Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen
Abstract
1. Introduction
2. Results and Discussion
2.1. General Procedure for the Synthesis of Thiourea Derivatives of Naproxen
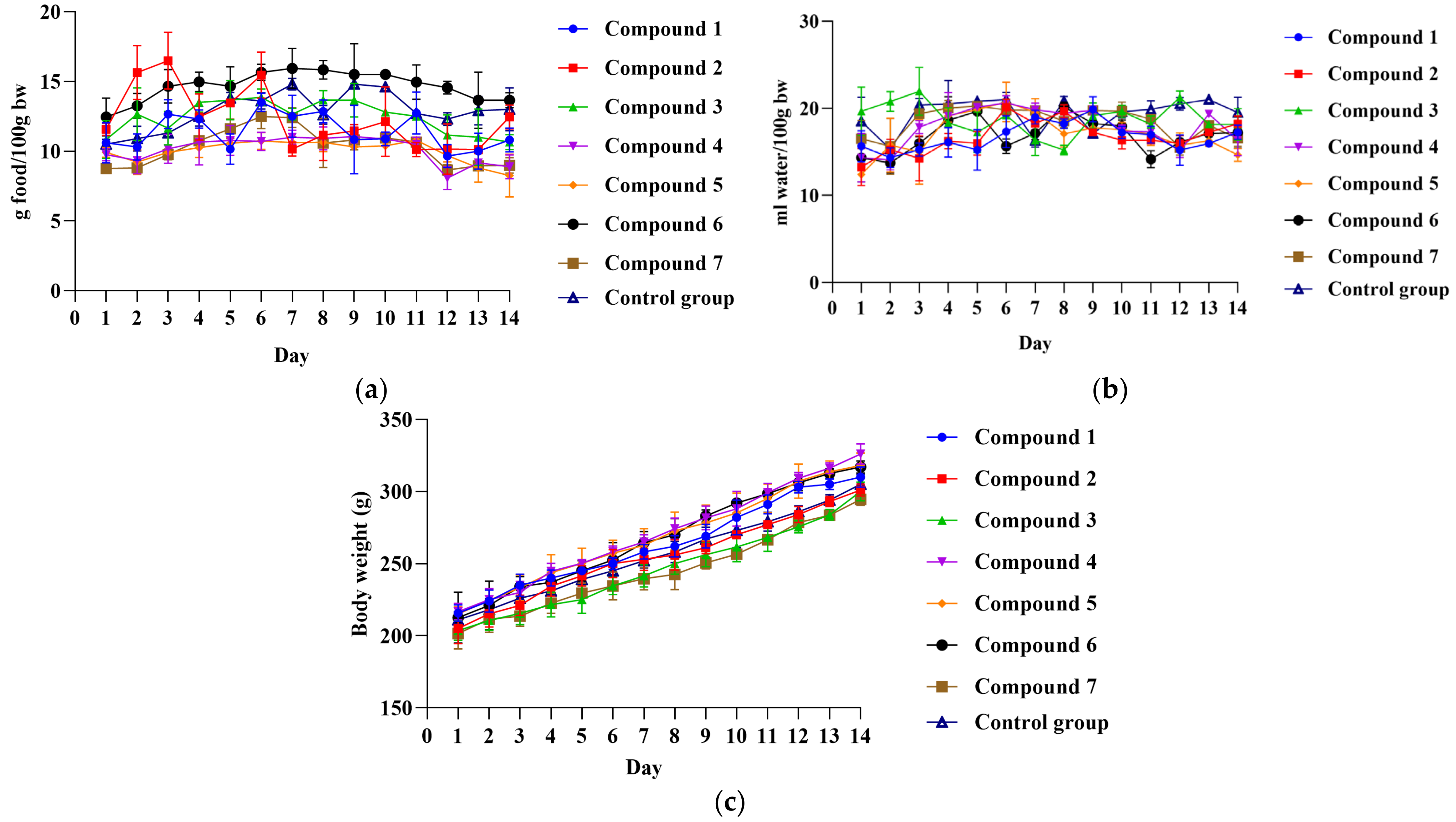
2.2. Acute Oral Toxicity Evaluation
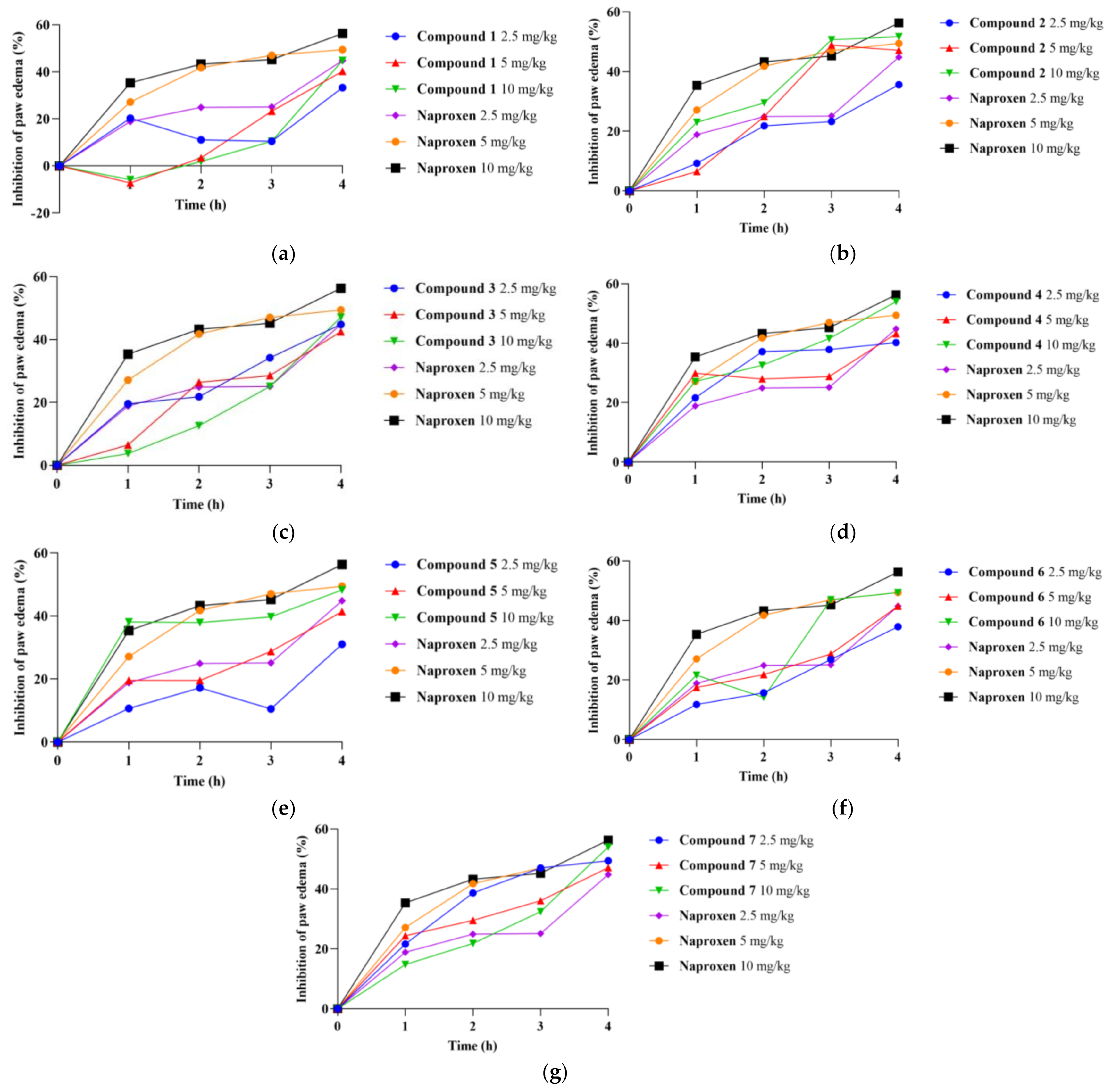
2.3. Carrageenan-Induced Paw Edema and Determination of the Anti-Inflammatory Activity
2.4. Investigation of COX-2 and 5-LOX Enzyme Inhibitory Properties
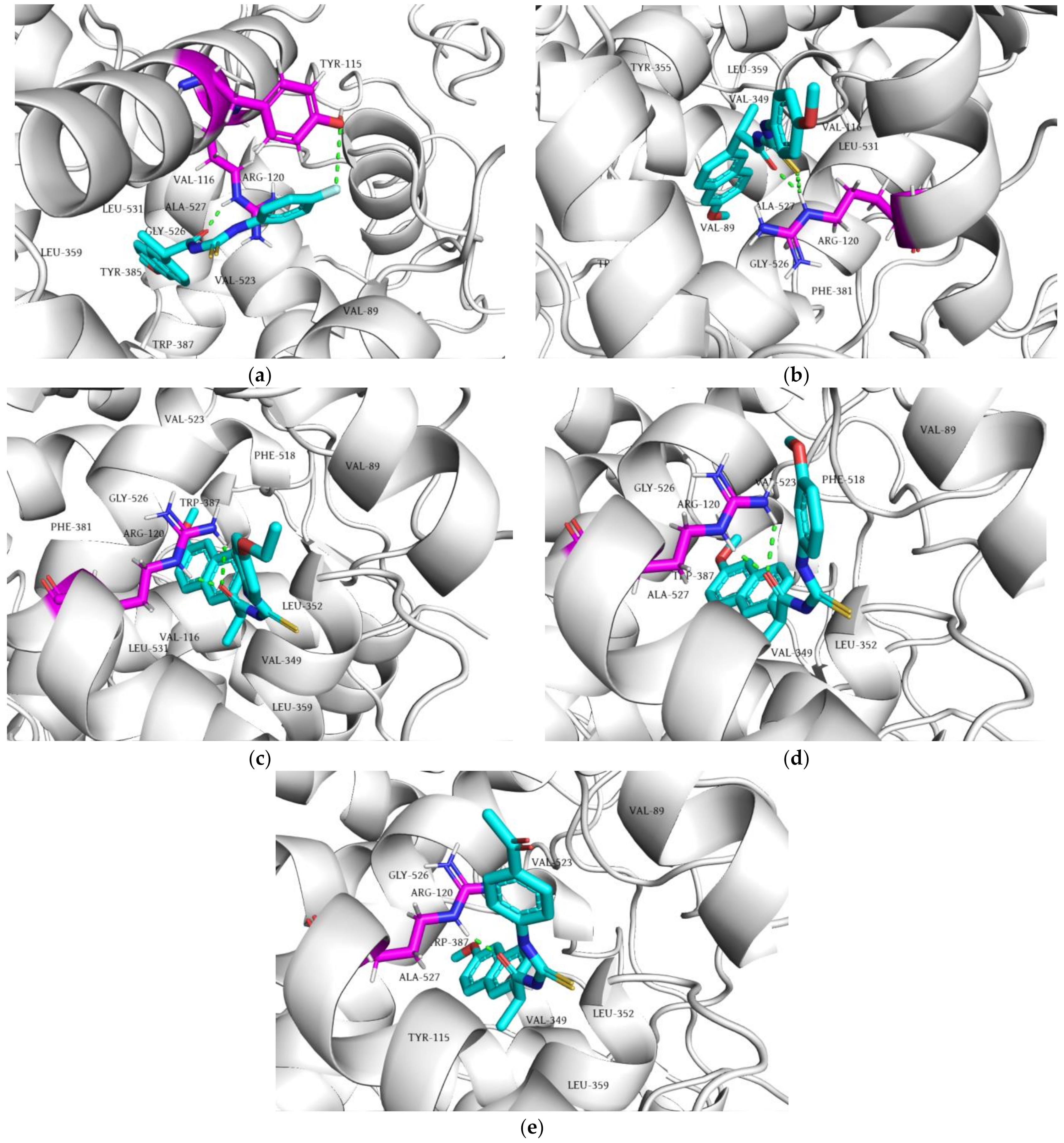
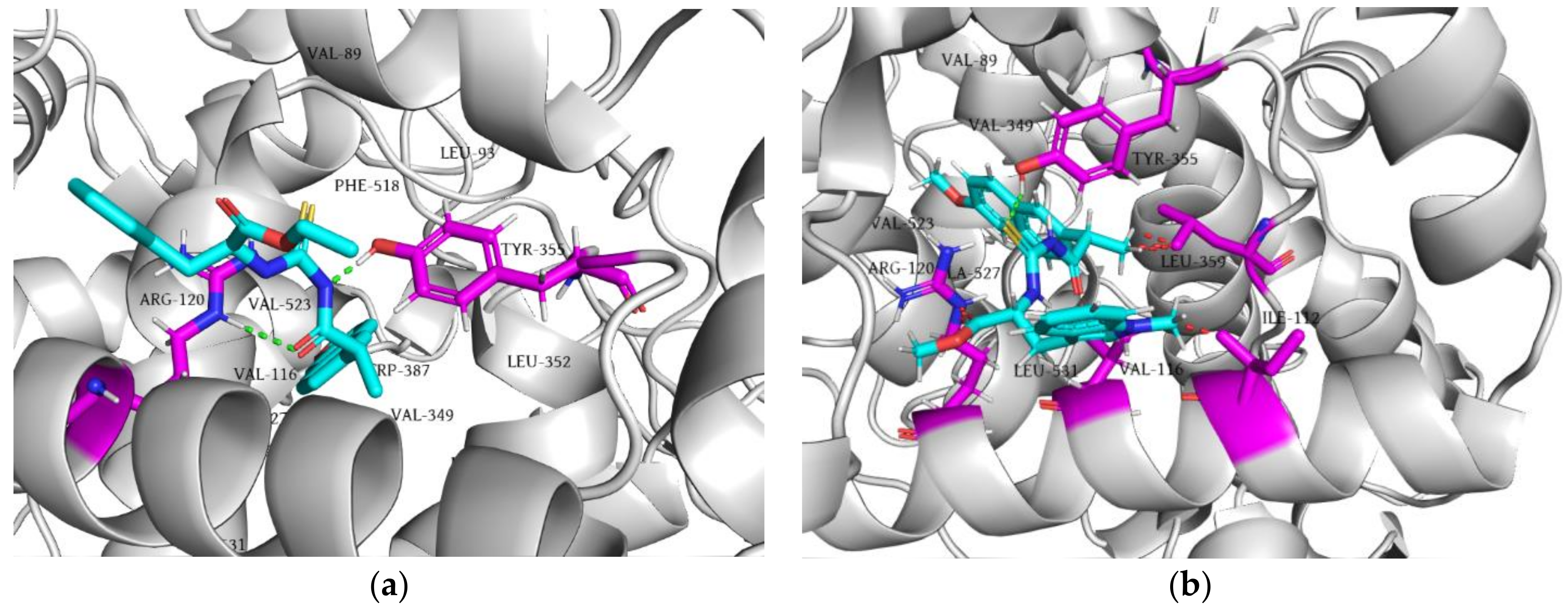
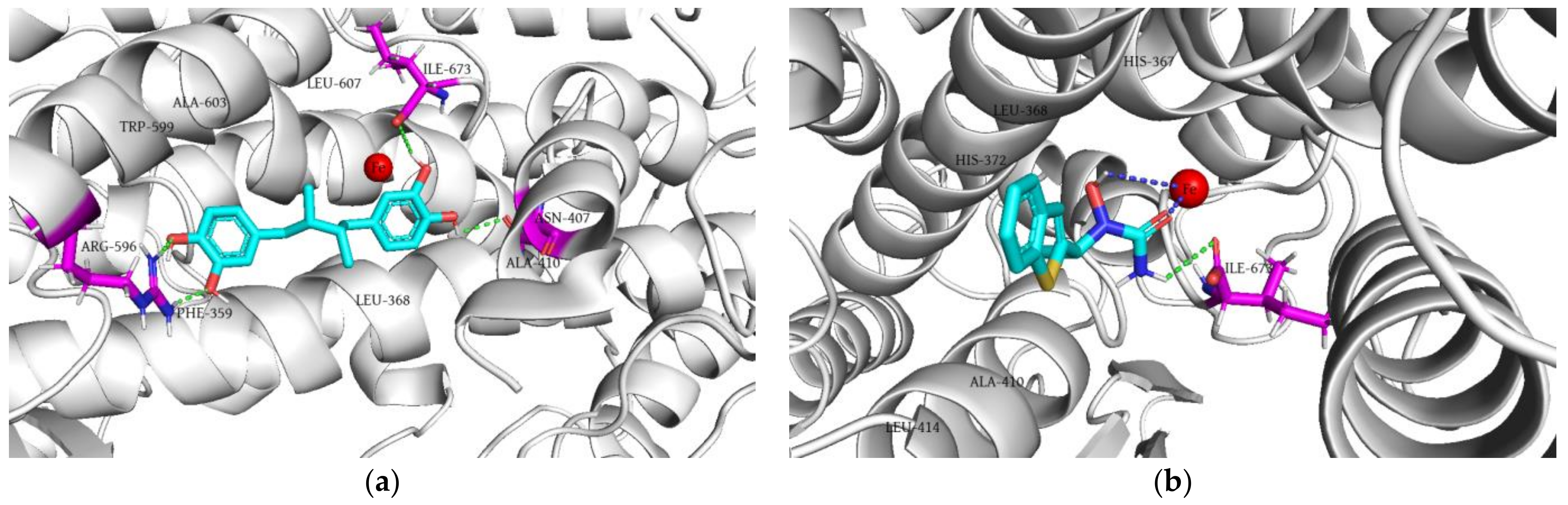
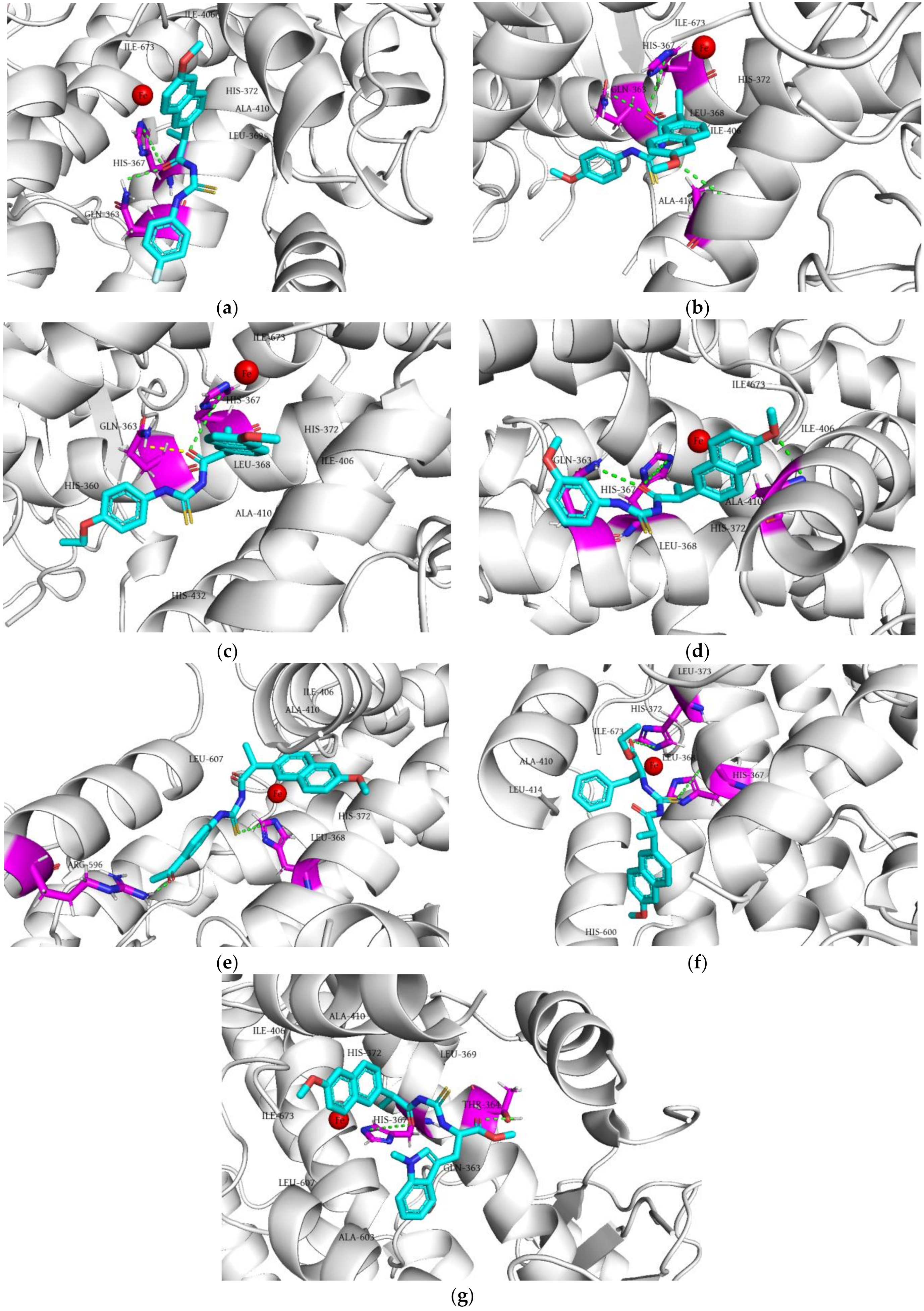
2.5. Molecular Docking Simulation
2.5.1. Molecular Docking of Tested Compounds into COX-2
2.5.2. Molecular Docking of the Tested Compounds into 5-LOX
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthetic Procedures
3.3. Evaluation of Toxicity Profile and Anti-Inflammatory Activity in Wistar Albino Rats
3.3.1. Acute Oral Toxicity Evaluation
3.3.2. Evaluation of Anti-Inflammatory Activity in Wistar Albino Rats
3.4. Investigation of COX-2 and 5-LOX Inhibitory Activity
3.5. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manjunathaiah Raghavendra, N.; Ramakrishna, K.; Sirisha, V.; Divya, P.; Venkateswara Rao, A. Computer aided discovery of potential anti-inflammatory (s)-naproxen analogs as COX-2 inhibitors. Med. Chem. 2013, 9, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Hasan, D.; Shono, A.; van Kalken, C.K.; van der Spek, P.J.; Krenning, E.P.; Kotani, T. A novel definition and treatment of hyperinflammation in COVID-19 based on purinergic signalling. Purinergic Signal. 2022, 18, 13–59. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: Implications for the benefit of high-dose intravenous vitamin C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective effects of ginger extract against glycation and oxidative stress-induced health complications: An in vitro study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal. Transduct. Target. Ther. 2021, 6, 1–30. [Google Scholar] [CrossRef]
- Krieg, P.; Fürstenberger, G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2014, 1841, 390–400. [Google Scholar] [CrossRef]
- Bruno, F.; Spaziano, G.; Liparulo, A.; Roviezzo, F.; Nabavi, S.M.; Sureda, A.; Filosa, R.; D’Agostino, B. Recent advances in the search for novel 5-lipoxygenase inhibitors for the treatment of asthma. Eur. J. Med. Chem. 2018, 153, 65–72. [Google Scholar] [CrossRef]
- Sarveswaran, S.; Chakraborty, D.; Chitale, D.; Sears, R.; Ghosh, J. Inhibition of 5-lipoxygenase selectively triggers disruption of c-Myc signaling in prostate cancer cells. J. Biol. Chem. 2015, 290, 4994–5006. [Google Scholar] [CrossRef]
- Khan, R.; Spagnoli, V.; Tardif, J.C.; L’Allier, P.L. Novel anti-inflammatory therapies for the treatment of atherosclerosis. Atherosclerosis 2015, 240, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, P.F.; Praticò, D. Overexpression of 5-lipoxygenase worsens the phenotype of a mouse model of tauopathy. Mol. Neurobiol. 2018, 55, 5926–5936. [Google Scholar] [CrossRef] [PubMed]
- Nejatian, N.; Häfner, A.K.; Shoghi, F.; Badenhoop, K.; Penna-Martinez, M. 5-Lipoxygenase (ALOX5): Genetic susceptibility to type 2 diabetes and vitamin D effects on monocytes. J. Steroid Biochem. Mol. Biol. 2019, 187, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Giannopoulos, P.F.; Ceballos-Diaz, C.; Golde, T.E.; Praticò, D. 5-lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of Alzheimer disease. Ann. Neurol. 2012, 72, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Gotoh, T.; Tomisato, W.; Mima, S.; Hoshino, T.; Hwang, H.J.; Takenaka, H.; Tsuchiya, T.; Mori, M.; Mizushima, T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell. Death Differ. 2004, 11, 1009–1016. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Tanaka, A.; Kato, S.; Amagase, K.; Satoh, H. Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clin. Chim. Acta 2010, 411, 459–466. [Google Scholar] [CrossRef]
- Shinu, P.; Sharma, M.; Gupta, G.L.; Mujwar, S.; Kandeel, M.; Kumar, M.; Nair, A.B.; Goyal, M.; Singh, P.; Attimarad, M.; et al. Computational Design, Synthesis, and Pharmacological Evaluation of Naproxen-Guaiacol Chimera for Gastro-Sparing Anti-Inflammatory Response by Selective COX2 Inhibition. Molecules 2022, 27, 6905. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Weisman, S.M. Clinical Pharmacology and Cardiovascular Safety of Naproxen. Am. J. Cardiovasc. Drugs 2017, 17, 97–107. [Google Scholar] [CrossRef]
- Domper Arnal, M.J.; Hijos-Mallada, G.; Lanas, A. Gastrointestinal and cardiovascular adverse events associated with NSAIDs. Expert. Opin. Drug. Saf. 2022, 21, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Han, M.İ.; Küçükgüzel, Ş.G. Anticancer and Antimicrobial Activities of Naproxen and Naproxen Derivatives. Mini Rev. Med. Chem. 2020, 20, 1300–1310. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Jishkariani, D.; Narindoshvili, T. Convenient synthesis of Ibuprofen and naproxen aminoacyl, dipeptidoyl and ester derivatives. Chem. Biol. Drug. Des. 2009, 73, 618–626. [Google Scholar] [CrossRef]
- Azizian, H.; Mousavi, Z.; Faraji, H.; Tajik, M.; Bagherzadeh, K.; Bayat, P.; Shafiee, A.; Almasirad, A. Arylhydrazone derivatives of naproxen as new analgesic and anti-inflammatory agents: Design, synthesis and molecular docking studies. J. Mol. Graph. Model. 2016, 67, 127–136. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, W.M.; El-Sayed, M.A.; Abdel-Aziz, N.I.; El-Azab, A.S.; Asiri, Y.A.; Abdel-Aziz, A.A. Structural alterations based on naproxen scaffold: Synthesis, evaluation of antitumor activity and COX-2 inhibition, and molecular docking. Eur. J. Med. Chem. 2018, 158, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, A.A.; Al-Harbi, L.M.; El-Gazzar, M.A.; Khowdiary, M.M.; Ouidate, A.; Alosaimi, A.M.; Elhamid Salim, A. Naproxenylamino acid derivatives: Design, synthesis, docking, QSAR and anti-inflammatory and analgesic activity. Biomed. Pharmacother. 2019, 116, 109024. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Marnett, A.B.; Crews, B.C.; Remmel, R.P.; Marnett, L.J. Ester and amide derivatives of the nonsteroidal antiinflammatory drug, indomethacin, as selective cyclooxygenase-2 inhibitors. J. Med. Chem. 2000, 43, 2860–2870. [Google Scholar] [CrossRef]
- Berk, B.; Aktay, G.; Yesilada, E.; Ertan, M. Synthesis and pharmacological activities of some new 2-[1-(6-methoxy-2-naphthyl)ethyl]-6-(substituted)benzylidene thiazolo[3,2-b]-1,2,4-triazole-5(6H)-one derivatives. Pharmazie 2001, 56, 613–616. [Google Scholar] [CrossRef]
- Ranatunge, R.R.; Augustyniak, M.E.; Dhawan, V.; Ellis, J.L.; Garvey, D.S.; Janero, D.R.; Letts, L.G.; Richardson, S.K.; Shumway, M.J.; Trocha, A.M.; et al. Synthesis and anti-inflammatory activity of a series of N-substituted naproxen glycolamides: Nitric oxide-donor naproxen prodrugs. Bioorg. Med. Chem. 2006, 14, 2589–2599. [Google Scholar] [CrossRef]
- Fernandes, J.; Singh, S.; Kumar, A.; Kumar, P. Synthesis, Analgesic and Anti-inflammatory Activity of Some Novel Derivatives of Naproxen. Res. J. Pharm. Tech. 2014, 7, 631–634. [Google Scholar]
- Levit, G.L.; Anikina, L.V.; Vikharev, Y.B.; Demin, A.M.; Safin, V.A.; Matveeva, T.V.; Krasnov, V.P. Synthesis and antiinflammatory and analgesic activity of naproxen amides with amino acid derivatives. Pharm. Chem. J. 2002, 36, 232–236. [Google Scholar] [CrossRef]
- Shakeel, A. Thiourea Derivatives in Drug Design and Medicinal Chemistry: A Short Review. J. Drug. Des. Med. Chem. 2016, 2, 10. [Google Scholar] [CrossRef]
- Calixto, S.D.; Simão, T.L.B.V.; Palmeira-Mello, M.V.; Viana, G.M.; Assumpção, P.W.M.C.; Rezende, M.G.; do Espirito Santo, C.C.; de Oliveira Mussi, V.; Rodrigues, C.R.; Lasunskaia, E.; et al. Antimycobacterial and anti-inflammatory activities of thiourea derivatives focusing on treatment approaches for severe pulmonary tuberculosis. Bioorg. Med. Chem. 2022, 53, 116506. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Zhang, T.; Zhu, H.; Qian, H.; Zhang, H.; Huang, W.; Gust, R. Design and synthesis of thiourea derivatives containing a benzo[5,6]cyclohepta[1,2-b]pyridine moiety as potential antitumor and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 2701–2704. [Google Scholar] [CrossRef]
- Pingaew, R.; Sinthupoom, N.; Mandi, P.; Prachayasittikul, V.; Cherdtrakulkiat, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and in silico study of bis-thiourea derivatives as anticancer, antimalarial and antimicrobial agents. Med. Chem. Res. 2017, 26, 3136–3148. [Google Scholar] [CrossRef]
- Ammar, Y.A.; Fayed, E.A.; Bayoumi, A.H.; Saleh, M.A.; El-Araby, M.E. Design and synthesis of pyridine-amide based compounds appended naproxen moiety as anti-microbial and anti-inflammatory agents. Am. J. Pharm. Tech. Res. 2015, 5, 245–273. [Google Scholar]
- Eissa, S.I.; Farrag, A.M.; Galeel, A.A. Non-carboxylic analogues of aryl propionic acid: Synthesis, anti-inflammatory, analgesic, antipyretic and ulcerogenic potential. Drug. Res. 2014, 64, 485–492. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; Moustafa, G.O.; El-Gazzar, M.A.; Abdel-Rahman, R.F.; Salim, A.E. Synthesis, comparative docking, and pharmacological activity of naproxen amino acid derivatives as possible anti-inflammatory and analgesic agents. Drug. Des. Dev. Ther. 2019, 13, 1773–1790. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Pal Singh, V. Licofelone-a novel analgesic and anti-inflammatory agent. Curr. Top. Med. Chem. 2007, 7, 251–263. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, W.; Li, Y.; Sun, Y.; Zhang, Y.; Ji, H.; Lai, Y. Darbufelone, a novel anti-inflammatory drug, induces growth inhibition of lung cancer cells both in vitro and in vivo. Cancer Chemother. Pharmacol. 2010, 66, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Sabe, V.T.; Ntombela, T.; Jhamba, L.A.; Maguire, G.E.; Govender, T.; Naicker, T.; Kruger, H.G. Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: A review. Eur. J. Med. Chem. 2021, 224, 113705. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.B.; Gupta, R. Contemporary Computational Applications and Tools in Drug Discovery. ACS Med. Chem. Lett. 2022, 13, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Mughal, E.U.; Ashraf, J.; Hussein, E.M.; Nazir, Y.; Alwuthaynani, A.S.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Ahmed, S.A. Design, synthesis, and structural characterization of thioflavones and thioflavonols as potential tyrosinase inhibitors: In vitro and in silico studies. ACS Omega 2022, 7, 17444–17461. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Anwar, S.; Raut, R.; Almatroudi, A.; Babiker, A.Y.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A. Therapeutic Potential of Myrrh, a Natural Resin, in Health Management through Modulation of Oxidative Stress, Inflammation, and Advanced Glycation End Products Formation Using In Vitro and In Silico Analysis. Appl. Sci. 2022, 12, 9175. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Nordin, N.A.; Chai, T.W.; Tan, B.L.; Choi, C.L.; Abd Halim, A.N.; Hussain, H.; Ngaini, Z. Novel synthetic monothiourea aspirin derivatives bearing alkylated amines as potential antimicrobial agents. J. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Karim, N.; Khan, I.; Khan, W.; Khan, I.; Khan, A.; Halim, S.A.; Khan, H.; Hussain, J.; Al-Harrasi, A. Anti-nociceptive and anti-inflammatory activities of asparacosin a involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: An in-vitro, in-vivo, and in-silico approach. Front. Immunol. 2019, 10, 581. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.D.; von Woedtke, T.; Wende, K. Non-steroidal anti-inflammatory drugs: Recent advances in the use of synthetic COX-2 inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Duggan, K.C.; Walters, M.J.; Musee, J.; Harp, J.M.; Kiefer, J.R.; Oates, J.A.; Marnett, L.J. Molecular basis for cyclooxygenase inhibition by the non-steroidal anti-inflammatory drug naproxen. J. Biol. Chem. 2010, 285, 34950–34959. [Google Scholar] [CrossRef]
- Pergola, C.; Werz, O. 5-Lipoxygenase inhibitors: A review of recent developments and patents. Expert. Opin. Ther. Pat. 2010, 20, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Kahnt, A.S.; Angioni, C.; Göbel, T.; Hofmann, B.; Roos, J.; Steinbrink, S.D.; Rörsch, F.; Thomas, D.; Geisslinger, G.; Zacharowski, K.; et al. Inhibitors of human 5-lipoxygenase potently interfere with prostaglandin transport. Front. Pharmacol. 2022, 12, 782584. [Google Scholar] [CrossRef]
- Ihsan, A.; Wang, X.; Huang, X.J.; Liu, Y.; Liu, Q.; Zhou, W.; Yuan, Z.H. Acute and subchronic toxicological evaluation of Mequindox in Wistar rats. Regul. Toxicol. Pharmacol. 2010, 57, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Salga, M.S.; Ali, H.M.; Abdulla, M.A.; Abdelwahab, S.I. Acute oral toxicity evaluations of some zinc (II) complexes derived from 1-(2-Salicylaldiminoethyl) piperazine schiff bases in rats. Int. J. Mol. Sci. 2012, 13, 1393–1404. [Google Scholar] [CrossRef]
- Rodríguez-Cal y Mayor, A.; Castañeda-Hernández, G.; Favari, L.; Martinez-Cruz, A.; Guízar-Sahagún, G.; Cruz-Antonio, L. Pharmacokinetics and anti-inflammatory effect of naproxen in rats with acute and subacute spinal cord injury. Naunyn Schmiedeb Arch. Pharmacol. 2020, 393, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Mićović, T.; Stanković, J.S.K.; Bauer, R.; Nöst, X.; Marković, Z.; Milenković, D.; Jakovljević, V.; Tomović, M.; Bradić, J.; Stešević, D.; et al. In vitro, in vivo and in silico evaluation of the anti-inflammatory potential of Hyssopus officinalis L. subsp. aristatus (Godr.) Nyman (Lamiaceae). J. Ethnopharmacol. 2022, 293, 115201. [Google Scholar] [CrossRef]
- COX2. Inhibitor Screening Kit (Fluorometric) (ab283401). Available online: https://www.abcam.com/products/assay-kits/cox2-inhibitor-screening-kit-fluorometric-ab283401.html (accessed on 27 February 2023).
- ab284521–5-Lipoxygenase Inhibitor Screening Kit (Fluorometric). Available online: https://www.abcam.com/ps/products/284/ab284521/documents/5-Lipoxygenase-Inhibitor-Screening-Kit-protocol-book-v2-ab284521%20(website).pdf (accessed on 27 February 2023).
- Protein Data Bank. Available online: http://www.rcsb.org/ (accessed on 26 March 2023).
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- MAKE Receptor 3.2.0.2: OpenEye Scientific Software, Santa Fe, USA. Available online: https://docs.eyesopen.com/applications/oedocking/make_receptor/make_receptor_setup.html (accessed on 28 March 2023).
- OMEGA 2.5.1.4: OpenEye Scientific Software, Santa Fe, NM. Available online: http://www.eyesopen.com/ (accessed on 28 March 2023).
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer Generation with OMEGA: Algorithm and Validation Using High Quality Structures from the Protein Databank and the Cambridge Structural Database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef]
- FRED 3.2.0.2: OpenEye Scientific Software, Santa Fe, NM. Available online: https://www.eyesopen.com/ (accessed on 28 March 2023).
- McGann, M. FRED pose prediction and virtual screening accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- McGann, M. FRED and HYBRID docking performance on standardized datasets. J. Comput. Aided Mol. Des. 2012, 26, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Bleylevens, I.W.; Bitorina, A.V.; Wichapong, K.; Nicolaes, G.A. Optimization of Compound Ranking for Structure-Based Virtual Ligand Screening Using an Established FRED–Surflex Consensus Approach. Chem. Biol. Drug. Des. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug. Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Carugo, O.; Pongor, S. A normalized root-mean-spuare distance for comparing protein three-dimensional structures. Protein Sci. 2001, 10, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
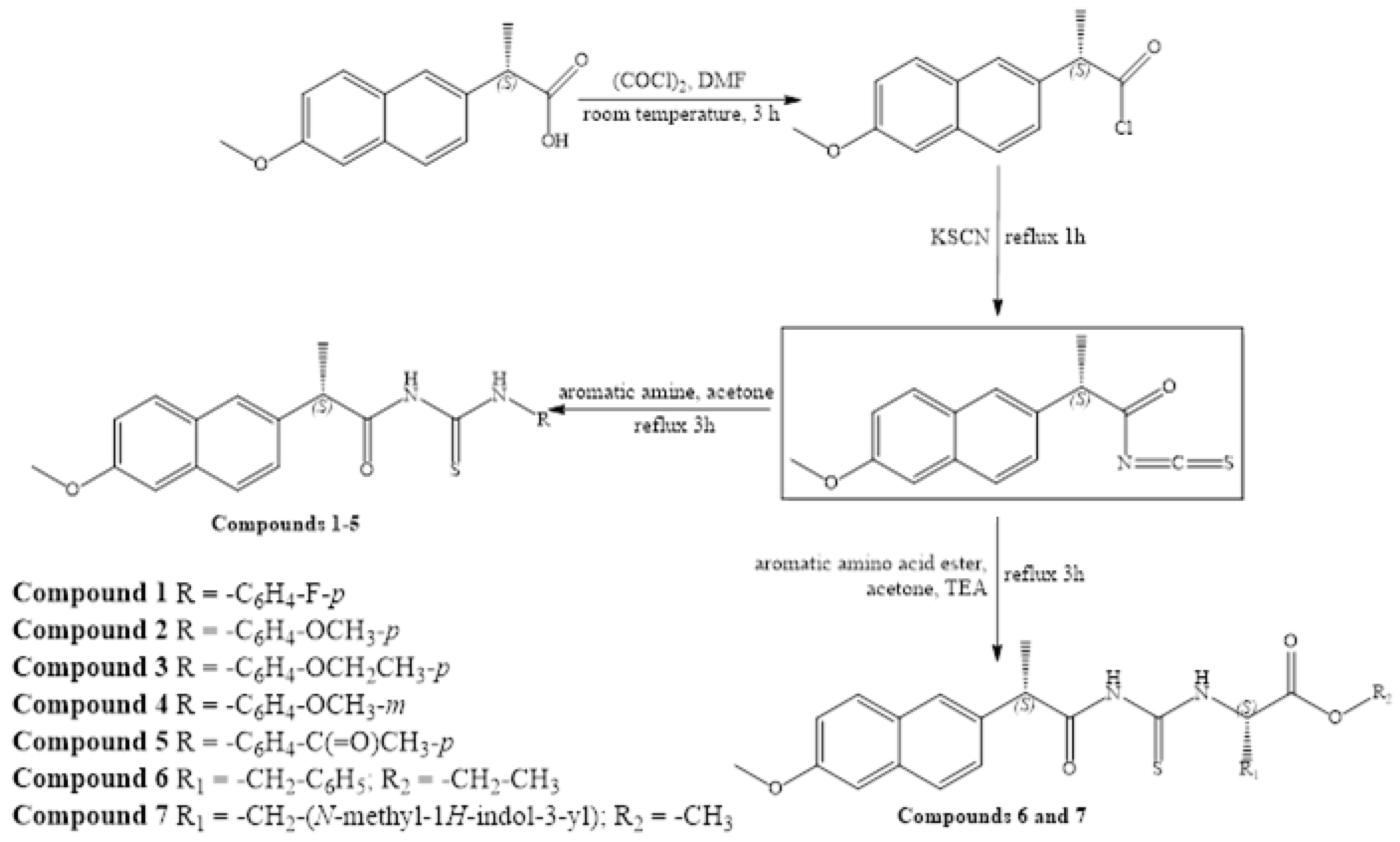

| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Control Group |
|---|---|---|---|---|---|---|---|---|
| Organ | ||||||||
| Kidney | 0.31 ± 0.03 | 0.34 ± 0.04 | 0.32 ± 0.06 | 0.33 ± 0.07 | 0.30 ± 0.03 | 0.35 ± 0.05 | 0.29 ± 0.03 | 0.33 ± 0.01 |
| Heart | 0.32 ± 0.02 | 0.33 ± 0.04 | 0.31 ± 0.03 | 0.30 ± 0.03 | 0.31 ± 0.03 | 0.34 ± 0.02 | 0.33 ± 0.03 | 0.31 ± 0.01 |
| Liver | 2.73 ± 0.24 | 3.07 ± 0.38 | 2.85 ± 0.17 | 2.45 ± 0.15 | 2.97 ± 0.33 | 3.01 ± 0.29 | 2.64 ± 0.46 | 2.91 ± 0.10 |
| Stomach | 0.54 ± 0.05 | 0.57 ± 0.04 | 0.50 ± 0.05 | 0.50 ± 0.05 | 0.53 ± 0.04 | 0.55 ± 0.03 | 0.50 ± 0.04 | 0.53 ± 0.04 |
| Rat Paw Thickness (mm) (% of Inhibition) | |||||
|---|---|---|---|---|---|
| Experimental Groups | 0 h | 1 h | 2 h | 3 h | 4 h |
| Compound 1 2.5 mg/kg | 4.57 ± 0.31 | 6.50 ± 0.40 (20.275%) | 6.50 ± 0.40 (11.111%) | 6.20 ± 0.30 (10.502%) | 5.53 ± 0.29 (33.333%) |
| Compound 1 5.0 mg/kg | 4.40 ± 0.36 | 7.00 ± 0.10 (−7.216%) | 6.50 ± 0.17 (3.448%) | 5.8 ± 0.20 (23.288%) | 5.27 ± 0.29 (40.230%) |
| Compound 1 10.0 mg/kg | 4.47 ± 0.51 | 7.03 ± 0.12 (−5.842%) | 6.60 ± 0.10 (1.916%) | 6.10 ± 0.17 (10.502%) | 5.27 ± 0.50 (44.800%) * |
| Compound 2 2.5 mg/kg | 4.37 ± 0.15 | 6.57 ± 1.16 (9.278%) | 6.07 ± 0.72 (21.839%) | 5.77 ± 0.42 (23.288%) | 5.30 ± 0.26 (35.632%) |
| Compound 2 5.0 mg/kg | 4.77 ± 0.21 | 7.03 ± 0.47 (6.529%) | 6.40 ± 0.53 (24.904%) | 5.70 ± 0.20 (48.858%) * | 5.53 ± 0.29 (47.126%) * |
| Compound 2 10.0 mg/kg | 4.63 ± 0.06 | 6.5 ± 0.40 (23.024%) | 6.17 ± 0.67 (29.502%) | 5.53 ± 0.15 (50.685%) * | 5.33 ± 0.25 (51.724%) * |
| Compound 3 2.5 mg/kg | 4.30 ± 0.28 | 6.25 ± 0.21 (19.588%) | 6.0 ± 0.57 (21.839%) | 5.50 ± 0.42 (34.247%) | 5.10 ± 0.14 (44.737%) |
| Compound 3 5.0 mg/kg | 4.13 ± 0.25 | 6.40 ± 0.79 (6.529%) | 5.73 ± 0.38 (26.437%) * | 5.44 ± 0.28 (28.584%) | 4.97 ± 0.42 (42.529%) * |
| Compound 3 10.0 mg/kg | 3.90 ± 0.20 | 6.23 ± 0.25 (3.780%) | 5.80 ± 0.26 (12.644%) | 5.27 ± 0.15 (25.114%) | 4.67 ± 0.38 (47.217%) * |
| Compound 4 2.5 mg/kg | 4.03 ± 0.06 | 5.93 ± 0.25 (21.649%) | 5.40 ± 0.10 (37.165%) * | 5.17 ± 0.15 (37.900%) | 4.90 ± 0.10 (40.523%) * |
| Compound 4 5.0 mg/kg | 4.40 ± 0.62 | 6.10 ± 0.44 (29.897%) * | 5.97 ± 0.25 (27.969%) | 5.70 ± 0.46 (28.767%) | 5.22 ± 0.50 (43.218%) * |
| Compound 4 10.0 mg/kg | 4.23 ± 0.06 | 6.00 ± 0.10 (27.148%) * | 5.70 ± 0.20 (32.567%) * | 5.30 ± 0.17 (41.553%) * | 4.90 ± 0.20 (54.013%) * |
| Compound 5 2.5 mg/kg | 3.57 ± 0.12 | 5.73 ± 0.12 (10.653%) | 5.37 ± 0.25 (17.241%) | 5.20 ± 0.20 (10.502%) | 4.57 ± 0.15 (31.034%) |
| Compound 5 5.0 mg/kg | 3.10 ± 0.00 | 5.05 ± 0.07 (19.588%) | 4.85 ± 0.07 (19.540%) | 4.40 ± 0.14 (28.767%) | 3.95 ± 0.07 (41.379%) |
| Compound 5 10.0 mg/kg | 4.00 ± 0.28 | 5.5 ± 0.71 (38.144%) * | 5.35 ± 0.64 (37.931%) * | 5.90 ± 0.71 (39.726%) | 4.75 ± 0.49 (48.276%) |
| Compound 6 2.5 mg/kg | 4.03 ± 0.06 | 6.17 ± 0.31 (11.753%) | 5.87 ± 0.23 (15.709%) | 5.37 ± 0.55 (26.941%) | 4.93 ± 0.21 (37.931%) |
| Compound 6 5.0 mg/kg | 4.47 ± 0.35 | 6.47 ± 0.57 (17.526%) | 6.17 ± 0.06 (21.839%) | 5.77 ± 0.06 (28.767%) | 5.27 ± 0.21 (44.724%) |
| Compound 6 10.0 mg/kg | 4.30 ± 0.62 | 6.20 ± 0.20 (21.649%) | 6.17 ± 0.23 (14.176%) | 5.27 ± 0.72 (47.103%) | 5.03 ± 0.76 (49.425%) * |
| Compound 7 2.5 mg/kg | 5.13 ± 0.29 | 7.03 ± 0.15 (21.649%) | 6.47 ± 0.21 (38.697%) * | 6.10 ± 0.20 (47.032%) * | 5.87 ± 0.21 (49.425%) * |
| Compound 7 5.0 mg/kg | 5.27 ± 0.40 | 7.10 ± 0.17 (24.399%) | 6.80 ± 0.20 (29.502%) * | 6.43 ± 0.32 (36.073%) | 6.03 ± 0.61 (47.126%) |
| Compound 7 10.0 mg/kg | 4.80 ± 0.53 | 6.87 ± 0.25 (14.777%) | 6.50 ± 0.36 (21.839%) | 6.03 ± 0.21 (32.420%) | 5.47 ± 0.38 (54.123%) * |
| 1% DMSO | 4.40 ± 0.08 | 6.83 ± 0.30 | 6.58 ± 0.30 | 6.23 ± 0.46 | 5.85 ± 0.31 |
| Naproxen 2.5 mg/kg | 4.23 ± 0.40 | 6.20 ± 0.10 (18.900%) | 5.87 ± 0.12 (24.904%) | 5.60 ± 0.17 (25.114%) | 5.03 ± 0.25 (44.828%) |
| Naproxen 5.0 mg/kg | 4.10 ± 0.10 | 5.87 ± 0.23 (27.148%) | 5.37 ± 0.31 (41.762%) | 5.07 ± 0.35 (47.083%) | 4.83 ± 0.21 (49.448%) |
| Naproxen 10.0 mg/kg | 4.17 ± 0.30 | 5.73 ± 0.20 (35.395%) | 5.40 ± 0.40 (43.295%) | 5.20 ± 0.30 (45.205%) | 4.80 ± 0.40 (56.322%) |
| Compound | COX-2 Percent of Inhibition | COX-2 (IC50 µM) | 5-LOX Percent of Inhibition | 5-LOX (IC50 µM) |
|---|---|---|---|---|
| 1 | 24.73 ± 1.11 | - | 34.25 ± 1.04 | 19.16 ± 0.96 |
| 2 | 18.77 ± 4.37 | - | 25.12 ± 1.83 | 39.77 ± 1.98 |
| 3 | 23.39 ± 0.57 | - | 47.02 ± 4.32 | 9.52 ± 1.51 |
| 4 | 37.71 ± 0.28 | - | 77.20 ± 0.26 | 0.30 ± 0.12 |
| 5 | 19.58 ± 4.55 | - | 46.19 ± 4.87 | 16.74 ± 1.79 |
| 6 | 23.99 ± 5.36 | - | 23.63 ± 2.14 | - |
| 7 | 24.23 ± 5.77 | - | 20.72 ± 4.19 | - |
| Naproxen | 72.15 ± 1.40 | 0.20 ± 0.02 | Not tested | Not tested |
| Celecoxib | 85.02 ± 0.31 | 0.07 ± 0.01 | Not tested | Not tested |
| Zileuton | Not tested | Not tested | 64.85 ± 0.39 | 0.36 ± 0.10 |
| Compound | COX-2 | 5-LOX |
|---|---|---|
| 1 | −14.40 | −7.70 |
| 2 | −14.90 | −7.47 |
| 3 | −14.13 | −6.74 |
| 4 | −14.94 | −8.39 |
| 5 | −14.42 | −7.30 |
| 6 | −8.76 | −7.77 |
| 7 | −9.41 | −7.98 |
| Naproxen | −13.13 | - |
| Zileuton | - | −8.77 |
| NDGA | - | −10.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeljković, N.; Dobričić, V.; Bošković, J.; Vesović, M.; Bradić, J.; Anđić, M.; Kočović, A.; Jeremić, N.; Novaković, J.; Jakovljević, V.; et al. Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen. Pharmaceuticals 2023, 16, 666. https://doi.org/10.3390/ph16050666
Nedeljković N, Dobričić V, Bošković J, Vesović M, Bradić J, Anđić M, Kočović A, Jeremić N, Novaković J, Jakovljević V, et al. Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen. Pharmaceuticals. 2023; 16(5):666. https://doi.org/10.3390/ph16050666
Chicago/Turabian StyleNedeljković, Nikola, Vladimir Dobričić, Jelena Bošković, Marina Vesović, Jovana Bradić, Marijana Anđić, Aleksandar Kočović, Nevena Jeremić, Jovana Novaković, Vladimir Jakovljević, and et al. 2023. "Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen" Pharmaceuticals 16, no. 5: 666. https://doi.org/10.3390/ph16050666
APA StyleNedeljković, N., Dobričić, V., Bošković, J., Vesović, M., Bradić, J., Anđić, M., Kočović, A., Jeremić, N., Novaković, J., Jakovljević, V., Vujić, Z., & Nikolić, M. (2023). Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen. Pharmaceuticals, 16(5), 666. https://doi.org/10.3390/ph16050666












