Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney
Abstract
1. Introduction
2. Results
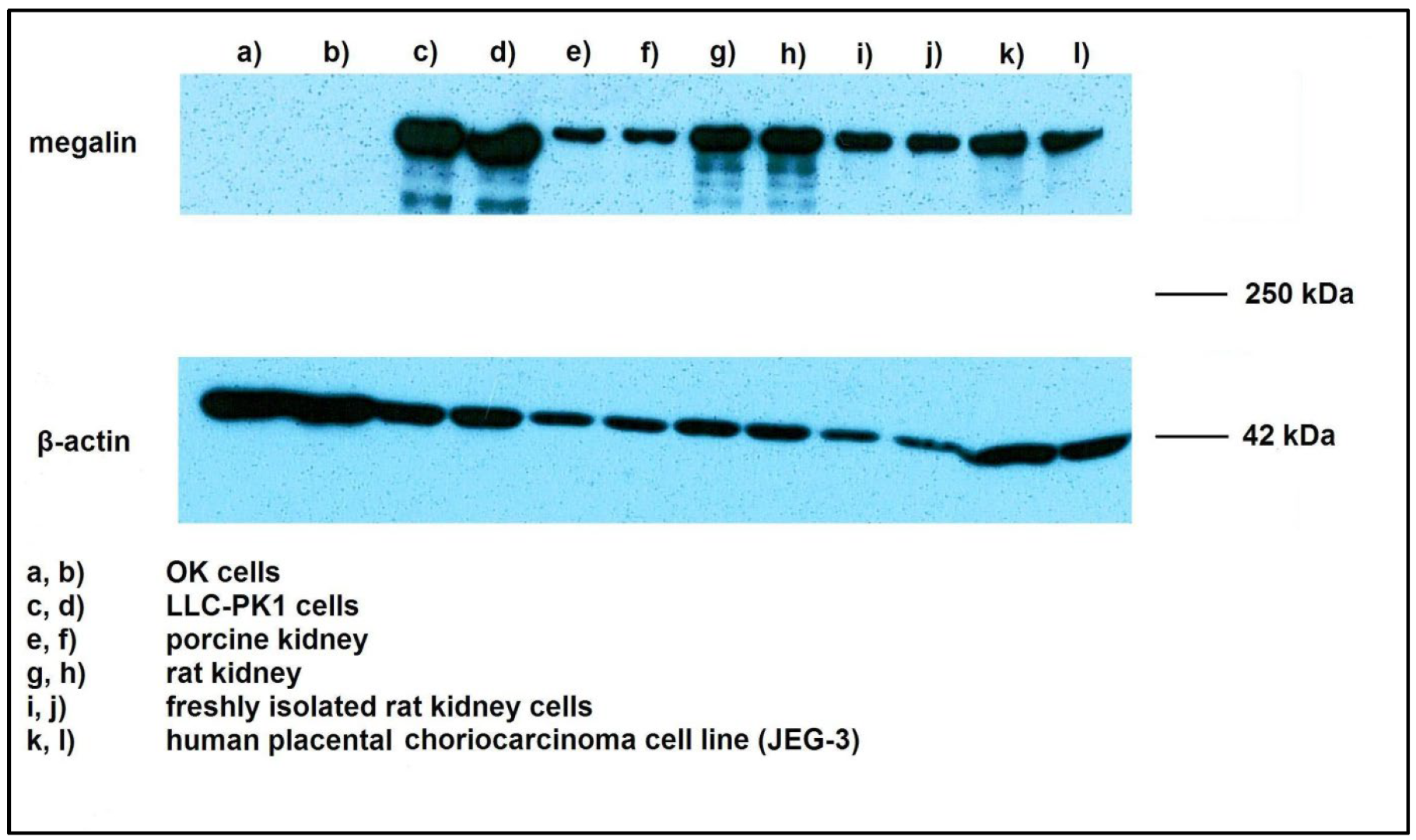
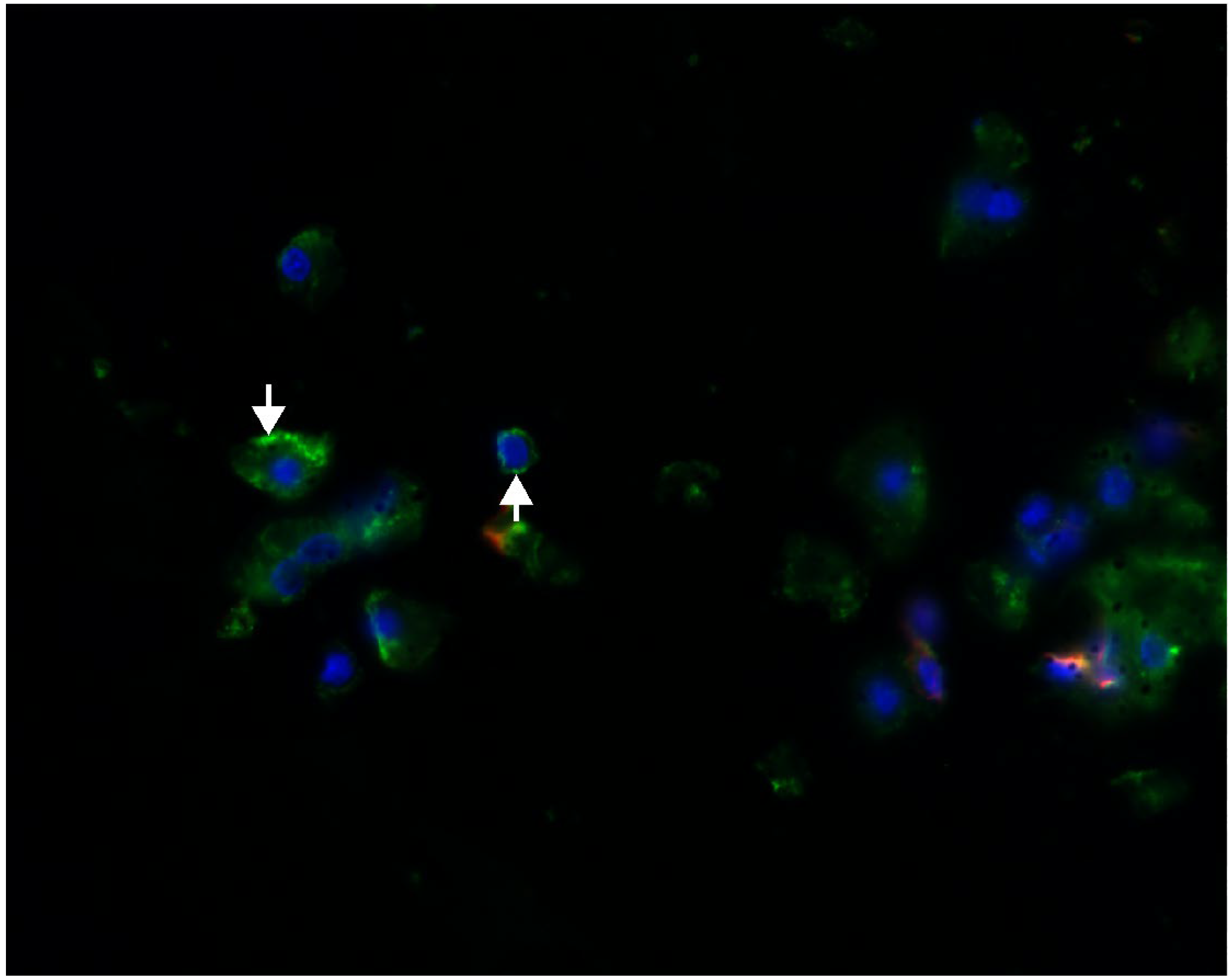
2.1. Megalin Expression Approvement
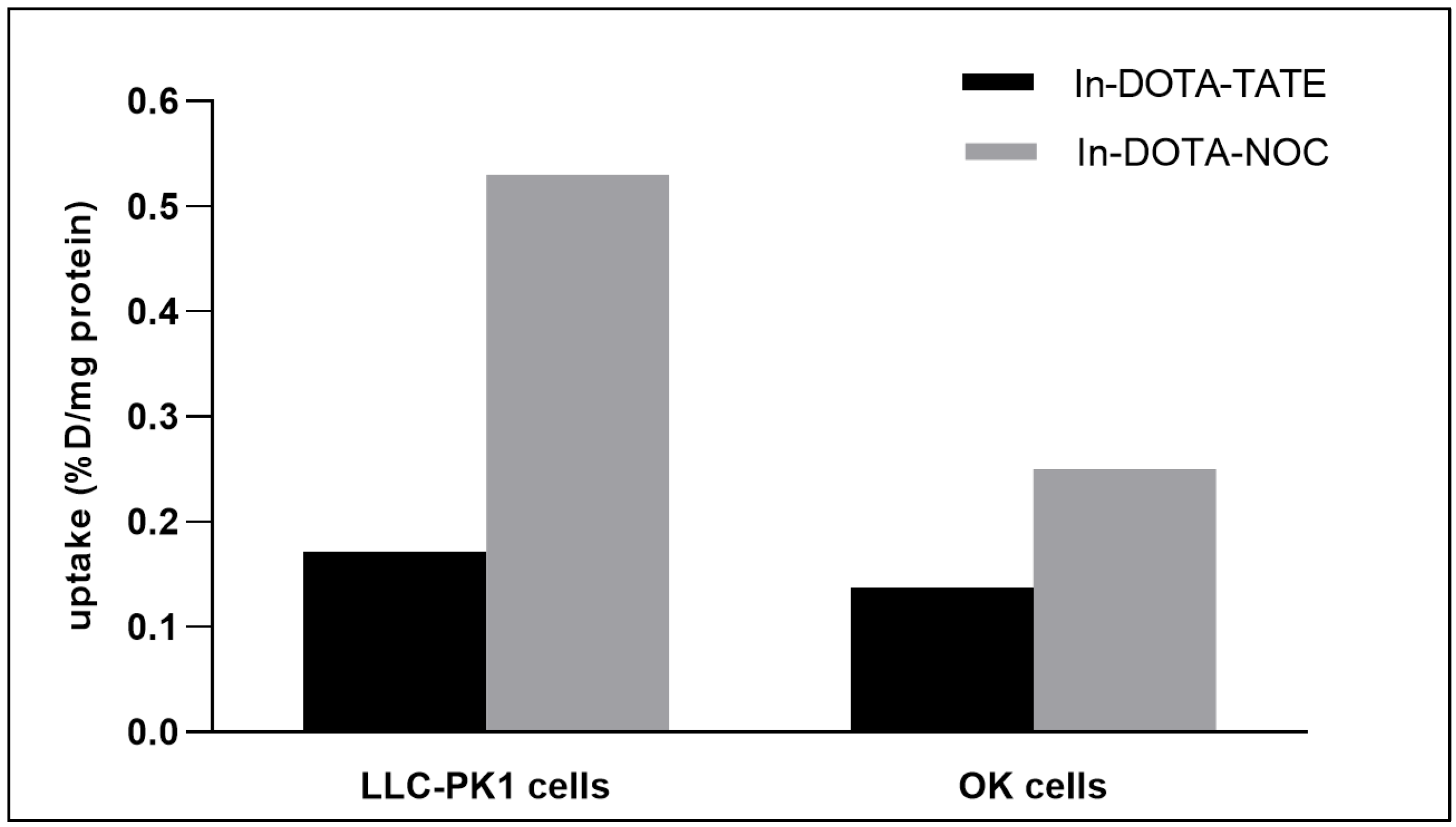
2.2. Evaluation of Cell Uptake In Vitro
3. Discussion
4. Materials and Methods
4.1. Western Blot Analysis
4.2. Immunohistochemistry
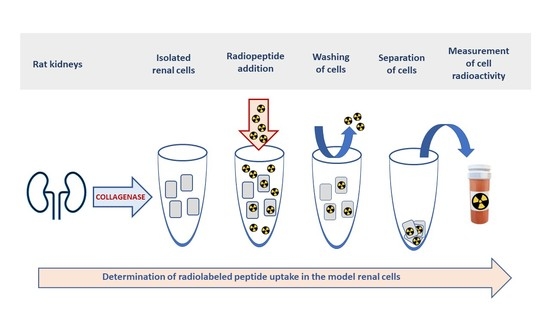
4.3. Experiments with the Isolated Rat Renal Cells
4.4. Radiolabeling of Peptides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohtavinejad, N.; Shafiee Ardestani, M.; Khalaj, A.; Pormohammad, A.; Najafi, R.; Bitarafan-Rajabi, A.; Hajiramezanali, M.; Amanlou, M. Application of radiolabeled peptides in tumor imaging and therapy. Life Sci. 2020, 258, 118206. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Gharibkandi, N.; Conlon, J.M.; Hosseinimehr, S.J. Strategies for improving stability and pharmacokinetic characteristics of radiolabeled peptides for imaging and therapy. Peptides 2020, 133, 170385. [Google Scholar] [CrossRef] [PubMed]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.F.A.; Naqvi, S.A.R.; Roohi, S.; Sherazi, T.A.; Rasheed, R. 177Lu-DOTA-coupled minigastrin peptides: Promising theranostic agents in neuroendocrine cancers. Mol. Biol. Rep. 2018, 45, 1759–1767. [Google Scholar] [CrossRef]
- Melis, M.; Krenning, E.P.; Bernard, B.F.; Barone, R.; Visser, T.J.; de Jong, M. Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur. J. Nucl. Med. Mol. Imaging. 2005, 32, 1136–1143. [Google Scholar] [CrossRef]
- Melis, M.; Krenning, E.P.; Bernard, B.F.; de Visser, M.; Rolleman, E.; de Jong, M. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: Species and gender differences. Nucl. Med. Biol. 2007, 34, 633–641. [Google Scholar] [CrossRef]
- Arano, Y. Renal brush border strategy: A developing procedure to reduce renal radioactivity levels of radiolabeled polypeptides. Nucl. Med. Biol. 2021, 92, 149–155. [Google Scholar] [CrossRef]
- Barone, R.; Van Der Smissen, P.; Devuyst, O.; Beaujean, V.; Pauwels, S.; Courtoy, P.J.; Jamar, F. Endocytosis of the somatostatin analogue, octreotide, by the proximal tubule-derived opossum kidney (OK) cell line. Kidney Int. 2005, 67, 969–976. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Lash, L.H.; Shivnani, A.; Mai, J.; Chinnaiyan, P.; Krause, R.J.; Elfarra, A.A. Renal cellular transport, metabolism, and cytotoxicity of S-(6-purinyl)glutathione, a prodrug of 6-mercaptopurine, and analogues. Biochem. Pharmacol. 1997, 54, 1341–1349. [Google Scholar] [CrossRef]
- Jones, D.P.; Sundby, G.B.; Ormstad, K.; Orrenius, S. Use of isolated kidney cells for study of drug metabolism. Biochem. Pharmacol. 1979, 28, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Racusen, L.C.; Monteil, C.; Sgrignoli, A.; Lucskay, M.; Marouillat, S.; Rhim, J.G.; Morin, J.P. Cell lines with extended in vitro growth potential from human renal proximal tubule: Characterization, response to inducers, and comparison with established cell lines. J. Lab. Clin. Med. 1997, 129, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Klaassen, C.D.; Aleksunes, L.M. Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacol. Rev. 2010, 62, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Ismail, A.; Doghish, A.S. Megalin, a multi-ligand endocytic receptor, and its participation in renal function and diseases: A review. Life Sci. 2022, 308, 120923. [Google Scholar] [CrossRef] [PubMed]
- Eshbach, M.L.; Weisz, O.A. Receptor-Mediated Endocytosis in the Proximal Tubule. Annu. Rev. Physiol. 2017, 79, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Birn, H.; Storm, T.; Weyer, K.; Nielsen, R. Endocytic receptors in the renal proximal tubule. Physiology 2012, 27, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Weyer, K.; Nielsen, R.; Petersen, S.V.; Christensen, E.I.; Rehling, M.; Birn, H. Renal uptake of 99mTc-dimercaptosuccinic acid is dependent on normal proximal tubule receptor-mediated endocytosis. J. Nucl. Med. 2013, 54, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Blaufox, M.D. Transport of 99mTc-MAG3 via rat renal organic anion. J. Nucl. Med. 2004, 45, 86–88. [Google Scholar]
- Cihlo, J.; Melicharová, L.; Petrik, M.; Laznickova, A.; Laznicek, M. Comparison of 111In-DOTA-NOC and 111I-DOTA-TATE distribution in the target and dose-limiting tissues: Conflicting results in vitro and in vivo. Anticancer. Res. 2008, 28, 2189–2195. [Google Scholar]
- Trejtnar, F.; Laznicek, M.; Laznickova, A.; Kopecky, M.; Petrik, M.; Béhé, M.; Schmidt, J.; Maecke, H.; Maina, T.; Nock, B. Biodistribution and elimination characteristics of two 111In-labeled CCK-2/gastrin receptor-specific peptides in rats. Anticancer. Res. 2007, 27, 907–912. [Google Scholar]
- Melicharova, L.; Laznickova, A.; Laznicek, M. Preclinical evaluation of gastrin derivatives labelled with 111In: Radiolabelling, affinity profile and pharmacokinetics in rats. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2014, 158, 544–551. [Google Scholar] [CrossRef]
- Masters, J.R.W. Cell line misidentification: The beginning of the end. Nat. Rev. Cancer 2010, 10, 441–448. [Google Scholar]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. 2007, 35, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Ahlin, G.; Hilgendorf, C.; Karlsson, J.; Szigyarto, C.A.; Uhlén, M.; Artursson, P. Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab. Dispos. 2009, 37, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Pietruck, F.; Kuhlmann, M.K.; Lange, B.; Feldkamp, T.; Herget-Rosenthal, S.; Rauen, U.; Burkhardt, G.; Kohler, H.; Philipp, T.; Kribben, A. Effect of quercetin on hypoxic injury in freshly isolated rat proximal tubules. J. Lab. Clin. Med. 2003, 142, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Nesslany, F.; Zennouche, N.; Simar-Meintières, S.; Talahari, I.; Nkili-Mboui, E.N.; Marzin, D. In vivo Comet assay on isolated kidney cells to distinguish genotoxic carcinogens from epigenetic carcinogens or cytotoxic compounds. Mutat. Res. 2007, 630, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Engbersen, R.; Masereeuw, R.; van Gestel, M.A.; van der Logt, E.M.; Smits, P.; Russel, F.G. Glibenclamide depletes ATP in renal proximal tubular cells by interfering with mitochondrial metabolism. Br. J. Pharmacol. 2005, 145, 1069–1075. [Google Scholar] [CrossRef]
- Shaw, S.; Marples, D. A rat kidney tubule suspension for the study of vasopressin-induced shuttling of AQP2 water channels. Am. J. Physiol. Renal. Physiol. 2002, 283, F1160–F1166. [Google Scholar] [CrossRef]
- Visarius, T.M.; Putt, D.A.; Schare, J.M.; Pegouske, D.M.; Lash, L.H. Pathways of glutathione metabolism and transport in isolated proximal tubular cells from rat kidney. Biochem. Pharmacol. 1996, 52, 259–272. [Google Scholar] [CrossRef]
- Juul, T.; Palm, F.; Nielsen, P.M.; Bertelsen, L.B.; Laustsen, C. Ex vivo hyperpolarized MR spectroscopy on isolated renal tubular cells: A novel technique for cell energy phenotyping. Magn. Reason. Med. 2017, 78, 457–461. [Google Scholar] [CrossRef]
- Haenen, H.E.; Rietjens, I.M.; Vervoort, J.; Temmink, J.H.; van Bladeren, P.J. In vitro metabolism of 5-fluoro-2-glutathionyl-nitrobenzene by kidney proximal tubular cells studied by 19F-NMR. Chem. Biol. Interact. 1995, 98, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Cai, H. Drug metabolism enzyme expression and activity in primary cultures of human proximal tubular cells. Toxicology 2008, 244, 56–65. [Google Scholar] [CrossRef]
- Trejtnar, F.; Novy, Z.; Petrik, M.; Laznickova, A.; Melicharova, L.; Vankova, M.; Laznicek, M. In vitro comparison of renal handling and uptake of two somatostatin receptor-specific peptides labeled with indium-111. Ann. Nucl. Med. 2008, 22, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Stolniceanu, C.R.; Nistor, I.; Bilha, S.C.; Constantin, V.; Simona, V.; Matovic, M.; Stefanescu, C.; Covic, A. Nephrotoxicity/renal failure after therapy with 90Yttrium- and 177Lutetium-radiolabeled somatostatin analogs in different types of neuroendocrine tumors: A systematic review. Nucl. Med. Commun. 2020, 41, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.Y.; Nielsen, R.; Birn, H.; Drumm, K.; Mildenberger, S.; Freudinger, R.; Moestrup, S.K.; Verroust, P.J.; Christensen, E.I.; Gekle, M. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000, 58, 1523–1533. [Google Scholar] [CrossRef]
- Christensen, E.I.; Nielsen, R. Role of megalin and cubilin in renal physiology and pathophysiology. Rev. Physiol. Biochem. Pharmacol. 2007, 158, 1–22. [Google Scholar]
- Donato, M.T.; Lahoz, A.; Castell, J.V.; Gómez-Lechón, M.J. Cell lines: A tool for in vitro drug metabolism studies. Curr. Drug Metab. 2008, 9, 1–11. [Google Scholar]
- Behrens, I.; Kamm, W.; Dantzig, A.H.; Kissel, T. Variation of peptide transporter (PepT1 and HPT1) expression in Caco-2 cells as a function of cell origin. J. Pharm. Sci. 2004, 93, 1743–1754. [Google Scholar] [CrossRef]
- Aiba, T.; Susa, M.; Fukumori, S.; Hashimoto, Y. The effects of culture conditions on CYP3A4 and MDR1 mRNA induction by 1,25-dihydroxyvitamin D3 in human intestinal cell lines, Caco-2 and LS180. Drug Metab. Pharmacokinet. 2005, 20, 268–274. [Google Scholar] [CrossRef]
- Odera, K.; Goto, S.; Takahashi, R. Age-related change of endocytic receptors megalin and cubilin in the kidney in rats. Biogerontology 2007, 8, 505–515. [Google Scholar] [CrossRef]
- Zheng, G.; Bachinsky, D.R.; Stamenkovic, I.; Strickland, D.K.; Brown, D.; Andres, G.; McCluskey, R.T. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP). J. Histochem. Cytochem. 1994, 42, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Verroust, P.J.; Birn, H.; Nielsen, R.; Kozyraki, R.; Christensen, E.I. The tandem endocytic receptors megalin and cubilin are important proteins in renal pathology. Kidney Int. 2002, 62, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Guzik, P.; Deberle, L.M.; Busslinger, S.D.; Landolt, T.; Schibli, R.; Müller, C. Design and Evaluation of Novel Albumin-Binding Folate Radioconjugates: Systematic Approach of Varying the Linker Entities. Mol. Pharm. 2022, 19, 963–973. [Google Scholar] [CrossRef]
- Garousi, J.; Vorobyeva, A.; Altai, M. Influence of Several Compounds and Drugs on the Renal Uptake of Radiolabeled Affibody Molecules. Molecules 2020, 25, 2673. [Google Scholar] [CrossRef] [PubMed]
- Geenen, L.; Nonnekens, J.; Konijnenberg, M.; Baatout, S.; De Jong, M.; Aerts, A. Overcoming nephrotoxicity in peptide receptor radionuclide therapy using [177Lu]Lu-DOTA-TATE for the treatment of neuroendocrine tumours. Nucl. Med. Biol. 2021, 102–103, 1–11. [Google Scholar] [CrossRef]
- Zhang, M.; Jacobson, O.; Kiesewetter, D.O.; Ma, Y.; Wang, Z.; Lang, L.; Tang, L.; Kang, F.; Deng, H.; Yang, W.; et al. Improving the Theranostic Potential of Exendin 4 by Reducing the Renal Radioactivity through Brush Border Membrane Enzyme-Mediated Degradation. Bioconjug. Chem. 2019, 30, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Bendre, S.; Zhang, Z.; Kuo, H.T.; Rousseau, J.; Zhang, C.; Merkens, H.; Roxin, Á.; Bénard, F.; Lin, K.S. Evaluation of Met-Val-Lys as a Renal Brush Border Enzyme-Cleavable Linker to Reduce Kidney Uptake of 68Ga-Labeled DOTA-Conjugated Peptides and Peptidomimetics. Molecules 2020, 25, 3854. [Google Scholar] [CrossRef]
- Liu, W.; Yu, W.R.; Carling, T.; Juhlin, C.; Rastad, J.; Ridefelt, P.; Akerström, G.; Hellman, P. Regulation of gp330/megalin expression by vitamins A and D. Eur. J. Clin. Invest. 1998, 28, 100–107. [Google Scholar] [CrossRef]
- Demeule, M.; Jodoin, J.; Beaulieu, E. Dexamethasone modulation of multidrug transporters in normal tissues. FEBS Lett. 1999, 442, 208–214. [Google Scholar] [CrossRef]
- Donner, M.G.; Keppler, D. Up-regulation of basolateral multidrug resistence protein 3 (Mrp3) in cholestatic rat liver. Hepatology 2001, 34, 351–359. [Google Scholar] [CrossRef]
- Payen, L.; Courtois, A.; Campion, J.P. Characterization and inhibition by a wide range of xenobiotics of organic anion excretion by primary human hepatocytes. Biochem. Pharmacol. 2000, 60, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Kubitz, R.; Warskulat, U.; Schmitt, M. Dexamethasone- and osmolarity—Dependent expression of the multidrug resistence protein 2 in cultured rat hepatocytes. Biochem. J. 1999, 340, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ivanyi, B.; Olsen, T.S. Immunohistochemical identification of tubular segments in percutaneous renal biopsies. Histochemistry 1991, 95, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.S.; Ruiz, A.; Gomez, L.M.; Miller, J.M.; Berman, N.E.; Stephens, E.B. APOBEC3G expression is restricted to epithelial cells of the proximal convoluted tubules and is not expressed in the glomeruli of macaques. J. Histochem. Cytochem. 2007, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dekker, B.G.; Arts, C.J.; De Ligny, C.L. Gel-chromatographic analysis of 99mTc-labeled human serum albumin prepared with Sn(II) as the reductant. Int. J. Appl. Radiat. Isot. 1982, 33, 1351–1357. [Google Scholar] [CrossRef] [PubMed]



| Radiopeptide | In Vitro | In Vivo |
|---|---|---|
| [111In]In-DOTA-TATE | 0.36 ± 0.10 | 3.08 ± 0.42 [19] |
| [111In]In-DOTA-NOC | 0.75 ± 0.09 | 2.84 ± 0.28 [19] |
| [111In]In-DTPA-MG0 | 2.52 ± 0.58 | 26.4 ± 14.6 [20] |
| [111In]In-DOTA-MG11 | 0.16 ± 0.15 | 0.84 ± 0.35 [21] |
| [111In]In-DOTA-MG45 | 0.26 ± 0.16 | 1.53 ± 1.35 [21] |
| [111In]In-DOTA-MG47 | 0.30 ± 0.03 | 1.84 ± 1.40 [21] |
| Young LLC-PK1 Cells | Older LLC-PK1 Cells | Rat Renal Cells | |
|---|---|---|---|
| Cellular uptake (%D/106 cells/30 min) | 0.036 ± 0.006 | 0.083 ± 0.015 ★ | 2.188 ± 0.411 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barta, P.; Nachtigal, P.; Maixnerova, J.; Zemankova, L.; Trejtnar, F. Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney. Pharmaceuticals 2023, 16, 696. https://doi.org/10.3390/ph16050696
Barta P, Nachtigal P, Maixnerova J, Zemankova L, Trejtnar F. Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney. Pharmaceuticals. 2023; 16(5):696. https://doi.org/10.3390/ph16050696
Chicago/Turabian StyleBarta, Pavel, Petr Nachtigal, Jana Maixnerova, Lenka Zemankova, and Frantisek Trejtnar. 2023. "Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney" Pharmaceuticals 16, no. 5: 696. https://doi.org/10.3390/ph16050696
APA StyleBarta, P., Nachtigal, P., Maixnerova, J., Zemankova, L., & Trejtnar, F. (2023). Validation of Freshly Isolated Rat Renal Cells as a Tool for Preclinical Assessment of Radiolabeled Receptor-Specific Peptide Uptake in the Kidney. Pharmaceuticals, 16(5), 696. https://doi.org/10.3390/ph16050696