Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase-Solubility Experiments
2.2. Characterization of Inclusion Complexes
2.2.1. Characterization of HPR-β-CYD/OMP Inclusion Complexes
2.2.2. Characterization of HPR-β-CYD/CURC Inclusion Complex
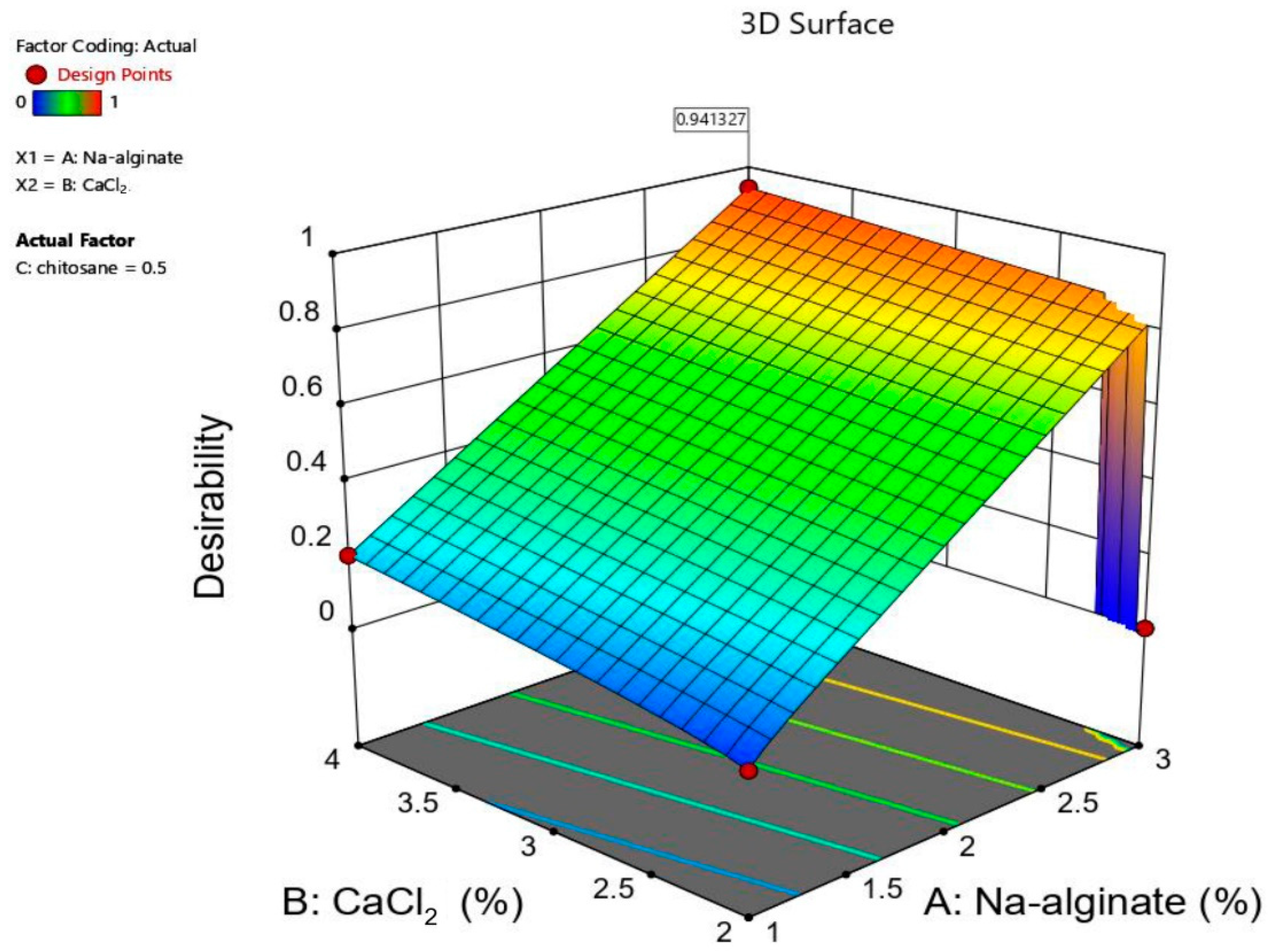
2.3. Formulation and Optimization using Two-Level Factorial Design
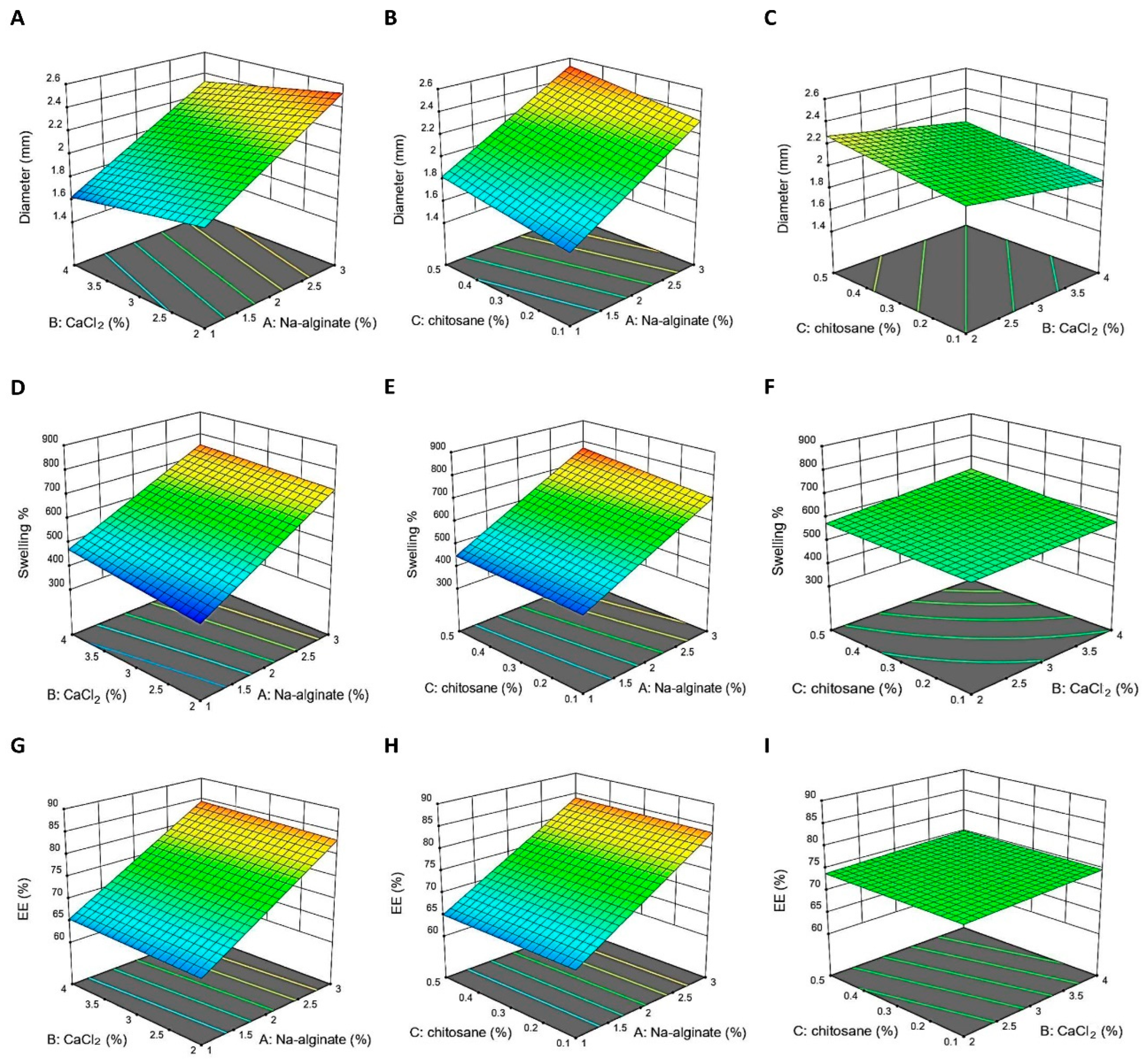
2.3.1. Beads Diameter
2.3.2. Swelling Behavior of the Beads
2.3.3. Drug Entrapment Efficiency (EE%)
2.4. Development of Optimization Models
2.4.1. Model Fit Report Describing the Impact of Sodium Alginate
2.4.2. Effect of Calcium Chloride (CaCl2)
2.4.3. The Effect of Coating the Beads with Chitosan
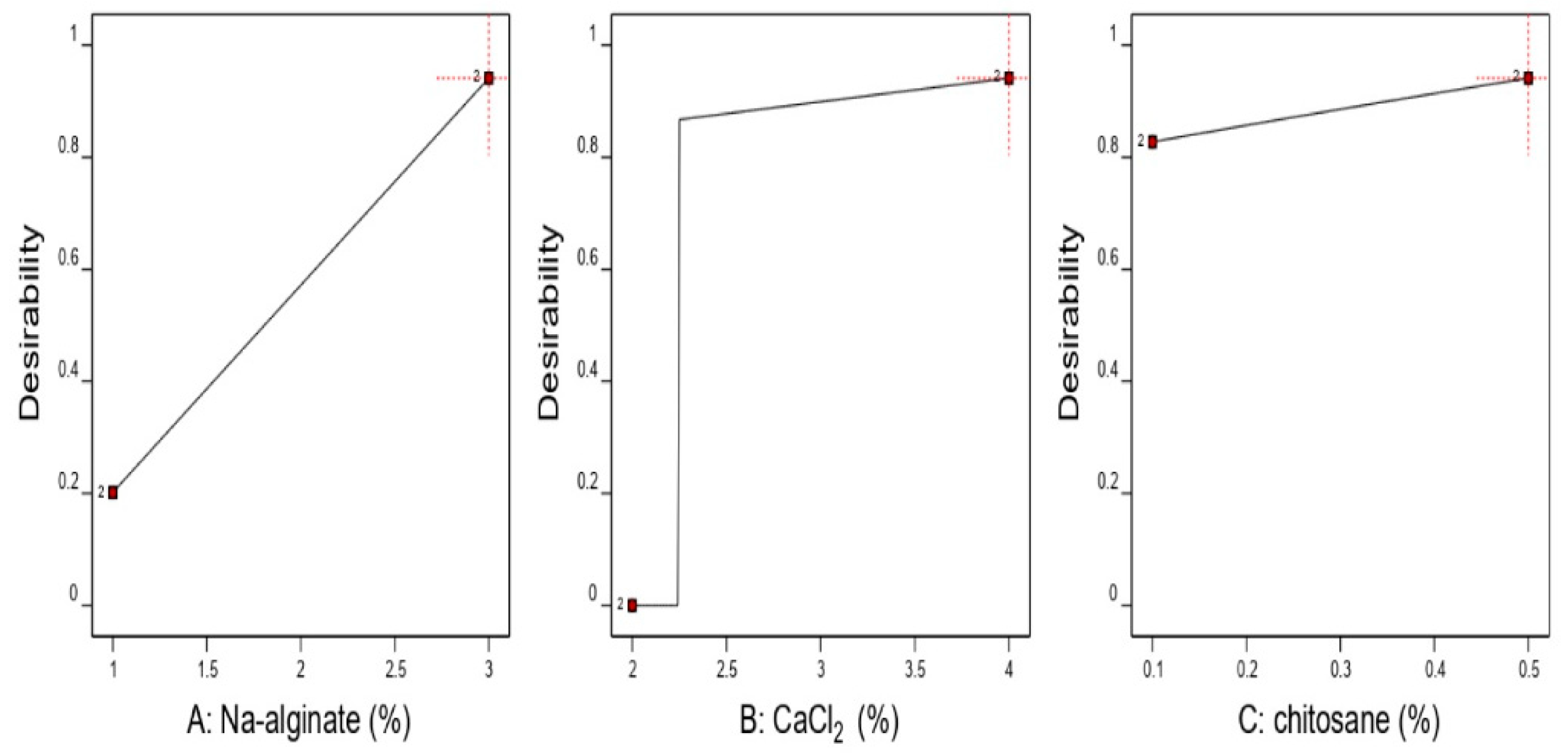
2.5. Selection of the Optimized Formulation
2.5.1. Morphology for the Optimal Formulation of the Hydrogel Beads
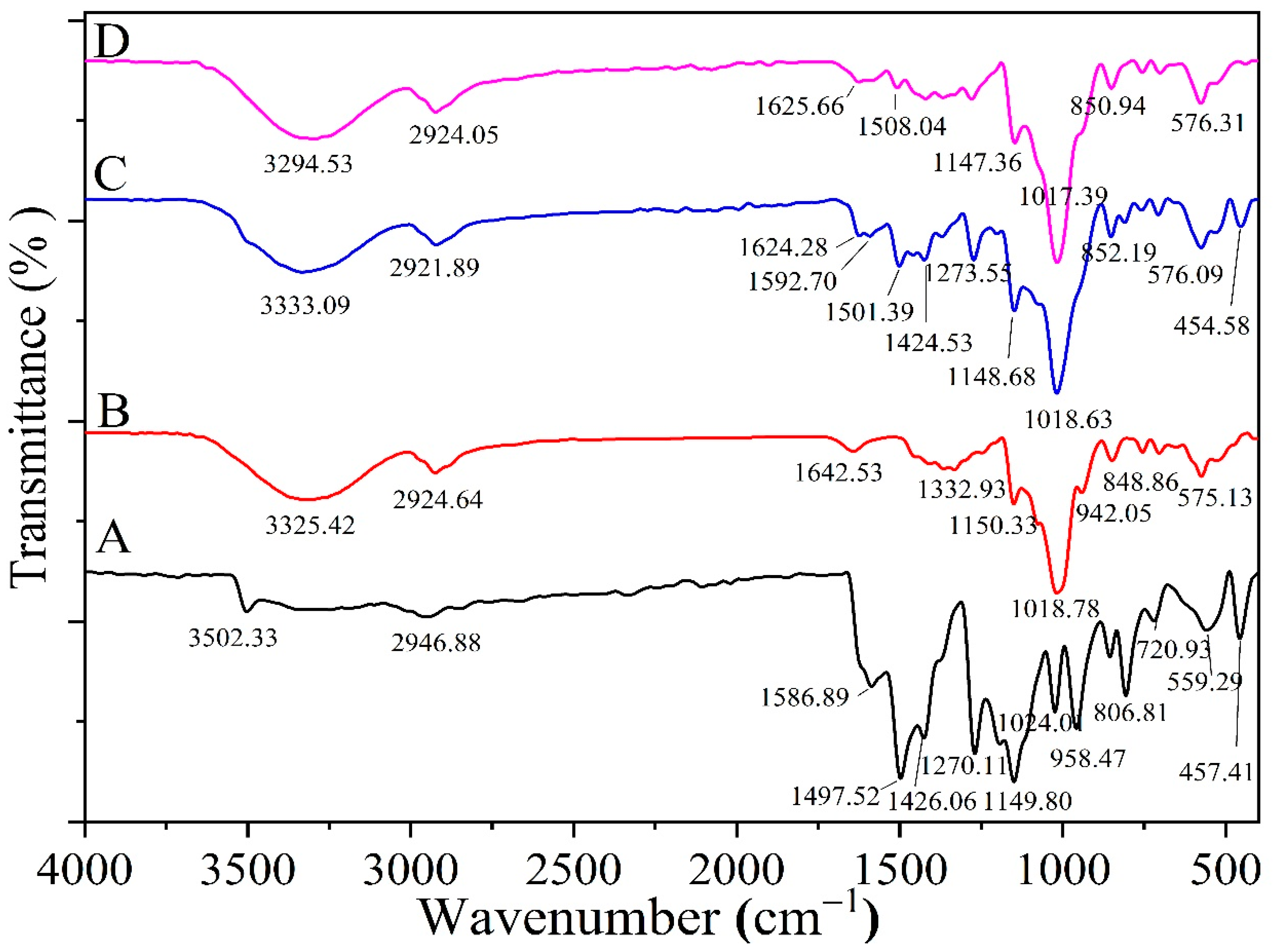
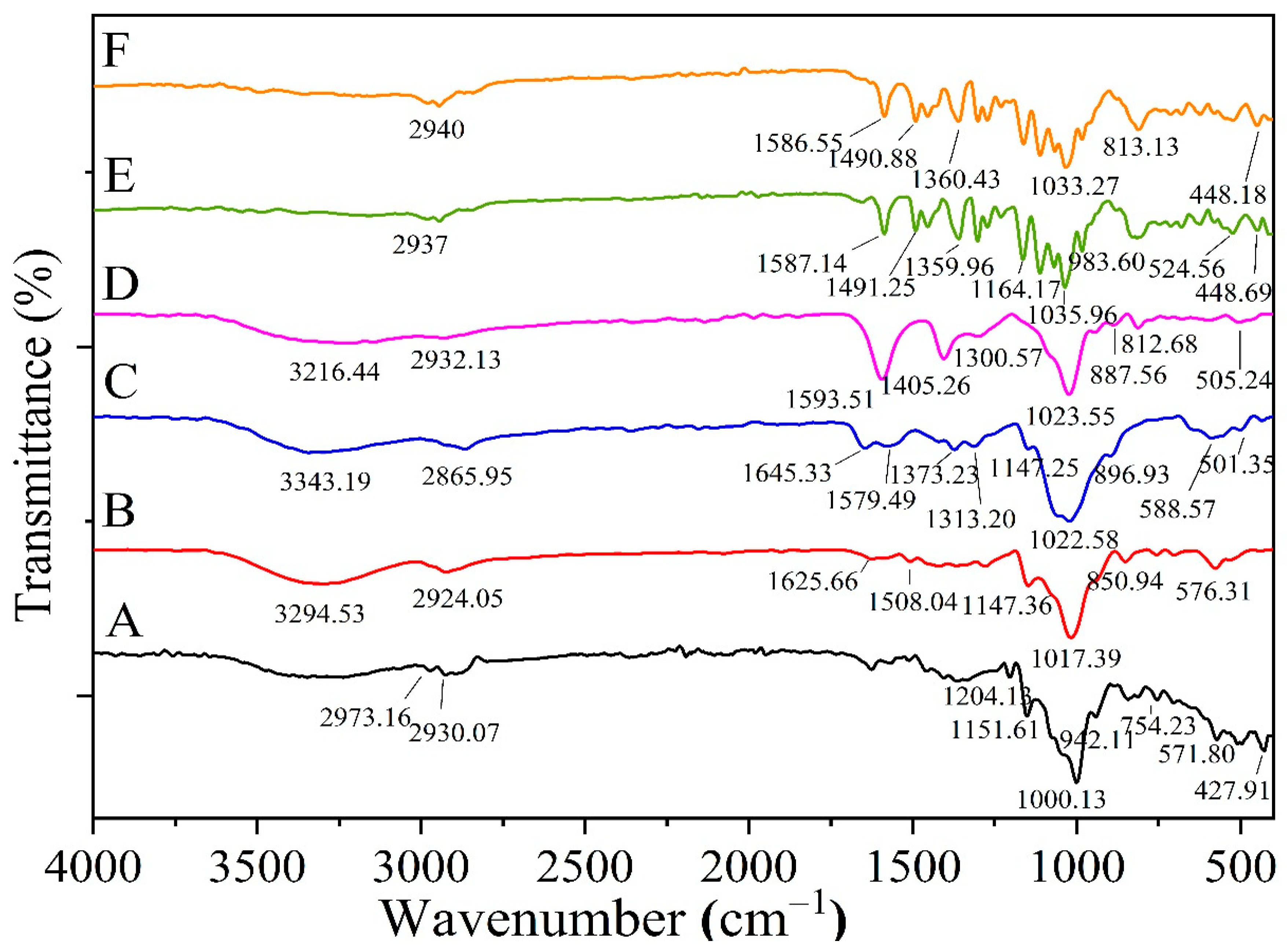
2.5.2. FTIR for Optimized Formula Beads
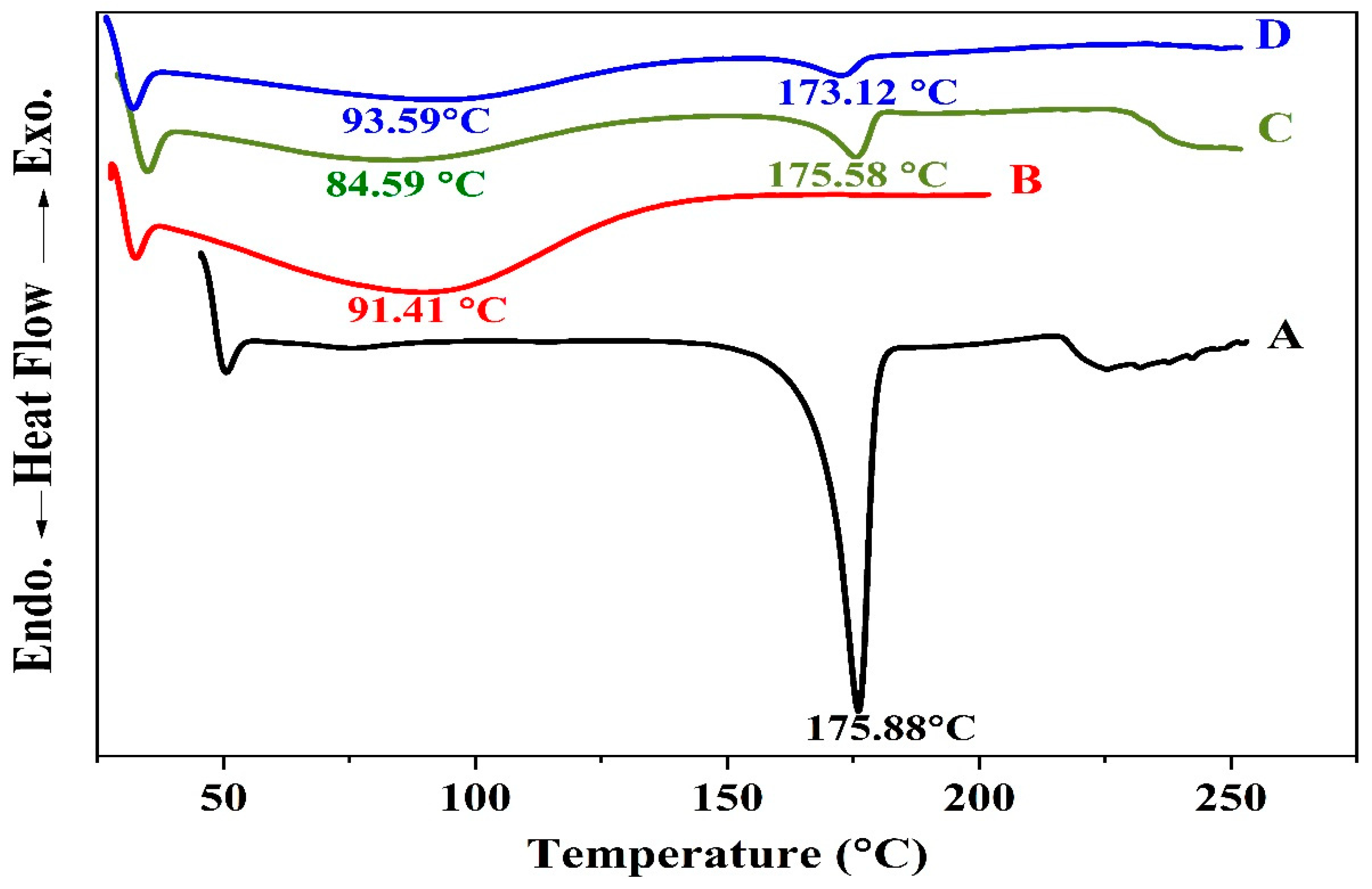
2.5.3. DSC Thermograms for the Optimized Formulation of the Coated Beads
2.5.4. In Vitro Release and Kinetic Release Study
2.5.5. Bio-Adhesion Test
2.6. In Vivo Treatment Efficacy
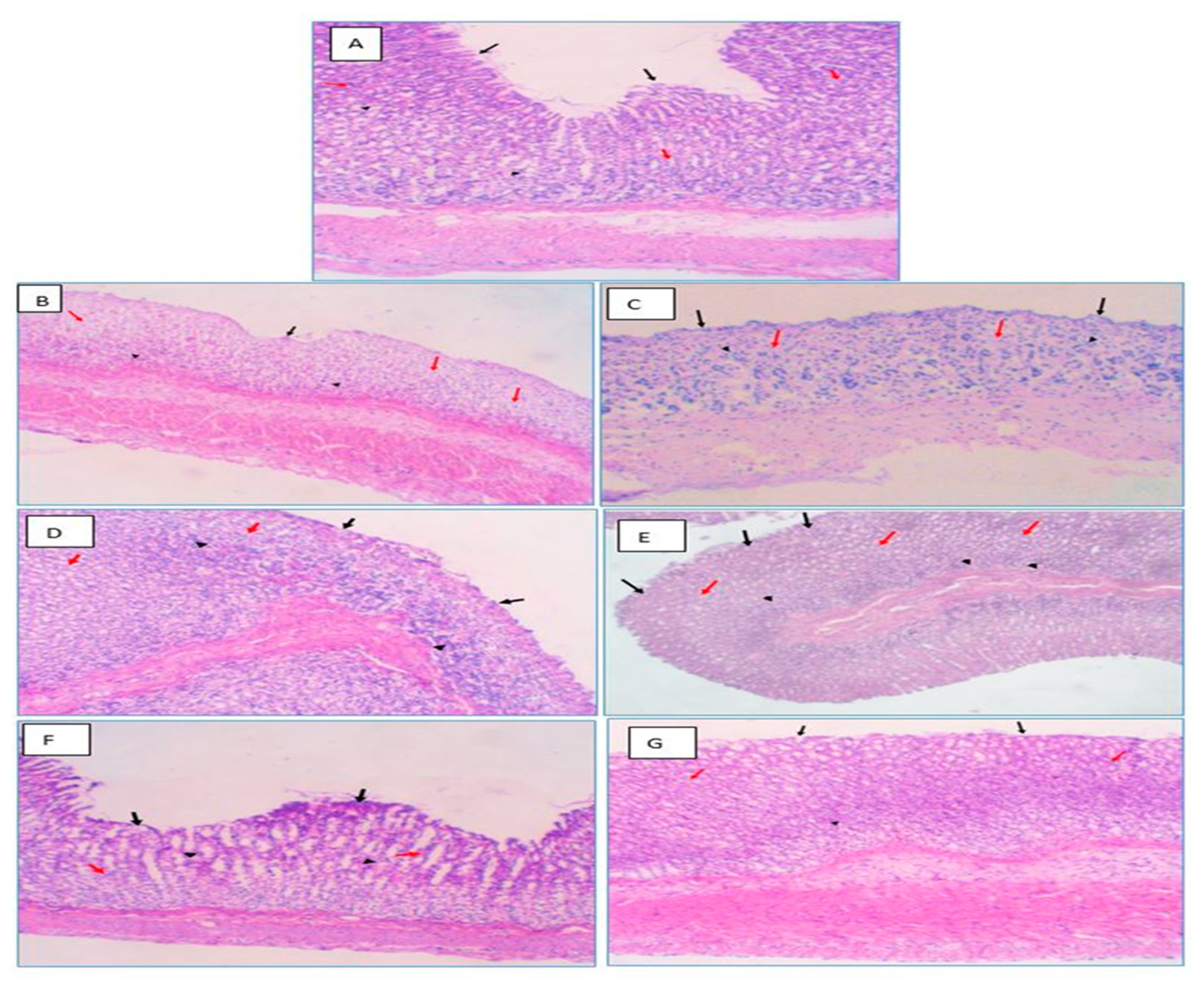
2.6.1. Assessment of Macroscopic Injury of Gastric Tissues
2.6.2. Histological Examination
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Phase Solubility Studies
3.2.2. Preparation of the HRP-β-CYD/Drug Inclusion Complex
3.2.3. Characterization of Drug Complex-HPR-β-CYD
3.2.4. Optimization of Formulation Variables
3.2.5. Preparation of Chitosan-Alginate Beads
3.3. Characteristics of the Beads
3.3.1. Bead Diameter Measurement
3.3.2. The Swelling Experiment
3.3.3. Encapsulation Efficiency (EE%)
3.4. Optimization Employing Two-Level Factorial Design
3.4.1. SEM Analysis for Optimized Formula Beads
3.4.2. FTIR Analysis for the Optimized Formula
3.4.3. DSC Studies for the Optimized Formula
3.4.4. In Vitro Drug Release from Optimized Formula Beads
3.4.5. Bio-Adhesion Test
3.4.6. In Vivo Study for Optimized Formula Hydrogel Beads
- (1)
- Vehicle control rats: received doses of CMC parallel to indomethacin.
- (2)
- PUD control: received indomethacin but no medication.
- (3)
- PUD + blank beads group.
- (4)
- PUD + free OMP group (20 mg/kg; orally: intragastric route).
- (5)
- PUD + CURC-only beads group (20 mg/kg; orally: intragastric route).
- (6)
- PUD + OMP-only beads group (20 mg/kg; orally: intragastric route).
- (7)
- PUD + beads loaded with CURC/OMP complex group (20 mg/kg, 20 mg/kg; orally: intragastric route).
3.4.7. Histological Assessment of Rat Gastric Tissues
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, X.; Ren, K.; Zhou, Z.; Dang, C.; Zhang, H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: A population-based study. BMC Gastroenterol. 2022, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Horny, H.-P.; Escribano, L.; Longley, B.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Rom, D.M. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika 1990, 77, 663–665. [Google Scholar] [CrossRef]
- Tuorkey, M.; Karolin, K. Anti-ulcer activity of curcumin on experimental gastric ulcer in rats and its effect on oxidative stress/antioxidant, IL-6 and enzyme activities. Biomed. Environ. Sci. 2009, 22, 488–495. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Karolin Kamel, A.-A. Comparative evaluation of the anti-ulcer activity of curcumin and omeprazole during the acute phase of gastric ulcer—Efficacy of curcumin in gastric ulcer prevention against omeprazole. Food Nutr. Sci. 2011, 2, 6622. [Google Scholar]
- Kerdsakundee, N.; Mahattanadul, S.; Wiwattanapatapee, R. Development and evaluation of gastroretentive raft forming systems incorporating curcumin-Eudragit®EPO solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Biopharm. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Sarisuta, N.; Tourtip, T.; Chuarcharoern, S. Chemical stability and mechanism of degradation of omeprazole. Thai J. Pharm. Sci. 1998, 22, 81–88. [Google Scholar]
- Figueiras, A.; Carvalho, R.A.; Ribeiro, L.; Torres-Labandeira, J.J.; Veiga, F.J. Solid-state characterization and dissolution profiles of the inclusion complexes of omeprazole with native and chemically modified $β$-cyclodextrin. Eur. J. Pharm. Biopharm. 2007, 67, 531–539. [Google Scholar] [CrossRef]
- Del Gaudio, P.; De Cicco, F.; Sansone, F.; Aquino, R.P.; Adami, R.; Ricci, M.; Giovagnoli, S. Alginate beads as a carrier for omeprazole/SBA-15 inclusion compound: A step towards the development of personalized paediatric dosage forms. Carbohydr. Polym. 2015, 133, 464–472. [Google Scholar] [CrossRef]
- Almurisi, S.H.; Doolaanea, A.A.; Akkawi, M.E.; Chatterjee, B.; Sarker, Z.I. Taste masking of paracetamol encapsulated in chitosan-coated alginate beads. J. Drug Deliv. Sci. Technol. 2020, 56, 101520. [Google Scholar] [CrossRef]
- Hochberg, J.; Tamhanea, A. Multiple Comparison Procedures; John Wiley & Sons, Inc.: New York, NY, USA, 1987. [Google Scholar]
- Mai NN, S.; Nakai, R.; Kawano, Y.; Hanawa, T. Enhancing the solubility of curcumin using a solid dispersion system with hydroxypropyl-$β$-cyclodextrin prepared by grinding, freeze-drying, and common solvent evaporation methods. Pharmacy 2020, 8, 203. [Google Scholar]
- Higuchi, T.; Lach, J.L. Investigation of Some Complexes Formed in Solution by Caffeine: V. Interactions Between Caffeine and P-Aminobenzoic Acid, M-Hydroxybenzoic Acid, Picric Acid, O-Phthalic Acid, Suberic Acid, and Valeric Acid. J. Am. Pharm. Assoc. 1954, 43, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.; Nada, A.H.; Ghorab, M.M.; Hammady, T.H. The Use of Some Additives for the Improvement of Griseofulvin Performance. Bull. Fac. Pharm. Cairo Univ. 2000, 38, 63–71. [Google Scholar]
- Jantarat, C.; Sirathanarun, P.; Ratanapongsai, S.; Watcharakan, P.; Sunyapong, S.; Wadu, A. Curcumin-hydroxypropyl-$β$-cyclodextrin inclusion complex preparation methods: Effect of common solvent evaporation, freeze drying, and pH shift on solubility and stability of curcumin. Trop. J. Pharm. Res. 2014, 13, 1215–1223. [Google Scholar] [CrossRef]
- Tang, P.; Ma, X.; Wu, D.; Li, S.; Xu, K.; Tang, B.; Li, H. Posaconazole/hydroxypropyl-$β$-cyclodextrin host--guest system: Improving dissolution while maintaining antifungal activity. Carbohydr. Polym. 2016, 142, 16–23. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.Y.; Ouyang, X.K. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Chatzitaki, A.-T.; Karavasili, C.; Katsamenis, O.L.; Tzetzis, D.; Mystiridou, E.; Bouropoulos, N.; Fatouros, D.G. Controlled release of 5-fluorouracil from alginate beads encapsulated in 3D printed pH-responsive solid dosage forms. AAPS PharmSciTech 2018, 19, 3362–3375. [Google Scholar] [CrossRef]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Retention and release of oil-in-water emulsions from filled hydrogel beads composed of calcium alginate: Impact of emulsifier type and pH. Soft Matter. 2015, 11, 2228–2236. [Google Scholar] [CrossRef]
- Anal, A.K.; Stevens, W.F. Chitosan--alginate multilayer beads for controlled release of ampicillin. Int. J. Pharm. 2005, 290, 45–54. [Google Scholar] [CrossRef]
- Omer, A.M.; Taher, M.A.; Hamed, A.M.; Ali, A.M.; Tamer, T.M.; Eldin, M.S.M. Development of smart alginate/chitosan grafted microcapsules for colon site-specific drug delivery. Egypt J. Chem. 2019, 62, 1037–1045. [Google Scholar] [CrossRef]
- Umaredkar, A.A.; Dangre, P.V.; Mahapatra, D.K.; Dhabarde, D.M. Fabrication of chitosan-alginate polyelectrolyte complexed hydrogel for controlled release of cilnidipine: A statistical design approach. Mater. Technol. 2020, 35, 697–707. [Google Scholar] [CrossRef]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate beads for colon specific delivery of self-emulsifying curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Hanna Pierre, A.; Gad Shadeed Ghonaim Hassan, M.; Ghorab Mamdouh, M. Optimization of Gabapentin Release and Targeting Absorption, Through Incorporation into Alginate Beads. Br. J. Pharm. Res. 2013, 3, 597–616. [Google Scholar] [CrossRef]
- Singh, A.; Mandal, U.K.; Narang, R.K. Development and characterization of enteric coated pectin pellets containing mesalamine and Saccharomyces boulardii for specific inflamed colon: In vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2021, 62, 102393. [Google Scholar] [CrossRef]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis: The updated Sydney system. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Alattar, A.; Alshaman, R.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Development of Novel pH-Sensitive Eudragit Coated Beads Containing Curcumin-Mesalamine Combination for Colon-Specific Drug Delivery. Gels 2023, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Elnashar, M.M.; Yassin, M.A.; Abdel Moneim, A.E.-F.; Bary, E.M.A. Surprising performance of alginate beads for the release of low-molecular-weight drugs. J. Appl. Polym. Sci. 2010, 116, 3021–3026. [Google Scholar] [CrossRef]
- Dai, Y.-N.; Li, P.; Zhang, J.-P.; Wang, A.; Wei, Q. Swelling characteristics and drug delivery properties of nifedipine-loaded pH sensitive alginate--chitosan hydrogel beads. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 86, 493–500. [Google Scholar] [CrossRef]
- Deshmukh, R.; Harwansh, R.K. Preformulation Considerations Development and Evaluation of Mesalamine Loaded Polysaccharide-Based Complex Mucoadhesive Beads for Colon Targeting. Indian J. Pharm. Educ. Res. 2021, 55, 95–106. [Google Scholar] [CrossRef]
- Malakar, J.; Nayak, A.K. Formulation and statistical optimization of multiple-unit ibuprofen-loaded buoyant system using 23-factorial design. Chem. Eng. Res. Des. 2012, 90, 1834–1846. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Zhang, Z. Calcium-carboxymethyl chitosan hydrogel beads for protein drug delivery system. J. Appl. Polym. Sci. 2007, 103, 3164–3168. [Google Scholar] [CrossRef]
- Mennini, N.; Furlanetto, S.; Cirri, M.; Mura, P. Quality by design approach for developing chitosan-Ca-alginate microspheres for colon delivery of celecoxib-hydroxypropyl-$β$-cyclodextrin-PVP complex. Eur. J. Pharm. Biopharm. 2012, 80, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Udhumansha, U.; Rathnam, G.; Ganesh, M.; Jang, H.T. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 840–850. [Google Scholar] [CrossRef]
- Mi, Y.; Su, R.; Fan, D.-D.; Zhu, X.-L.; Zhang, W.-N. Preparation of N, O-carboxymethyl chitosan coated alginate microcapsules and their application to Bifidobacterium longum BIOMA 5920. Mater. Sci. Eng. C 2013, 33, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; Bueno, P.V.A.; Almeida, E.A.M.S.; Rodrigues, F.H.; Rubira, A.F.; Muniz, E.C. Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int. J. Biol. Macromol. 2013, 57, 174–184. [Google Scholar] [CrossRef]
- Thakral, N.K.; Ray, A.R.; Majumdar, D.K. Eudragit S-100 entrapped chitosan microspheres of valdecoxib for colon cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2691–2699. [Google Scholar] [CrossRef]








| Run | SA (%) | CaCl2 (%) | Chitosan (%) | Diameter (mm) | Swelling% | EE% |
|---|---|---|---|---|---|---|
| F1 | 1 | 2 | 0.50 | 2.00 ± 0.32 | 408.00 ± 4.3 | 60.85 ± 1.01 |
| F2 | 1 | 4 | 0.10 | 1.50 ± 0.08 | 455.56 ± 7.0 | 64.89 ± 3.62 |
| F3 | 1 | 4 | 0.50 | 1.60 ± 0.16 | 488.24 ± 5.0 | 66.50 ± 0.94 |
| F4 | 1 | 2 | 0.10 | 1.80 ± 0.14 | 400.00 ± 8.5 | 67.10 ± 1.95 |
| F5 | 3 | 4 | 0.10 | 2.20 ± 0.08 | 708.09 ± 3.5 | 80.88 ± 0.93 |
| F6 | 3 | 2 | 0.50 | 2.50 ± 0.21 | 743.14 ± 2.7 | 83.20 ± 0.80 |
| F7 | 3 | 2 | 0.10 | 2.40 ± 0.08 | 700.00 ± 3.6 | 83.81 ± 1.73 |
| F8 | 3 | 4 | 0.50 | 2.60 ± 0.24 | 800.00 ± 6.2 | 87.44 ± 1.88 |
| Scoring | |||||
|---|---|---|---|---|---|
| Groups | Chronic Lymphoplasmacytic Infiltration | Neutrophils Attacking the Glands | Areas of Atrophying Glands | Intestinal Metaplasia | |
| A | Group 1 (Normal) | 0 | 0 | 0 | 0 |
| B | Group 2 (PUD Control received no treatment) | 2 | 0 | 3 | 0 |
| C | Group 3 (PUD + blank beads) | 2 | 0 | 3 | 0 |
| D | Group 4 (PUD + free OMP) | 2 | 0 | 2 | 0 |
| E | Group 5 (PUD + CURC-only beads) | 2 | 0 | 2 | 0 |
| F | Group 6 (PUD + OMP-only beads) | 2 | 0 | 1 | 0 |
| G | Group 7 (PUD + OMP/CURC-loaded beads) | 1 | 0 | 0 | 0 |
| Factors | Independent Variables | Low | High | |
|---|---|---|---|---|
| X1 | Na Alginate | % | 1 | 3 |
| X2 | Ca Chloride | % | 2 | 4 |
| X3 | Chitosan | % | 0.1 | 0.5 |
| Response | ||||
| Y1 | Diameter | Mm | ||
| Y2 | Swelling | % | ||
| Y3 | Entrapment Efficiency (EE) | % | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heikal, E.J.; Kaoud, R.M.; Gad, S.; Mokhtar, H.I.; Aldahish, A.A.; Alzlaiq, W.A.; Zaitone, S.A.; Moustafa, Y.M.; Hammady, T.M. Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals 2023, 16, 795. https://doi.org/10.3390/ph16060795
Heikal EJ, Kaoud RM, Gad S, Mokhtar HI, Aldahish AA, Alzlaiq WA, Zaitone SA, Moustafa YM, Hammady TM. Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals. 2023; 16(6):795. https://doi.org/10.3390/ph16060795
Chicago/Turabian StyleHeikal, Eman J., Rashad M. Kaoud, Shadeed Gad, Hatem I. Mokhtar, Afaf A. Aldahish, Wafa Ali Alzlaiq, Sawsan A. Zaitone, Yasser M. Moustafa, and Taha M. Hammady. 2023. "Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers" Pharmaceuticals 16, no. 6: 795. https://doi.org/10.3390/ph16060795
APA StyleHeikal, E. J., Kaoud, R. M., Gad, S., Mokhtar, H. I., Aldahish, A. A., Alzlaiq, W. A., Zaitone, S. A., Moustafa, Y. M., & Hammady, T. M. (2023). Design and Optimization of Omeprazole-Curcumin-Loaded Hydrogel Beads Coated with Chitosan for Treating Peptic Ulcers. Pharmaceuticals, 16(6), 795. https://doi.org/10.3390/ph16060795









