Combination of Niclosamide and Pirfenidone Alleviates Pulmonary Fibrosis by Inhibiting Oxidative Stress and MAPK/Nf-κB and STATs Regulated Genes
Abstract
1. Introduction
2. Results
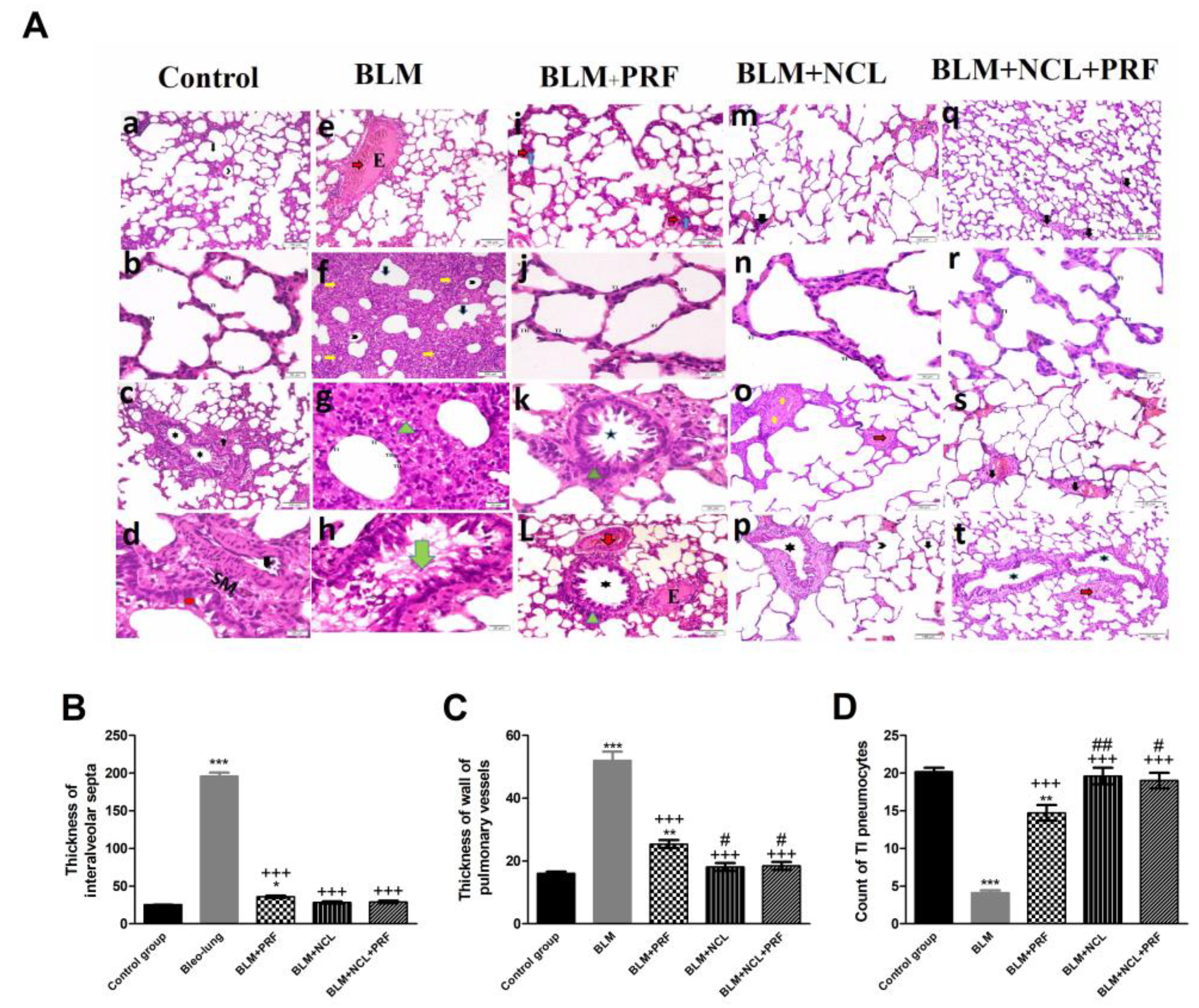
2.1. Amelioration of Lung Pathohistology
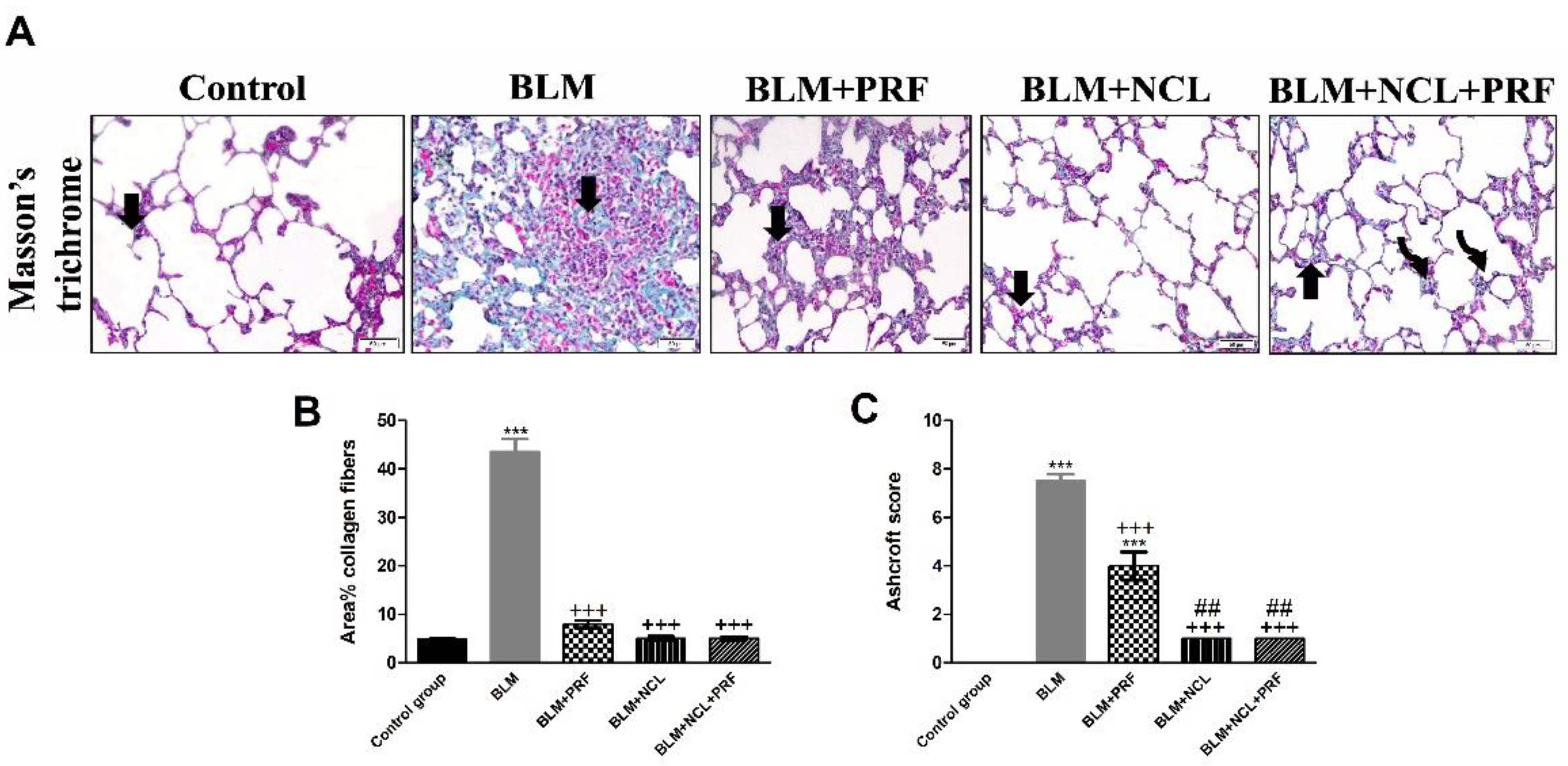
2.2. Effect on Extracellular Matrix Deposition
2.3. Immunostaining and Quantification of α-SMA and Caspase-3
2.4. Amelioration of Oxidative Stress by NCL and PRF
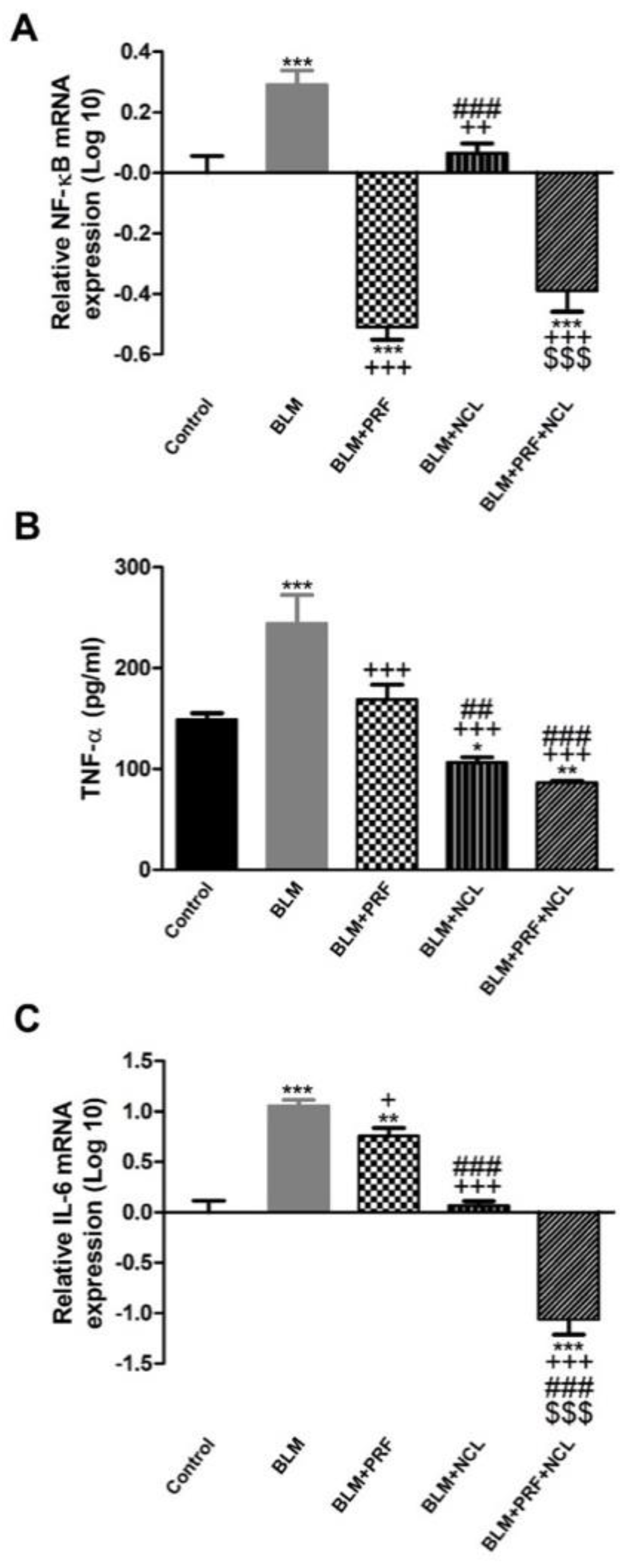
2.5. The Effect of BLM, NCL and PRF on the Regulation of Inflammatory Mediators
2.6. NCL and PRF Protection against Lung Fibrosis through Inhibition of STATs and MAPK Pathways
3. Discussion
4. Methods
4.1. Drugs
4.2. Animals and Grouping
4.3. Histopathological Examination
4.4. Immunohistochemical Staining
4.5. Caspase-3 and α-SMA Immunostaining
4.6. MDA, SOD, GSH-Px and TNF-α Measurements
4.7. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S.-Y. Pulmonary pathology of early phase SARS-COV-2 pneumonia. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Rangarajan, S.; Locy, M.L.; Luckhardt, T.R.; Thannickal, V.J. Targeted therapy for idiopathic pulmonary fibrosis: Where to now? Drugs 2016, 76, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef]
- Roque, W.; Cuevas-Mora, K.; Romero, F. Mitochondrial quality control in age-related pulmonary fibrosis. Int. J. Mol. Sci. 2020, 21, 643. [Google Scholar] [CrossRef]
- Figarola, J.L.; Singhal, J.; Singhal, S.; Kusari, J.; Riggs, A. Bioenergetic modulation with the mitochondria uncouplers SR4 and niclosamide prevents proliferation and growth of treatment-naive and vemurafenib-resistant melanomas. Oncotarget 2018, 9, 36945–36965. [Google Scholar] [CrossRef] [PubMed]
- Sadrkhanloo, M.; Entezari, M.; Orouei, S.; Ghollasi, M.; Rezaei, S.; Hejazi, E.S.; Kakavand, A.; Saebfar, H.; Hashemi, M.; Goharrizi, M.A.S.B. STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacol. Res. 2022, 182, 106311. [Google Scholar] [CrossRef]
- Singh, S.; Weiss, A.; Goodman, J.; Fisk, M.; Kulkarni, S.; Lu, I.; Gray, J.; Smith, R.; Sommer, M.; Cheriyan, J. Niclosamide—A promising treatment for COVID-19. Br. J. Pharmacol. 2022, 179, 3250–3267. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, M.; Winuthayanon, S.; MacLean, J.A.; Hayashi, K. Niclosamide targets the dynamic progression of macrophages for the resolution of endometriosis in a mouse model. Commun. Biol. 2022, 5, 1225. [Google Scholar]
- Gan, C.; Wang, Y.; Xiang, Z.; Liu, H.; Tan, Z.; Xie, Y.; Yao, Y.; Ouyang, L.; Gong, C.; Ye, T. Niclosamide-loaded nanoparticles (Ncl-NPs) reverse pulmonary fibrosis in vivo and in vitro. J. Adv. Res. 2022; in press. [Google Scholar]
- Boyapally, R.; Pulivendala, G.; Bale, S.; Godugu, C. Niclosamide alleviates pulmonary fibrosis in vitro and in vivo by attenuation of epithelial-to-mesenchymal transition, matrix proteins & Wnt/β-catenin signaling: A drug repurposing study. Life Sci. 2019, 220, 8–20. [Google Scholar] [PubMed]
- Li, C.; Pei, X.; Zheng, F.; Cao, K.; Ren, D. Niclosamide Ethanolamine Salt Attenuated Idiopathic Pulmonary Fibrosis by Suppresses TGF-β1 Mediated Epithelial-Mesenchymal Transition. In B64. Mechanistic Advances in Lung Fibrosis; American Thoracic Society: New York, NY, USA, 2020; p. A4053. [Google Scholar]
- Wanas, H.; El Shereef, Z.; Rashed, L.; Aboulhoda, B.E. Ticagrelor Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Rats by the Inhibition of TGF-beta1/Smad3 and PI3K/AKT/mTOR Pathways. Curr. Mol. Pharm. 2022, 15, 227–238. [Google Scholar] [CrossRef]
- Yu, T.-W.; Anderson, D. Reactive oxygen species-induced DNA damage and its modification: A chemical investigation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1997, 379, 201–210. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Periasamy, V.S.; Athinarayanan, J.; Elango, R. Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem. Biol. Interact. 2016, 247, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mungunsukh, O.; Griffin, A.J.; Lee, Y.H.; Day, R.M. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L696–L703. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Sousa, A.M.; Liu, T.; Guevara, O.; Stevens, J.; Fanburg, B.L.; Gaestel, M.; Toksoz, D.; Kayyali, U.S. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J. Cell Biochem. 2007, 100, 1581–1592. [Google Scholar]
- Vijayan, V.; Mueller, S.; Baumgart-Vogt, E.; Immenschuh, S. Heme oxygenase-1 as a therapeutic target in inflammatory disorders of the gastrointestinal tract. World J. Gastroenterol. 2010, 16, 3112–3119. [Google Scholar] [PubMed]
- Pan, X.; Cao, X.; Li, N.; Xu, Y.; Wu, Q.; Bai, J.; Yin, Z.; Luo, L.; Lan, L. Forsythin inhibits lipopolysaccharide-induced inflammation by suppressing JAK-STAT and p38 MAPK signalings and ROS production. Inflamm. Res. 2014, 63, 597–608. [Google Scholar]
- Paithankar, J.G.; Saini, S.; Dwivedi, S.; Sharma, A.; Chowdhuri, D.K. Heavy metal associated health hazards: An interplay of oxidative stress and signal transduction. Chemosphere 2021, 262, 128350. [Google Scholar]
- Zhang, S.; Yan, S.; Lu, K.; Qiu, S.; Chen, X.D.; Wu, W.D. Spray freeze dried niclosamide nanocrystals embedded dry powder for high dose pulmonary delivery. Powder Technol. 2023, 415, 118168. [Google Scholar] [CrossRef]
- Cairns, D.M.; Dulko, D.; Griffiths, J.K.; Golan, Y.; Cohen, T.; Trinquart, L.; Price, L.L.; Beaulac, K.R.; Selker, H.P. Efficacy of niclosamide vs placebo in SARS-CoV-2 respiratory viral clearance, viral shedding, and duration of symptoms among patients with mild to moderate COVID-19: A phase 2 randomized clinical trial. JAMA Netw. Open 2022, 5, e2144942. [Google Scholar] [CrossRef]
- Kunzelmann, K. Getting hands on a drug for COVID-19: Inhaled and Intranasal Niclosamide. Lancet Reg. Health Eur. 2021, 4, 100094. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Gorial, F.I.; Saadi, S.J.; Maulood, M.F.; Hashim, H.A.; Alnuaimi, A.S. A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID-19 management. Ann. Med. Surg. 2021, 69, 102779. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alzahrani, K.J.; Alexiou, A.; Batiha, G.E.-S. Niclosamide for COVID-19: Bridging the gap. Mol. Biol. Rep. 2021, 48, 8195–8202. [Google Scholar] [CrossRef]
- Prather, G.R.; MacLean, J.A.; Shi, M.; Boadu, D.K.; Paquet, M.; Hayashi, K. Niclosamide as a potential nonsteroidal therapy for endometriosis that preserves reproductive function in an experimental mouse model. Biol. Reprod. 2016, 95, 74. [Google Scholar] [CrossRef]
- Gyamfi, J.; Lee, Y.-H.; Min, B.S.; Choi, J. Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis. Sci. Rep. 2019, 9, 11336. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Ward, T.; Mao, Y.; Bockhorn, J.; Liu, X.; Wang, G.; Pegram, M.; Shen, K. Niclosamide inhibits epithelial-mesenchymal transition and tumor growth in lapatinib-resistant human epidermal growth factor receptor 2-positive breast cancer. Int. J. Biochem. Cell Biol. 2016, 71, 12–23. [Google Scholar] [CrossRef]
- Quinlan, T.; Spivack, S.; Mossman, B. Regulation of antioxidant enzymes in lung after oxidant injury. Environ. Health Perspect. 1994, 102, 79–87. [Google Scholar] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health Part B 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Basu, A.; Biswas, J.; Bhattacharya, S. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol. Cell. Biochem. 2015, 405, 243–256. [Google Scholar] [CrossRef]
- Hua-Huy, T.; Le-Dong, N.-N.; Duong-Quy, S.; Bei, Y.; Rivière, S.; Tiev, K.-P.; Nicco, C.; Chéreau, C.; Batteux, F.; Dinh-Xuan, A.T. Increased exhaled nitric oxide precedes lung fibrosis in two murine models of systemic sclerosis. J. Breath Res. 2015, 9, 036007. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Zaiman, A.; Champion, H.C. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L24–L33. [Google Scholar] [CrossRef]
- Amara, N.; Goven, D.; Prost, F.; Muloway, R.; Crestani, B.; Boczkowski, J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFβ1-induced fibroblast differentiation into myofibroblasts. Thorax 2010, 65, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Vittal, R.; Jones, T.; Jagirdar, R.; Luckhardt, T.R.; Horowitz, J.C.; Pennathur, S.; Martinez, F.J.; Thannickal, V.J. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009, 15, 1077–1081. [Google Scholar] [CrossRef]
- Venkatadri, R.; Iyer, A.K.V.; Ramesh, V.; Wright, C.; Castro, C.A.; Yakisich, J.S.; Azad, N. MnTBAP inhibits bleomycin-induced pulmonary fibrosis by regulating VEGF and Wnt signaling. J. Cell. Physiol. 2017, 232, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Larios, J.M.; Budhiraja, R.; Fanburg, B.L.; Thannickal, V.J. Oxidative Protein Cross-linking Reactions Involvingl-Tyrosine in Transforming Growth Factor-β1-stimulated Fibroblasts. J. Biol. Chem. 2001, 276, 17437–17441. [Google Scholar] [CrossRef]
- Lai, J.-l.; Liu, Y.-h.; Liu, C.; Qi, M.-p.; Liu, R.-n.; Zhu, X.-f.; Zhou, Q.-g.; Chen, Y.-y.; Guo, A.-z.; Hu, C.-m. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Zhu, H.; Pu, D.; Di, Q.; Zhao, X.; Ji, F.; Li, H.; Zhao, Z.; Gao, J.; Xiao, W.; Chen, W. Cirsitakaoside isolated from Premna szemaoensis reduces LPS-induced inflammatory responses in vitro and in vivo. Int. Immunopharmacol. 2018, 59, 384–390. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Jeong, M.; Lee, K.-T.; Jang, D.S.; Choi, J.-H. Isocyperol, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced inflammatory responses via suppression of the NF-κB and STAT3 pathways and ROS stress in LPS-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2016, 38, 61–69. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.-Y.; Choi, Y.H. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int. J. Mol. Med. 2012, 30, 204–210. [Google Scholar]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Shin, E.M.; Guo, L.Y.; Youn, U.J.; Bae, K.; Kang, S.S.; Zou, L.B.; Kim, Y.S. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur. J. Pharmacol. 2008, 586, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; Lim, M.H.; Chang, W.; Lim, S.; Choi, S. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef]
- Montero, P.; Milara, J.; Roger, I.; Cortijo, J. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int. J. Mol. Sci. 2021, 22, 6211. [Google Scholar] [CrossRef] [PubMed]
- Bousoik, E.; Montazeri Aliabadi, H. “Do we know jack” about JAK? A closer look at JAK/STAT signaling pathway. Front. Oncol. 2018, 8, 287. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Kojima, J.; Takasaka, N.; Ito, S.; Fujii, S.; Hara, H.; Yanagisawa, H.; Kobayashi, K.; Tsurushige, C.; Kawaishi, M. Insufficient autophagy in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L56–L69. [Google Scholar] [CrossRef]
- Morin, F.; Kavian, N.; Nicco, C.; Cerles, O.; Chéreau, C.; Batteux, F. Niclosamide prevents systemic sclerosis in a reactive oxygen species–induced mouse model. J. Immunol. 2016, 197, 3018–3028. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeño, J.M.; Ortega-Prieto, A.M.; Bussani, R. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Moodley, Y.P.; Misso, N.L.; Scaffidi, A.K.; Fogel-Petrovic, M.; McAnulty, R.J.; Laurent, G.J.; Thompson, P.J.; Knight, D.A. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am. J. Respir. Cell Mol. Biol. 2003, 29, 490–498. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, F.; Zhang, C.; Geng, X.; Syeda, M.Z.; Du, X.; Shao, Z.; Hua, W.; Li, W.; Chen, Z. Therapeutic Effects of the Bcl-2 Inhibitor on Bleomycin-induced Pulmonary Fibrosis in Mice. Front. Mol. Biosci. 2021, 8, 645846. [Google Scholar]
- Adamson, I.Y.; Bowden, D.H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am. J. Pathol. 1974, 77, 185. [Google Scholar]
- Farkas, L.; Farkas, D.; Ask, K.; Möller, A.; Gauldie, J.; Margetts, P.; Inman, M.; Kolb, M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Investig. 2009, 119, 1298–1311. [Google Scholar] [CrossRef]
- Hagimoto, N.; Kuwano, K.; Miyazaki, H.; Kunitake, R.; Fujita, M.; Kawasaki, M.; Kaneko, Y.; Hara, N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am. J. Respir. Cell Mol. Biol. 1997, 17, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lawson, W.E.; Polosukhin, V.V.; Pozzi, A.; Blackwell, T.S.; Litingtung, Y.; Chiang, C. Inhibitor of differentiation 1 promotes endothelial survival in a bleomycin model of lung injury in mice. Am. J. Pathol. 2007, 171, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Polunovsky, V.A.; Chen, B.; Henke, C.; Snover, D.; Wendt, C.; Ingbar, D.H.; Bitterman, P.B. Role of mesenchymal cell death in lung remodeling after injury. J. Clin. Investig. 1993, 92, 388–397. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Deshane, J.S.; Ryan, A.J.; Thannickal, V.J.; Carter, A.B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 2016, 44, 582–596. [Google Scholar]
- Drakopanagiotakis, F.; Xifteri, A.; Tsiambas, E.; Karameris, A.; Tsakanika, K.; Karagiannidis, N.; Mermigkis, D.; Polychronopoulos, V.; Bouros, D. Decreased apoptotic rate of alveolar macrophages of patients with idiopathic pulmonary fibrosis. Pulm. Med. 2012, 2012, 981730. [Google Scholar]
- Huang, L.S.; Natarajan, V. Sphingolipids in pulmonary fibrosis. Adv. Biol. Regul. 2015, 57, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sime, P.J.; Xing, Z.; Graham, F.L.; Csaky, K.G.; Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Investig. 1997, 100, 768–776. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Hecker, L.; Kurundkar, D.; Kurundkar, A.; Liu, H.; Jin, T.-H.; Desai, L.; Bernard, K.; Thannickal, V.J. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Investig. 2013, 123, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Nishimura, Y.; Nishiike-Wada, T.; Wada, Y.; Miura, Y.; Otsuki, T.; Iguchi, H. Long-lasting production of TGF-β1 by alveolar macrophages exposed to low doses of asbestos without apoptosis. Int. J. Immunopathol. Pharmacol. 2007, 20, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid. Med. Cell Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Araya, J.; Minagawa, S.; Hara, H.; Saito, N.; Kadota, T.; Sato, N.; Yoshida, M.; Tsubouchi, K.; Kurita, Y.; et al. Involvement of PARK2-Mediated Mitophagy in Idiopathic Pulmonary Fibrosis Pathogenesis. J. Immunol. 2016, 197, 504–516. [Google Scholar] [CrossRef]
- Alasadi, A.; Chen, M.; Swapna, G.V.T.; Tao, H.; Guo, J.; Collantes, J.; Fadhil, N.; Montelione, G.T.; Jin, S. Effect of mitochondrial uncouplers niclosamide ethanolamine (NEN) and oxyclozanide on hepatic metastasis of colon cancer. Cell Death Dis. 2018, 9, 215. [Google Scholar] [CrossRef]
- Kumar, R.; Coronel, L.; Somalanka, B.; Raju, A.; Aning, O.A.; An, O.; Ho, Y.S.; Chen, S.; Mak, S.Y.; Hor, P.Y.; et al. Mitochondrial uncoupling reveals a novel therapeutic opportunity for p53-defective cancers. Nat. Commun. 2018, 9, 3931. [Google Scholar] [CrossRef]
- Yu, Q.S.; Xin, H.R.; Qiu, R.L.; Deng, Z.L.; Deng, F.; Yan, Z.J. Niclosamide: Drug repurposing for human chondrosarcoma treatment via the caspase-dependent mitochondrial apoptotic pathway. Am. J. Transl. Res. 2020, 12, 3688–3701. [Google Scholar]
- Kurita, Y.; Araya, J.; Minagawa, S.; Hara, H.; Ichikawa, A.; Saito, N.; Kadota, T.; Tsubouchi, K.; Sato, N.; Yoshida, M.; et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir. Res. 2017, 18, 114. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yu, W.; Guo, F. Pirfenidone suppresses bleomycin-induced pulmonary fibrosis and periostin expression in rats. Exp. Med. 2018, 16, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Al-Gareeb, A.; Gorial, F.; Mahmood, A. The Anti-Rheumatoid Activity of Niclosamide in Collagen-Induced Arthritis in Rats. Arch. Rheumatol. 2019, 34, 426–433. [Google Scholar] [CrossRef]
- Toda, M.; Mizuguchi, S.; Minamiyama, Y.; Takemura, S.; Yamamoto, H.; Aota, T.; Nishiyama, N. Pirfenidone Suppresses Pulmonary Fibrosis Through Regulation of Alveolar Macrophage Polarization. Free Radic. Biol. Med. 2016, 100, S62. [Google Scholar] [CrossRef]
- Guan, R.; Wang, X.; Zhao, X.; Song, N.; Zhu, J.; Wang, J.; Wang, J.; Xia, C.; Chen, Y.; Zhu, D.; et al. Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation. Sci. Rep. 2016, 6, 35696. [Google Scholar] [CrossRef]
- Hubner, R.H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–511. [Google Scholar] [CrossRef]
- Al Hamdan, A.S.; Alghamdi, A.A.; Alyousif, G.F.; Hamza, F.A.; Shafey, M.M.; AlAmri, A.M.; Sunki, A.A. Evaluating the prevalence and the risk factors of gram-negative multi-drug resistant bacteria in Eastern Saudi Arabia. Infect. Drug Resist. 2022, 15, 475–490. [Google Scholar] [CrossRef]
- Kaur, M.; Sodhi, R.K.; Jyothi, V.G.S.; Sree, V.H.; Singh, P.K.; Mehra, N.K.; Khatri, D.K.; Srivastava, S.; Singh, S.B.; Madan, J. Brain targeting drug delivery systems for the management of brain disorders: Molecular targets and nanotechnological strategies. In Multifunctional Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 289–345. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanas, H.; Elbadawy, H.M.; Almikhlafi, M.A.; Hamoud, A.E.; Ali, E.N.; Galal, A.M. Combination of Niclosamide and Pirfenidone Alleviates Pulmonary Fibrosis by Inhibiting Oxidative Stress and MAPK/Nf-κB and STATs Regulated Genes. Pharmaceuticals 2023, 16, 697. https://doi.org/10.3390/ph16050697
Wanas H, Elbadawy HM, Almikhlafi MA, Hamoud AE, Ali EN, Galal AM. Combination of Niclosamide and Pirfenidone Alleviates Pulmonary Fibrosis by Inhibiting Oxidative Stress and MAPK/Nf-κB and STATs Regulated Genes. Pharmaceuticals. 2023; 16(5):697. https://doi.org/10.3390/ph16050697
Chicago/Turabian StyleWanas, Hanaa, Hossein M. Elbadawy, Mohannad A. Almikhlafi, Amany E. Hamoud, Eid N. Ali, and Amr M. Galal. 2023. "Combination of Niclosamide and Pirfenidone Alleviates Pulmonary Fibrosis by Inhibiting Oxidative Stress and MAPK/Nf-κB and STATs Regulated Genes" Pharmaceuticals 16, no. 5: 697. https://doi.org/10.3390/ph16050697
APA StyleWanas, H., Elbadawy, H. M., Almikhlafi, M. A., Hamoud, A. E., Ali, E. N., & Galal, A. M. (2023). Combination of Niclosamide and Pirfenidone Alleviates Pulmonary Fibrosis by Inhibiting Oxidative Stress and MAPK/Nf-κB and STATs Regulated Genes. Pharmaceuticals, 16(5), 697. https://doi.org/10.3390/ph16050697




