Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease
Abstract
1. Introduction
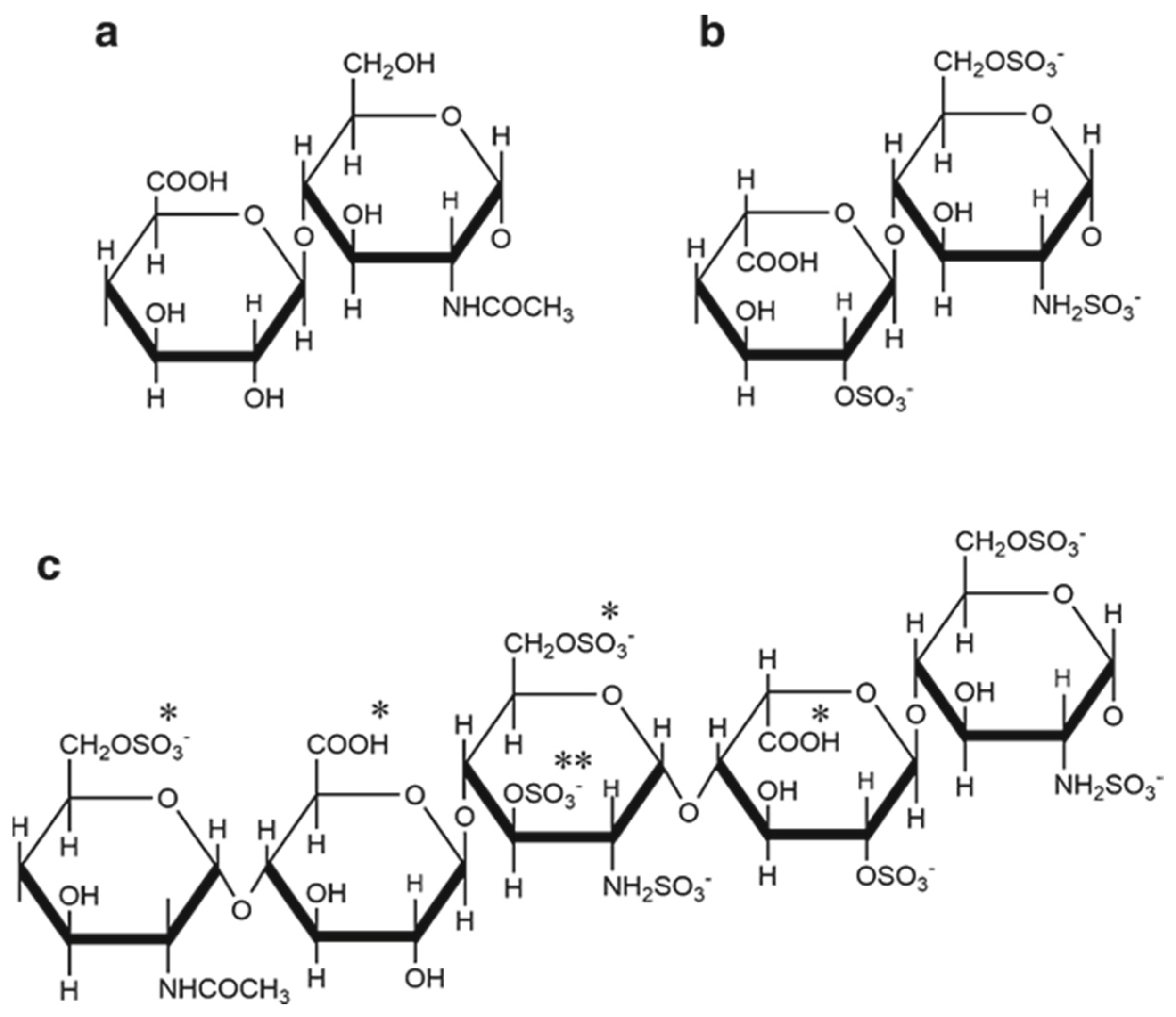
2. Heparin Structure and Anticoagulant Activity
3. Heparin Binding Partners
4. Heparin Binding Proteins That Neutralise the Anticoagulant Activity of Heparin
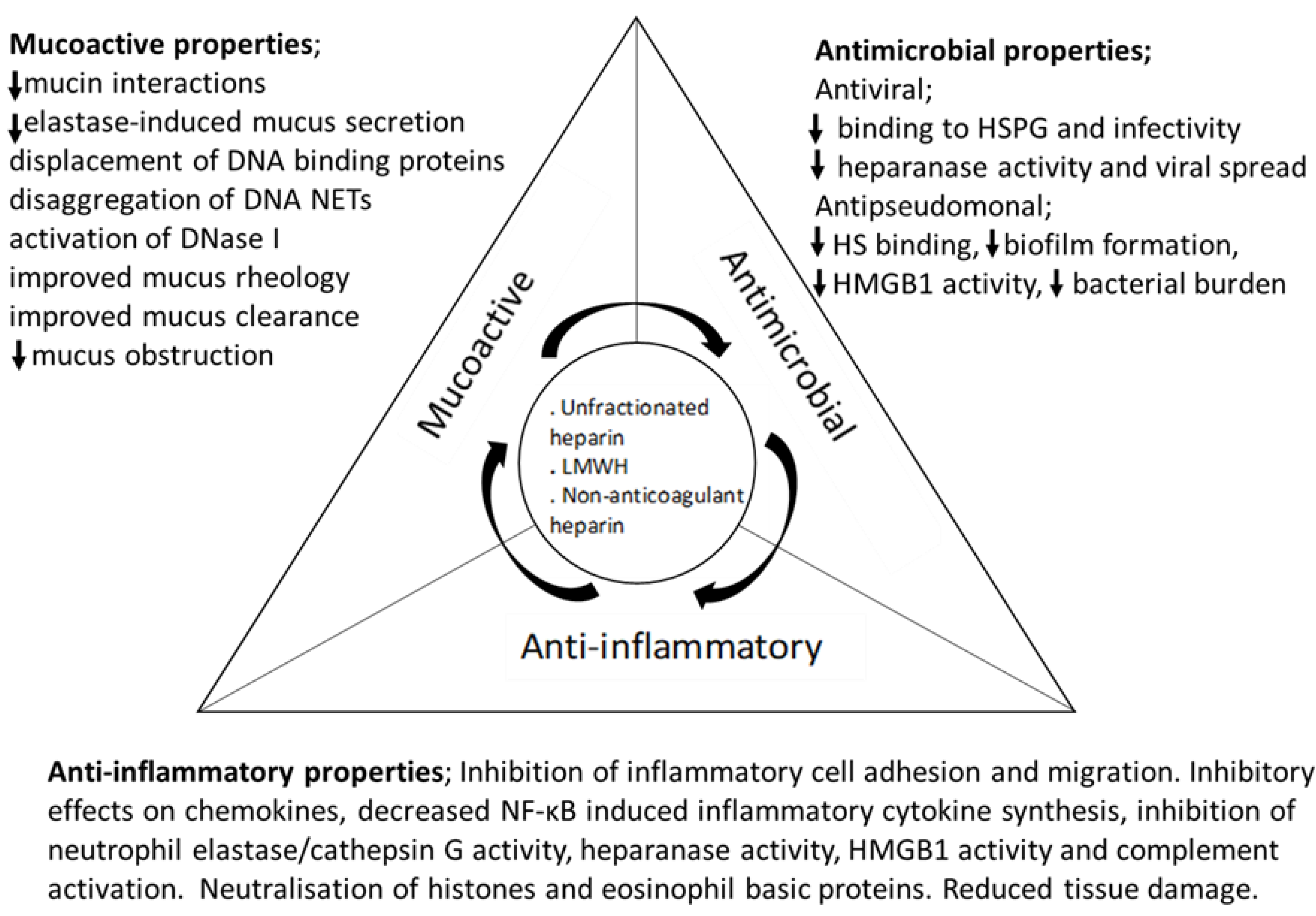
5. The Anti-Inflammatory Effects of Heparin
5.1. Inhibition of Chemokine Activity
5.2. Inhibition of Leukocyte Adhesion to Endothelial Cells
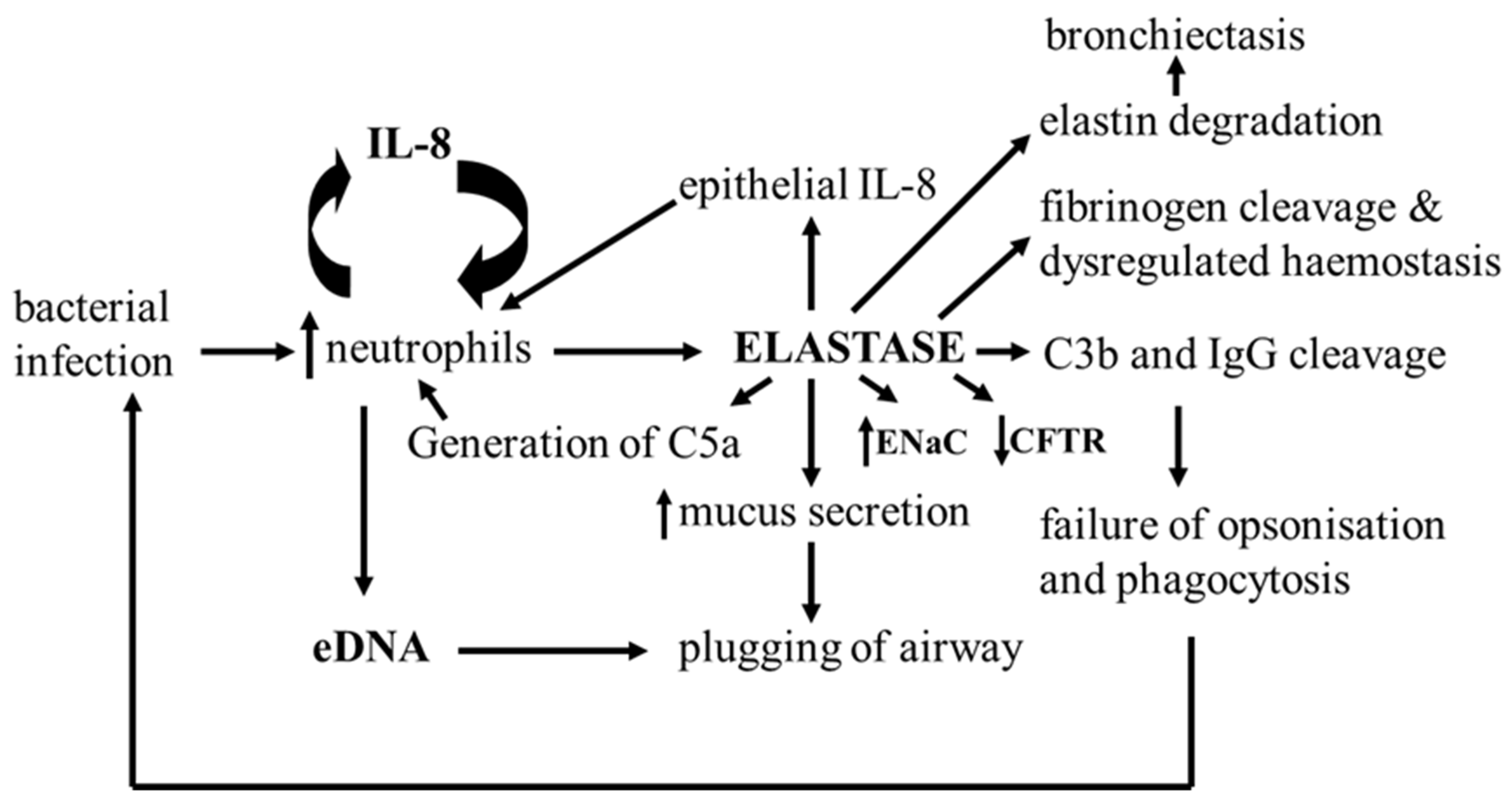
5.3. Inhibition of Neutrophil Elastase Activity
5.4. Inhibition of High Mobility Group Box1 (HMGB1)
5.5. Inhibition of Heparanase Activity
5.6. Inhibition of Complement Activation
6. Mucoactive Effects of Heparin and Heparin Derivatives
7. Heparin as a Systemic Anticoagulant in COVID-19
8. Inhaled Heparin as an Anticoagulant, Anti-Viral, Anti-Inflammatory, and Mucoactive Therapy in COVID-19
8.1. The Anti-Viral Properties of Heparin in COVID-19
8.2. Inflammation and the Potential Anti-Inflammatory Effects of Heparin and Derivatives in COVID-19
8.3. Effects of Heparin on DNA NETS, Histones, Neutrophil Elastase, Alveolar Damage and Fluid Exudation in COVID-19
9. Inhaled Heparin for ARDS and ALI
10. Inhaled Heparin for Inflammatory Lung Disease in Asthma, Cystic Fibrosis and COPD
Anti-Viral Effects
11. Inhaled Heparin in Asthma
12. Inhaled Heparin in COPD
13. Inhaled Heparin in Cystic Fibrosis
13.1. Antimicrobial Effects
13.2. Anti-Inflammatory Effects of Heparin and Derivatives in CF
Clinical Trials of Inhaled Heparin in CF
14. Alternative Formulations of Heparin
15. Dose-Dependent Effects of Inhaled Heparin on Systemic Anticoagulation
16. Potential Side Effects of Inhaled Heparin Therapy
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2017, 34, 351–361. [Google Scholar] [CrossRef]
- Jeske, W.; Kouta, A.; Farooqui, A.; Siddiqui, F.; Rangnekar, V.; Niverthi, M.; Laddu, R.; Hoppensteadt, D.; Iqbal, O.; Walenga, J.; et al. Bovine Mucosal Heparins Are Comparable to Porcine Mucosal Heparin at USP Potency Adjusted Levels. Front. Med. 2019, 5, 360. [Google Scholar] [CrossRef]
- Ange, K.S.; Onishi, A.; Fuming, Z.; Sun, X.; Lin, L.; Mori, D.; Zhang, F.; Dordick, J.S.; Fareed, J.; Hoppensteadt, D.; et al. Analysis of Heparins Derived from Bovine Tissues and Comparison to Porcine Intestinal Heparins. Clin. Appl. Thromb. Hemost. 2016, 22, 520–527. [Google Scholar] [CrossRef]
- Mulloy, B.; Hogwood, J.; Gray, E.; Lever, R.; Page, C.P. Pharmacology of Heparin and Related Drugs. Pharmacol. Rev. 2016, 68, 76–141. [Google Scholar] [CrossRef]
- Gray, E.; Hogwood, J.; Mulloy, B. The Anticoagulant and Antithrombotic Mechanisms of Heparin. In Heparin—A Century of Progress; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 207, pp. 43–61. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and Low-Molecular-Weight Heparin Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest 2001, 119 (Suppl. 1), 64S–94S. [Google Scholar] [CrossRef]
- Gray, E.; Mulloy, B. Biosimilar low molecular weight heparin products. J. Thromb. Haemost. 2009, 7, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Merli, G.J.; Groce, J.B. Pharmacological and clinical differences between low-molecular-weight heparins: Implications for prescribing practice and therapeutic interchange. Pharm. Ther. 2010, 35, 95–105. [Google Scholar]
- Baytas, S.N.; Varghese, S.S.; Jin, W.; Yu, Y.; He, P.; Douaisi, M.; Zhang, F.; Brodfuehrer, P.; Xia, K.; Dordick, J.S.; et al. Preparation of Low Molecular Weight Heparin from a Remodeled Bovine Intestinal Heparin. J. Med. Chem. 2021, 64, 2242–2253. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.A.; Maroney, S.A.; Martinez, N.D.; Mast, A.E. Major Reservoir for Heparin-Releasable TFPIα (Tissue Factor Pathway Inhibitor α) Is Extracellular Matrix. Arter. Thromb. Vasc. Biol. 2021, 41, 1942–1955. [Google Scholar] [CrossRef] [PubMed]
- Jaques, L.B. Heparins-anionic polyelectrolyte drugs. Pharmacol. Rev. 1979, 31, 96–166. [Google Scholar]
- Warkentin, T.E.; Levine, M.N.; Hirsh, J.; Horsewood, P.; Roberts, R.S.; Gent, M.; Kelton, J.G. Heparin-Induced Thrombocytopenia in Patients Treated with Low-Molecular-Weight Heparin or Unfractionated Heparin. N. Engl. J. Med. 1995, 332, 1330–1336. [Google Scholar] [CrossRef]
- Ahmed, I.; Majeed, A.; Powell, R. Heparin induced thrombocytopenia: Diagnosis and management update. Postgrad. Med. J. 2007, 83, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Coombe, D.R. Heparin Mimetics: Their Therapeutic Potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Khan, N.; Lindenbauer, A.; Nguyen, T.-H. When Will Fondaparinux Induce Thrombocytopenia? Bioconjug. Chem. 2022, 33, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Ori, A.; Wilkinson, M.; Fernig, D.G. A Systems Biology Approach for the Investigation of the Heparin/Heparan Sulfate Interactome. J. Biol. Chem. 2011, 286, 19892–19904. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mulloy, B. Current structural biology of the heparin interactome. Curr. Opin. Struct. Biol. 2015, 34, 17–25. [Google Scholar] [CrossRef]
- Chudasama, S.L.; Espinasse, B.; Hwang, F.; Qi, R.; Joglekar, M.; Afonina, G.; Wiesner, M.R.; Welsby, I.J.; Ortel, T.L.; Arepally, G.M. Heparin modifies the immunogenicity of positively charged proteins. Blood 2010, 116, 6046–6053. [Google Scholar] [CrossRef]
- Schroeder, M.; Hogwood, J.; Gray, E.; Mulloy, B.; Hackett, A.-M.; Johansen, K.B. Protamine neutralisation of low molecular weight heparins and their oligosaccharide components. Anal. Bioanal. Chem. 2011, 399, 763–771. [Google Scholar] [CrossRef]
- Giangrande, P.L.F. Fondaparinux (Arixtra): A new anticoagulant. Int. J. Clin. Pract. 2002, 56, 615–617. [Google Scholar] [PubMed]
- Walz, D.A.; Wu, V.Y.; de Lamo, R.; Dene, H.; McCoy, L.E. Primary structure of human platelet factor 4. Thromb. Res. 1977, 11, 893–898. [Google Scholar] [CrossRef]
- Sobczak, A.I.; Pitt, S.J.; Stewart, A.J. Glycosaminoglycan Neutralization in Coagulation Control. Arter. Thromb. Vasc. Biol. 2018, 38, 1258–1270. [Google Scholar] [CrossRef]
- Mitsuguro, M.; Okamoto, A.; Shironouchi, Y.; Sano, M.; Miyata, S.; Neki, R.; Araki, T.; Hamamoto, T.; Yoshimatsu, J.; Miyata, T. Effects of factor VIII levels on the APTT and anti-Xa activity under a therapeutic dose of heparin. Int. J. Hematol. 2015, 101, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.K.; Calzetta, L.; Cardaci, V.; di Toro, S.; Page, C.P.; Cazzola, M. Inhaled nebulised unfractionated heparin improves lung function in moderate to very severe COPD: A pilot study. Pulm. Pharmacol. Ther. 2018, 48, 88–96. [Google Scholar] [CrossRef]
- Hogwood, J.; Gray, E.; Komorowicz, E.; Varjú, I.; Varga, Z.; Kolev, K.; Longstaff, C. Neutralisation of the anti-coagulant effects of heparin by histones in blood plasma and purified systems. Thromb. Haemost. 2016, 115, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Prins, M.; Levine, M.N.; Hirsh, J. Heparin binding to plasma proteins, an important mechanism for heparin resistance. Thromb. Haemost. 1992, 67, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Kuschert, G.S.V.; Coulin, F.; Power, C.A.; Proudfoot, A.E.I.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N.C. Glycosaminoglycans Interact Selectively with Chemokines and Modulate Receptor Binding and Cellular Responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.C.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, M.A.; Guimond, S.E.; Rudd, T.R.; Fernig, D.G.; Turnbull, J.E.; Yates, E.A. The Activities of Heparan Sulfate and its Analogue Heparin are Dictated by Biosynthesis, Sequence, and Conformation. Connect. Tissue Res. 2008, 49, 140–144. [Google Scholar] [CrossRef]
- Veraldi, N.; Hughes, A.J.; Rudd, T.R.; Thomas, H.B.; Edwards, S.W.; Hadfield, L.; Skidmore, M.A.; Siligardi, G.; Cosentino, C.; Shute, J.K.; et al. Heparin derivatives for the targeting of multiple activities in the inflammatory response. Carbohydr. Polym. 2015, 117, 400–407. [Google Scholar] [CrossRef]
- Koenig, A.; Norgard-Sumnicht, K.; Linhardt, R.; Varki, A. Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J. Clin. Investig. 1998, 101, 877–889. [Google Scholar] [CrossRef]
- Lever, R.; Lo, W.T.; Faraidoun, M.; Amin, V.; Brown, R.A.; Gallagher, J.; Page, C.P. Size-fractionated heparins have differential effects on human neutrophil function in vitro. Br. J. Pharmacol. 2007, 151, 837–843. [Google Scholar] [CrossRef]
- Lever, R.; Smailbegovic, A.; Page, C.P. Locally available heparin modulates inflammatory cell recruitment in a manner independent of anticoagulant activity. Eur. J. Pharmacol. 2010, 630, 137–144. [Google Scholar] [CrossRef]
- Diamond, M.S.; Alon, R.; Parkos, C.A.; Quinn, M.; Springer, T.A. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1). J. Cell. Biol. 1995, 130, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Sans, M.; Soriano, A.; Reverter, J.C.; Anderson, D.C.; Piqué, J.M.; Panés, J. Heparin attenuates TNF-alpha induced inflammatory response through a CD11b dependent mechanism. Gut 2000, 47, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.L.; Stone, P.J.; Nugent, M.A. New Insights into the Inhibition of Human Neutrophil Elastase by Heparin. Biochemistry 2006, 45, 9104–9120. [Google Scholar] [CrossRef]
- Rao, N.V.; Argyle, B.; Xu, X.; Reynolds, P.R.; Walenga, J.M.; Prechel, M.; Prestwich, G.D.; MacArthur, R.B.; Walters, B.B.; Hoidal, J.R.; et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am. J. Physiol. Physiol. 2010, 299, C97–C110. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.A. Mechanisms of glycosaminoglycan activation of the serpins in hemostasis. J. Thromb. Haemost. 2003, 1, 1535–1549. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gowda, L.R. Alpha-1-proteinase inhibitor is a heparin binding serpin: Molecular interactions with the Lys rich cluster of helix-F domain. Biochimie 2008, 90, 749–761. [Google Scholar] [CrossRef]
- Fath, M.A.; Wu, X.; Hileman, R.E.; Linhardt, R.J.; Kashem, M.A.; Nelson, R.M.; Wright, C.D.; Abraham, W.M. Interaction of Secretory Leukocyte Protease Inhibitor with Heparin Inhibits Proteases Involved in Asthma. J. Biol. Chem. 1998, 273, 13563–13569. [Google Scholar] [CrossRef]
- Magna, M.; Pisetsky, D.S. The Role of HMGB1 in the Pathogenesis of Inflammatory and Autoimmune Diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alkazmi, L.; Habotta, O.A.; Batiha, G.E.-S. High-mobility group box 1 (HMGB1) in COVID-19: Extrapolation of dangerous liaisons. Inflammopharmacology 2022, 30, 811–820. [Google Scholar] [CrossRef]
- Li, L.; Ling, Y.; Huang, M.; Yin, T.; Gou, S.-M.; Zhan, N.-Y.; Xiong, J.-X.; Wu, H.-S.; Yang, Z.-Y.; Wang, C.-Y. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine 2015, 72, 36–42. [Google Scholar] [CrossRef]
- Griffin, K.L.; Fischer, B.M.; Kummarapurugu, A.B.; Zheng, S.; Kennedy, T.P.; Rao, N.V.; Foster, W.M.; Voynow, J.A. 2-O, 3-O-Desulfated Heparin Inhibits Neutrophil Elastase–Induced HMGB-1 Secretion and Airway Inflammation. Am. J. Respir. Cell. Mol. Biol. 2014, 50, 684–689. [Google Scholar] [CrossRef]
- Lever, R.; Rose, M.J.; McKenzie, E.A.; Page, C.P. Heparanase induces inflammatory cell recruitment in vivo by promoting adhesion to vascular endothelium. Am. J. Physiol. Cell Physiol. 2014, 306, C1184–C1190. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Ilan, N.; Naggi, A.; Casu, B. Heparanase: Structure, Biological Functions, and Inhibition by Heparin-Derived Mimetics of Heparan Sulfate. Curr. Pharm. Des. 2007, 13, 2057–2073. [Google Scholar] [CrossRef]
- Cassinelli, G.; Torri, G.; Naggi, A. Non-Anticoagulant Heparins as Heparanase Inhibitors. Adv. Exp. Med. Biol. 2020, 1221, 493–522. [Google Scholar] [CrossRef]
- Naggi, A.; Casu, B.; Perez, M.; Torri, G.; Cassinelli, G.; Penco, S.; Pisano, C.; Giannini, G.; Ishai-Michaeli, R.; Vlodavsky, I. Modulation of the Heparanase-inhibiting Activity of Heparin through Selective Desulfation, Graded N-Acetylation, and Glycol Splitting. J. Biol. Chem. 2005, 280, 12103–12113. [Google Scholar] [CrossRef] [PubMed]
- Pandya, P.H.; Wilkes, D.S. Complement System in Lung Disease. Am. J. Respir. Cell. Mol. Biol. 2014, 51, 467–473. [Google Scholar] [CrossRef]
- Edens, R.; Linhardt, R.J.; Bell, C.S.; Weiler, J.M. Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacology 1994, 27, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.M.; Lindhardt, R.J. Comparison of the activity of polyanions and polycations on the classical and alternative pathways of complement. Immunopharmacology 1989, 17, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Linhardt, R.J.; Rice, K.G.; Kim, Y.S.; Engelken, J.D.; Weiler, J.M. Homogeneous, structurally defined heparin-oligosaccharides with low anticoagulant activity inhibit the generation of the amplification pathway C3 convertase in vitro. J. Biol. Chem. 1988, 263, 13090–13096. [Google Scholar] [CrossRef]
- Weiler, J.M.; Edens, R.E.; Linhardt, R.J.; Kapelanski, D.P. Heparin and modified heparin inhibit complement activation in vivo. J. Immunol. 1992, 148, 3210–3215. [Google Scholar] [CrossRef]
- Kim, J.-S.; Okamoto, K.; Rubin, B.K. Pulmonary Function Is Negatively Correlated with Sputum Inflammatory Markers and Cough Clearability in Subjects with Cystic Fibrosis But Not Those with Chronic Bronchitis. Chest 2006, 129, 1148–1154. [Google Scholar] [CrossRef]
- Broughton-Head, V.J.; Shur, J.; Carroll, M.P.; Smith, J.R.; Shute, J.K. Unfractionated heparin reduces the elasticity of sputum from patients with cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L1240–L1249. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Stein, R.T. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed]
- Romanato, M.; Cameo, M.S.; Bertolesi, G.; Baldini, C.; Calvo, J.C.; Calvo, L. Heparan sulphate: A putative decondensing agent for human spermatozoa in vivo. Hum. Reprod. 2003, 18, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Manfredi, A.A.; Rovere-Querini, P.; D’Angelo, A.; Maugeri, N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol. Res. 2017, 123, 146–156. [Google Scholar] [CrossRef]
- Lelliott, P.M.; Momota, M.; Shibahara, T.; Lee, M.S.J.; Smith, N.I.; Ishii, K.J.; Coban, C. Heparin induces neutrophil elastase-dependent vital and lytic NET formation. Int. Immunol. 2020, 32, 359–368. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Gaston, B.; Hunt, J. Acid stress in the pathology of asthma. J. Allergy Clin. Immunol. 2004, 113, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.X.; Ostedgaard, L.S.; Hoegger, M.J.; Moninger, T.O.; Karp, P.H.; McMenimen, J.D.; Choudhury, B.; Varki, A.; Stoltz, D.A.; Welsh, M.J. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J. Clin. Investig. 2016, 126, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J. Role of Enzymes from Inflammatory Cells on Airway Submucosal Gland Secretion. Respiration 1991, 58, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. Coagulopathy of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 1358–1364. [Google Scholar] [CrossRef]
- Overton, P.M.; Toshner, M.; Mulligan, C.; Vora, P.; Nikkho, S.; de Backer, J.; Lavon, B.R.; Klok, F.A. The PVRI Innovative Drug Development Initiative Pulmonary thromboembolic events in COVID-19—A systematic literature review. Pulm. Circ. 2022, 12, e12113. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Glas, G.J.; VAN DER Sluijs, K.F.; Schultz, M.J.; Hofstra, J.H.; VAN DER Poll, T.; Levi, M. Bronchoalveolar hemostasis in lung injury and acute respiratory distress syndrome. J. Thromb. Haemost. 2013, 11, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Sadeghipour, P.; Kakavand, H.; Aghakouchakzadeh, M.; Kordzadeh-Kermani, E.; Van Tassell, B.W.; Gheymati, A.; Ariannejad, H.; Hosseini, S.H.; Jamalkhani, S.; et al. Recent Randomized Trials of Antithrombotic Therapy for Patients with COVID-19. JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 77, 1903–1921. [Google Scholar] [CrossRef]
- Schulman, S.; Sholzberg, M.; Spyropoulos, A.C.; Zarychanski, R.; Resnick, H.E.; Bradbury, C.A.; Connors, J.M.; Falanga, A.; Iba, T.; Kaatz, S.; et al. ISTH guidelines for antithrombotic treatment in COVID-19. J. Thromb. Haemost. 2022, 20, 2214–2225. [Google Scholar] [CrossRef]
- Swan, D.; Carrier, M.; Lisman, T.; Thachil, J. Heparin–Messias or Verschlimmbesserung? J. Thromb. Haemost. 2021, 19, 2373–2382. [Google Scholar] [CrossRef]
- Alroomi, M.; Alsaber, A.; Al-Bader, B.; Almutairi, F.; Malhas, H.; Pan, J.; Zhanna, K.D.; Ramadhan, M.; Al Saleh, M.; Abdullah, M.; et al. In-hospital Mortality Rates in SARS-CoV-2 Patients Treated with Enoxaparin and Heparin. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221131802. [Google Scholar] [CrossRef] [PubMed]
- Ayerbe, L.; Risco, C.; Ayis, S. The association between treatment with heparin and survival in patients with COVID-19. J. Thromb. Thrombolysis 2020, 50, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, P.; Bergamaschi, L.; D’Angelo, E.C.; Donati, F.; Giannella, M.; Tedeschi, S.; Pascale, R.; Bartoletti, M.; Tesini, G.; Biffi, M.; et al. Preliminary Experience with Low Molecular Weight Heparin Strategy in COVID-19 Patients. Front. Pharmacol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation with In-Hospital Survival among Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- White, D.; MacDonald, S.; Bull, T.; Hayman, M.; de Monteverde-Robb, R.; Sapsford, D.; Lavinio, A.; Varley, J.; Johnston, A.; Besser, M.; et al. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis 2020, 50, 287–291. [Google Scholar] [CrossRef]
- Desborough, M.J.; Doyle, A.J.; Griffiths, A.; Retter, A.; Breen, K.A.; Hunt, B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb. Res. 2020, 193, 1–4. [Google Scholar] [CrossRef]
- Riker, R.R.; May, T.L.; Fraser, G.L.; Gagnon, D.J.; Bandara, M.; Zemrak, W.R.; Seder, D.B. Heparin-induced thrombocytopenia with thrombosis in COVID-19 adult respiratory distress syndrome. Res. Pract. Thromb. Haemost. 2020, 4, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Werlein, C.; Acker, T.; Aepfelbacher, M.; Amann, K.U.; Baretton, G.; Barth, P.; Bohle, R.M.; Büttner, A.; Büttner, R.; et al. Organ manifestations of COVID-19: What have we learned so far (not only) from autopsies? Virchows Arch. 2022, 481, 139–159. [Google Scholar] [CrossRef]
- Van Haren, F.M.P.; Page, C.; Laffey, J.G.; Artigas, A.; Camprubi-Rimblas, M.; Nunes, Q.; Smith, R.; Shute, J.; Carroll, M.; Tree, J.; et al. Nebulised heparin as a treatment for COVID-19: Scientific rationale and a call for randomised evidence. Crit. Care 2020, 24, 454. [Google Scholar] [CrossRef] [PubMed]
- Conzelmann, C.; Müller, J.A.; Perkhofer, L.; Sparrer, K.M.J.; Zelikin, A.N.; Münch, J.; Kleger, A. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin. Med. 2020, 20, e218–e221. [Google Scholar] [CrossRef] [PubMed]
- van Haren, F.M.P.; van Loon, L.M.; Steins, A.; Smoot, T.L.; Sas, C.; Staas, S.; Vilaseca, A.B.; Barbera, R.A.; Vidmar, G.; Beccari, H.; et al. Inhaled nebulised unfractionated heparin for the treatment of hospitalised patients with COVID-19: A multicentre case series of 98 patients. Br. J. Clin. Pharmacol. 2022, 88, 2802–2813. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Smith, R.J.; Campbell, D.J.; Moran, J.L.; Doig, G.S.; Rechnitzer, T.; MacIsaac, C.M.; Simpson, N.; van Haren, F.M.P.; Ghosh, A.N.; et al. Nebulised heparin for patients with or at risk of acute respiratory distress syndrome: A multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Olapour, A.; Rashidi, M.; Foroush, F.J.; Akhoondzadeh, R.; Hosseini, N. Effect of Nebulized Heparin on Weaning off Intubated Patients with Acute Respiratory Distress Syndrome (ARDS) Admitted to Intensive Care Unit (ICU): A Randomized Clinical Trial. Anesthesiol. Pain Med. 2021, 11, e115938. [Google Scholar] [CrossRef] [PubMed]
- van Haren, F.M.; Richardson, A.; Yoon, H.; Artigas, A.; Laffey, J.G.; Dixon, B.; Smith, R.; Vilaseca, A.B.; Barbera, R.A.; Ismail, T.I.; et al. INHALEd nebulised unfractionated HEParin for the treatment of hospitalised patients with COVID-19 (INHALE-HEP): Protocol and statistical analysis plan for an investigator-initiated international metatrial of randomised studies. Br. J. Clin. Pharmacol. 2021, 87, 3075–3091. [Google Scholar] [CrossRef]
- van Haren, F.M.P.; Laffey, J.G.; Artigas, A.; Page, C.; Schultz, M.J.; Cosgrave, D.; McNicholas, B.; Smoot, T.L.; Nunes, Q.; Richardson, A.; et al. Can nebulised HepArin Reduce morTality and time to Extubation in patients with COVID-19 Requiring invasive ventilation Meta-Trial (CHARTER-MT): Protocol and statistical analysis plan for an investigator-initiated international meta-trial of prospective randomised clinical studies. Br. J. Clin. Pharmacol. 2022, 88, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.R.; Calpin, P.; Kernan, M.; Kelly, C.; Casey, S.; Murphy, D.; Alvarez-Iglesias, A.; Giacomini, C.; Cody, C.; Curley, G.; et al. The CHARTER-Ireland trial: Can nebulised heparin reduce acute lung injury in patients with SARS-CoV-2 requiring advanced respiratory support in Ireland: A study protocol and statistical analysis plan for a randomised control trial. Trials 2022, 23, 774. [Google Scholar] [CrossRef]
- Erelel, M.; Kaskal, M.; Akbal-Dagistan, O.; Issever, H.; Dagistanli, A.S.; Balkanci, H.; Oguz, M.S.; Qarayeva, A.; Culha, M.; Erturk, A.; et al. Early Effects of Low Molecular Weight Heparin Therapy with Soft-Mist Inhaler for COVID-19-Induced Hypoxemia: A Phase IIb Trial. Pharmaceutics 2021, 13, 1768. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Paiardi, G.; Richter, S.; Oreste, P.; Urbinati, C.; Rusnati, M.; Wade, R.C. The binding of heparin to spike glycoprotein inhibits SARS-CoV-2 infection by three mechanisms. J. Biol. Chem. 2022, 298, 101507. [Google Scholar] [CrossRef] [PubMed]
- Tree, J.A.; Turnbull, J.E.; Buttigieg, K.R.; Elmore, M.J.; Coombes, N.; Hogwood, J.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; et al. Unfractionated heparin inhibits live wild type SARS-CoV-2 cell infectivity at therapeutically relevant concentrations. Br. J. Pharmacol. 2021, 178, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Noseda, A.; Barbieri, P. Roneparstat: Development, Preclinical and Clinical Studies. Adv. Exp. Med. Biol. 2020, 1221, 523–538. [Google Scholar] [CrossRef]
- Agelidis, A.; Shukla, D. Heparanase, Heparan Sulfate and Viral Infection. Heparanase 2020, 1221, 759–770. [Google Scholar] [CrossRef]
- Xiang, J.; Lu, M.; Shi, M.; Cheng, X.; Kwakwa, K.A.; Davis, J.L.; Su, X.; Bakewell, S.J.; Zhang, Y.; Fontana, F.; et al. Heparanase Blockade as a Novel Dual-Targeting Therapy for COVID-19. J. Virol. 2022, 96, e0005722. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, L.; Immenschuh, P.; Weil, T.; Conzelmann, C.; Almeida-Hernández, Y.; Hoffmann, M.; Kempf, A.; Nehlmeier, I.; Lotke, R.; Petersen, M.; et al. Native and activated antithrombin inhibits TMPRSS2 activity and SARS-CoV-2 infection. J. Med. Virol. 2022, 95, e28124. [Google Scholar] [CrossRef]
- Kowal-Vern, A.; Dennis, A.J.; Bourdon, P.; Casey, L.E.; Latenser, B.A. Bronchoalveolar lavage and plasma Antithrombin and cytokines in inhalation and burn injury: A pilot study. Int. J. Burns Trauma 2020, 10, 255–262. [Google Scholar] [PubMed]
- Salje, H.; Kiem, C.T.; Lefrancq, N.; Courtejoie, N.; Bosetti, P.; Paireau, J.; Andronico, A.; Hozé, N.; Richet, J.; Dubost, C.-L.; et al. Estimating the burden of SARS-CoV-2 in France. Science 2020, 369, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, X.; Xiong, L.; Cai, K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: A systematic review and meta-analysis. J. Med. Virol. 2020, 92, 1449–1459. [Google Scholar] [CrossRef]
- Khalil, B.A.; Elemam, N.M.; Maghazachi, A.A. Chemokines and chemokine receptors during COVID-19 infection. Comput. Struct. Biotechnol. J. 2021, 19, 976–988. [Google Scholar] [CrossRef]
- Voiriot, G.; Dorgham, K.; Bachelot, G.; Fajac, A.; Morand-Joubert, L.; Parizot, C.; Gerotziafas, G.; Farabos, D.; Trugnan, G.; Eguether, T.; et al. Identification of bronchoalveolar and blood immune-inflammatory biomarker signature associated with poor 28-day outcome in critically ill COVID-19 patients. Sci. Rep. 2022, 12, 9502. [Google Scholar] [CrossRef]
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.; Idzikowski, E.; et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 2021, 54, 797–814.e6. [Google Scholar] [CrossRef]
- Schall, T.J.; Bacon, K.B.; Camp, R.D.; Kaspari, J.W.; Goeddel, D.V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 1993, 177, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Zaid, Y.; Doré, É.; Dubuc, I.; Archambault, A.-S.; Flamand, O.; Laviolette, M.; Flamand, N.; Boilard, E.; Flamand, L. Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19. J. Allergy Clin. Immunol. 2021, 148, 368–380.e3. [Google Scholar] [CrossRef] [PubMed]
- Dorgham, K.; Quentric, P.; Gökkaya, M.; Marot, S.; Parizot, C.; Sauce, D.; Guihot, A.; Luyt, C.-E.; Schmidt, M.; Mayaux, J.; et al. Distinct cytokine profiles associated with COVID-19 severity and mortality. J. Allergy Clin. Immunol. 2021, 147, 2098–2107. [Google Scholar] [CrossRef]
- Mehta, P.; Fajgenbaum, D.C. Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate. Curr. Opin. Rheumatol. 2021, 33, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, R.S.; Uruchurtu, A.S.S.; Siggins, M.K.; Liew, F.; Russell, C.D.; Moore, S.C.; Fairfield, C.; Carter, E.; Abrams, S.; Short, C.-E.; et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 2021, 6, eabg9873. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, L.; Fossali, T.; Frangipane, V.; Bozzini, S.; Morosini, M.; D’Amato, M.; Lettieri, S.; Urtis, M.; Di Toro, A.; Saracino, L.; et al. Broncho-alveolar inflammation in COVID-19 patients: A correlation with clinical outcome. BMC Pulm. Med. 2020, 20, 301. [Google Scholar] [CrossRef]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2022, 12, 808797. [Google Scholar] [CrossRef]
- Shi, C.; Tingting, W.; Li, J.-P.; Sullivan, M.A.; Wang, C.; Wang, H.; Deng, B.; Zhang, Y. Comprehensive Landscape of Heparin Therapy for COVID-19. Carbohydr. Polym. 2021, 254, 117232. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Wang, H.; Yang, C.; Cai, F.; Zeng, F.; Cheng, F.; Liu, Y.; Zhou, T.; Deng, B.; et al. The Potential of Low Molecular Weight Heparin to Mitigate Cytokine Storm in Severe COVID-19 Patients: A Retrospective Cohort Study. Clin. Transl. Sci. 2020, 13, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Buijsers, B.; Yanginlar, C.; de Nooijer, A.; Grondman, I.; Maciej-Hulme, M.L.; Jonkman, I.; Janssen, N.A.F.; Rother, N.; de Graaf, M.; Pickkers, P.; et al. Increased Plasma Heparanase Activity in COVID-19 Patients. Front. Immunol. 2020, 11, 575047. [Google Scholar] [CrossRef]
- Mummery, R.S.; Rider, C.C. Characterization of the Heparin-Binding Properties of IL-6. J. Immunol. 2000, 165, 5671–5679. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Gambardella, J.; Morelli, M.B.; Wang, X.; Marfella, R.; Santulli, G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? A Comprehensive Evaluation of Clinical and Basic Evidence. J. Clin. Med. 2020, 9, 1417. [Google Scholar] [CrossRef]
- Potje, S.R.; Costa, T.J.; Fraga-Silva, T.F.; Martins, R.B.; Benatti, M.N.; Almado, C.E.; de Sá, K.S.; Bonato, V.L.; Arruda, E.; Louzada-Junior, P.; et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021, 276, 119376. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, F.V.S.; Kubes, P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood 2019, 133, 2178–2185. [Google Scholar] [CrossRef]
- Ackermann, M.; Anders, H.-J.; Bilyy, R.; Bowlin, G.L.; Daniel, C.; De Lorenzo, R.; Egeblad, M.; Henneck, T.; Hidalgo, A.; Hoffmann, M.; et al. Patients with COVID-19: In the dark-NETs of neutrophils. Cell. Death Differ. 2021, 28, 3125–3139. [Google Scholar] [CrossRef]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.N.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Cremonesi, G.; Khan, A.; Mantelli, F.; Allegretti, M.; Balk, R. Neutrophil activation and neutrophil extracellular traps (NETs) in COVID-19 ARDS and immunothrombosis. Eur. J. Immunol. 2023, 53, e2250010. [Google Scholar] [CrossRef] [PubMed]
- Melero, I.; Villalba-Esparza, M.; Recalde-Zamacona, B.; Jiménez-Sánchez, D.; Teijeira, Á.; Argueta, A.; García-Tobar, L.; Álvarez-Gigli, L.; Sainz, C.; Garcia-Ros, D.; et al. Neutrophil Extracellular Traps, Local IL-8 Expression, and Cytotoxic T-Lymphocyte Response in the Lungs of Patients with Fatal COVID-19. Chest 2022, 162, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.; Huckriede, J.; Wichapong, K.; Reutelingsperger, C.; Nicolaes, G.A.F. The role of extracellular histones in COVID-19. J. Intern. Med. 2022, 293, 275–292. [Google Scholar] [CrossRef]
- Jiang, P.; Jin, Y.; Sun, M.; Jiang, X.; Yang, J.; Lv, X.; Wen, Z. Extracellular histones aggravate inflammation in ARDS by promoting alveolar macrophage pyroptosis. Mol. Immunol. 2021, 135, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wildhagen, K.C.A.A.; de Frutos, P.G.; Reutelingsperger, C.P.; Schrijver, R.; Aresté, C.; Ortega-Gomez, A.; Deckers, N.M.; Hemker, C.; Soehnlein, O.; Nicolaes, G.A.F. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014, 123, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Hogwood, J.; Pitchford, S.; Mulloy, B.; Page, C.; Gray, E. Heparin and non-anticoagulant heparin attenuate histone-induced inflammatory responses in whole blood. PLoS ONE 2020, 15, e0233644. [Google Scholar] [CrossRef] [PubMed]
- Cardelli, M.; Pierpaoli, E.; Marchegiani, F.; Marcheselli, F.; Piacenza, F.; Giacconi, R.; Recchioni, R.; Casoli, T.; Stripoli, P.; Provinciali, M.; et al. Biomarkers of cell damage, neutrophil and macrophage activation associated with in-hospital mortality in geriatric COVID-19 patients. Immun. Ageing 2022, 19, 65. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.F.; Asakura, T.; Meinig, S.L.; Torres-Castillo, J.L.; Hagan, R.S.; Gabillard-Lefort, C.; Murphy, M.P.; Thorne, L.B.; Borczuk, A.; Reeves, E.P.; et al. Protease-anti-protease compartmentalization in SARS-CoV-2 ARDS: Therapeutic implications. Ebiomedicine 2022, 77, 103894. [Google Scholar] [CrossRef]
- Ceccato, A.; Camprubí-Rimblas, M.; Campaña-Duel, E.; Areny-Balagueró, A.; Morales-Quinteros, L.; Artigas, A. Anticoagulant Treatment in Severe ARDS COVID-19 Patients. J. Clin. Med. 2022, 11, 2695. [Google Scholar] [CrossRef]
- Schultz, M.J.; Determann, R.M.; Royakkers, A.A.N.M.; Wolthuis, E.K.; Korevaar, J.C.; Levi, M.M. Bronchoalveolar Activation of Coagulation and Inhibition of Fibrinolysis during Ventilator-Associated Lung Injury. Crit. Care Res. Pract. 2012, 2012, 961784. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Santamaria, J.D.; Campbell, D.J. A phase 1 trial of nebulised heparin in acute lung injury. Crit. Care 2008, 12, R64. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Schultz, M.J.; Hofstra, J.J.; Campbell, D.J.; Santamaria, J.D. Nebulized heparin reduces levels of pulmonary coagulation activation in acute lung injury. Crit. Care 2010, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.; Schultz, M.J.; Smith, R.; Fink, J.B.; Santamaria, J.D.; Campbell, D.J. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: A randomized controlled trial. Crit. Care 2010, 14, R180. [Google Scholar] [CrossRef]
- Glas, G.J.; Neto, A.S.; Horn, J.; Cochran, A.; Dixon, B.; Elamin, E.M.; Faraklas, I.; Dissanaike, S.; Miller, A.C.; Schultz, M.J. Nebulized heparin for patients under mechanical ventilation: An individual patient data meta-analysis. Ann. Intensiv. Care 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Bandeshe, H.; Boots, R.; Dulhunty, J.; Dunlop, R.; Holley, A.; Jarrett, P.; Gomersall, C.D.; Lipman, J.; Lo, T.; O’Donoghue, S.; et al. Is inhaled prophylactic heparin useful for prevention and management of pneumonia in ventilated ICU patients? J. Crit. Care 2016, 35, 231–239. [Google Scholar] [CrossRef]
- Yamaya, M.; Kikuchi, A.; Sugawara, M.; Nishimura, H. Anti-inflammatory effects of medications used for viral infection-induced respiratory diseases. Respir. Investig. 2023, 61, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Nakagome, K.; Nagata, M. Innate Immune Responses by Respiratory Viruses, Including Rhinovirus, During Asthma Exacerbation. Front. Immunol. 2022, 13, 865973. [Google Scholar] [CrossRef]
- Guo-Parke, H.; Linden, D.; Weldon, S.; Kidney, J.C.; Taggart, C.C. Deciphering Respiratory-Virus-Associated Interferon Signaling in COPD Airway Epithelium. Medicina 2022, 58, 121. [Google Scholar] [CrossRef] [PubMed]
- Coultas, J.A.; Cafferkey, J.; Mallia, P.; Johnston, S.L. Experimental Antiviral Therapeutic Studies for Human Rhinovirus Infections. J. Exp. Pharmacol. 2021, 13, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.-M.; Garratt, L.W.; Gill, E.E.; Lee, A.H.Y.; Agudelo-Romero, P.; Sutanto, E.N.; Iosifidis, T.; Rosenow, T.; Turvey, S.E.; Lassmann, T.; et al. Rhinovirus Infection Drives Complex Host Airway Molecular Responses in Children with Cystic Fibrosis. Front. Immunol. 2020, 11, 1327. [Google Scholar] [CrossRef]
- Price, A.S.; Kennedy, J.L. T-helper 2 mechanisms involved in human rhinovirus infections and asthma. Ann. Allergy Asthma Immunol. 2022, 129, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosales, N.; Kasi, A.S.; McCracken, C.E.; Silva, G.L.; Starks, M.; Stecenko, A.; Guglani, L. Impact of viral respiratory infections on pulmonary exacerbations in children with cystic fibrosis. Pediatr. Pulmonol. 2022, 58, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.; Harper-Owen, R.; Bhowmik, A.; Jeffries, D.; Wedzicha, J. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 2000, 16, 677–683. [Google Scholar] [CrossRef]
- Khan, A.G.; Pichler, J.; Rosemann, A.; Blaas, D. Human Rhinovirus Type 54 Infection via Heparan Sulfate Is Less Efficient and Strictly Dependent on Low Endosomal pH. J. Virol. 2007, 81, 4625–4632. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Shute, J.K.; Puxeddu, E.; Calzetta, L. Therapeutic use of heparin and derivatives beyond anticoagulation in patients with bronchial asthma or COPD. Curr. Opin. Pharmacol. 2018, 40, 39–45. [Google Scholar] [CrossRef]
- Shastri, M.D.; Peterson, G.M.; Stewart, N.; Sohal, S.S.; Patel, R.P. Non-anticoagulant derivatives of heparin for the management of asthma: Distant dream or close reality? Expert. Opin. Investig. Drugs 2013, 23, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Kianian, F.; Kadkhodaee, M.; Sadeghipour, H.R.; Karimian, S.M.; Seifi, B. An overview of high-mobility group box 1, a potent pro-inflammatory cytokine in asthma. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef] [PubMed]
- Brims, F.J.H.; Chauhan, A.J.; Higgins, B.; Shute, J.K. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax 2009, 64, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- de Boer, J.D.; Majoor, C.J.; van’t Veer, C.; Bel, E.H.; van der Poll, T. Asthma and coagulation. Blood 2012, 119, 3236–3244. [Google Scholar] [CrossRef]
- Shastri, M.D.; Peterson, G.M.; Patel, R.P. Redefining Approaches to Asthma: Bridging the Gap Between Heparin and Its Anti-inflammatory Activities. Curr. Allergy Asthma Rep. 2017, 17, 70. [Google Scholar] [CrossRef]
- Mousavi, S.; Moradi, M.; Khorshidahmad, T.; Motamedi, M. Anti-Inflammatory Effects of Heparin and Its Derivatives: A Systematic Review. Adv. Pharmacol. Sci. 2015, 2015, 507151. [Google Scholar] [CrossRef] [PubMed]
- Monagle, K.; Ryan, A.; Hepponstall, M.; Mertyn, E.; Ignjatovic, V.; Newall, F. Inhalational use of antithrombotics in humans: Review of the literature. Thromb. Res. 2015, 136, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gonzalez, B.J.; Danta, I. Prevention of Exercise-induced Bronchoconstriction by Inhaled Low-molecular-weight Heparin. Am. J. Respir. Crit. Care Med. 1999, 160, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Fal, A.M.; Kraus-Filarska, M.; Miecielica, J.; Małolepszy, J. Mechanizmy działania heparyny małoczasteczkowej nebulizowanej u chorych na astme oskrzelowa [Mechanisms of action of nebulized low molecular weights heparin in patients with bronchial asthma]. Pol. Merkur. Lekarski. 2003, 15, 543–545, In Polish (abstract in English). [Google Scholar] [PubMed]
- Bendstrup, K.; Jensen, J. Inhaled heparin is effective in exacerbations of asthma. Respir. Med. 2000, 94, 174–175. [Google Scholar] [CrossRef]
- Motamed, H.; Verki, M.M.; Nematollahi, A.V.; Hesam, S. Evaluation of efficacy of nebulized low molecular weight heparin as an adjunctive extra treatment for acute mild-moderate asthma attack; a randomized clinical trial study. Pulm. Pharmacol. Ther. 2021, 68, 102037. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Song, Q.; Cheng, W.; Chen, P. The role of HMGB1/RAGE/TLR4 signaling pathways in cigarette smoke-induced inflammation in chronic obstructive pulmonary disease. Immunity Inflamm. Dis. 2022, 10, e711. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, X.; Zhong, X. Role of RAGE and its ligand HMGB1 in the development of COPD. Postgrad. Med. 2022, 134, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Ashoor, T.M.; Hasseb, A.M.; Esmat, I.M. Nebulized heparin and salbutamol versus salbutamol alone in acute exacerbations of chronic obstructive pulmonary disease requiring mechanical ventilation: A double-blind randomized controlled trial. Korean J. Anesthesiol. 2020, 73, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Brackenborough, K.; Ellis, H.; Flight, W.G. Respiratory Viruses and Cystic Fibrosis. Semin. Respir. Crit. Care Med. 2023, 44, 196–208. [Google Scholar] [CrossRef]
- Billard, L.; Le Berre, R.; Pilorgé, L.; Payan, C.; Héry-Arnaud, G.; Vallet, S. Viruses in cystic fibrosis patients’ airways. Crit. Rev. Microbiol. 2017, 43, 690–708. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Bomberger, J.M. Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract. Front. Immunol. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed]
- van Ewijk, B.E.; Wolfs, T.F.W.; Aerts, P.C.; Van Kessel, K.P.M.; Fleer, A.; Kimpen, J.L.L.; Van Der Ent, C.K. RSV Mediates Pseudomonas aeruginosa Binding to Cystic Fibrosis and Normal Epithelial Cells. Pediatr. Res. 2007, 61, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Zappala, C.; Chandan, S.; George, N.; Faoagali, J.; Boots, R.J. The antimicrobial effect of heparin on common respiratory pathogens. Crit. Care Resusc. 2007, 9, 157–160. [Google Scholar] [PubMed]
- Lorè, N.I.; Veraldi, N.; Riva, C.; Sipione, B.; Spagnuolo, L.; De Fino, I.; Melessike, M.; Calzi, E.; Bragonzi, A.; Naggi, A.; et al. Synthesized Heparan Sulfate Competitors Attenuate Pseudomonas aeruginosa Lung Infection. Int. J. Mol. Sci. 2018, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Lozano-Iturbe, V.; Girón, R.M.; Vazquez-Espinosa, E.; Rodriguez, D.; Merayo-Lloves, J.; Vazquez, F.; Quirós, L.M.; García, B. Glycosaminoglycans are differentially involved in bacterial binding to healthy and cystic fibrosis lung cells. J. Cyst. Fibros. 2019, 18, e19–e25. [Google Scholar] [CrossRef]
- Sharma, L.; Wu, J.; Patel, V.; Sitapara, R.; Rao, N.V.; Kennedy, T.P.; Mantell, L.L. Partially-desulfated heparin improves survival in Pseudomonas pneumonia by enhancing bacterial clearance and ameliorating lung injury. J. Immunotoxicol. 2014, 11, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gauthier, A.G.; Kennedy, T.P.; Wang, H.; Velagapudi, U.K.; Talele, T.T.; Lin, M.; Wu, J.; Daley, L.; Yang, X.; et al. 2-O, 3-O desulfated heparin (ODSH) increases bacterial clearance and attenuates lung injury in cystic fibrosis by restoring HMGB1-compromised macrophage function. Mol. Med. 2021, 27, 79. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.; Weldon, S.; Elborn, S.; Downey, D.G.; Taggart, C. The Effect of CFTR Modulators on Airway Infection in Cystic Fibrosis. Int. J. Mol. Sci. 2022, 23, 3513. [Google Scholar] [CrossRef]
- Zhou-Suckow, Z.; Duerr, J.; Hagner, M.; Agrawal, R.; Mall, M.A. Airway mucus, inflammation and remodeling: Emerging links in the pathogenesis of chronic lung diseases. Cell. Tissue Res. 2017, 367, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Mall, M.A. Mucus obstruction and inflammation in early cystic fibrosis lung disease: Emerging role of the IL-1 signaling pathway. Pediatr. Pulmonol. 2019, 54, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Reihill, J.A.; Moreland, M.; Jarvis, G.E.; McDowell, A.; Einarsson, G.G.; Elborn, J.S.; Martin, S.L. Bacterial proteases and haemostasis dysregulation in the CF lung. J. Cyst. Fibros. 2017, 16, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Zheng, S.; Kummarapurugu, A.B. Glycosaminoglycans as Multifunctional Anti-Elastase and Anti-Inflammatory Drugs in Cystic Fibrosis Lung Disease. Front. Pharmacol. 2020, 11, 1011. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, R.D.; Elkin, M.; Rapraeger, A.C.; Ilan, N.; Vlodavsky, I. Heparanase regulation of cancer, autophagy and inflammation: New mechanisms and targets for therapy. FEBS J. 2017, 284, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.T.; Fitch, J.; Jaramillo, L.; Shrestha, C.L.; Robledo-Avila, F.; Zhang, S.; Palacios, S.; Woodley, F.; Hayes, D.; Partida-Sanchez, S.; et al. Whole-blood transcriptomic responses to lumacaftor/ivacaftor therapy in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 245–254. [Google Scholar] [CrossRef]
- Alekseeva, A.; Mazzini, G.; Giannini, G.; Naggi, A. Structural features of heparanase-inhibiting non-anticoagulant heparin derivative Roneparstat. Carbohydr. Polym. 2017, 156, 470–480. [Google Scholar] [CrossRef]
- Marcos, V.; Zhou-Suckow, Z.; Yildirim, A.; Bohla, A.; Hector, A.; Vitkov, L.; Krautgartner, W.D.; Stoiber, W.; Griese, M.; Eickelberg, O.; et al. Free DNA in Cystic Fibrosis Airway Fluids Correlates with Airflow Obstruction. Mediat. Inflamm. 2015, 2015, 408935. [Google Scholar] [CrossRef] [PubMed]
- Serisier, D.J.; Carroll, M.P.; Shute, J.K.; Young, S.A. Macrorheology of cystic fibrosis, chronic obstructive pulmonary disease & normal sputum. Respir. Res. 2009, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Davies, J. Clinical trial research in focus: Ensuring new cystic fibrosis drugs fulfil their potential. Lancet Respir. Med. 2017, 5, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.K.; Forsyth, C.; Hockey, P.M.; Carroll, M.P. Anti-inflammatory effects of nebulised heparin in cystic fibrosis [abstract]. Am. J. Respir. Crit. Care Med. 2000, 161, A75. [Google Scholar]
- Bendstrup, K.E.; Chambers, C.B.; Jensen, J.I.; Newhouse, M.T. Lung Deposition and Clearance of Inhaled 99mTc-Heparin in Healthy Volunteers. Am. J. Respir. Crit. Care Med. 1999, 160, 1653–1658. [Google Scholar] [CrossRef]
- Bendstrup, K.; Gram, J.B.; Jensen, J.; Dancey, D.; Tullis, E.; Heslegrave, R.; Thornley, K.; Hanly, P. Effect of inhaled heparin on lung function and coagulation in healthy volunteers. Eur. Respir. J. 2002, 19, 606–610. [Google Scholar] [CrossRef]
- Ledson, M.; Gallagher, M.; Hart, C.; Walshaw, M. Nebulized heparin in Burkholderia cepacia colonized adult cystic fibrosis patients. Eur. Respir. J. 2001, 17, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Serisier, D.J.; Shute, J.K.; Hockey, P.M.; Higgins, B.; Conway, J.; Carroll, M.P. Inhaled heparin in cystic fibrosis. Eur. Respir. J. 2006, 27, 354–358. [Google Scholar] [CrossRef]
- Sagel, S.D.; Chmiel, J.F.; Konstan, M.W. Sputum Biomarkers of Inflammation in Cystic Fibrosis Lung Disease. Proc. Am. Thorac. Soc. 2007, 4, 406–417. [Google Scholar] [CrossRef]
- Ordoñez, C.L.; Kartashov, A.I.; Wohl, M.E.B. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J. Pediatr. 2004, 145, 689–692. [Google Scholar] [CrossRef]
- Shur, J.; Nevell, T.G.; Ewen, R.J.; Price, R.; Smith, A.; Barbu, E.; Conway, J.H.; Carroll, M.P.; Shute, J.K.; Smith, J.R. Cospray-dried unfractionated heparin with L-leucine as a dry powder inhaler mucolytic for cystic fibrosis therapy. J. Pharm. Sci. 2008, 97, 4857–4868. [Google Scholar] [CrossRef] [PubMed]
- Shur, J.; Nevell, T.G.; Shute, J.K.; Smith, J.R. The Spray Drying of Unfractionated Heparin: Optimization of the Operating Parameters. Drug. Dev. Ind. Pharm. 2008, 34, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Yildiz-Pekoz, A.; Ozsoy, Y. Inhaled Heparin: Therapeutic Efficacy and Recent Formulations. J. Aerosol Med. Pulm. Drug. Deliv. 2017, 30, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Baglin, T.; Barrowcliffe, T.W.; Cohen, A.; Greaves, M. The British Committee for Standards in Haematology Guidelines on the use and monitoring of heparin. Br. J. Haematol. 2006, 133, 19–34. [Google Scholar] [CrossRef]
- Mulloy, B.; Gray, E.; Barrowcliffe, T.W. Characterization of unfractionated heparin: Comparison of materials from the last 50 years. Thromb. Haemost. 2000, 84, 1052–1056. [Google Scholar] [PubMed]
- Jaques, L. INTRAPULMONARY HEPARIN A New Procedure for Anticoagulant Therapy. Lancet 1976, 308, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Markart, P.; Nass, R.; Ruppert, C.; Hundack, L.; Wygrecka, M.; Korfei, M.; Boedeker, R.H.; Staehler, G.; Kroll, H.; Scheuch, G.; et al. Safety and tolerability of inhaled heparin in idiopathic pulmonary fibrosis. J. Aerosol Med. Pulm. Drug. Deliv. 2010, 23, 161–172. [Google Scholar] [CrossRef]
- McCarthy, S.D.; González, H.E.; Higgins, B.D. Future Trends in Nebulized Therapies for Pulmonary Disease. J. Pers. Med. 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shute, J.K. Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease. Pharmaceuticals 2023, 16, 584. https://doi.org/10.3390/ph16040584
Shute JK. Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease. Pharmaceuticals. 2023; 16(4):584. https://doi.org/10.3390/ph16040584
Chicago/Turabian StyleShute, Janis Kay. 2023. "Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease" Pharmaceuticals 16, no. 4: 584. https://doi.org/10.3390/ph16040584
APA StyleShute, J. K. (2023). Heparin, Low Molecular Weight Heparin, and Non-Anticoagulant Derivatives for the Treatment of Inflammatory Lung Disease. Pharmaceuticals, 16(4), 584. https://doi.org/10.3390/ph16040584



