Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways
Abstract
1. Introduction
2. Results
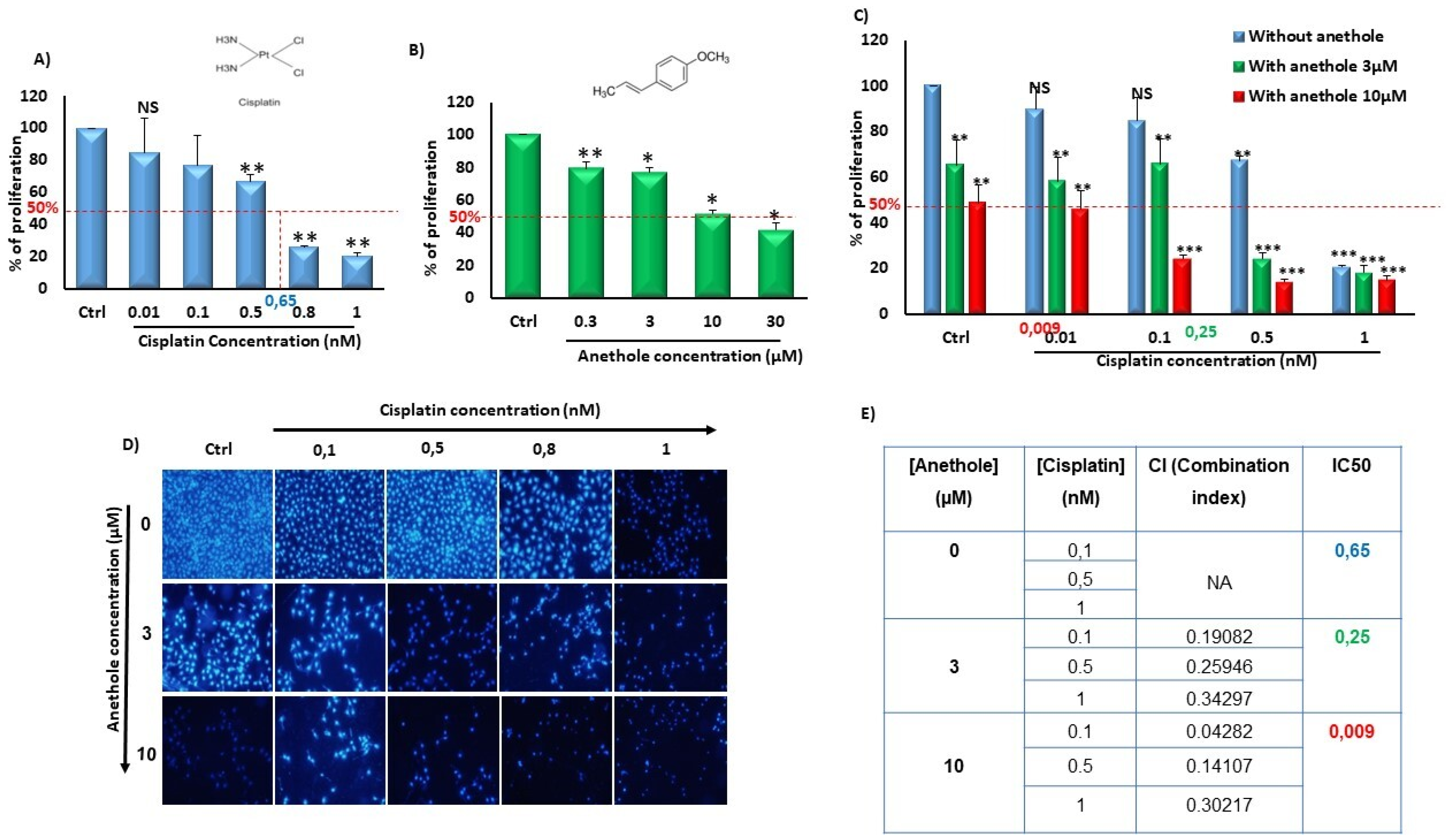
2.1. Synergistic Effect of Anethole and Cisplatin on Inhibition of Oral Cancer Cell Viability
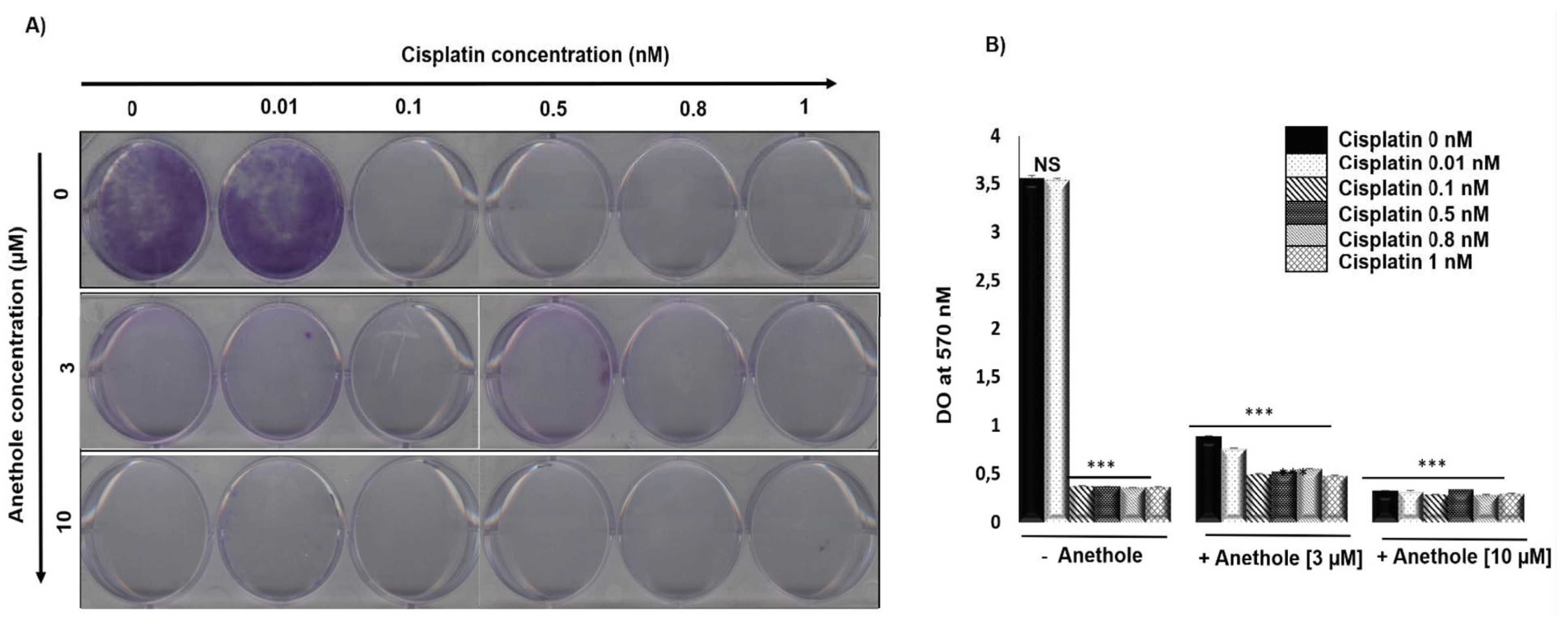
2.2. The Combination of Anethole and Cisplatin Potentiates the Inhibition of Colony Formation in Oral Cancer Cells
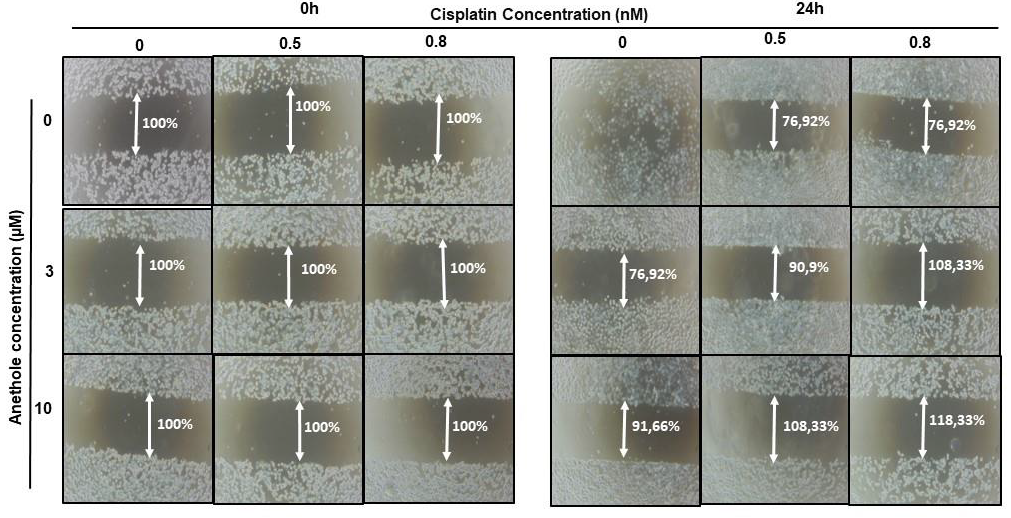
2.3. Anethole Potentiates Cisplatin on Inhibition of Cell Migration in Oral Cancer Cells
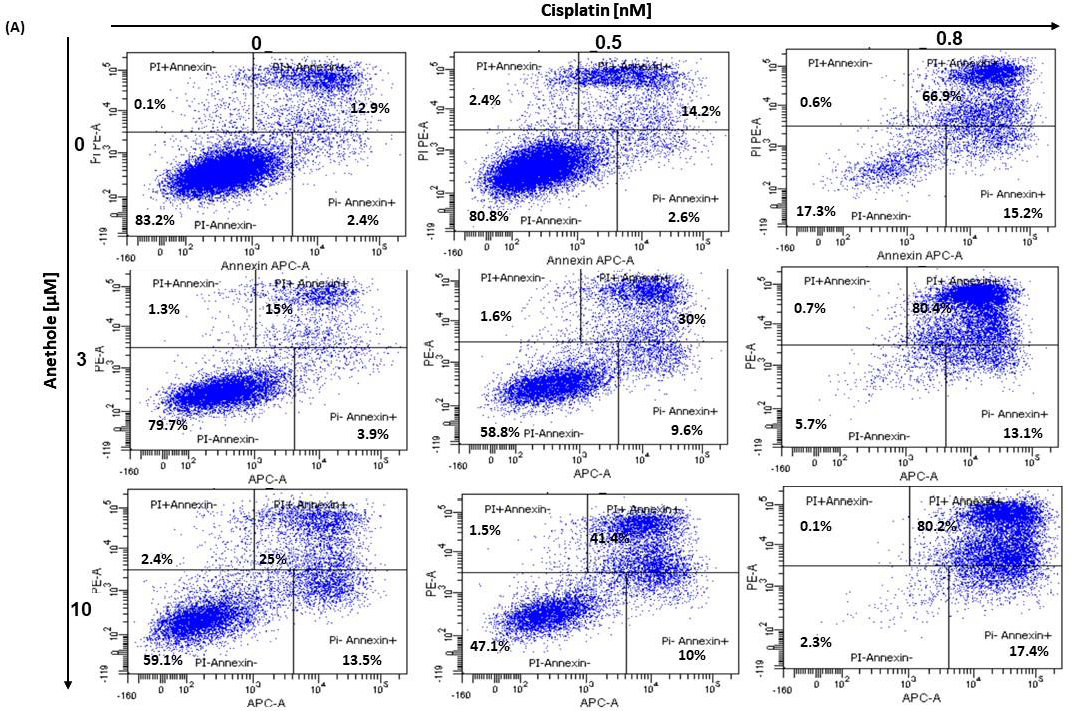
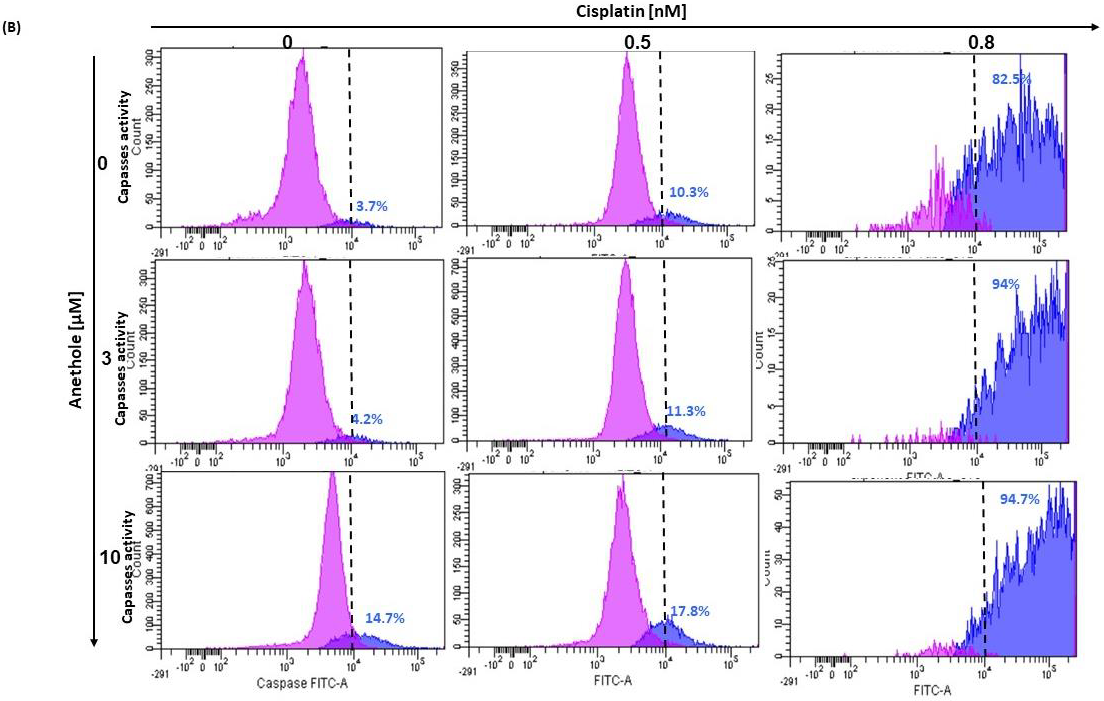
2.4. Anethole Synergy with Cisplatin in the Induction of Apoptosis via Caspase Activation
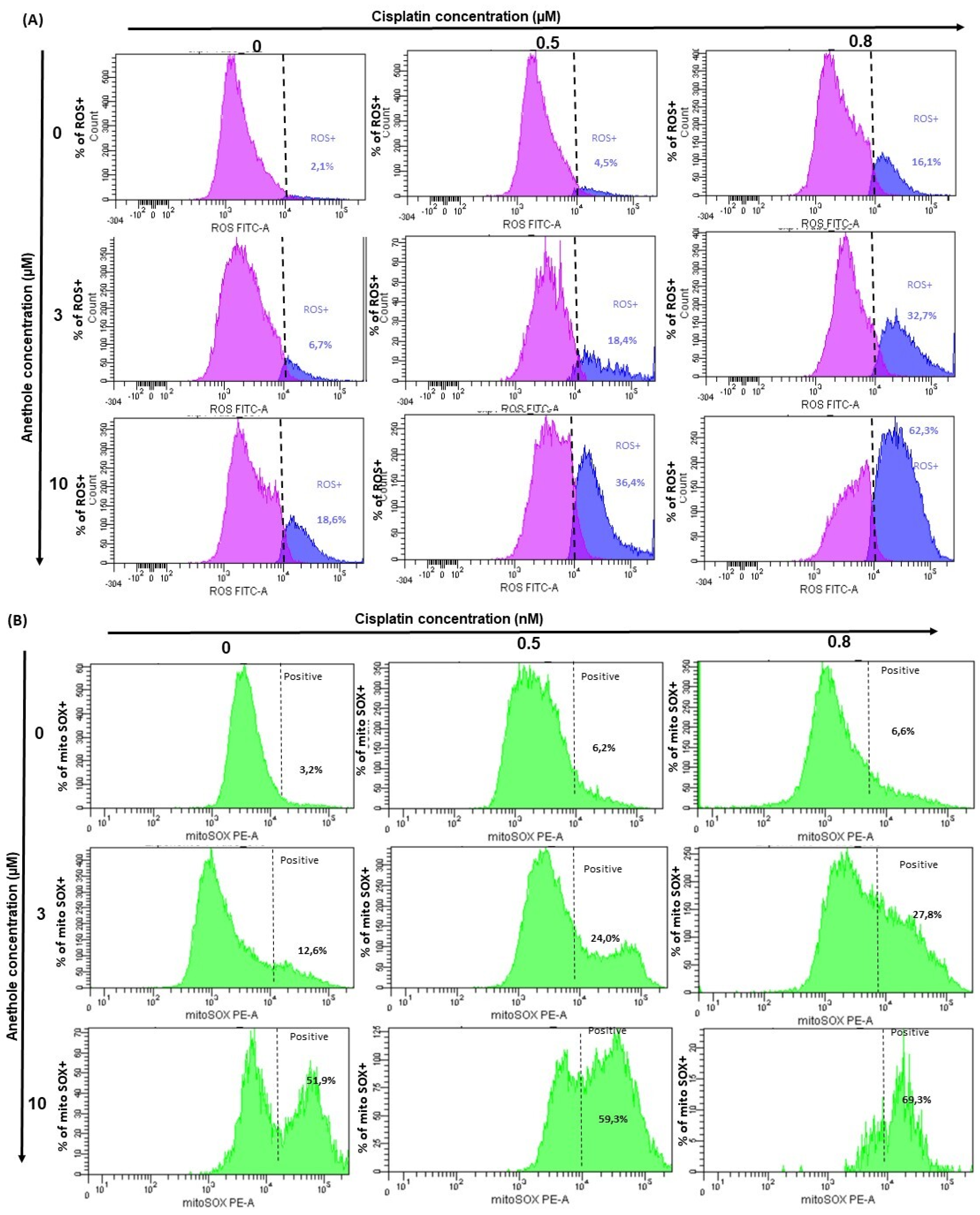
2.5. Anethole Potentiates Oxidative Stress Induced by Cisplatin
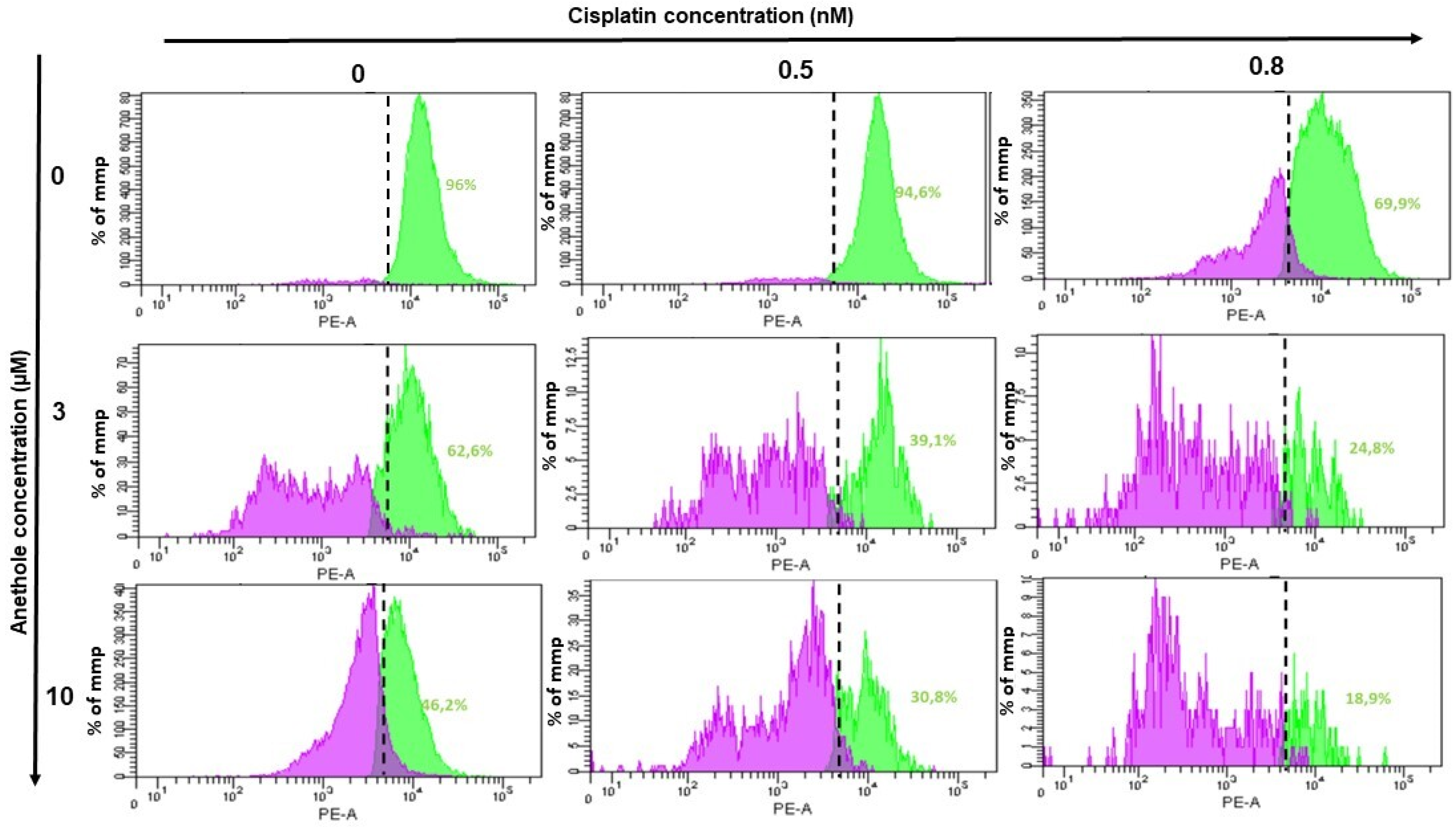
2.6. Anethole Synergy with Cisplatin in the Induction of Mitochondrial Membrane Potential (ΔΨm)
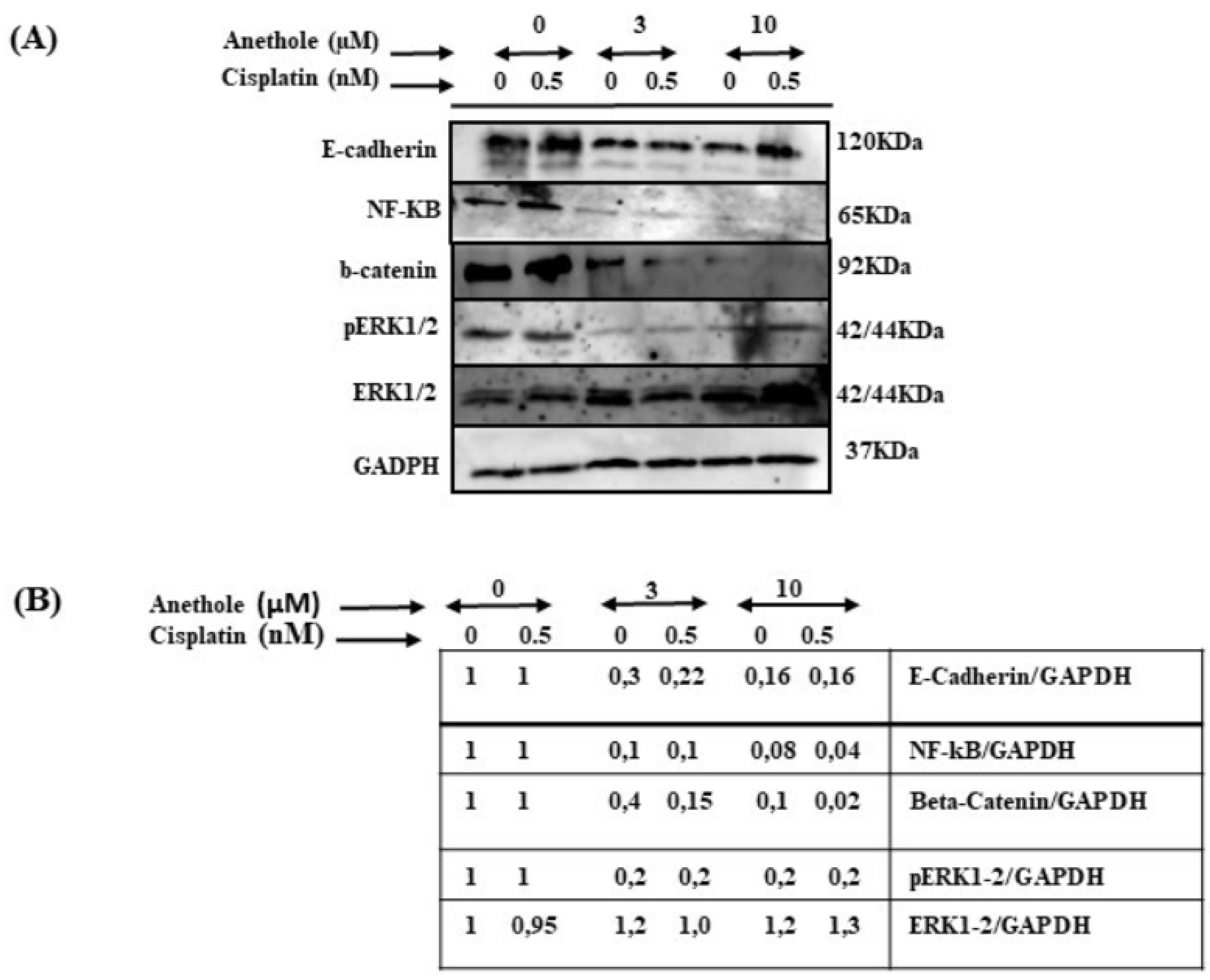
2.7. Anethole Synergy with Cisplatin in Inhibition of Many Cancers Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture Conditions
4.2. Drugs
4.3. MTT Assay
4.4. Nuclear Staining by Hoechst Assay
4.5. LDH Activity
4.6. Colony Formation Assay by Crystal Violet Staining
4.7. Wound Healing Assay
4.8. Cell Apoptosis Assay by Annexin V-FITC and Propidium Iodide
4.9. Cell Autophagy Assay
4.10. Determination of ROS Levels
4.11. Determination of MitoSOX Levels
4.12. ΔΨm (Mitochondrial Membrane Potential) Assay
4.13. Western Blot and Antibodies
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelazius, R.; Gervickas, A.; Petronis, Z.; Vaiciunaite, E. Epidemiology of primary oral cancer diagnostics among dentists and physicians in Lithuania. Stomatologija 2019, 21, 83–91. [Google Scholar] [PubMed]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Wünsch-Filho, V.; et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Popkin, B.M. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nat. Rev. Cancer 2007, 7, 61–67. [Google Scholar] [CrossRef]
- Fund, W.C.R. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; American Institute for Cancer Research: Arlington, WV, USA, 2018; Available online: https://www.wcrf.org/dietandcancer (accessed on 1 February 2021).
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for Oral Cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef]
- L’Esperance, S.; Bachvarova, M.; Tetu, B.; Mes-Masson, A.M.; Bachvarov, D. Global gene expression analysis of early response to chemotherapy treatment in ovarian cancer spheroids. BMC Genom. 2008, 9, 99. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular mechanisms of cisplatin resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Sun, C.Y.; Zhang, Q.Y.; Zheng, G.J.; Feng, B. Phytochemicals: Current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother. 2019, 110, 518–527. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Xiaokaiti, Y.; Li, X. Natural Product Regulates Autophagy in Cancer. Adv. Exp. Med. Biol. 2020, 1207, 709–724. [Google Scholar] [CrossRef]
- Villegas, C.; Perez, R.; Sterner, O.; Gonzalez-Chavarria, I.; Paz, C. Curcuma as an adjuvant in colorectal cancer treatment. Life Sci. 2021, 286, 120043. [Google Scholar] [CrossRef]
- Castillo, R.L.; Herrera, E.A.; Gonzalez-Candia, A.; Reyes-Farias, M.; de la Jara, N.; Pena, J.P.; Carrasco-Pozo, C. Quercetin Prevents Diastolic Dysfunction Induced by a High-Cholesterol Diet: Role of Oxidative Stress and Bioenergetics in Hyperglycemic Rats. Oxidative Med. Cell. Longev. 2018, 2018, 7239123. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Flores, S.E.; Rial-Hermida, M.I.; Ramirez, J.C.; Pazos, A.; Concheiro, A.; Alvarez-Lorenzo, C.; Peralta, R.D. Microemulsions for Colorectal Cancer Treatments. General Considerations and Formulation of Methotrexate. Mini Rev. Med. Chem. 2016, 16, 498–508. [Google Scholar] [CrossRef]
- Sung, B.; Prasad, S.; Yadav, V.R.; Aggarwal, B.B. Cancer Cell Signaling Pathways Targeted by Spice-Derived Nutraceuticals. Nutr. Cancer 2012, 64, 173–197. [Google Scholar] [CrossRef]
- Chainy, G.B.N.; Manna, S.K.; Chaturvedi, M.M.; Aggarwal, B.B. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: Effect on NF-κB, AP-1, JNK, MAPKK and apoptosis. Oncogene 2000, 19, 2943–2950. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Costache, I.-I.; Miron, A. Anethole and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [CrossRef]
- Dinesh, D. Sudha antidepressant-like activity of trans-anethole in unstressed mice and stressed mice. Asian J. Pharm. Clin. Res. 2019, 12, 121–127. [Google Scholar] [CrossRef]
- Marinov, V.; Valcheva-Kuzmanova, S. Review on the pharmacological activities of anethole. Scr. Sci. Pharm. 2015, 2, 14. [Google Scholar] [CrossRef]
- Contant, C.; Rouabhia, M.; Loubaki, L.; Chandad, F.; Semlali, A. Anethole induces anti-oral cancer activity by triggering apoptosis, autophagy and oxidative stress and by modulation of multiple signaling pathways. Sci. Rep. 2021, 11, 13087. [Google Scholar] [CrossRef]
- Shahbazian, S.; Akbarzadeh, A.; Torabi, S.; Omidi, M. Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D. Biotechnol. Lett. 2015, 37, 1355–1359. [Google Scholar] [CrossRef]
- Choo, E.J.; Rhee, Y.H.; Jeong, S.J.; Kim, H.S.; Ko, H.S.; Kim, J.H.; Kwon, T.R.; Jung, J.H.; Kim, J.H.; Lee, H.J.; et al. Anethole Exerts Antimetatstaic Activity via Inhibition of Matrix Metalloproteinase 2/9 and AKT/Mitogen-Activated Kinase/Nuclear Factor Kappa B Signaling Pathways. Biol. Pharm. Bull. 2011, 34, 41–46. [Google Scholar] [CrossRef]
- Elkady, A.I. Anethole Inhibits the Proliferation of Human Prostate Cancer Cells via Induction of Cell Cycle Arrest and Apoptosis. Anti Cancer Agents Med. Chem. 2018, 18, 216–236. [Google Scholar] [CrossRef]
- Chen, C.H.; Degraffenried, L.A. Anethole suppressed cell survival and induced apoptosis in human breast cancer cells independent of estrogen receptor status. Phytomedicine 2012, 19, 763–767. [Google Scholar] [CrossRef]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer. Res. 2012, 32, 4843–4850. [Google Scholar]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Li, Y.; Mao, W. Berberine in combination with cisplatin suppresses breast cancer cell growth through induction of DNA breaks and caspase-3-dependent apoptosis. Oncol. Rep. 2016, 36, 567–572. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Yu, C.; Zhao, G.; Zhou, J.; Zhang, G.; Li, M.; Jiang, D.; Quan, Z.; Zhang, Y. Synergistic inhibitory effects of capsaicin combined with cisplatin on human osteosarcoma in culture and in xenografts. J. Exp. Clin. Cancer Res. 2018, 37, 251. [Google Scholar] [CrossRef]
- Hu, H.; Luo, L.; Liu, F.; Zou, D.; Zhu, S.; Tan, B.; Chen, T. Anti-cancer and Sensibilisation Effect of Triptolide on Human Epithelial Ovarian Cancer. J. Cancer 2016, 7, 2093–2099. [Google Scholar] [CrossRef]
- Sorenson, C.M.; Eastman, A. Influence of cis-diamminedichloroplatinum(II) on DNA synthesis and cell cycle progression in excision repair proficient and deficient Chinese hamster ovary cells. Cancer Res. 1988, 48, 6703–6707. [Google Scholar]
- Siddik, Z.H. Biochemical and Molecular Mechanisms of Cisplatin Resistance. Cancer Treat. Res. 2002, 112, 263–284. [Google Scholar] [CrossRef]
- Itamochi, H.; Kigawa, J.; Akeshima, R.; Sato, S.; Kamazawa, S.; Takahashi, M.; Kanamori, Y.; Suzuki, M.; Ohwada, M.; Terakawa, N. Mechanisms of Cisplatin Resistance in Clear Cell Carcinoma of the Ovary. Oncology 2002, 62, 349–353. [Google Scholar] [CrossRef]
- Boice, A.; Bouchier-Hayes, L. Targeting apoptotic caspases in cancer. Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118688. [Google Scholar] [CrossRef]
- Canman, C.E.; Kastan, M.B. Induction of apoptosis by tumor suppressor genes and oncogenes. Semin. Cancer Biol. 1995, 6, 17–25. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The Cytotoxicity of Kahweol in HT-29 Human Colorectal Cancer Cells Is Mediated by Apoptosis and Suppression of Heat Shock Protein 70 Expression. Biomol. Ther. 2015, 23, 128–133. [Google Scholar] [CrossRef]
- Arumugam, P.; Sampathkumar, B.; Perumalsamy, H.; Balusamy, S.R.; Ramesh, V.; Sundaravadevel, S. Synergistic effect of anethole and doxorubicin alleviates cell proliferation, cell cycle arrest, and ER stress and promotes ROS-mediated apoptosis in triple-negative breast cancer cells. J. Biochem. Mol. Toxicol. 2021, 35, e22928. [Google Scholar] [CrossRef]
- Levi, L.E.; Lalla, R.V. Dental Treatment Planning for the Patient with Oral Cancer. Dent. Clin. N. Am. 2018, 62, 121–130. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Asati, V.; Bharti, S.K. MEK inhibitors in oncology: A patent review (2015-Present). Expert Opin. Ther. Pat. 2017, 27, 887–906. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Loubaki, L.; Rouabhia, M.; Al Zahrani, M.; Al Amri, A.; Semlali, A. Oxidative Stress and Autophagy Mediate Anti-Cancer Properties of Cannabis Derivatives in Human Oral Cancer Cells. Cancers 2022, 14, 4924. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semlali, A.; Ajala, I.; Beji, S.; Al-Zharani, M.M.; Rouabhia, M. Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways. Pharmaceuticals 2023, 16, 700. https://doi.org/10.3390/ph16050700
Semlali A, Ajala I, Beji S, Al-Zharani MM, Rouabhia M. Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways. Pharmaceuticals. 2023; 16(5):700. https://doi.org/10.3390/ph16050700
Chicago/Turabian StyleSemlali, Abdelhabib, Ikram Ajala, Sarra Beji, Mohammed Mousa Al-Zharani, and Mahmoud Rouabhia. 2023. "Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways" Pharmaceuticals 16, no. 5: 700. https://doi.org/10.3390/ph16050700
APA StyleSemlali, A., Ajala, I., Beji, S., Al-Zharani, M. M., & Rouabhia, M. (2023). Synergistic Effect of Anethole and Platinum Drug Cisplatin against Oral Cancer Cell Growth and Migration by Inhibiting MAPKase, Beta-Catenin, and NF-κB Pathways. Pharmaceuticals, 16(5), 700. https://doi.org/10.3390/ph16050700









