Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies
Abstract
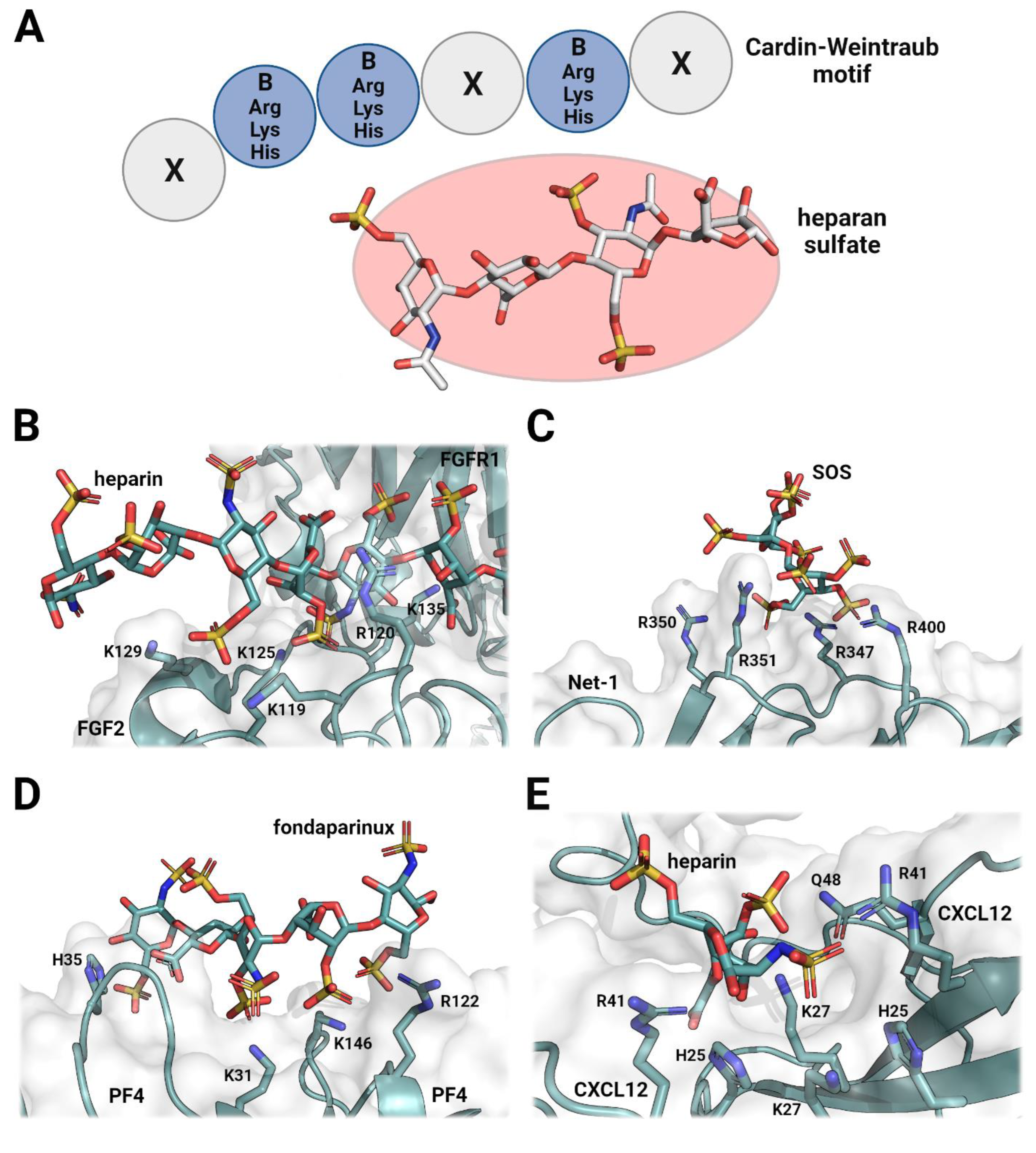
1. Introduction
2. The Role of Heparan Sulfates in Cellular Signaling and Organization
3. Heparin Mimetics Influence Extracellular Matrix Organization

Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stickens, D.; Zak, B.M.; Rougier, N.; Esko, J.D.; Werb, Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development 2005, 132, 5055–5068. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wei, G.; Shi, Z.; Dryer, L.; Esko, J.D.; Wells, D.E.; Matzuk, M.M. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev. Biol. 2000, 224, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Gubbiotti, M.A. Extracellular matrix: The driving force of mammalian diseases. Matrix Biol. 2018, 71–72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, M.C.Z.; Hughes, A.; Rudd, T.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kett, W.C.; Severin, I.C.; Agyekum, I.; Duan, J.; Amster, I.J.; Proudfoot, A.E.I.; Coombe, D.R.; Woods, R.J. The interaction of heparin tetrasaccharides with chemokine CCL5 is modulated by sulfation pattern and PH. J. Biol. Chem. 2015, 290, 15421–15436. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Esko, J.D. Demystifying heparan sulfate-protein interactions. Annu. Rev. Biochem. 2014, 83, 129–157. [Google Scholar] [CrossRef]
- Maaroufi, R.M.; Jozefowicz, M.; Tapon-Bretaudière, J.; Fischer, A.M. Mechanism of thrombin inhibition by antithrombin and heparin cofactor II in the presence of heparin. Biomaterials 1997, 18, 203–211. [Google Scholar] [CrossRef]
- Raitman, I.; Huang, M.L.; Williams, S.A.; Friedman, B.; Godula, K.; Schwarzbauer, J.E. Heparin-fibronectin interactions in the development of extracellular matrix insolubility. Matrix Biol. 2018, 67, 107–122. [Google Scholar] [CrossRef]
- Vallet, S.D.; Berthollier, C.; Ricard-Blum, S. The glycosaminoglycan interactome 2.0. Am. J. Physiol.-Cell Physiol. 2022, 322, C1271–C1278. [Google Scholar] [CrossRef]
- Litov, L.; Petkov, P.; Rangelov, M.; Ilieva, N.; Lilkova, E.; Todorova, N.; Krachmarova, E.; Malinova, K.; Gospodinov, A.; Hristova, R.; et al. Molecular mechanism of the anti-inflammatory action of heparin. Int. J. Mol. Sci. 2021, 22, 10730. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Doherty, G.G.; See, N.W.; Gandhi, N.S.; Ferro, V. From cancer to COVID-19: A perspective on targeting heparan sulfate-protein interactions. Chem. Rec. 2021, 21, 3087–3101. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Aliter, K.F.; Kar, S.; Mottamal, M. Sulfonated nonsaccharide heparin mimetics are potent and noncompetitive inhibitors of human neutrophil elastase. ACS Omega 2021, 6, 12699–12710. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Coombe, D. Heparin mimetics: Their therapeutic potential. Pharmaceuticals 2017, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Afosah, D.K.; Al-Horani, R.A.; Sankaranarayanan, N.V.; Desai, U.R. Potent, selective, allosteric inhibition of human plasmin by sulfa non-saccharide glycosaminoglycan mimetics. J. Med. Chem. 2017, 60, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Nahain, A.A.; Ignjatovic, V.; Monagle, P.; Tsanaktsidis, J.; Ferro, V. Heparin mimetics with anticoagulant activity. Med. Res. Rev. 2018, 38, 1582–1613. [Google Scholar] [CrossRef]
- Gockel, L.M.; Heyes, M.; Li, H.; Al Nahain, A.; Gorzelanny, C.; Schlesinger, M.; Holdenrieder, S.; Li, J.P.; Ferro, V.; Bendas, G. Inhibition of tumor-host cell interactions using synthetic heparin mimetics. ACS Appl. Mater. Interfaces 2021, 13, 7080–7093. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, S.P.; Li, J.Y.; Chen, P.C.; Lee, Y.Z.; Li, K.M.; Zarivach, R.; Sun, Y.J.; Sue, S.C. Integrative Model to coordinate the oligomerization and aggregation mechanisms of CCL5. J. Mol. Biol. 2020, 432, 1143–1157. [Google Scholar] [CrossRef]
- Heide, F.; Legare, S.; To, V.; Gupta, M.; Gabir, H.; Imhof, T.; Moya-Torres, A.; McDougall, M.; Meier, M.; Koch, M.; et al. Heparins mediate the multimer assembly of secreted noggin. Protein Sci. 2022, 31, 1–13. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Xu, Y.; Brandt, S.; Mandelkow, M.; Raschke, R.; Strobel, U.; Delcea, M.; Zhou, W.; Liu, J.; Greinacher, A. Characterization of the interaction between platelet factor 4 and homogeneous synthetic low molecular weight heparins. J. Thromb. Haemost. 2020, 18, 390–398. [Google Scholar] [CrossRef]
- Cardin, A.D.; Weintraub, H.J. Molecular modeling of protein-glycosaminoglycan interactions. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1989, 9, 21–32. [Google Scholar] [CrossRef]
- Friedrich, U.; Blom, A.M.; Dahlbäck, B.; Villoutreix, B.O. Structural and energetic characteristics of the heparin-binding site in antithrombotic Protein, C. J. Biol. Chem. 2001, 276, 24122–24128. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Beraud, M.; Raynal, N.; Farndale, R.W.; Ruggiero, F. Structural requirements for heparin/heparan sulfate binding to type V collagen. J. Biol. Chem. 2006, 281, 25195–25204. [Google Scholar] [CrossRef]
- Billings, P.C.; Yang, E.; Mundy, C.; Pacifici, M. Domains with highest heparan sulfate–binding affinity reside at opposite ends in BMP2/4 versus BMP5/6/7: Implications for function. J. Biol. Chem. 2018, 293, 14371–14383. [Google Scholar] [CrossRef]
- Sandoval, D.R.; Gomez Toledo, A.; Painter, C.D.; Tota, E.M.; Sheikh, M.O.; West, A.M.V.; Frank, M.M.; Wells, L.; Xu, D.; Bicknell, R.; et al. Proteomics-based screening of the endothelial heparan sulfate interactome reveals that C-Type lectin 14a (CLEC14A) is a heparin-binding protein. J. Biol. Chem. 2020, 295, 2804–2821. [Google Scholar] [CrossRef]
- Schlessinger, J.; Plotnikov, A.N.; Ibrahimi, O.A.; Eliseenkova, A.V.; Yeh, B.K.; Yayon, A.; Linhardt, R.J.; Mohammadi, M. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in fgfr binding and dimerization. Mol. Cell 2000, 6, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.C.; Schwab, R.A.; El Omari, K.; Bishop, B.; Iverson, E.J.; Malinauskas, T.; Dubey, R.; Qian, M.; Covey, D.F.; Gilbert, R.J.C.; et al. Hedgehog-interacting protein is a multimodal antagonist of hedgehog signalling. Nat. Commun. 2021, 12, 7171. [Google Scholar] [CrossRef] [PubMed]
- Paine-Saunders, S.; Viviano, B.L.; Economides, A.N.; Saunders, S. Heparan sulfate proteoglycans retain noggin at the cell surface. A potential mechanism for shaping bone morphogenetic protein gradients. J. Biol. Chem. 2002, 277, 2089–2096. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.C.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef]
- Kogut, M.M.; Marcisz, M.; Samsonov, S.A. Modeling glycosaminoglycan–protein complexes. Curr. Opin. Struct. Biol. 2022, 73. [Google Scholar] [CrossRef] [PubMed]
- Zagris, N. Extracellular matrix in development of the early embryo. Micron 2001, 32, 427–438. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Meier, M.; Gupta, M.; Akgül, S.; McDougall, M.; Imhof, T.; Nikodemus, D.; Reuten, R.; Moya-Torres, A.; To, V.; Ferens, F.; et al. The dynamic nature of Netrin-1 and the structural basis for glycosaminoglycan fragment-induced filament formation. Nat. Commun. 2023, 14, 1226. [Google Scholar] [CrossRef]
- Cai, Z.; Yarovoi, S.V.; Zhu, Z.; Rauova, L.; Hayes, V.; Lebedeva, T.; Liu, Q.; Poncz, M.; Arepally, G.; Cines, D.B.; et al. Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat. Commun. 2015, 6, 8277. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.W.; Cho, Y.; Sachpatzidis, A.; Fan, C.; Hodsdon, M.E.; Lolis, E. Structural and functional basis of CXCL12 (Stromal Cell-Derived Factor-1α) binding to heparin. J. Biol. Chem. 2007, 282, 10018–10027. [Google Scholar] [CrossRef] [PubMed]
- Crijns, H.; Adyns, L.; Ganseman, E.; Cambier, S.; Vandekerckhove, E.; Pörtner, N.; Vanbrabant, L.; Struyf, S.; Gerlza, T.; Kungl, A.; et al. Affinity and specificity for binding to glycosaminoglycans can be tuned by adapting peptide length and sequence. Int. J. Mol. Sci. 2022, 23, 447. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Proudfoot, A.E.I. The biological relevance of chemokine–proteoglycan interactions. Biochem. Soc. Trans. 2006, 34, 422–426. [Google Scholar] [CrossRef]
- Jägers, C.; Roelink, H. Association of sonic hedgehog with the extracellular matrix requires its zinc-coordination center. BMC Mol. Cell Biol. 2021, 22, 1–18. [Google Scholar] [CrossRef]
- Nesterenko, A.M.; Orlov, E.E.; Ermakova, G.V.; Ivanov, I.A.; Semenyuk, P.I.; Orlov, V.N.; Martynova, N.Y.; Zaraisky, A.G. Affinity of the heparin binding motif of noggin1 to heparan sulfate and its visualization in the embryonic tissues. Biochem. Biophys. Res. Commun. 2015, 468, 331–336. [Google Scholar] [CrossRef]
- Graf, F.; Horn, P.; Ho, A.D.; Boutros, M.; Maercker, C. The extracellular matrix proteins type I collagen, type III collagen, fibronectin, and laminin 421 stimulate migration of cancer cells. FASEB J. 2021, 35, e21692. [Google Scholar] [CrossRef]
- Artinger, M.; Gerken, O.J.; Purvanov, V.; Legler, D.F. Distinct fates of chemokine and surrogate molecule gradients: Consequences for CCR7-guided dendritic cell migration. Front. Immunol. 2022, 13, 913366. [Google Scholar] [CrossRef] [PubMed]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Segerer, S.; Johnson, Z.; Rek, A.; Baltus, T.; von Hundelshausen, P.; Kungl, A.J.; Proudfoot, A.E.I.; Weber, C.; Nelson, P.J. The basic residue cluster 55KKWVR59 in CCL5 is required for in vivo biologic function. Mol. Immunol. 2009, 46, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.A.; McGovern, A.; Baldwin, A.K.; Baldock, C.; Kielty, C.M. Fibrillin-1 mutations causing weill-marchesani syndrome and acromicric and geleophysic dysplasias disrupt heparan sulfate interactions. PLoS ONE 2012, 7, e48634. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Namba, K.; Mutai, H.; Usui, S.; Miyanaga, Y.; Kaneko, H.; Matsunaga, T. A mutation in the heparin-binding site of noggin as a novel mechanism of proximal symphalangism and conductive hearing loss. Biochem. Biophys. Res. Commun. 2014, 447, 496–502. [Google Scholar] [CrossRef]
- Schwaninger, R.; Rentsch, C.A.; Wetterwald, A.; Van Der Horst, G.; Van Bezooijen, R.L.; Van Der Pluijm, G.; Löwik, C.W.G.M.; Ackermann, K.; Pyerin, W.; Hamdy, F.C.; et al. Lack of noggin expression by cancer cells is a determinant of the osteoblast response in bone metastases. Am. J. Pathol. 2007, 170, 160–175. [Google Scholar] [CrossRef]
- Feeley, B.T.; Krenek, L.; Liu, N.; Hsu, W.K.; Gamradt, S.C.; Schwarz, E.M.; Huard, J.; Lieberman, J.R. Overexpression of noggin inhibits bmp-mediated growth of osteolytic prostate cancer lesions. Bone 2006, 38, 154–166. [Google Scholar] [CrossRef]
- Ouahoud, S.; Hardwick, J.C.H.; Hawinkels, L.J.A.C. Extracellular bmp antagonists, multifaceted orchestrators in the tumor and its microenvironment. Int. J. Mol. Sci. 2020, 21, 3888. [Google Scholar] [CrossRef]
- Dyer, D.P.; Salanga, C.L.; Volkman, B.F.; Kawamura, T.; Handel, T.M. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 2015, 26, 312–326. [Google Scholar] [CrossRef]
- Fujita, M.; Davari, P.; Takada, Y.K.; Takada, Y. Stromal cell-derived factor-1 (CXCL12) activates integrins by direct binding to an allosteric ligand-binding site (site 2) of integrins without CXCR4. Biochem. J. 2018, 475, 723–732. [Google Scholar] [CrossRef]
- Mayfosh, A.J.; Nguyen, T.K.; Hulett, M.D. The heparanase regulatory network in health and disease. Int. J. Mol. Sci. 2021, 22, 11096. [Google Scholar] [CrossRef]
- Bhattacharya, U.; Gutter-Kapon, L.; Kan, T.; Boyango, I.; Barash, U.; Yang, S.M.; Liu, J.J.; Gross-Cohen, M.; Sanderson, R.D.; Shaked, Y.; et al. Heparanase and chemotherapy synergize to drive macrophage activation and enhance tumor growth. Cancer Res. 2020, 80, 57–68. [Google Scholar] [CrossRef]
- Barash, U.; Zohar, Y.; Wildbaum, G.; Beider, K.; Nagler, A.; Karin, N.; Ilan, N.; Vlodavsky, I. heparanase enhances myeloma progression via CXCL10 downregulation. Leukemia 2014, 28, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Jayatilleke, K.M.; Hulett, M.D. Heparanase and the hallmarks of cancer. J. Transl. Med. 2020, 18, 453. [Google Scholar] [CrossRef] [PubMed]
- Coombe, D.R.; Gandhi, N.S. Heparanase: A challenging cancer drug target. Front. Oncol. 2019, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Rauch, U. Extracellular matrix components associated with remodeling processes in brain. Cell. Mol. Life Sci. 2004, 61, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Méneret, A.; Franz, E.A.; Trouillard, O.; Oliver, T.C.; Zagar, Y.; Robertson, S.P.; Welniarz, Q.; Gardner, R.J.M.; Gallea, C.; Srour, M.; et al. Mutations in the Netrin-1 gene cause congenital mirror movements. J. Clin. Investig. 2017, 127, 3923–3936. [Google Scholar] [CrossRef] [PubMed]
- Loka, R.S.; Song, Z.; Sletten, E.T.; Kayal, Y.; Vlodavsky, I.; Zhang, K.; Nguyen, H.M. Heparan sulfate mimicking glycopolymer prevents pancreatic β cell destruction and suppresses inflammatory cytokine expression in islets under the challenge of upregulated heparanase. ACS Chem. Biol. 2022, 17, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Feng, J.; Rao, Y.; Xu, Z.; Zu, J.; Wang, H.; Zhang, Z.; Chen, H. Artificial extracellular matrix composed of heparin-mimicking polymers for efficient anticoagulation and promotion of endothelial cell proliferation. ACS Appl. Mater. Interfaces 2022, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Abdelfadiel, E.I.; Afosah, D.K.; Morla, S.; Sistla, J.C.; Mohammed, B.; Martin, E.J.; Sakagami, M.; Brophy, D.F.; Desai, U.R. A synthetic heparin mimetic that allosterically inhibits factor XIa and reduces thrombosis in vivo without enhanced risk of bleeding. J. Thromb. Haemost. 2019, 17, 2110–2122. [Google Scholar] [CrossRef] [PubMed]
- Kiyan, Y.; Tkachuk, S.; Kurselis, K.; Shushakova, N.; Stahl, K.; Dawodu, D.; Kiyan, R.; Chichkov, B.; Haller, H. Heparanase-2 protects from LPS-mediated endothelial injury by inhibiting TLR4 signalling. Sci. Rep. 2019, 9, 13591. [Google Scholar] [CrossRef]
- Shamdani, S.; Chantepie, S.; Flageollet, C.; Henni-Chebra, N.; Jouan, Y.; Eymard, F.; Hay, E.; Cohen-Solal, M.; Papy-Garcia, D.; Chevalier, X.; et al. Heparan sulfate functions are altered in the osteoarthritic cartilage. Arthritis Res. Ther. 2020, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.; Besse, B.; Doubre, H.; Charles-Nelson, A.; Aquilanti, S.; Izadifar, A.; Azarian, R.; Monnet, I.; Lamour, C.; Descourt, R.; et al. Anti-tumour effect of low molecular weight heparin in localised lung cancer: A phase III clinical trial. Eur. Respir. J. 2018, 52, 1801220. [Google Scholar] [CrossRef]
- Cai, Z.; Teng, L.; Zhou, J.; Yan, Y.; Zhang, Y.; Lv, G.; Chen, J. Design and synthesis of a native heparin disaccharide grafted poly-2-aminoethyl methacrylate glycopolymer for inhibition of melanoma cell metastasis. Int. J. Biol. Macromol. 2019, 126, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Yin, S.; Xu, S.; Ran, G.; Deng, M.; Mei, L.; Tang, X.; Rao, J.; Li, M.; Zhang, Z.; et al. Low molecular weight heparin-coated and dendrimer-based core-shell nanoplatform with enhanced immune activation and multiple anti-metastatic effects for melanoma treatment. Theranostics 2019, 9, 337–354. [Google Scholar] [CrossRef]
- Feeley, B.T.; Liu, N.Q.; Conduah, A.H.; Krenek, L.; Roth, K.; Dougall, W.C.; Huard, J.; Dubinett, S.; Lieberman, J.R. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and rank: Fc administration. J. Bone Miner. Res. 2006, 21, 1571–1580. [Google Scholar] [CrossRef]
- Nagata, K.; Kumasaka, K.; Browne, K.D.; Li, S.; St-Pierre, J.; Cognetti, J.; Marks, J.; Johnson, V.E.; Smith, D.H.; Pascual, J.L. Unfractionated heparin after tbi reduces in vivo cerebrovascular inflammation, brain edema and accelerates cognitive recovery. J. Trauma Acute Care Surg. 2016, 81, 1088–1094. [Google Scholar] [CrossRef]
- Al Faruque, H.; Kang, J.H.; Hwang, S.R.; Sung, S.; Alam, M.M.; Sa, K.H.; Nam, E.J.; Byun, Y.R.; Kang, Y.M. Stepwise inhibition of T cell recruitment at post-capillary venules by orally active desulfated heparins in inflammatory arthritis. PLoS ONE 2017, 12, e0176110. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.; Rai, B.; Dombrowski, C.; Lee, J.L.J.; Lim, Z.X.H.; Bramono, D.S.; Ling, L.; Bell, T.; Hinkley, S.; Nathan, S.S.; et al. Affinity-selected heparan sulfate for bone repair. Biomaterials 2013, 34, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heide, F.; Koch, M.; Stetefeld, J. Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies. Pharmaceuticals 2023, 16, 471. https://doi.org/10.3390/ph16030471
Heide F, Koch M, Stetefeld J. Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies. Pharmaceuticals. 2023; 16(3):471. https://doi.org/10.3390/ph16030471
Chicago/Turabian StyleHeide, Fabian, Manuel Koch, and Jörg Stetefeld. 2023. "Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies" Pharmaceuticals 16, no. 3: 471. https://doi.org/10.3390/ph16030471
APA StyleHeide, F., Koch, M., & Stetefeld, J. (2023). Heparin Mimetics and Their Impact on Extracellular Matrix Protein Assemblies. Pharmaceuticals, 16(3), 471. https://doi.org/10.3390/ph16030471




