[212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors
Abstract
1. Introduction
2. Results
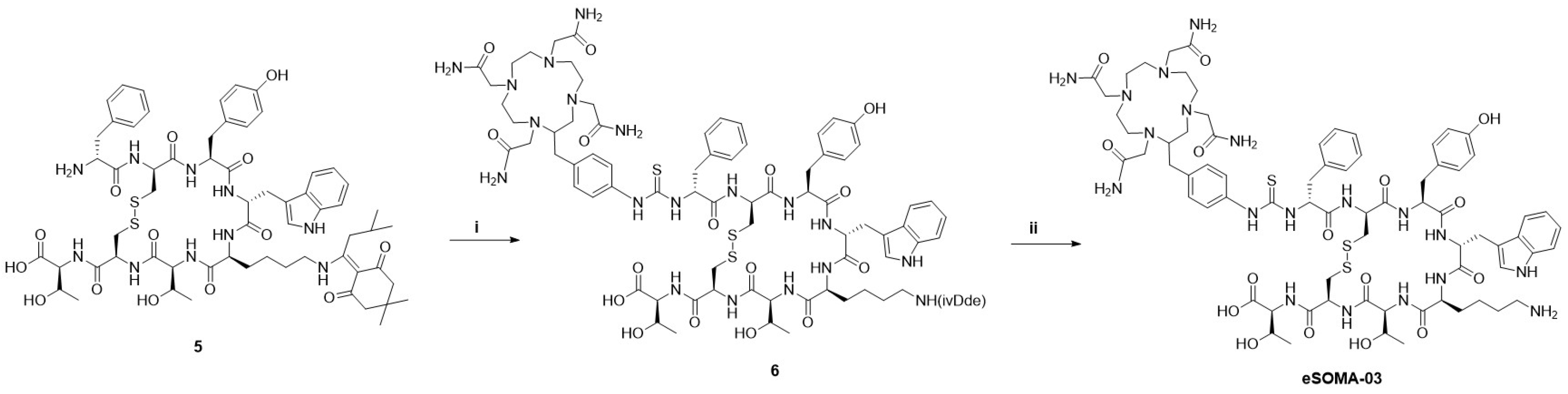
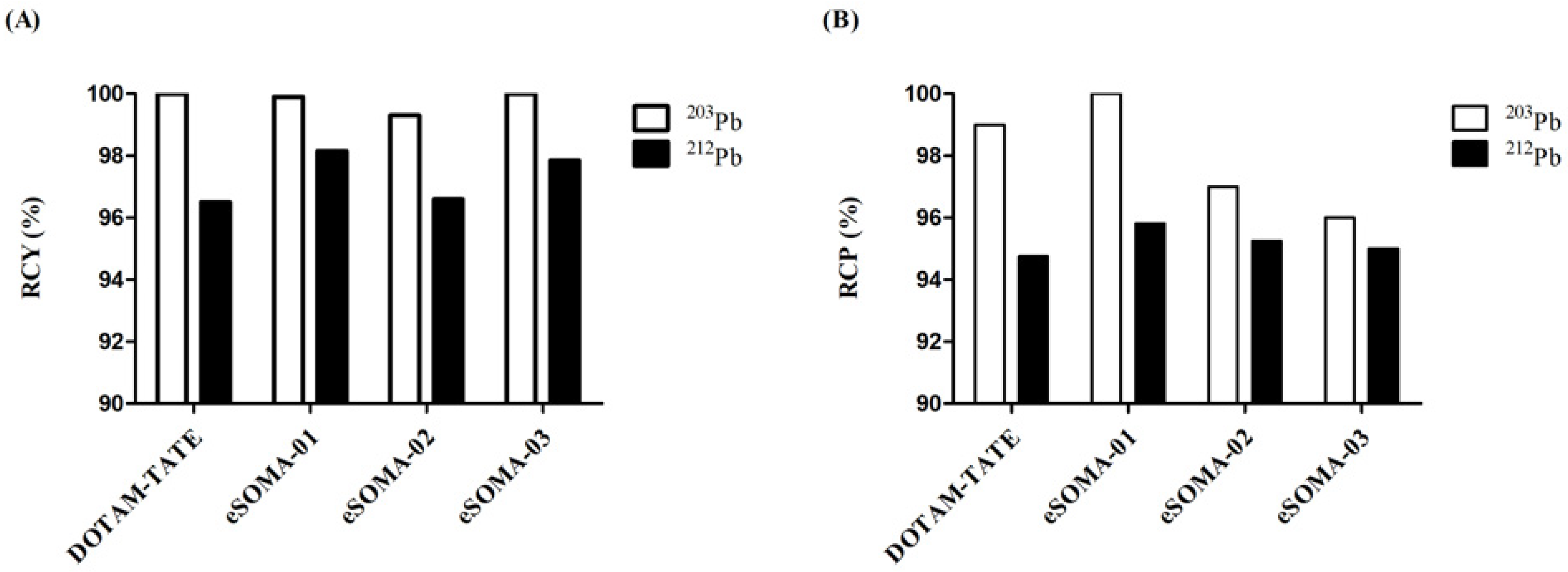
2.1. Chemistry and Radiochemistry
2.2. In Vitro Characterization of DOTAM-TATE, eSOMA-01, eSOMA-02, and eSOMA-03
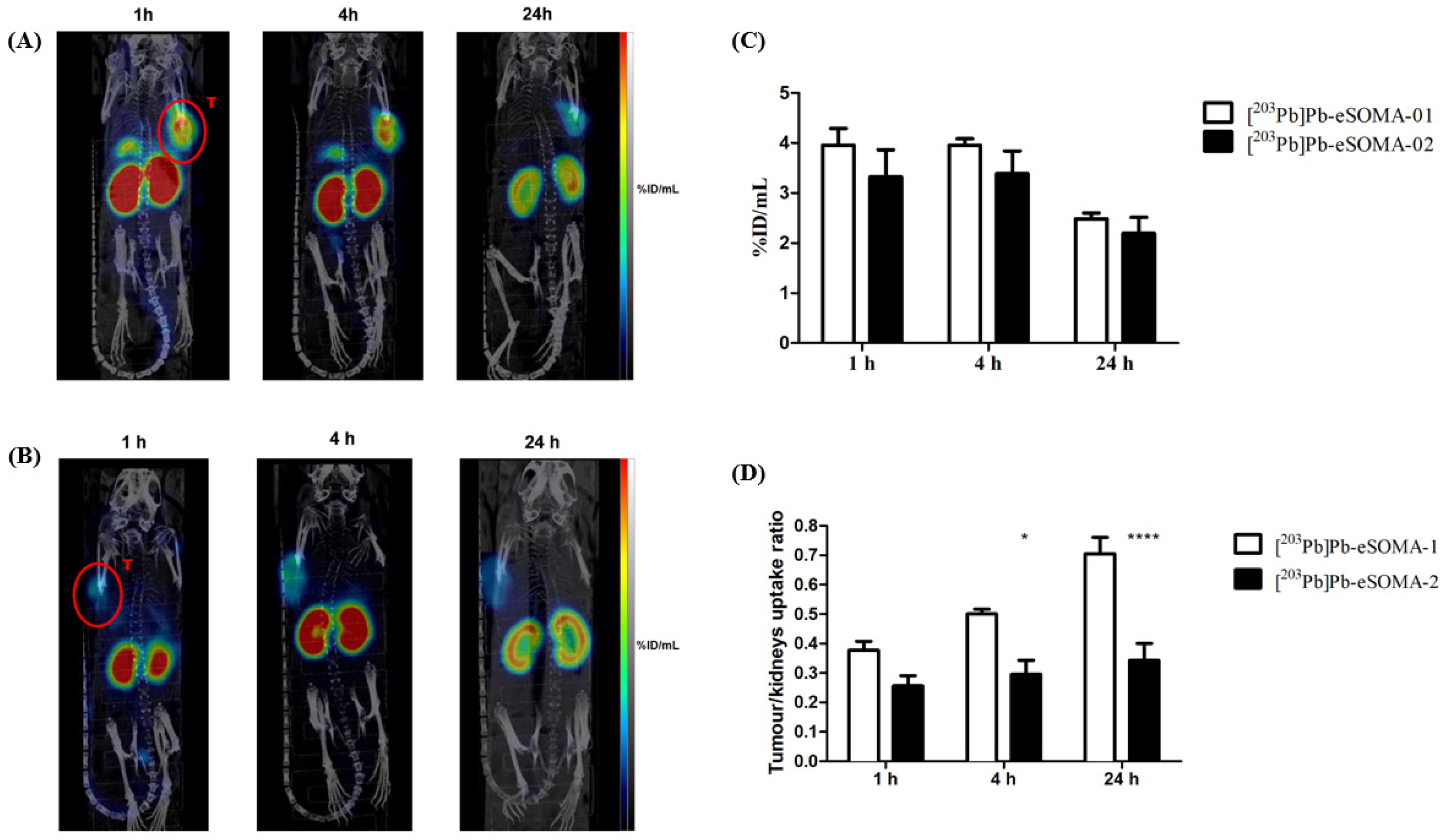
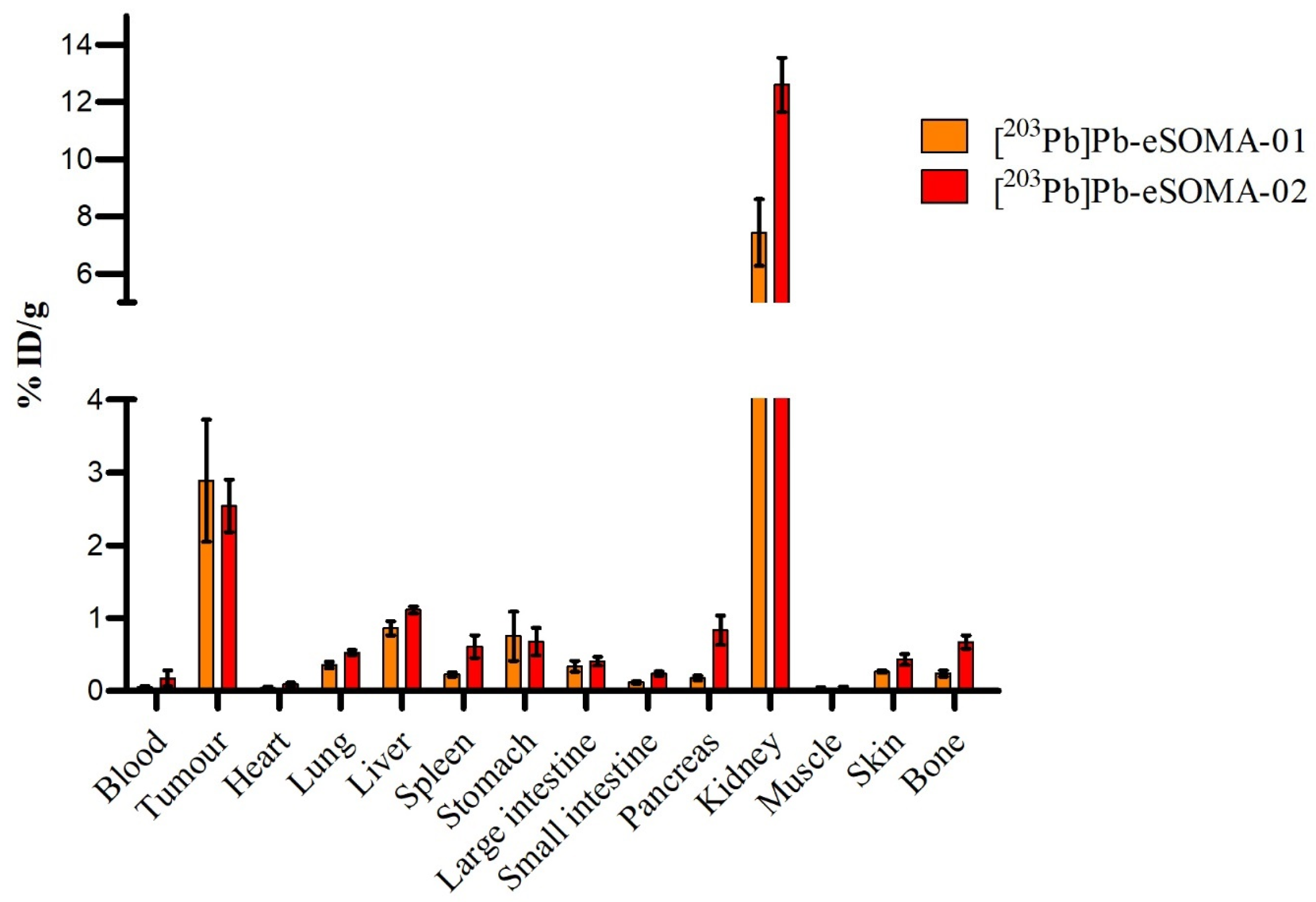
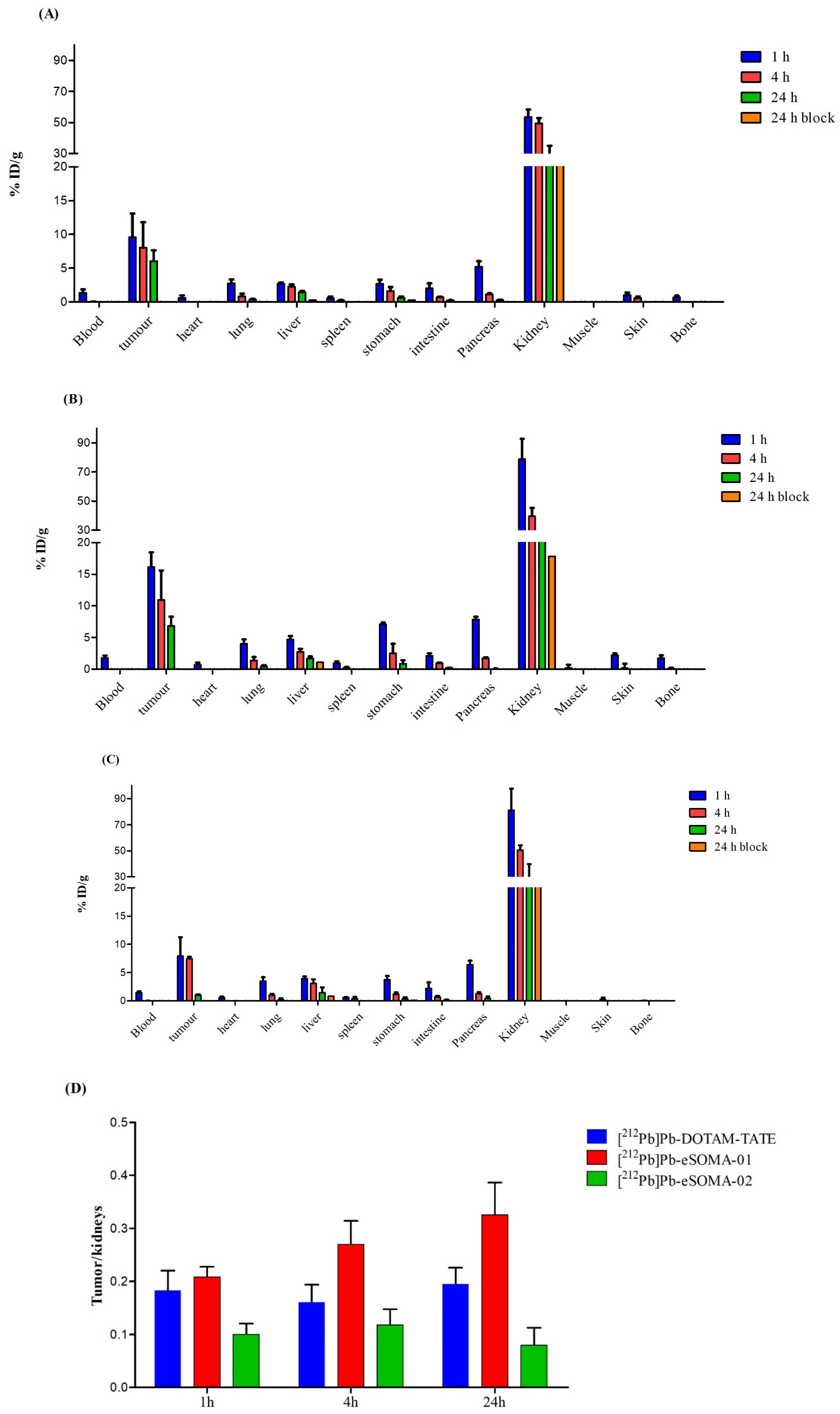
2.3. In Vivo Preclinical Evaluation
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. HPLC Conditions
4.3. Synthetic Method
4.3.1. d-Phe-cyclo[Cys-Tyr(tBu)-d-Trp(Boc)-Lys(Boc)-Thr(tBu)-Cys]-Thr(tBu)-resin (4)
4.3.2. DO3AM-d-Phe-cyclo[Cys-Tyr-d-Trp-Lys-Thr-Cys]-Thr-OH (DOTAM-TATE)
4.3.3. DO3AM-Amcha-d-Phe-cyclo[Cys-Tyr-d-Trp-Lys-Thr-Cys]-Thr-OH (eSOMA-01)
4.3.4. DO3AM-Pip-d-Phe-cyclo[Cys-Tyr-d-Trp-Lys-Thr-Cys]-Thr-OH (eSOMA-02)
4.3.5. d-Phe-cyclo[Cys-Tyr-d-Trp-Lys(ivDde)-Thr-Cys]-Thr (5)
4.3.6. TCMC-Bn-thiourea-(d-Phe-cyclo[Cys-Tyr-d-Trp-Lys(ivDde)-Thr-Cys]-Thr-OH (6)
4.3.7. TCMC-Bn-thiourea-(d-Phe-cyclo[Cys-Tyr-d-Trp-Lys-Thr-Cys]-Thr-OH (eSOMA-03)
4.4. Complexation with the Lead ICP Standard
4.5. Radiolabeling with 203Pb
4.6. Radiolabeling with 212Pb
4.7. In Vitro Stability
4.8. LogD7.4
4.9. Binding Affinity
4.10. Cell Culture
4.11. Animal Models
4.12. In Vivo Single Photon Emission Computed Topology/Computer Tomography (SPECT/CT)
4.13. Ex-Vivo Biodistribution with Lead-203
4.14. Biodistribution Study with Lead-212
4.15. Statistical Analysis
4.16. Dosimetry
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergsma, H.; van Vliet, E.I.; Teunissen, J.J.; Kam, B.L.; de Herder, W.W.; Peeters, R.P.; Krenning, E.P.; Kwekkeboom, D.J. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Prac. Res. Clin. Gastroenterol. 2012, 26, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Capello, A.; Krenning, E.P.; Breeman, W.A.P.; Bernard, B.F.; De Jong, M. Peptide receptor radionuclide therapy in vitro using [111In-DTPA0]octreotide. J. Nucl. Med. 2003, 44, 98–104. [Google Scholar]
- Janson, E.T.; Westlin, J.E.; Ohrvall, U.; Oberg, K.; Lukinius, A. Nuclear localization of 111In after intravenous injection of [111In-DTPA-D-Phe1]-octreotide in patients with neuroendocrine tumors. J. Nucl. Med. 2000, 41, 1514–1518. [Google Scholar]
- Valkema, R.; de Jong, M.; Bakker, W.H.; Breeman, W.A.; Kooij, P.P.; Lugtenburg, P.J.; de Jong, F.H.; Christiansen, A.; Kam, B.L.; de Herder, W.W.; et al. Phase I study of peptide receptor radionuclide therapy with [111In-DTPA0]octreotide: The rotterdam experience. Semin. Nucl. Med. 2002, 32, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Cwikla, J.B.; Sankowski, A.; Seklecka, N.; Buscombe, J.R.; Nasierowska-Guttmejer, A.; Jeziorski, K.G.; Mikolajczak, R.; Pawlak, D.; Stepien, K.; Walecki, J. Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): A phase II study. Ann. Oncol. 2009, 21, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Imhof, A.; Brunner, P.; Marincek, N.; Briel, M.; Schindler, C.; Rasch, H.; Walter, M.A. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J. Clin. Oncol. 2011, 29, 2416–2423. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; De Herder, W.W.; Kam, B.L.; Van Eijck, C.H.; Van Essen, M.; Kooij, P.P.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177Lu- DOTA0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-Related Quality of Life in Patients with Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Kunikowska, J.; Królicki, L. Targeted α-Emitter Therapy of Neuroendocrine Tumors. Semin. Nucl. Med. 2020, 50, 171–176. [Google Scholar] [CrossRef]
- Nayak, T.K.; Norenberg, J.P.; Anderson, T.L.; Prossnitz, E.R.; Stabin, M.G.; Atcher, R.W. Somatostatin-receptor-targeted α-emitting 213Bi is therapeutically more effective than β−-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl. Med. Biol. 2007, 34, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-M.; Beaumier, P.; Axworthy, D.; Atcher, R.; Fritzberg, A. Pretargeted radioimmunotherapy in tumored mice using an in vivo 212Pb/212Bi generator. Nucl. Med. Biol. 2005, 32, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, C.L.; Anderson-Berg, W.T.; Brechbiel, M.W.; Mirzadeh, S.; Gansow, O.A.; Strand, M. Improved in vivo stability and tumor targeting of bismuth-labeled antibody. Cancer Res. 1990, 50, 4221–4226. [Google Scholar]
- Maumela, H.; Hancock, R.D.; Carlton, L.; Reibenspies, J.H.; Wainwright, K.P. The Amide Oxygen as a Donor Group. Metal Ion Complexing Properties of Tetra-N-acetamide Substituted Cyclen: A Crystallographic, NMR, Molecular Mechanics, and Thermodynamic Study. J. Am. Chem. Soc. 1995, 117, 6698–6707. [Google Scholar] [CrossRef]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and Imaging of212Pb-TCMC-Trastuzumab After Intraperitoneal Administration in Ovarian Cancer Patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Tworowska, I.; Esfandiari, R.; Torgue, J.; Hurt, J.; Shafie, A.; Núñez, R. Targeted α-Emitter Therapy with 212Pb-DOTAMTATE for the Treatment of Metastatic SSTR-Expressing Neuroendocrine Tumors: First-in-Humans Dose-Escalation Clinical Trial. J. Nucl. Med. 2022, 63, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Mier, W.; Eritja, R.; Mohammed, A.; Haberkorn, U.; Eisenhut, M. Preparation and Evaluation of Tumor-Targeting Peptide−Oligonucleotide Conjugates. Bioconjugate Chem. 2000, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Stallons, T.A.R.; Saidi, A.; Tworowska, I.; Delpassand, E.S.; Torgue, J.J. Preclinical Investigation of 212Pb-DOTAMTATE for Peptide Receptor Radionuclide Therapy in a Neuroendocrine Tumor Model. Mol. Cancer Ther. 2019, 18, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.L.; Dadachova, E.; Milenic, D.E.; Garmestani, K.; Wu, C.; Brechbiel, M.W. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl. Med. Biol. 2000, 27, 93–100. [Google Scholar] [CrossRef]
- dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kratochwil, C. Development and dosimetry of 203 Pb/212 Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef]
- Amouroux, G.; Pan, J.; Jenni, S.; Zhang, C.; Zhang, Z.; Hundal-Jabal, N.; Colpo, N.; Liu, Z.; Bénard, F.; Lin, K.-S. Imaging Bradykinin B1 Receptor with 68Ga-Labeled [des-Arg10]Kallidin Derivatives: Effect of the Linker on Biodistribution and Tumor Uptake. Mol. Pharm. 2015, 12, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Wuest, F. 18F-Labeled Peptides: The Future Is Bright. Molecules 2014, 19, 20536–20556. [Google Scholar] [CrossRef] [PubMed]
- Larenkov, A.; Mitrofanov, I.; Pavlenko, E.; Rakhimov, M. Radiolysis-Associated Decrease in Radiochemical Purity of 177Lu-Radiopharmaceuticals and Comparison of the Effectiveness of Selected Quenchers against This Process. Molecules 2023, 28, 1884. [Google Scholar] [CrossRef] [PubMed]
- Nawar, W.W. Reaction Mechanisms in the Radiolysis of Peptides, Polypeptides, and Protein. J. Agric. Food Chem. 1978, 26, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Simat, T.J.; Steinhart, H. Oxidation of Free Tryptophan and Tryptophan Residues in Peptides and Proteins. J. Agric. Food Chem. 1998, 46, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Edwards, D.S. Stabilization of 90Y-Labeled DOTA-Biomolecule Conjugates Using Gentisic Acid and Ascorbic Acid. Bioconjugate Chem. 2001, 12, 554–558. [Google Scholar] [CrossRef]
- Wang, W.-F.; Schuchmann, M.N.; Schuchmann, H.-P.; Knolle, W.; von Sonntag, J.; von Sonntag, C. Radical Cations in the OH-Radical-Induced Oxidation of Thiourea and Tetramethylthiourea in Aqueous Solution. J. Am. Chem. Soc. 1998, 121, 238–245. [Google Scholar] [CrossRef]
- Schottelius, M.; Šimeček, J.; Hoffmann, F.; Willibald, M.; Schwaiger, M.; Wester, H.-J. Twins in spirit—Episode I: Comparative preclinical evaluation of [68Ga]DOTATATE and [68Ga]HA-DOTATATE. EJNMMI Res. 2015, 5, 22. [Google Scholar] [CrossRef]
- Wang, Q.; Graham, K.; Schauer, T.; Fietz, T.; Mohammed, A.; Liu, X.; Hoffend, J.; Haberkorn, U.; Eisenhut, M.; Mier, W. Pharmacological properties of hydrophilic and lipophilic derivatives of octreotate. Nucl. Med. Biol. 2004, 31, 21–30. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef]
- Teunissen, J.J.M.; Kwekkeboom, D.J.; de Jong, M.; Esser, J.P.; Valkema, R.; Krenning, E.P. Peptide receptor radionuclide therapy. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 595–616. [Google Scholar] [CrossRef]
- Tworowska, I.; Stallons, T.; Saidi, A.; Wagh, N.; Rojas-Quijano, F.; Jurek, P.; Kiefer, G.; Torgue, J.; Delpassand, E. Abstract LB-259: Pb203-AR-RMX conjugates for image-guided TAT of neuroendocrine tumors (NETs). Cancer Res. 2017, 73, LB–259. [Google Scholar] [CrossRef]
- Taylor, J.E.; Theveniau, M.A.; Bashirzadeh, R.; Reisine, T.; Eden, P.A. Detection of somatostatin receptor subtype 2 (SSTR2) in established tumors and tumor cell lines: Evidence for SSTR2 heterogeneity. Peptides 1994, 15, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Vegt, E.; Melis, M.; Eek, A.; de Visser, M.; Brom, M.; Oyen, W.J.G.; Gotthardt, M.; de Jong, M.; Boerman, O.C. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, J.P.; Krenning, B.J.; Konings, I.R.; Kusewitt, D.F.; Nayak, T.K.; Anderson, T.L.; de Jong, M.; Garmestani, K.; Brechbiel, M.W.; Kvols, L.K. 213Bi-[DOTA0, Tyr3]Octreotide Peptide Receptor Radionuclide Therapy of Pancreatic Tumors in a Preclinical Animal Model. Clin. Cancer Res. 2006, 12, 897–903. [Google Scholar] [CrossRef]
- Tworowska, I.; Wagh, N.; Delpassand, E.S.; Rojas-Quijano, F.; Jurek, P.; Kiefer, G.E.; Amal, S. Treatment of Cancer Cells Overexpressing Somatostatin Receptors Using Ocreotide Derivatives Chelated to Radioisotopes. WO2018132751A1, 12 January 2018. [Google Scholar]
- Burlikowska, K.; Stryjak, I.; Bogusiewicz, J.; Kupcewicz, B.; Jaroch, K.; Bojko, B. Comparison of Metabolomic Profiles of Organs in Mice of Different Strains Based on SPME-LC-HRMS. Metabolites 2020, 10, 255. [Google Scholar] [CrossRef]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat. Oncol. 2021, 16, 43. [Google Scholar] [CrossRef]
- Tworowska, I.; Stallons, T.; Delpassand, E.; Torgue, J.; Saidi, A.; Jurek, P.; Sgouros, G. Theranostic 203/212Pb-labeled octreotate analogs (AlphaMedix TM) and their preclinical characterization for the Phase I clinical studies in neuroendocrine cancer patients. Nucl. Med. Biol. 2019, 72–73, S19–S20. [Google Scholar] [CrossRef]
- Hobbs, R.F.; Song, H.; Huso, D.L.; Sundel, M.H.; Sgouros, G. A nephron-based model of the kidneys for macro-to-micro α-particle dosimetry. Phys. Med. Biol. 2012, 57, 4403–4424. [Google Scholar] [CrossRef]
- Schmitt, A.; Bernhardt, P.; Nilsson, O.; Ahlman, H.; Kölby, L.; Maecke, H.R.; Forssell-Aronsson, E. Radiation therapy of small cell lung cancer with 177Lu-DOTA-Tyr3-octreotate in an animal model. J. Nucl. Med. 2004, 45, 1542–1548. [Google Scholar]
- Hammond, P.J.; Wade, A.F.; Gwilliam, M.E.; Peters, A.M.; Myers, M.J.; Gilbey, S.G.; Bloom, S.R.; Calam, J. Amino acid infusion blocks renal tubular uptake of an indium-labelled somatostatin analogue. Br. J. Cancer 1993, 67, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Vegt, E.; De Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Valkema, R.; de Jong, M.; Kooij, P.P.; Krenning, E.P. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Breeman, W.; de Zanger, R.; Chan, H.; Blois, E. Alternative method to determine Specific Activity of 177Lu by HPLC. Curr. Radiopharm. 2015, 8, 119–122. [Google Scholar] [CrossRef]
- Keenan, M.A.; Stabin, M.G.; Segars, W.P.; Fernald, M.J. RADAR Realistic Animal Model Series for Dose Assessment. J. Nucl. Med. 2010, 51, 471–476. [Google Scholar] [CrossRef]
- Sgouros, G.; Roeske, J.C.; Mcdevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of α-Particle Emitters for Targeted Radionuclide Therapy*. J. Nucl. Med. 2017, 51, 311–328. [Google Scholar] [CrossRef]
- Gear, J.I.; Cox, M.G.; Gustafsson, J.; Gleisner, K.S.; Murray, I.; Glatting, G.; Konijnenberg, M.; Flux, G.D. EANM practical guidance on uncertainty analysis for molecular radiotherapy absorbed dose calculations. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2456–2474. [Google Scholar] [CrossRef]



| Compound | LogD7.4 b | Stability in PBS(%) a | Stability in PBS(%) a |
|---|---|---|---|
| 1 h 4 h 24 h | 1 h 4 h 24 h | ||
| [203Pb]Pb-DOTAM-TATE | −1.41 ± 0.01 | 99 99 99 | 99 99 99 |
| [203Pb]Pb-eSOMA-01 | −1.99 ± 0.03 | 100 100 100 | 100 100 100 |
| [203Pb]Pb-eSOMA-02 | −1.88 ± 0.03 | 97 97 97 | 97 97 97 |
| [203Pb]Pb-eSOMA-03 | −1.36 ± 0.06 | 70 40 15 | 85 70 60 |
| Compound | Stability in PBS(%) a | Stability in PBS(%) a |
|---|---|---|
| 1 h 4 h 24 h | 1 h 4 h 24 h | |
| [212Pb]Pb-DOTAM-TATE | 99 98 95 | 99 99 97 |
| [212Pb]Pb-eSOMA-01 | 99 97 94 | 96 96 93 |
| [212Pb]Pb-eSOMA-02 | 97 95 95 | 99 99 96 |
| Compound | IC50 (nM) |
|---|---|
| DOTA-TATE | 3.70 ± 0.18 a |
| DOTAM-TATE | 0.91 ± 0.18 a |
| eSOMA-01 | 2.54 ± 0.20 a |
| eSOMA-02 | 2.53 ± 0.19 a |
| eSOMA-03 | 3.11 ± 0.05 a |
| [natPb]Pb-DOTAM-TATE | 7.60 b |
| [natPb]Pb-eSOMA-01 | 5.29 b |
| [natPb]Pb-eSOMA-02 | 6.83 b |
| [natPb]Pb-eSOMA-03 | 5.64 b |
| Tissue | [212Pb]Pb-DOTAM-TATE | [212Pb]Pb-eSOMA-01 | [212Pb]Pb-eSOMA-02 |
|---|---|---|---|
| Tumor | 26.61 ± 19.27 | 35.49 ± 9.89 | 14.73 ± 5.73 |
| Lungs | 2.06 ± 0.29 | 3.20 ± 0.40 | 2.55 ± 0.28 |
| Liver | 6.93 ± 0.64 | 9.03 ± 2.24 | 8.46 ± 1.96 |
| Spleen | 0.56 ± 0.14 | 0.73 ± 0.19 | 1.17 ± 0.49 |
| Stomach wall | 4.42 ± 1.30 | 5.78 ± 0.77 | 2.92 ± 0.33 |
| Large Intestine | 1.64 ± 0.30 | 2.03 ± 0.26 | 1.74 ± 0.39 |
| Pancreas | 3.65 ± 0.28 | 5.40 ± 0.14 | 4.36 ± 0.28 |
| Kidneys | 140.03 ± 17.95 | 121.73 ± 14.57 | 147.44 ± 36.20 |
| K/T | 5.26 ± 3.90 | 3.43 ± 1.10 | 10.01 ± 4.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapeau, D.; Koustoulidou, S.; Handula, M.; Beekman, S.; de Ridder, C.; Stuurman, D.; de Blois, E.; Buchatskaya, Y.; van der Schilden, K.; de Jong, M.; et al. [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals 2023, 16, 985. https://doi.org/10.3390/ph16070985
Chapeau D, Koustoulidou S, Handula M, Beekman S, de Ridder C, Stuurman D, de Blois E, Buchatskaya Y, van der Schilden K, de Jong M, et al. [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals. 2023; 16(7):985. https://doi.org/10.3390/ph16070985
Chicago/Turabian StyleChapeau, Dylan, Sofia Koustoulidou, Maryana Handula, Savanne Beekman, Corrina de Ridder, Debra Stuurman, Erik de Blois, Yulia Buchatskaya, Karlijn van der Schilden, Marion de Jong, and et al. 2023. "[212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors" Pharmaceuticals 16, no. 7: 985. https://doi.org/10.3390/ph16070985
APA StyleChapeau, D., Koustoulidou, S., Handula, M., Beekman, S., de Ridder, C., Stuurman, D., de Blois, E., Buchatskaya, Y., van der Schilden, K., de Jong, M., Konijnenberg, M. W., & Seimbille, Y. (2023). [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals, 16(7), 985. https://doi.org/10.3390/ph16070985







