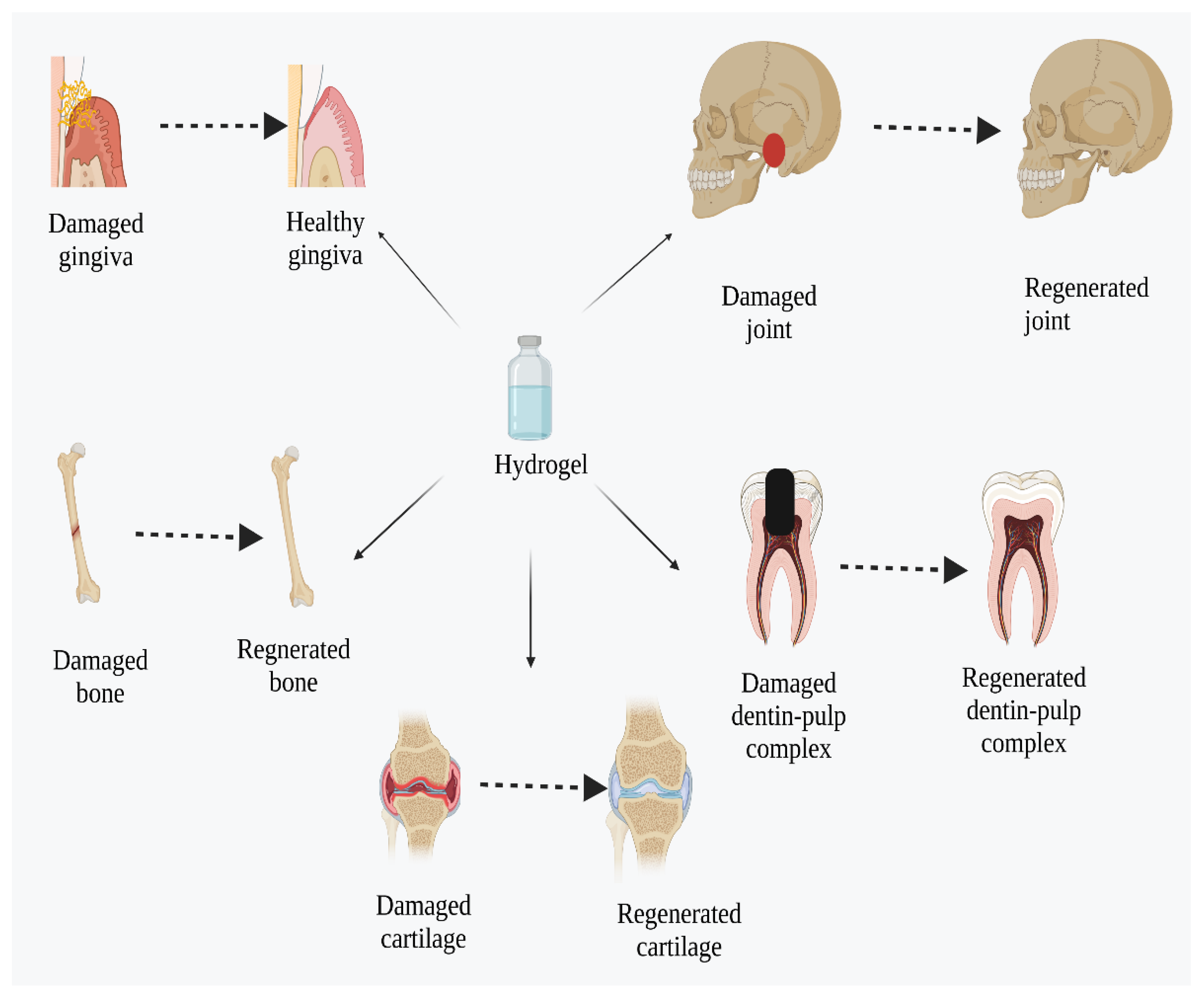
10. Application of Hydrogels in Dental and Osseous Regeneration
10.1. Dentin-Pulp Complex Regeneration
10.2. Cementum Regeneration
10.3. Gingival Tissue Regeneration
10.4. Periodontal Regeneration
10.5. Bone Regeneration
10.6. Cartilage Repair
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanholder, R.; Domínguez-Gil, B.; Busic, M.; Cortez-Pinto, H.; Craig, J.C.; Jager, K.J.; Mahillo, B.; Stel, V.S.; Valentin, M.O.; Zoccali, C.; et al. Organ donation and transplantation: A multi-stakeholder call to action. Nat. Rev. Nephrol. 2021, 17, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Atala, A. Advances in tissue and organ replacement. Curr. Stem. Cell. Res. Ther. 2008, 3, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.Y.; Sha’ban, M.J.R. Regeneration of Human Body Parts via Tissue Engineering and Regenerative Medicine: A Brief Insight into the Technology from Islamic Perspective. Revel. Sci. 2015, 5, 10–17. [Google Scholar]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nanotoday 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Jain, A.; Bansal, R. Applications of regenerative medicine in organ transplantation. J. Pharm. Bioallied. Sci. 2015, 7, 188–194. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- Dayoub, J.C.; Cortese, F.; Anžič, A.; Grum, T.; de Magalhães, J.P. The effects of donor age on organ transplants: A review and implications for aging research. Exp. Gerontol. 2018, 110, 230–240. [Google Scholar] [CrossRef]
- Lancet, S.C. Organ trafficking and transplant tourism and commercialism: The Declaration of Istanbul. Clin. J. Am. Soc. Nephrol. 2008, 372, 5–6. [Google Scholar]
- Evans, R.W.; Manninen, D.L.; Garrison, L.P.; Maier, A.M. Donor availability as the primary determinant of the future of heart transplantation. JAMA 1986, 255, 1892–1898. [Google Scholar] [CrossRef]
- Thorne, M.C. Responding to radiation accidents: What more do we need to know? J. Radiol. Prot. 2022, 42, 031003. [Google Scholar] [CrossRef]
- Millán, G.O. Organ donation and presumed consent. Objetions to its implementation in Mexico Donación de órganos y consentimiento tácito. Med. Ética 2019, 1, 151–180. [Google Scholar]
- Sevari, S.P.; Shahnazi, F.; Chen, C.; Mitchell, J.C.; Ansari, S.; Moshaverinia, A. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J. Biomed. Mater. Res. A. 2020, 108, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Abdel Nasser Atia, G.; Shalaby, H.K.; Zehravi, M.; Ghobashy, M.M.; Ahmad, Z.; Khan, F.S.; Dey, A.; Rahman, M.H.; Joo, S.W.; Barai, H.R. Locally Applied Repositioned Hormones for Oral Bone and Periodontal Tissue Engineering: A Narrative Review. Polymers 2022, 14, 2964. [Google Scholar] [CrossRef]
- Patil, A.S.; Merchant, Y.; Nagarajan, P. Tissue engineering of craniofacial tissues—A review. J. Regen. Med. Tissue Eng. 2013, 2, 1–20. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive manufacturing of material scaffolds for bone regeneration: Toward application in the clinics. Adv. Func. Mat. 2021, 31, 2006967. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Seagroves, J.T.; Chen, C.; Shah, K.; Aghaloo, T.; Wu, B.M.; Bencharit, S.; Moshaverinia, A. Dental and orofacial mesenchymal stem cells in craniofacial regeneration: The prosthodontist’s point of view. J. Prosthet. Dent. 2017, 118, 455–461. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110716. [Google Scholar] [CrossRef]
- Gazzaneo, I.; Vieira, G.C.; Pérez, A.R.; Alves, F.R.; Gonçalves, L.S.; Mdala, I.; Siqueira, J.F., Jr.; Rôças, I.N. Root canal disinfection by single-and multiple-instrument systems: Effects of sodium hypochlorite volume, concentration, and retention time. J. Endod. 2019, 45, 736–741. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Ishikawa, I.; Ando, T.; Okano, T. Tissue engineering in periodontal tissue. Anat. Rec. 2014, 297, 16–25. [Google Scholar] [CrossRef]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Betz, R.R. Limitations of autograft and allograft: New synthetic solutions. Orthopedics 2002, 25, S561–S570. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Joint Surg. Am. 2008, 90, 36–42. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Shue, L.; Yufeng, Z.; Mony, U. Biomaterials for periodontal regeneration: A review of ceramics and polymers. Biomatter 2012, 2, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.N.; Cavalu, S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef]
- Lee, T.C.; Niederer, P. Basic Engineering for Medics and Biologists: An ESEM Primer; IOS Press: Amsterdam, The Netherlands, 2010; Volume 152. [Google Scholar]
- Zhou, G.; Growth, T. Host Responses to Biomaterials and Anti-Inflammatory Design—A Brief Review. Macromol. Biosci. 2018, 18, 1800112. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.W. Emerging properties of hydrogels in tissue engineering. J. Tissue Eng. 2018, 9, 2041731418768285. [Google Scholar] [CrossRef]
- Chung, S.; King, M.W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 2011, 58, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 9, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Paul, A.; Shakesheff, K.M. Injectable scaffolds for tissue regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar] [CrossRef]
- He, S.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Injectable biodegradable polymer composites based on poly (propylene fumarate) crosslinked with poly (ethylene glycol)-dimethacrylate. Biomaterials 2000, 21, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.R.; Lu, L.; Kim, C.; Zhu, X.; Dhert, W.J.A.; Currier, B.L.; Yaszemski, M.J. Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly (propylene fumarate). J. Biomed. Mater. Res. Part A 2006, 77, 103–111. [Google Scholar] [CrossRef]
- Shung, A.K.; Behravesh, E.; Jo, S.; Mikos, A.G. Crosslinking characteristics of and cell adhesion to an injectable poly (propylene fumarate-co-ethylene glycol) hydrogel using a water-soluble crosslinking system. Tissue Eng. 2003, 9, 243–254. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, Biodegradable Hydrogels for Tissue Engineering Applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Gavasane, A.J.; Pawar, H.A. Synthetic biodegradable polymers used in controlled drug delivery system: An overview. Clin. Pharmacol. Biopharm. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Timmer, M.D.; Ambrose, C.G.; Mikos, A.G. In vitro degradation of polymeric networks of poly (propylene fumarate) and the crosslinking macromer poly (propylene fumarate)-diacrylate. Biomaterials 2003, 24, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L.J. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef]
- Moghadam, E.T.; Yazdanian, M.; Alam, M.; Tebyanian, H.; Tafazoli, A.; Tahmasebi, E.; Ranjbar, R.; Yazdanian, A.; Seifalian, A. Current natural bioactive materials in bone and tooth regeneration in dentistry: A comprehensive overview. J. Mater. Res. Technol. 2021, 13, 2078–2114. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Wieckiewicz, M.; Owczarek, A.; Wezgowiec, J. Natural polymers for the maintenance of oral health: Review of recent advances and perspectives. Int. J. Mol. Sci. 2021, 22, 10337. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Yan, X.-Z.; van den Beucken, J.; Cai, X.; Yu, N.; Jansen, J.A.; Yang, F. Periodontal tissue regeneration using enzymatically solidified chitosan hydrogels with or without cell loading. Tissue Eng. Part A 2015, 21, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A. Gingival mesenchymal stem cell (GMSC) Delivery system based on RGD-coupled alginate hydrogel with antimicrobial properties: A novel treatment modality for peri-implantitis. J. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef]
- Devillard, R.; Rémy, M.; Kalisky, J.; Bourget, J.M.; Kérourédan, O.; Siadous, R.; Bareille, R.; Amédée-Vilamitjana, J.; Chassande, O.; Fricain, J.C. In vitro assessment of a collagen/alginate composite scaffold for regenerative endodontics. Int. Endod. J. 2017, 50, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Dobie, K.; Smith, G.; Sloan, A.J.; Smith, A.J. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Niloy, K.K.; Gulfam, M.; Compton, K.B.; Li, D.; Huang, G.T.J.; Lowe, T.L. Methacrylated hyaluronic acid–based hydrogels maintain stemness in human dental pulp stem cells. Regen. Eng. Transl. Med. 2020, 6, 262–272. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef]
- Huang, C.; Soenen, S.J.; van Gulck, E.; Vanham, G.; Rejman, J.; Van Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 2012, 33, 962–969. [Google Scholar] [CrossRef]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug delivery systems based on titania nanotubes and active agents for enhanced osseointegration of bone implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar] [CrossRef]
- Ding, L.; Huang, Q.; Li, H.; Wang, Z.; Fu, X.; Zhang, B. Controlled gelatinization of potato parenchyma cells under excess water condition: Structural and in vitro digestion properties of starch. Food Funct. 2019, 10, 5312–5322. [Google Scholar] [CrossRef]
- Katoch, A.; Choudhury, A.R. Understanding the rheology of novel guar-gellan gum composite hydrogels. Mater. Lett. 2020, 263, 127234. [Google Scholar] [CrossRef]
- Morrison, W.R.; Tester, R.F.; Snape, C.E.; Law, R.; Gidley, M.J. Swelling and gelatinization of cereal starches. IV: Some effects of lipid-complexed amylose and free amylose in waxy and normal barley starches. Cereal Chem. 1993, 70, 385–391. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X.J. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Nigam, P.; Singh, D. Enzyme and microbial systems involved in starch processing. Enzym. Microb. Technol. 1995, 17, 770–778. [Google Scholar] [CrossRef]
- Ali, A.; Rehman, A.; Shehzad, Q.; Khan, S.; Karim, A.; Afzal, N.; Hussain, A.; Yang, F.; Xia, W.J. Development and Characterization of Nanoemulsions Incorporating Tuna Fish Oil. Int. J. Res. Agric. Sci. 2020, 7, 2348–3997. [Google Scholar]
- Bhattacharjee, A.; Bose, S. 3D printed hydroxyapatite—Zn2+ functionalized starch composite bone grafts for orthopedic and dental applications. Mater. Design 2022, 221, 110903. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V.J. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macromol. 2017, 104, 799–812. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kulkarni, A.D.; Belgamwar, V.S.; Surana, S.J. Xyloglucan for drug delivery applications. In Fundamental Biomaterials: Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–169. [Google Scholar]
- Muscolino, E.; Di Stefano, A.B.; Trapani, M.; Sabatino, M.A.; Giacomazza, D.; Moschella, F.; Cordova, A.; Toia, F.; Dispenza, C. Injectable xyloglucan hydrogels incorporating spheroids of adipose stem cells for bone and cartilage regeneration. Mater. Sci. Eng. C 2021, 131, 112545. [Google Scholar] [CrossRef]
- Hirun, N.; Tantishaiyakul, V.; Sangfai, T.; Ouiyangkul, P.; Li, L. In situ mucoadhesive hydrogel based on methylcellulose/xyloglucan for periodontitis. J. Sol-Gel Sci. Technol. 2019, 89, 531–542. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Tamura, A.; Arisaka, Y.; Masuda, H.; Yoda, T.; Yui, N. Cyclodextrin-based supramolecular complexes of osteoinductive agents for dental tissue regeneration. Pharmaceutics 2021, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhao, Y.-M.; Zhang, R.; Jin, T.; Sun, H.-H.; Wu, Z.-F.; Jin, Y. Periodontal regeneration using novel glycidyl methacrylated dextran (Dex-GMA)/gelatin scaffolds containing microspheres loaded with bone morphogenetic proteins. J. Control. Release 2007, 121, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L. Toxicological properties of carrageenan. Agents Actions 1991, 32, 46–51. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. A. 2010, 92, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, S.M.; Popa, E.G.; Reis, R.L.; Marques, A.P.; Gomes, M.E. Fabrication of endothelial cell-laden carrageenan microfibers for microvascularized bone tissue engineering applications. Biomacromolecules 2014, 15, 2849–2860. [Google Scholar] [CrossRef]
- Ocampo, J.I.G.; de Paula, M.M.M.; Bassous, N.J.; Lobo, A.O.; Orozco, C.P.O.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Acta Biomater. 2019, 83, 425–434. [Google Scholar] [CrossRef]
- Feng, W.; Feng, S.; Tang, K.; He, X.; Jing, A.; Liang, G.J. A novel composite of collagen-hydroxyapatite/kappa-carrageenan. J. Alloys Compd. 2017, 693, 482–489. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R.J. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef]
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.-T.V.; Thomas, S.; Huang, Y.-C.; Kong, Z.-L. Therapeutic effects of antibiotics loaded cellulose nanofiber and κ-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037. [Google Scholar] [CrossRef]
- Li, D.; Hu, X.; Zhang, S. Biodegradation of graphene-based nanomaterials in blood plasma affects their biocompatibility, drug delivery, targeted organs and antitumor ability. Biomaterials 2019, 202, 12–25. [Google Scholar] [CrossRef]
- Yasui, T.; Matsuki, J.; Sasaki, T.; Yamamori, M.J. Amylose and lipid contents, amylopectin structure, and gelatinisation properties of waxy wheat (Triticum aestivum) starch. J. Cereal Sci. 1996, 24, 131–137. [Google Scholar] [CrossRef]
- Weaver, C.L.; Cui, X.T. Directed neural stem cell differentiation with a functionalized graphene oxide nanocomposite. Adv. Healthc. Mater. 2015, 4, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Pańczyszyn, E.; Jaśko, M.; Miłek, O.; Niedziela, M.; Męcik-Kronenberg, T.; Hoang-Bujnowicz, A.; Zięba, M.; Adamus, G.; Kowalczuk, M.; Osyczka, A.M.; et al. Gellan gum hydrogels cross-linked with carbodiimide stimulates vacuolation of human tooth-derived stem cells in vitro. Toxicol. Vitr. 2021, 73, 105111. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Jolly, R.; Zia, I.; Saad Umar, M.; Owais, M.; Shakir, M. Bioactive Gum Arabic/κ-Carrageenan-Incorporated Nano-Hydroxyapatite Nanocomposites and Their Relative Biological Functionalities in Bone Tissue Engineering. ACS Omega 2020, 5, 11279–11290. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Pavithra, U.; Sivaranjani, V.; Balasubramanian, N.; Sakthivel, K.M.; Pruncu, C.I. A novel Ag/carrageenan–gelatin hybrid hydrogel nanocomposite and its biological applications: Preparation and characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104257. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X.J. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef]
- Aminu, N.; Chan, S.-Y.; Yam, M.-F.; Toh, S.-M.J. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019, 570, 118659. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C.J. Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- González Ocampo, J.I.; Bassous, N.; Ossa Orozco, C.P.; Webster, T.J. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J. Biomed. Mater. Res. A 2018, 106, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-L.; Jung, G.-Y.; Yoon, J.-H.; Han, J.-S.; Park, Y.-J.; Kim, D.-G.; Zhang, M.; Kim, D.-J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Smith, J.; Smith, A.; Shelton, R.; Cooper, P.J. Dental pulp cell behavior in biomimetic environments. J. Dent. Res. 2015, 94, 1552–1559. [Google Scholar] [CrossRef]
- Almeida, L.D.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E.J. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar] [CrossRef]
- Zhu, N.; Chatzistavrou, X.; Ge, L.; Qin, M.; Papagerakis, P.; Wang, Y.J. Biological properties of modified bioactive glass on dental pulp cells. J Dent. 2019, 83, 18–26. [Google Scholar] [CrossRef]
- Rosenoer, V.M.; Oratz, M.; Rothschild, M.A. Albumin: Structure, Function and Uses; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Anbouhi, T.S.; Esfidvajani, E.M.; Nemati, F.; Haghighat, S.; Sari, S.; Attar, F.; Pakaghideh, A.; Sohrabi, M.J.; Mousavi, S.E.; Falahati, M.J. Albumin binding, anticancer and antibacterial properties of synthesized zero valent iron nanoparticles. Int. J. Nanomed. 2019, 14, 243. [Google Scholar] [CrossRef]
- Bernards, M.T.; Qin, C.; Jiang, S. MC3T3-E1 cell adhesion to hydroxyapatite with adsorbed bone sialoprotein, bone osteopontin, and bovine serum albumin. Colloids Surf. B Biointerfaces 2008, 64, 236–247. [Google Scholar] [CrossRef]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-based hydrogels for regenerative engineering and cell transplantation. Biotechnol. Bioeng. 2019, 116, 3457–3468. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Xiong, S.; Tian, T.; Liu, T.; Sun, Y.J. Chitosan-stablized bovine serum albumin nanoparticles having ability to control the release of NELL-1 protein. Int. J. Biol. Macromol. 2018, 109, 672–680. [Google Scholar] [CrossRef]
- Hirose, M.; Tachibana, A.; Tanabe, T. Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Mater. Sci. Eng. C 2010, 30, 664–669. [Google Scholar] [CrossRef]
- Li, P.-S.; Liang Lee, I.; Yu, W.-L.; Sun, J.-S.; Jane, W.-N.; Shen, H.-H. A Novel Albumin-Based Tissue Scaffold for Autogenic Tissue Engineering Applications. Sci. Rep. 2014, 4, 5600. [Google Scholar] [CrossRef] [PubMed]
- El Blidi, O.; El Omari, N.; Balahbib, A.; Ghchime, R.; El Menyiy, N.; Ibrahimi, A.; Ben Kaddour, K.; Bouyahya, A.; Chokairi, O.; Barkiyou, M. Extraction methods, characterization and biomedical applications of collagen: A review. Biointerface Res. Appl. Chem. 2021, 11, 13587–13613. [Google Scholar]
- Radhakrishnan, S.; Nagarajan, S.; Bechelany, M.; Kalkura, S. Collagen based biomaterials for tissue engineering applications: A review. In Lecture Notes in Earth System Sciences; Springer: Cham, Switzerland, 2020; pp. 3–22. [Google Scholar]
- Struillou, X.; Fruchet, A.; Rakic, M.; Badran, Z.; Réthoré, G.; Sourice, S.; Fellah, B.H.; Le Guéhennec, L.; Gauthier, O.; Weiss, P. Evaluation of a hydrogel membrane on bone regeneration in furcation periodontal defects in dogs. Dent. Mater. J. 2018, 37, 825–834. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.-J.; Min, K.-S. Effects of proanthocyanidin, a crosslinking agent, on physical and biological properties of collagen hydrogel scaffold. Restor. Dent. Endod. 2016, 41, 296–303. [Google Scholar] [CrossRef]
- Momose, T.; Miyaji, H.; Kato, A.; Ogawa, K.; Yoshida, T.; Nishida, E.; Murakami, S.; Kosen, Y.; Sugaya, T.; Kawanami, M. Collagen hydrogel scaffold and fibroblast growth factor-2 accelerate periodontal healing of class II furcation defects in dog. Open Dent. J. 2016, 10, 347. [Google Scholar] [CrossRef]
- Zuo, Y.; Xiao, W.; Chen, X.; Tang, Y.; Luo, H.; Fan, H. Bottom-up approach to build osteon-like structure by cell-laden photocrosslinkable hydrogel. Chem. Commun. 2012, 48, 3170–3172. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 2544–2553. [Google Scholar] [CrossRef]
- He, X.-T.; Li, X.; Xia, Y.; Yin, Y.; Wu, R.-X.; Sun, H.-H.; Chen, F.-M. Building capacity for macrophage modulation and stem cell recruitment in high-stiffness hydrogels for complex periodontal regeneration: Experimental studies in vitro and in rats. Acta Biomater. 2019, 88, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Riedelová-Reicheltová, Z.; Brynda, E.; Riedel, T. Fibrin nanostructures for biomedical applications. Physiol. Res. 2016, 65, S263. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.C.; Frese, J.; Sachweh, J.S.; Diamantouros, S.; Koch, S.; Schmitz-Rode, T.; Jockenhoevel, S. The Use of Fibrin as an Autologous Scaffold Material for Cardiovascular Tissue Engineering Applications: From In Vitro to In Vivo Evaluation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 2186–2189. [Google Scholar]
- Kretlow, J.D.; Young, S.; Klouda, L.; Wong, M.; Mikos, A.G. Injectable biomaterials for regenerating complex craniofacial tissues. Adv. Mater. 2009, 21, 3368–3393. [Google Scholar] [CrossRef] [PubMed]
- Rajangam, T.; An, S.S.A. Fibrinogen and fibrin based micro and nano scaffolds incorporated with drugs, proteins, cells and genes for therapeutic biomedical applications. Int. J. Nanomed. 2013, 8, 3641–3662. [Google Scholar]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wnek, G.E.; Carr, M.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of nanofiber fibrinogen structures. Nano Lett. 2003, 3, 213–216. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Development of novel fibrinogen nanoparticles by two-step co-acervation method. Int. J. Biol. Macromol. 2010, 47, 37–43. [Google Scholar] [CrossRef]
- Swartz, D.D.; Russell, J.A.; Andreadis, S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. -Heart Circ. Physiol. 2005, 288, H1451–H1460. [Google Scholar] [CrossRef]
- Rajangam, T.; Paik, H.-J.; An, S.-S.A. Fabricating fibrinogen microfibers with aligned nanostructure, as biodegradable threads for tissue engineering. Bull. Korean Chem. Soc. 2012, 33, 2075–2078. [Google Scholar] [CrossRef]
- Rajangam, T.; Paik, H.-J.; An, S.S.A. Development of fibrinogen microspheres as a biodegradable carrier for tissue engineering. BioChip J. 2011, 5, 175–183. [Google Scholar] [CrossRef]
- Kaplan, D.; Adams, W.W.; Farmer, B.; Viney, C. Silk Polymers: Materials Science and Biotechnology; ACS Publications: Washington, DC, USA, 1993. [Google Scholar]
- Wray, L.S.; Rnjak-Kovacina, J.; Mandal, B.B.; Schmidt, D.F.; Gil, E.S.; Kaplan, D.L. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials 2012, 33, 9214–9224. [Google Scholar] [CrossRef]
- Wang, X.; Kluge, J.A.; Leisk, G.G.; Kaplan, D.L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 2008, 29, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Souron, J.-B.; Petiet, A.; Decup, F.; Tran, X.V.; Lesieur, J.; Poliard, A.; Le Guludec, D.; Letourneur, D.; Chaussain, C.; Rouzet, F. Pulp cell tracking by radionuclide imaging for dental tissue engineering. Tissue Eng. Part C Methods 2014, 20, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.F.P.; Pérez-Guerrero, J.A.; Gomes, M.J.P.; Cavalcante, F.L.; Souza Filho, M.S.M.; Castro, S., II. Development and characterization of poultry collagen-based hybrid hydrogels for bone regeneration. Acta Cir. Bras. 2022, 37, e370302. [Google Scholar] [CrossRef]
- Kakudo, N.; Morimoto, N.; Ogawa, T.; Taketani, S.; Kusumoto, K. FGF-2 combined with bilayer artificial dermis composed of collagen matrix prompts generation of fat pad in subcutis of mice. Med. Mol. Morphol. 2019, 52, 73–81. [Google Scholar] [CrossRef]
- Bekhouche, M.; Bolon, M.; Charriaud, F.; Lamrayah, M.; Da Costa, D.; Primard, C.; Costantini, A.; Pasdeloup, M.; Gobert, S.; Mallein-Gerin, F.J. Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J. Mater. Chem. B 2020, 8, 8422–8432. [Google Scholar] [CrossRef]
- Burger, D.; Beaumont, M.; Rosenau, T.; Tamada, Y. Porous Silk Fibroin/Cellulose Hydrogels for Bone Tissue Engineering via a Novel Combined Process Based on Sequential Regeneration and Porogen Leaching. Molecules 2020, 25, 5097. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. Biodegradable PEG-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lu, L.-X.; Shi, J.-C.; Wang, H.-F.; Xiao, Z.-D.; Huang, N.-P. Introducing RGD peptides on PHBV films through PEG-containing cross-linkers to improve the biocompatibility. Biomacromolecules 2011, 12, 551–559. [Google Scholar] [CrossRef]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in situ forming collagen–PEG hydrogel for tissue regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Saghebasl, S.; Davaran, S.; Rahbarghazi, R.; Montaseri, A.; Salehi, R.; Ramazani, A.J. Polymer edition. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering. J. Biomater. Sci. Polym. Ed. 2018, 29, 1185–1206. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Hälg, G.A.; Thoma, D.S.; Hämmerle, C.H.F. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin. Oral Implant. Res. 2009, 20, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Pandya, M.; Rufaihah, A.J.; Rosa, V.; Tong, H.J.; Seliktar, D.; Toh, W.S. Modulation of dental pulp stem cell odontogenesis in a tunable PEG-fibrinogen hydrogel system. Stem Cells Int. 2015, 2015, 525367. [Google Scholar] [CrossRef] [PubMed]
- Ghandforoushan, P.; Hanaee, J.; Aghazadeh, Z.; Samiei, M.; Navali, A.M.; Khatibi, A.; Davaran, S. Novel nanocomposite scaffold based on gelatin/PLGA-PEG-PLGA hydrogels embedded with TGF-β1 for chondrogenic differentiation of human dental pulp stem cells in vitro. Int. J. Biol. Macromol. 2022, 201, 270–287. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Gołda-Cępa, M.; Krok-Borkowicz, M.; Brzychczy-Włoch, M.; Pamuła, E.; Simka, W. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater. Sci. Eng. C 2019, 94, 998–1008. [Google Scholar] [CrossRef]
- Buyuksungur, S.; Hasirci, V.; Hasirci, N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J. Biomed. Mater. Res. Part A 2021, 109, 2425–2437. [Google Scholar] [CrossRef]
- Qodratnama, R.; Serino, L.P.; Cox, H.C.; Qutachi, O.; White, L.J. Formulations for modulation of protein release from large-size PLGA microparticles for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Can, E.; Bucak, S.; Kınacı, E.; Çalıkoğlu, A.C.; Köse, G.T. Polybutylene Succinate (PBS)–Polycaprolactone (PCL) Blends Compatibilized with Poly (ethylene oxide)-block-poly (propylene oxide)-block-poly (ethylene oxide)(PEO-PPO-PEO) Copolymer for Biomaterial Applications. Polym. Plast. Technol. Eng. 2014, 53, 1178–1193. [Google Scholar] [CrossRef]
- Klouda, L.J. Biopharmaceutics. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Shiehzadeh, V.; Aghmasheh, F.; Shiehzadeh, F.; Joulae, M.; Kosarieh, E.; Shiehzadeh, F.J. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: New method and three case reports. Indian J. Dent. Res. 2014, 25, 248. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, S.; Lee, S.S.; Rajendran, A.K.; Kim, I.; Hwang, N.S.; Rangasamy, J. Addition of lactoferrin and substance P in a chitin/PLGA-CaSO(4) hydrogel for regeneration of calvarial bone defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112172. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Wadajkar, A.; Santimano, S.; Ahn, C.; Zhu, Q.; Opperman, L.A.; Bellinger, L.L.; Yang, J.; Nguyen, K.T. Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle. J. Investig. Clin. Dent. 2016, 7, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, N.; Zhang, T.; Sun, Q.; Han, B.; Yu, T. A Tetra-PEG Hydrogel Based Aspirin Sustained Release System Exerts Beneficial Effects on Periodontal Ligament Stem Cells Mediated Bone Regeneration. Front. Chem. 2019, 7, 682. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S.J. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X.J. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Sant, S.; Hwang, C.M.; Lee, S.H.; Khademhosseini, A. Hybrid PGS–PCL microfibrous scaffolds with improved mechanical and biological properties. J. Tissue Eng. Regen. Med. 2011, 5, 283–291. [Google Scholar] [CrossRef]
- Kharaziha, M.; Nikkhah, M.; Shin, S.-R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef]
- Osaheni, A.O.; Finkelstein, E.B.; Mather, P.T.; Blum, M.M. Synthesis and characterization of a zwitterionic hydrogel blend with low coefficient of friction. Acta Biomater. 2016, 46, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.; Watson, B.M.; Kasper, F.K.; Mikos, A.G. Building bridges: Leveraging interdisciplinary collaborations in the development of biomaterials to meet clinical needs. Adv. Mater. 2012, 24, 4995–5013. [Google Scholar] [CrossRef] [PubMed]
- Fahimipour, F.; Dashtimoghadam, E.; Hasani-Sadrabadi, M.M.; Vargas, J.; Vashaee, D.; Lobner, D.C.; Kashi, T.S.J.; Ghasemzadeh, B.; Tayebi, L. Enhancing cell seeding and osteogenesis of MSCs on 3D printed scaffolds through injectable BMP2 immobilized ECM-Mimetic gel. Dent. Mater. 2019, 35, 990–1006. [Google Scholar] [CrossRef]
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of bacterial cellulose as a carrier of BMP-2 for bone regeneration in a rabbit frontal sinus model. Materials 2019, 12, 2489. [Google Scholar] [CrossRef]
- Covarrubias, C.; Cádiz, M.; Maureira, M.; Celhay, I.; Cuadra, F.; von Marttens, A.J. Bionanocomposite scaffolds based on chitosan–gelatin and nanodimensional bioactive glass particles: In vitro properties and in vivo bone regeneration. J. Biomater. Appl. 2018, 32, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Trbakovic, A.; Hedenqvist, P.; Mellgren, T.; Ley, C.; Hilborn, J.; Ossipov, D.; Ekman, S.; Johansson, C.B.; Jensen-Waern, M.; Thor, A. A new synthetic granular calcium phosphate compound induces new bone in a sinus lift rabbit model. J. Dent. 2018, 70, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bastami, F.; Paknejad, Z.; Jafari, M.; Salehi, M.; Rad, M.R.; Khojasteh, A. Fabrication of a three-dimensional β-tricalcium-phosphate/gelatin containing chitosan-based nanoparticles for sustained release of bone morphogenetic protein-2: Implication for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 481–491. [Google Scholar] [CrossRef]
- Ansari, S.; Sarrion, P.; Hasani-Sadrabadi, M.M.; Aghaloo, T.; Wu, B.M.; Moshaverinia, A. Regulation of the fate of dental-derived mesenchymal stem cells using engineered alginate-GelMA hydrogels. J. Biomed. Mater. Res. A. 2017, 105, 2957–2967. [Google Scholar] [CrossRef]
- Thorpe, A.; Creasey, S.; Sammon, C.; Le Maitre, C. Hydroxyapatite nanoparticle injectable hydrogel scaffold to support osteogenic differentiation of human mesenchymal stem cells. Eur. Cells Mater. 2016, 32, 1–23. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Freeman, C.; Farthing, P.; Callaghan, J.; Hatton, P.V.; Brook, I.M.; Sammon, C.; Le Maitre, C.L. In vivo safety and efficacy testing of a thermally triggered injectable hydrogel scaffold for bone regeneration and augmentation in a rat model. Oncotarget 2018, 9, 18277. [Google Scholar] [CrossRef]
- Piard, C.; Baker, H.; Kamalitdinov, T.; Fisher, J. Bioprinted osteon-like scaffolds enhance in vivo neovascularization. Biofabrication 2019, 11, 025013. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A.J. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, K.M.; Soliman, I.E.; Mabrouk, M.; ElShebiney, S.; Beherei, H.H.; Aboelnasr, M.A.; Das, D.B. Novel polysaccharide hybrid scaffold loaded with hydroxyapatite: Fabrication, bioactivity, and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Simon, K.A.; Mosadegh, B.; Minn, K.T.; Lockett, M.R.; Mohammady, M.R.; Boucher, D.M.; Hall, A.B.; Hillier, S.M.; Udagawa, T.; Eustace, B.K. Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system. Biomaterials 2016, 95, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; De Borggraeve, W.; Encinas, M.V.; Matsuhiro, B.; Müller, R. Preparation and characterization of hydrogels based on homopolymeric fractions of sodium alginate and PNIPAAm. Carbohydr. Polym. 2013, 92, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Güven, O.; Şen, M.; Karadağ, E.; Saraydın, D. Chemistry. A review on the radiation synthesis of copolymeric hydrogels for adsorption and separation purposes. Radiat. Phys. Chem. 1999, 56, 381–386. [Google Scholar] [CrossRef]
- Singhal, R.; Gupta, K. A review: Tailor-made hydrogel structures (classifications and synthesis parameters). Polym. Plast. Technol. Eng. 2016, 55, 54–70. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as drug delivery systems; pros and cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Dietrich, T.; Ower, P.; Tank, M.; West, N.X.; Walter, C.; Needleman, I.; Hughes, F.J.; Wadia, R.; Milward, M.R.; Hodge, P.J. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions–implementation in clinical practice. Br. Dent. J. 2019, 226, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Mikos, A.G. Preparation methods and structure of hydrogels. In Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–26. [Google Scholar]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs. 2022, 20, 364. [Google Scholar] [CrossRef]
- Kim, D.; Lee, A.E.; Xu, Q.; Zhang, Q.; Le, A.D. Gingiva-derived mesenchymal stem cells: Potential application in tissue engineering and regenerative medicine—A comprehensive review. Front. Immunol. 2021, 12, 667221. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef] [PubMed]
- Jeoh, T.; Wong, D.E.; Strobel, S.A.; Hudnall, K.; Pereira, N.R.; Williams, K.A.; Arbaugh, B.M.; Cunniffe, J.C.; Scher, H.B. How alginate properties influence in situ internal gelation in crosslinked alginate microcapsules (CLAMs) formed by spray drying. PLoS ONE 2021, 16, e0247171. [Google Scholar] [CrossRef]
- Valle, K.Z.M.; Saucedo Acuña, R.A.; Ríos Arana, J.V.; Lobo, N.; Rodriguez, C.; Cuevas-Gonzalez, J.C.; Tovar-Carrillo, K.L. Natural film based on pectin and allantoin for wound healing: Obtaining, characterization, and rat model. BioMed Res. Int. 2020, 2020, 6897497. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Maleki, H.; Larraneta, E.; Fajardo, A.R.; Nik, A.B.; Shavandi, A.; Sheikhi, A.; Ghorbanpour, M.; Farokhi, M.; Govindh, P. Status and future scope of plant-based green hydrogels in biomedical engineering. Appl. Mater. Today 2019, 16, 213–246. [Google Scholar] [CrossRef]
- Sikder, A.; Ghosh, S. Hydrogen-bonding regulated assembly of molecular and macromolecular amphiphiles. Mater. Chem. Front. 2019, 3, 2602–2616. [Google Scholar] [CrossRef]
- Ailincai, D.; Gavril, G.; Marin, L. Polyvinyl alcohol boric acid–A promising tool for the development of sustained release drug delivery systems. Mater. Sci. Eng. C 2020, 107, 110316. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.J.H.b.o.n.p. The physical and chemical properties of hydrogels based on natural polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. [Google Scholar]
- Zhang, X.; Xiang, J.; Hong, Y.; Shen, L. Recent Advances in Design Strategies of Tough Hydrogels. Macromol. Rapid Commun. 2022, 43, 2200075. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Zhang, Y.; Cao, X.; Luo, Z. Dual physically crosslinked nanocomposite hydrogels reinforced by poly (N-vinylpyrrolidone) grafted cellulose nanocrystal with high strength, toughness, and rapid self-recovery. Cellulose 2020, 27, 9913–9925. [Google Scholar] [CrossRef]
- Ravichandran, G.; Rengan, A.K. A Retrospective Analysis in the Facet of Biomedical Engineering; Wiley: Hoboken, NJ, USA, 2021; pp. 201–246. [Google Scholar] [CrossRef]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable Biomaterials for Dental Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef]
- Dubey, S.; Mody, N.; Sharma, R.; Agrawal, U.; Vyas, S.P. Nanobiomaterials: Novel nanoplatforms for protein and peptide delivery. In Nanobiomaterials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 111–146. [Google Scholar]
- Bhatnagar, D.; Simon, M.; Rafailovich, M.H. Hydrogels for regenerative medicine. In Recent Advances in Biopolymers; IntechOpen: London, UK, 2016; p. 105. [Google Scholar]
- Budama-Kilinc, Y.; Cakir-Koc, R.; Aslan, B.; Özkan, B.; Mutlu, H.; Üstün, E. Hydrogels in regenerative medicine. In Biomaterials in Regenerative Medicine; Dobrzanski, L.A., Ed.; IntechOpen Limited: London, UK, 2018; pp. 277–301. [Google Scholar]
- Abbasian, V.; Emadi, R.; Kharaziha, M. Biomimetic nylon 6-baghdadite nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 109, 110549. [Google Scholar] [CrossRef]
- Kang, A.; Park, J.; Ju, J.; Jeong, G.S.; Lee, S.-H. Cell encapsulation via microtechnologies. Biomaterials 2014, 35, 2651–2663. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chen, C.; Akiyama, K.; Xu, X.; Chee, W.W.; Schricker, S.R.; Shi, S. Encapsulated dental-derived mesenchymal stem cells in an injectable and biodegradable scaffold for applications in bone tissue engineering. J Biomed. Mater. Res. A. 2013, 101, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Asti, A.; Gioglio, L. Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation. Int. J. Artif. Organs 2014, 37, 187–205. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Y.; Guan, J.; Guan, J.; Ye, T.; Chen, Y.; Zhu, Y.; Zhou, P.; Cui, W. Electrospun fibers: An innovative delivery method for the treatment of bone diseases. Expert Opin. Drug Deliv. 2020, 17, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.K.; Alford, A.I.; Goldstein, S.A.; Stegemann, J.P. Synergistic enhancement of ectopic bone formation by supplementation of freshly isolated marrow cells with purified MSC in collagen–chitosan hydrogel microbeads. Connect. Tissue Res. 2016, 57, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Katakura, O.; Morimoto, N.; Akiyoshi, K.; Kasugai, S. Effects of cholesterol-bearing pullulan (CHP)-nanogels in combination with prostaglandin E1 on wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 55–60. [Google Scholar] [CrossRef]
- Seo, B.-B.; Choi, H.; Koh, J.-T.; Song, S.-C. Sustained BMP-2 delivery and injectable bone regeneration using thermosensitive polymeric nanoparticle hydrogel bearing dual interactions with BMP-2. J. Control. Release 2015, 209, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Markova, N.; Rashkov, I. Electrospun non-woven nanofibrous hybrid mats based on chitosan and PLA for wound-dressing applications. Macromol. Biosci. 2009, 9, 102–111. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W. Advanced electrospun hydrogel fibers for wound healing. Compos. Part B Eng. 2021, 223, 109101. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Y.; Wang, H.; Zhang, H.; Zhang, X.; Zhao, Y. Conductive polymer hydrogel microfibers from multiflow microfluidics. Small 2019, 15, 1805162. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef]
- Kirchmajer, D.M.; Gorkin, I.R. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117. [Google Scholar] [CrossRef]
- Hu, M.; Deng, R.; Schumacher, K.M.; Kurisawa, M.; Ye, H.; Purnamawati, K.; Ying, J.Y. Hydrodynamic spinning of hydrogel fibers. Biomaterials 2010, 31, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Jeznach, O.; Kołbuk, D.; Sajkiewicz, P.J.J.o.B.M.R.P.A. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed. Mater. Res. A 2018, 106, 2762–2776. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta Biomater. 2020, 113, 164–176. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Li, C.; Weir, M.D.; Wang, P.; Reynolds, M.A.; Zhao, L.; Xu, H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater. Sci. Eng. C 2016, 69, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Toh, W. Injectable hydrogels in dentistry: Advances and promises. Austin J. Dent. 2014, 1, 1001. [Google Scholar]
- Risbud, M.V.; Shapiro, I.M. Stem cells in craniofacial and dental tissue engineering. Orthod. Craniofac. Res. 2005, 8, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morsi, Y.; Wang, Y.; Li, Y.; Ramakrishna, S. Review scaffold design and stem cells for tooth regeneration. Jpn. Dent. Sci. Rev. 2013, 49, 14–26. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef]
- Huang, G.T.J. Pulp and dentin tissue engineering and regeneration: Current progress. Regen. Med. 2009, 4, 697–707. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.M.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Mellati, A.; Akhtari, J. Injectable hydrogels: A review of injectability mechanisms and biomedical applications. Res. Mol. Med. (RMM) 2019, 6, 1–14. [Google Scholar] [CrossRef]
- Sordi, M.B.; Cruz, A.; Fredel, M.C.; Magini, R.; Sharpe, P.T. Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112055. [Google Scholar] [CrossRef]
- Hashemi-Beni, B.; Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Khademi, A.A. Tissue engineering: Dentin–pulp complex regeneration approaches (A review). Tissue Cell 2017, 49, 552–564. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration. J. Dent. Res. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- Sharma, S.; Srivastava, D.; Grover, S.; Sharma, V. Biomaterials in tooth tissue engineering: A review. J. Clin. Diagn. Res. 2014, 8, 309–315. [Google Scholar] [CrossRef]
- Mao, J.J.; Kim, S.G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative endodontics: Barriers and strategies for clinical translation. Dent. Clin. North Am. 2012, 56, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of human cementum: Its structure, function, and development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Oortgiesen, D.A.; Bronckers, A.L.; Yang, F.; Walboomers, X.F.; Jansen, J.A. Enhanced periodontal tissue regeneration by periodontal cell implantation. J. Clin. Periodontol. 2013, 40, 698–706. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.P.; Mathias-Santamaria, I.F.; Ferraz, L.F.F.; Casarin, R.C.V.; Romito, G.A.; Sallum, E.A.; Pini-Prato, G.P.; Casati, M.Z. Rethinking the decision-making process to treat gingival recession associated with non-carious cervical lesions. Braz. Oral Res. 2021, 35, 96. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Sulijaya, B.; Yamada-Hara, M.; Tsuzuno, T.; Tabeta, K.; Yamazaki, K. Gingival epithelial barrier: Regulation by beneficial and harmful microbes. Tissue Barriers 2019, 7, e1651158. [Google Scholar] [CrossRef]
- Zatta da Silva, T.; Margonar, R.; Silveira Faeda, R.; de Oliveira, A.; Cavalcanti de Souza, I.; dos Santos, P.L.; Pereira Queiroz, T. Hyaluronic acid for repairing interdental papilla in esthetic area: Case report. Rev. Clín. Periodoncia Implantol. Rehabil. Oral 2019, 12, 157–158. [Google Scholar] [CrossRef]
- Li, D.; Zhao, L.; Cong, M.; Liu, L.; Yan, G.; Li, Z.; Li, B.; Yu, W.; Sun, H.; Yang, B. Injectable thermosensitive chitosan/gelatin-based hydrogel carried erythropoietin to effectively enhance maxillary sinus floor augmentation in vivo. Dent. Mater. 2020, 36, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Abboud, A.R.; Ali, A.M.; Youssef, T. Preparation and characterization of insulin-loaded injectable hydrogels as potential adjunctive periodontal treatment. Dent. Med. Probl. 2020, 57, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Mu, R.; Chen, F.; Wei, X.; Zhu, L.; Han, B.; Yu, H.; Bi, B.; Chen, B.; Wang, Q.; et al. Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.H.; Chang, Y.L.; Wang, M.L.; Chuang, J.H.; Yang, Y.C.; Tai, M.C.; Wang, C.Y.; Liu, Y.Y.; Li, H.Y.; Chen, J.T.; et al. Promoting Induced Pluripotent Stem Cell-driven Biomineralization and Periodontal Regeneration in Rats with Maxillary-Molar Defects using Injectable BMP-6 Hydrogel. Sci. Rep. 2018, 8, 114. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.B.; Mano, J.F. Polymer-based microparticles in tissue engineering and regenerative medicine. Biotechnol. Prog. 2011, 27, 897–912. [Google Scholar] [CrossRef]
- Cheng, W.; Ding, Z.; Zheng, X.; Lu, Q.; Kong, X.; Zhou, X.; Lu, G.; Kaplan, D.L. Injectable hydrogel systems with multiple biophysical and biochemical cues for bone regeneration. Biomater. Sci. 2020, 8, 2537–2548. [Google Scholar] [CrossRef]
- Cooper, C.; Snow, S.; McAlindon, T.E.; Kellingray, S.; Stuart, B.; Coggon, D.; Dieppe, P.A. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000, 43, 995–1000. [Google Scholar] [CrossRef]
- Leslie, S.K.; Cohen, D.J.; Sedlaczek, J.; Pinsker, E.J.; Boyan, B.D.; Schwartz, Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials 2013, 34, 8172–8184. [Google Scholar] [CrossRef]
- Daley, E.L.; Coleman, R.M.; Stegemann, J.P. Biomimetic microbeads containing a chondroitin sulfate/chitosan polyelectrolyte complex for cell-based cartilage therapy. J. Mater. Chem. B 2015, 3, 7920–7929. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Hillel, A.T.; Taube, J.M.; Cornish, T.C.; Sharma, B.; Halushka, M.; McCarthy, E.F.; Hutchins, G.M.; Elisseeff, J.H. Characterization of human mesenchymal stem cell-engineered cartilage: Analysis of its ultrastructure, cell density and chondrocyte phenotype compared to native adult and fetal cartilage. Cells Tissues Organs 2010, 191, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, A.; Mao, J.J. Tissue-engineered neogenesis of human-shaped mandibular condyle from rat mesenchymal stem cells. J. Dent. Res. 2003, 82, 951–956. [Google Scholar] [CrossRef] [PubMed]

| Natural polymers |
|
| Synthetic polymers | Polyglycolic acid, polycaprolactone, etc. |
| Ceramics | Hydroxyapatite, bio glass, β-tricalcium phosphates, etc. |
| Hybrid |
|
| Metals | Gold, silver, titanium, etc. |
| Application | Advantages |
|---|---|
| Drug delivery |
|
| Detoxification |
|
| Immune modulation |
|
| Tissue engineering |
|
| Polysaccharides | Proteins |
|---|---|
| Alginate | Collagen |
| Starch | Gelatin |
| Cellulose | Silk |
| Chitosan | Fibrin |
| Cyclodextrin | Albumin |
| Dextran | |
| Gum polysaccharides | |
| Pectin | |
| Pullulan | |
| Heparin | |
| Chondroitin sulfate |
| Plants | Mucilage, Pectin, Hemicellulose, Gums Cellulose, Glucomannan, Starch |
|---|---|
| Algae | carrageenans, alginates |
| Animals | cellulose, glycosaminoglycans, hyaluronic acid, chitosan, Chitin |
| Bacteria | cellulose, Dextran |
| Fungal | yeast glucans, chitosan, chitin, pollulan, Elsinan |
| Natural Polysaccharide | Delivery System | Outcome | Ref |
|---|---|---|---|
| Carrageenan | Nano-HA/gum Arabic/k-carrageenan composite | Without cytotoxicity, osteoblast-like cells exhibit substantial osteogenic signals. | [88] 2020 |
| Carrageenan | Ag/carrageenan/gelatin nanocomposite | The unique Ag/carrageenan-gelatin hydrogel’s antimicrobial, drug carriers, and antitumor capabilities | [89] 2021 |
| 54646N-carboxyethyl chitosan/ hyaluronic acid-aldehyde | N-carboxyethyl chitosan/hyaluronic acid-aldehyde loaded with nano hydroxyapatite | preserving alveolar ridge integrity and facilitating soft tissue healing process | [90] 2020 |
| regenerated cellulose (rCL) nanofibers/chitosan (CS) | Regenerated cellulose (rCL) nano fibers chitosan (CS) hydrogel | The rCL/CS scaffold aided bio mineralization and increased the survival, adherence, and multiplication of preosteoblast cells (MC3T3-E1). | [91] 2021 |
| Chitosan/hyaluronic acid | Chitosan/hyaluronic acid nano pearl composite | RUNX2, OCN, and OPN gene expression increases. The best results were achieved with 10 wt% and 25 wt% nano pearl. | [92] 2020 |
| chitosan | Chitosan nanohydrogel/poly-ε-caprolactone (PCL) loaded with nanotriclosan and flurbiprofen | The combination of anti-microbial and anti-inflammation properties resulted in a remarkable treatment outcome. | [93] 2019 |
| Gelatin/Alginate | Gelatin-alginate-graphene oxide nano framework | Amplification of the transcription of osteoblast enhancing factors and ALP | [94] 2019 |
| Carrageenan | Carrageenan/whitlockite nano composite hydrogel | Improved osteogenic development and ALP expression | [82] 2019 |
| Carrageenan | Carrageenan/nanohydroxyapatite composite scaffold | Enhancement of osteogenic differentiation without the use of pharmacological drugs | [95] 2018 |
| Chitosan | Chitosan gold nanoparticles mixed with peroxisome proliferator-activated ligand | Optimizing the outcome of implant placement in diabetic patients (bone development and mineralization) | [96] 2017 |
| Alginate/chitosan | Alginate/chitosan loaded with nanohydroxylapatite | Increased hydroxyapatite levels stimulate MC3T3 cell development and calcification. | [97] 2015 |
| Alginate | Alginate hydrogel mixed with bovine dental pulp extracellular matrix (pECM) | Accelerated differentiation in the mineralizing environment lead to mineralization at the hydrogel’s perimeter. HA hydrogels integrating PL enhanced cell functions and hDPSC mineralized matrix formation. | [98] |
| Hyaluronic acid hydrogel | Photo crosslinking of methacrylated HA incorporated with PL | Accelerated differentiation in the mineralizing environment lead to time-dependent mineral deposition at the hydrogel’s perimeter. HA hydrogels integrating PL enhanced cell functions and hDPSC mineralized matrix formation. | [99] |
| Chitosan | Ag-blended bioactive glass micro particles mixed with chitosan (Ag-BG/CS). | Ag-BG/CS enhanced the odontogenic differentiation capability of lipopolysaccharide-increased inflammatory reactivity in dental pulp cells and shown antimicrobial and anti-inflammatory activities | [100] |
| Natural Protein | Delivery System | Experiment Design | Outcome | Reference |
|---|---|---|---|---|
| Collagen | Collagen hydrogel loaded with Rat pulp cells marked with indium-111-oxine | Implantation in the rat emptied pulp chamber. | Functioning fibroblasts, neovasculature, and nerve fibers were seen in the collagen hydrogel one month after insertion. | [131] |
| Collagen | Blends with nano keratin, and hydroxyapatite | Histomorphometry on critical size defects in rat calvaria | bio-compatibility, biodegradability, and increased density of newly formed bone | [132] |
| Gelatin | Cross-linked gelatin hydrogel micro particles were encapsulated with fibroblast growth factor 2 (FGF-2) and mixed with collagen sponge pieces | Detection of expression of DSPP (Dentin Sialophosphoprotein) | Regulated FGF2 release from gelatin hydrogels resulted in the production of dentin-like particulates with dentin defects above exposed pulp. | [133] |
| Fibrin | Incorporation of clindamycin loaded Poly (D, L) Lactic Acid nanoparticles (CLIN-loaded PLA NPs). | Cell viability and antimicrobial assay | Fibrin hydrogels incorporating CLIN-loaded PLA NPs reduced bacterial colonization and had an antimicrobial property towards E. faecalis. In cellularized hydrogels, DPSC survival and type I collagen production were comparable to the unmodified groups. | [134] |
| Silk Fibroin | Silk Fibroin/Cellulose Hydrogel | Cell viability | The hydrogels promote MC3T3 cell development into osteoblasts and are predicted to be a promising matrix for osteogenesis. | [135] |
| Hydrogel | Delivery Vehicle | Experiment Design | Outcome | Reference |
|---|---|---|---|---|
| Polylactic polyglycolic acid–polyethylene glycol (PLGA-PEG) | Clinical Trial | A biological strategy may create a conditions favorable to therapeutic regeneration of dental and paradental tissues. | [148] | |
| PLGA | Lactoferrin and substance P in a chitin/PLGA-CaSO4 hydrogel | Clavarial rat defect | In mice, clavarial bone regeneration was enhanced compared to controls. | [149] |
| PEG | PEG–maleate–citrate (PEGMC) (45% w/v), acrylic acid (AA) cross linker (5% w/v), 2.20-Azobis (2-methylpropionamidine)dihydrochloride (AAPH) photo-initiator (0.1% w/v), | Cell viability | [150] | |
| PEG | A Tetra-PEG Hydrogel Based Aspirin Sustained Release System | In vitro and in vivo analyses | When periodontal ligament stem cells (PDLSCs) were co-incubated with hydrogel materials, in vitro tests revealed that cell growth was somewhat aided and osteogenic development was significantly enhanced. Furthermore, an in vivo investigation revealed that the aspirin controlled release approach greatly aided PDLSCs-mediated bone defect healing. | [151] |
| Poly-Nisopropylacrylamide (NIPAAm) | NIPAAm cross-linked by PEG-DMA | DSPP in the outer cell layer. | DPSCs in the construct’s outermost surface developed into odontoblast-like cells, whilst DPSCs in the inner layer remained stem cells. In vivo, blood vessel-rich pulp-like tissues were created. | [152] |
| Material | Application | Outcomes | Reference | |
|---|---|---|---|---|
| Alginate-Matrigel hydrogel encapsulated with bioactive glass micro particles | In vitro (human dental pulp MSCs) | Despite a decrease in elasticity due to the incorporation of bioactive glass microparticles, the incorporation of Matrigel in the hydrogel combination promotes MSC osteogenic differentiation. | [12] | |
| 3D-printed heparin-collagen network enclosing MSCs, reinforced with -TCP nanoscale framework, and complexed with human bone morphogenetic protein type 2 (rhBMP-2) | In vitro (human dental pulp MSCs); In vivo (rat dorsum defects) | In vitro: the capability of heparin-conjugated collagen matrix to retain rhBMP-2 bioactivity and improve MSC survival and osteogenic growth. MSCs’ osteogenic differentiation capacity and the formation of ectopic osteogenesis in vivo | [159] | |
| Bacterial cellulose encapsulated with bone morphogenetic protein type 2 (BMP-2) | In vivo (frontal sinus lift rabbit model) | Bacterial cellulose has demonstrated great biocompatibility. Bacterial cellulose, when combined with BMP-2, aided bone repair while also acting as a barrier membrane and a drug release prolonger, as indicated by histological and immunohistochemical tests. | [160] | |
| Chitosan-Gelatin hydrogel incorporating nanodimensional bioactive glass particles | Human dental pulp MSCs in vitro; rat femoral deformities in vivo | Biocompatibility and ability to generate bone-like apatite crystallization in vitro In vivo, the chitosan-gelatin hydrogel containing 5% bioactive glass nanostructures generated the highest bone regeneration outcomes. | [161] | |
| Composite bisphosphonate-linked hyaluronic acid-calcium phosphate hydrogel | In vivo (sinus lift rabbit model) | In a histomorphometric analysis, the synthetic granular calcium phosphate material and deproteinized cow mineral xenograft stimulated more bone repair than the hyaluronic acid-calcium phosphate hydrogel. | [162] | |
| Gelatin-coated β-tricalcium phosphate (βTCP) scaffolds with rhBMP-2-loaded chitosan nanoparticles delivery system | In vitro (human buccal fat pad MSCs) | Gelatin-coated TCP scaffolding with rhBMP-2-loaded chitosan nanoparticles promoted cell survival and adhesion while progressively releasing rhBMP-2 at a therapeutic dosage that allowed MSCs to develop into osteoblasts. | [163] | |
| Alginate-gelatin methacrylate (GelMA) hydrogel | In vitro (human gingival MSCs and human bone marrow MSCs) | Because of the hydrogel’s reduced flexibility, the addition of GelMA to alginate impairs the hydrogel osteogenic development beginning of encapsulated MSCs. The biological characteristics of alginateGelMA, as well as the existence of inductive cues, govern MSC differentiation into osteoblasts. | [164] | |
| Crosslinked pNIPAM-co-DMAc hydrogel loaded with hydroxyapatite nanoparticles | In vitro (commercial human MSCs); In vivo (rat femur defects) | Commercial human MSCs’ capacity to drive osteogenic development in vitro; in vivo: biocompatibility, capacity to combine with surrounding structures, and enhanced accumulation of early indicators of bone regeneration. | [165,166] | |
| 3D-bioprinted biphasic osteon-like framework comprising human mesenchymal stem cells (hMSCs) and human umbilical vein endothelial cells (HUVECs) enclosed in a fibrin-polycaprolactone hydrogel | In vitro (commercial hMSCs and HUVECs) In vivo (rat cranial bone defects) | in vitro; Significant increase in transcription of angiogenic biomarkers In vivo: histological analysis of explanted biomaterials demonstrated a boost in the quantity of blood vessels per square meter (the capacity to stimulate angiogenesis) in the three-dimensional bioprinting osteon-like framework. | [167] | |
| Sodium alginate/hydroxyethylcellulose/ hydroxyapatite composite | Semi-synthetic | In vitro (commercial human MSCs); In vivo (rat femur defects) | The capacity of the hydrogel composite scaffolds to support hMSC cell survival and proliferation in vitro. Histological studies demonstrated neo-osteogenesis to heal the damaged sites 6 weeks following scaffold placement. | [168,169] |
| 3D polyvinyl alcohol-tetraethylorthosilicatealginate-calcium oxide biocomposite cryogels | Semi-synthetic | In vivo (rat cranial bone defects) | The bone defect is allowed to repair during a 4-week period while its components are recirculated from the defect area. Osteoblastic function at the injured area, with a 2 to 4 week surge in development towards the osteoblastic lineage and osteoblast maturation. | [170] |
| Triblock poly(ethylene glycol)-poly (ε-caprolactone)-poly(ethylene glycol) copolymer, collagen and nanohydroxyapatite | Semi-synthetic, injectable | In vivo (rabbit calvarial bone defects) | After 4, 12, and 20 weeks, bone regeneration was evaluated. The creation of new bone tissue from the border of defects and the surface of native bone towards the center was established by radiological and pathological investigations. Non-loading defects have a great potentiality for correction via minimally invasive surgical procedures. | [171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atia, G.A.N.; Shalaby, H.K.; Ali, N.G.; Morsy, S.M.; Ghobashy, M.M.; Attia, H.A.N.; Barai, P.; Nady, N.; Kodous, A.S.; Barai, H.R. New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals 2023, 16, 702. https://doi.org/10.3390/ph16050702
Atia GAN, Shalaby HK, Ali NG, Morsy SM, Ghobashy MM, Attia HAN, Barai P, Nady N, Kodous AS, Barai HR. New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals. 2023; 16(5):702. https://doi.org/10.3390/ph16050702
Chicago/Turabian StyleAtia, Gamal Abdel Nasser, Hany K. Shalaby, Naema Goda Ali, Shaimaa Mohammed Morsy, Mohamed Mohamady Ghobashy, Hager Abdel Nasser Attia, Paritosh Barai, Norhan Nady, Ahmad S. Kodous, and Hasi Rani Barai. 2023. "New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review" Pharmaceuticals 16, no. 5: 702. https://doi.org/10.3390/ph16050702
APA StyleAtia, G. A. N., Shalaby, H. K., Ali, N. G., Morsy, S. M., Ghobashy, M. M., Attia, H. A. N., Barai, P., Nady, N., Kodous, A. S., & Barai, H. R. (2023). New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals, 16(5), 702. https://doi.org/10.3390/ph16050702









