The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review
Abstract
1. Introduction
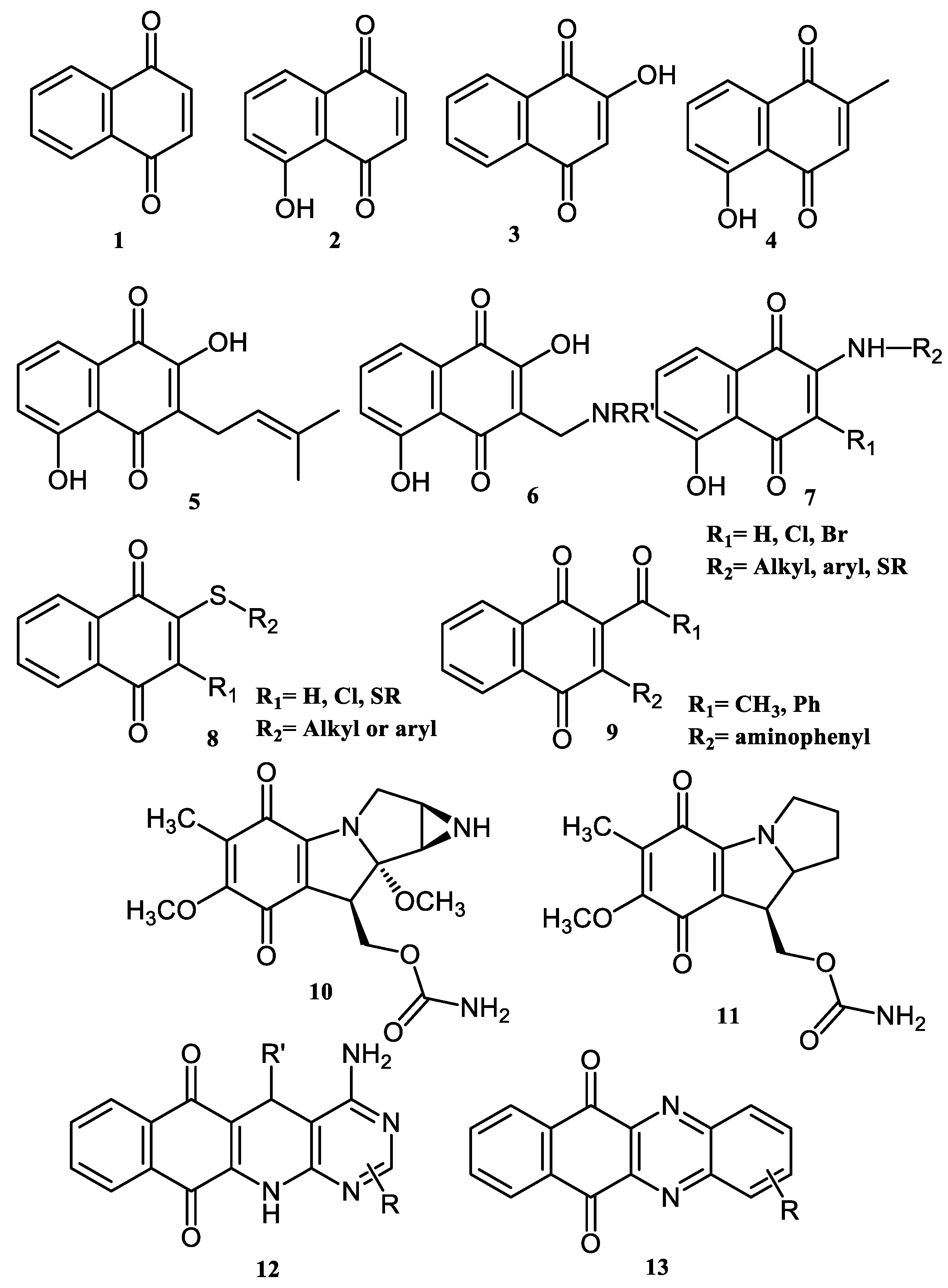
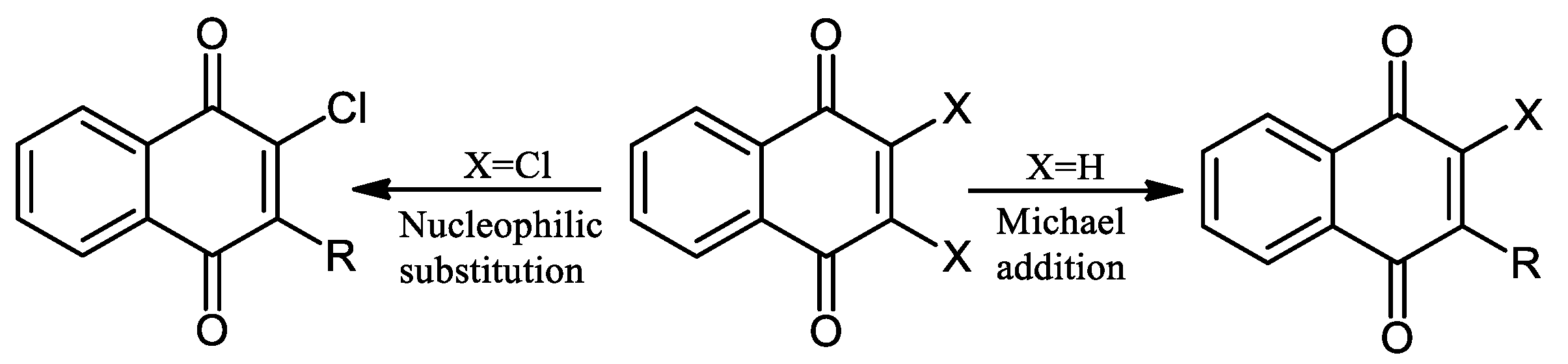
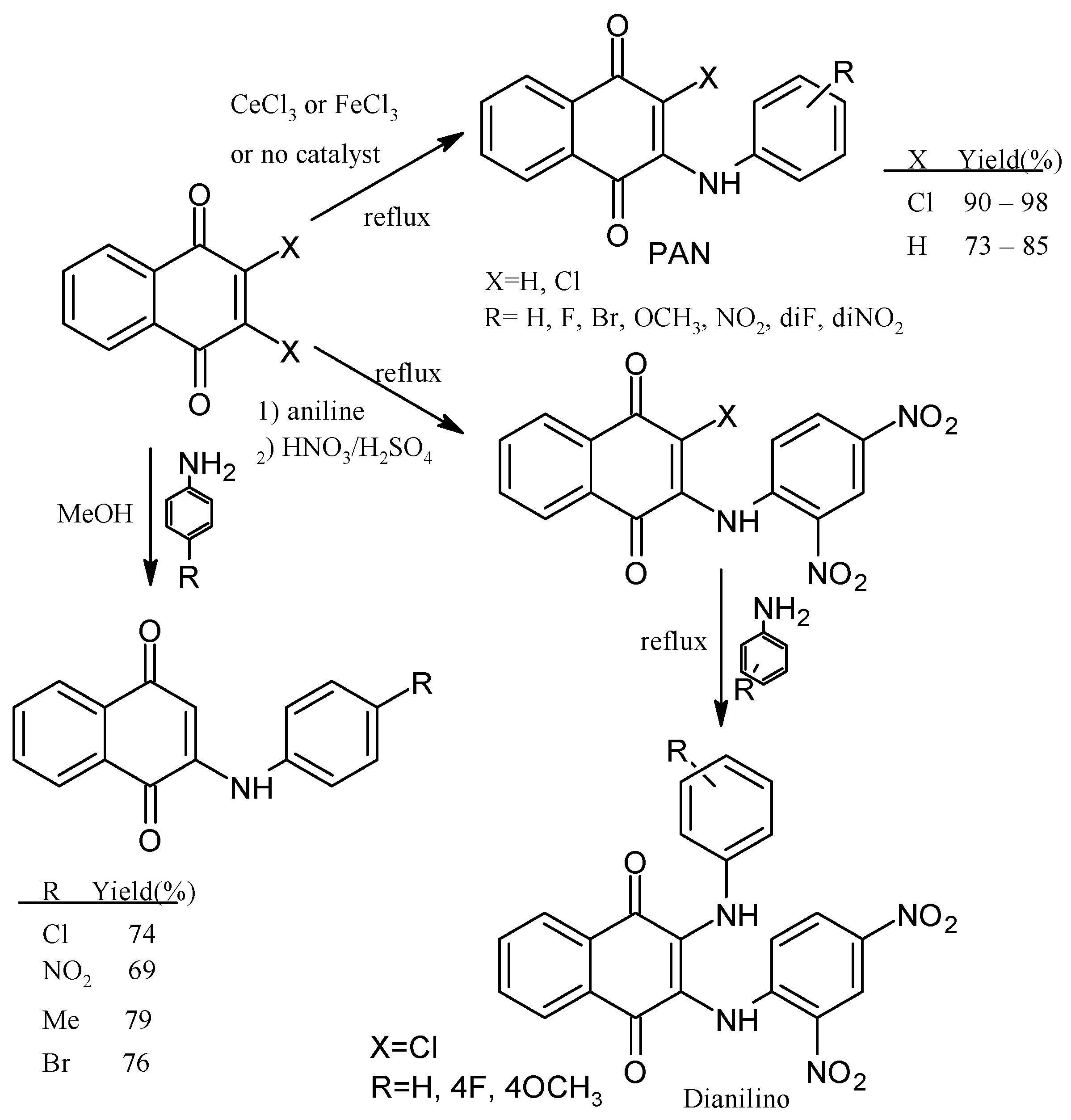
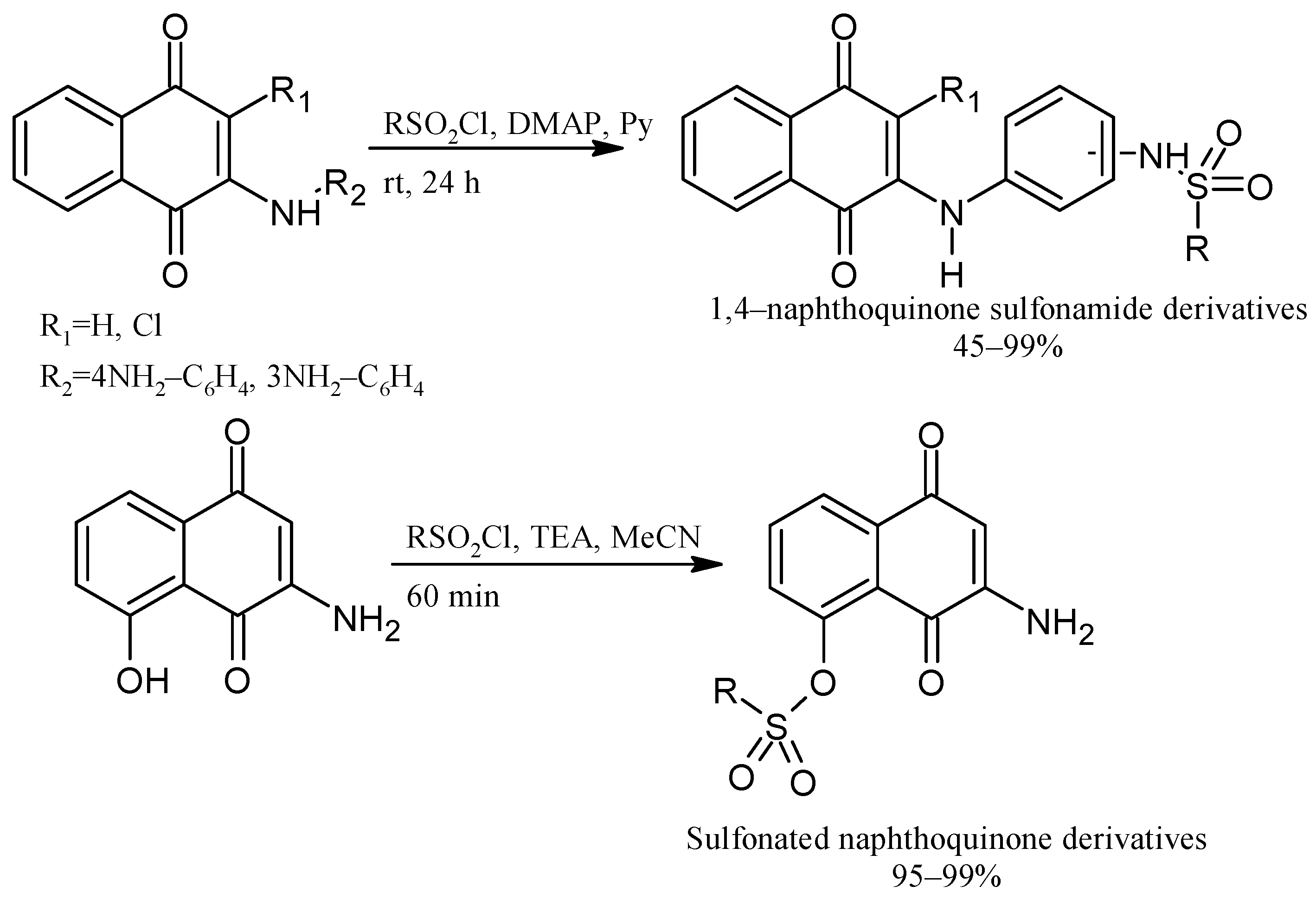
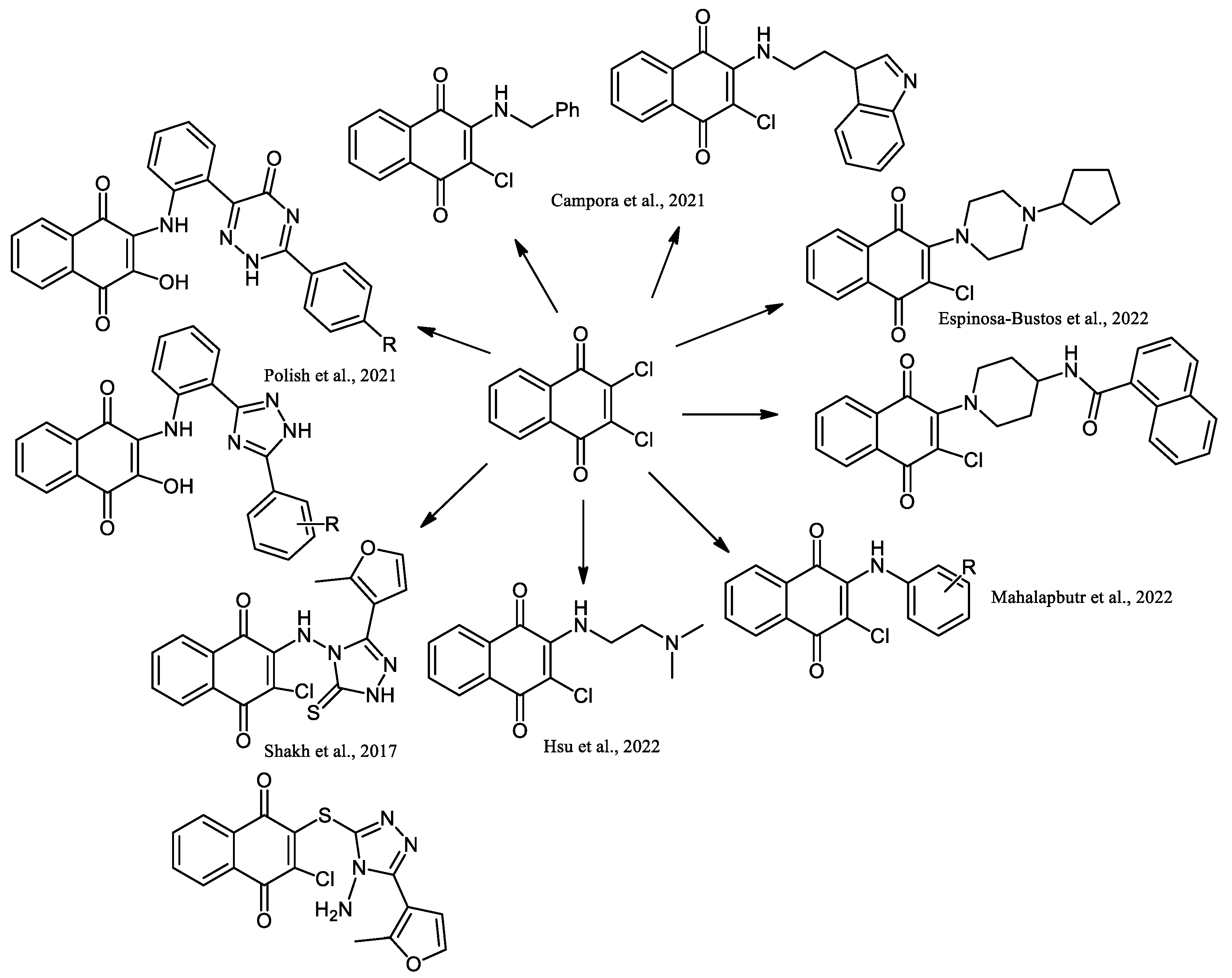
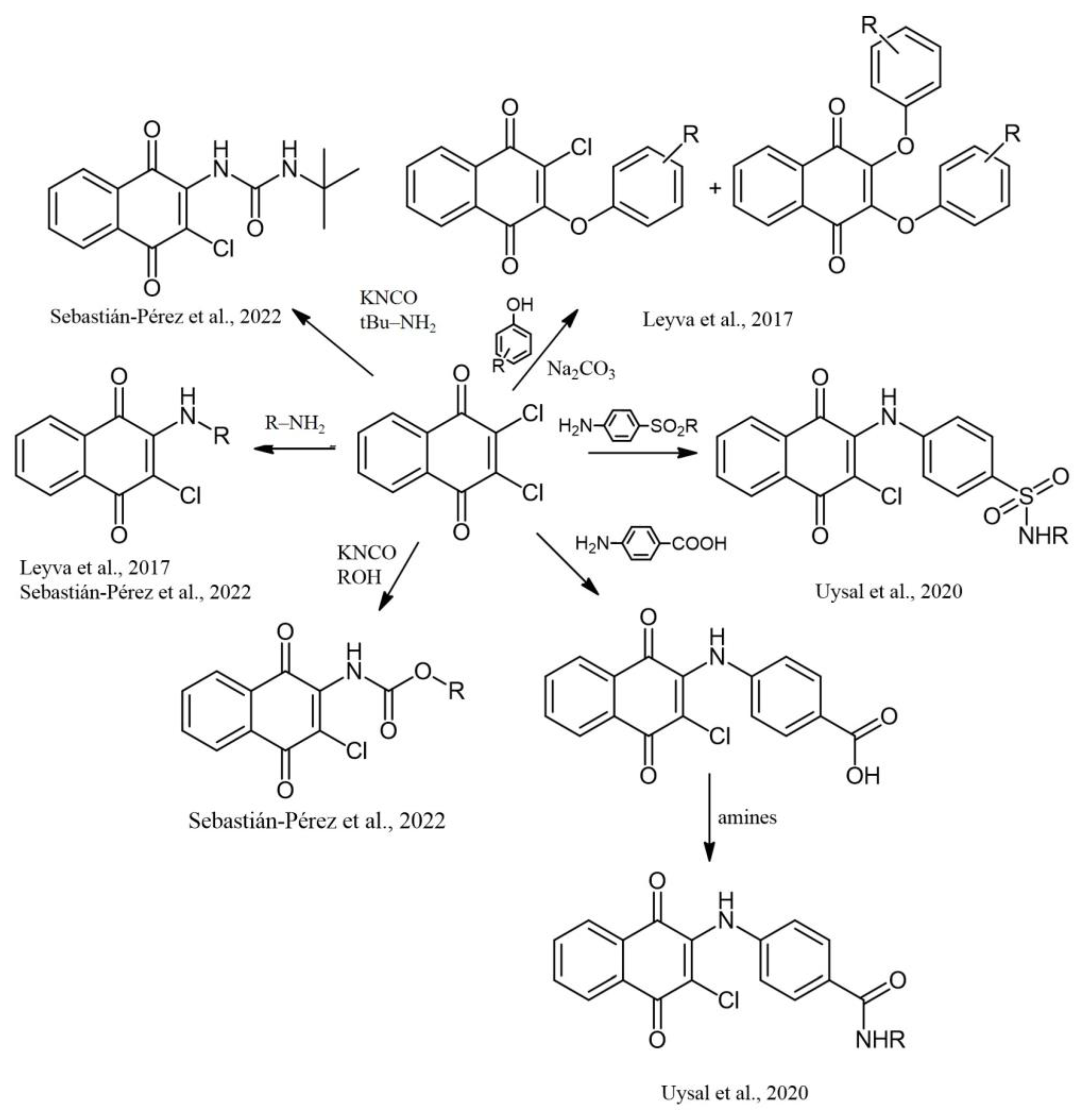
2. Chemical Modification of NQs Structures with Nitrogen Groups
3. Pharmaceutical Relevance and Evidence on the Antitumoral and Antibacterial Effects of NQs in Preclinical Assays
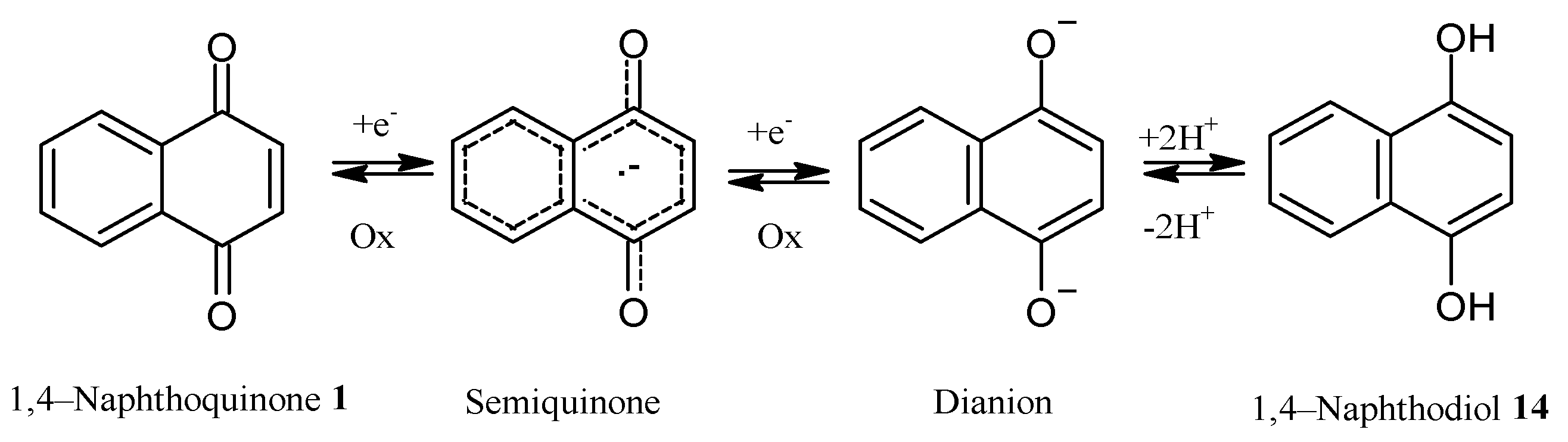
3.1. Antitumoral and Antimicrobial Mechanisms of NQs
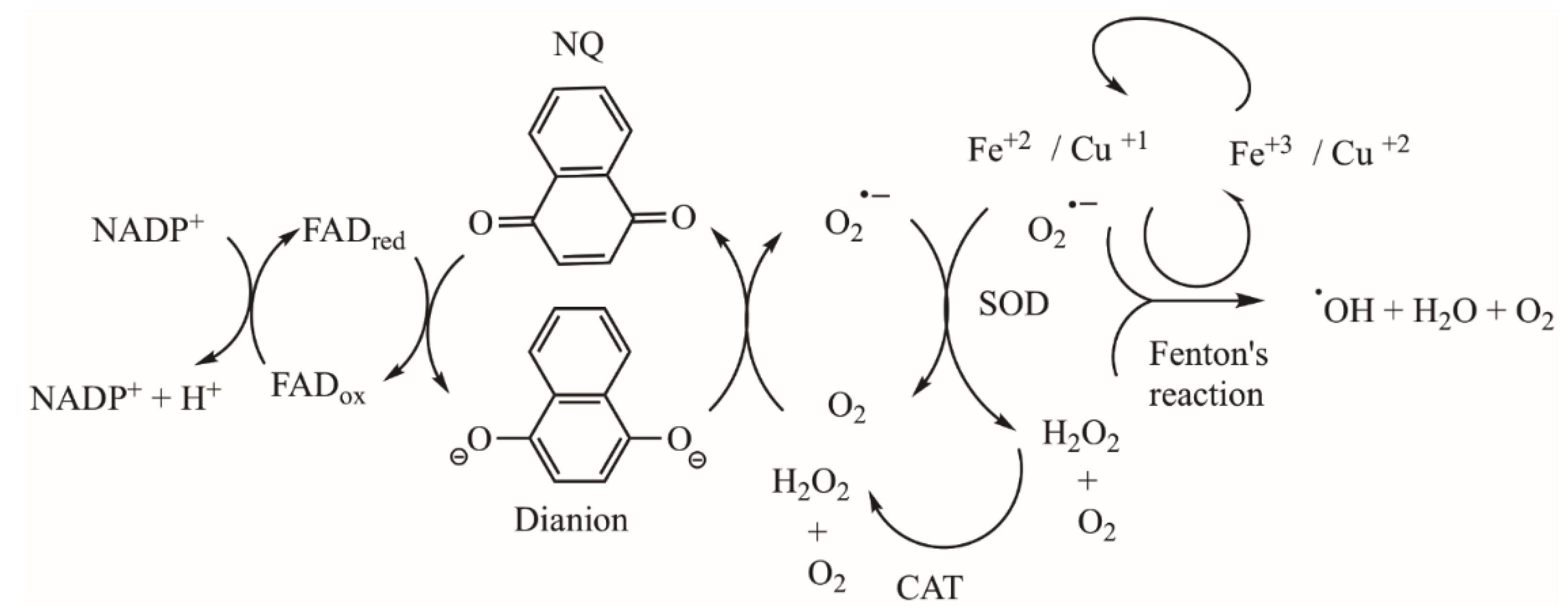
REDOX Imbalance (ROS), Alteration of Mitochondrial Respiration, and Other Mechanisms Induced by NQs in Tumor Cells
3.2. NQs Alter the ROS Levels and Membrane Integrity and Can Chelate Metals Ions in Bacteria Cells
3.3. Computational Studies in the Search for NQ Mechanisms against Cancer Cells and Bacteria
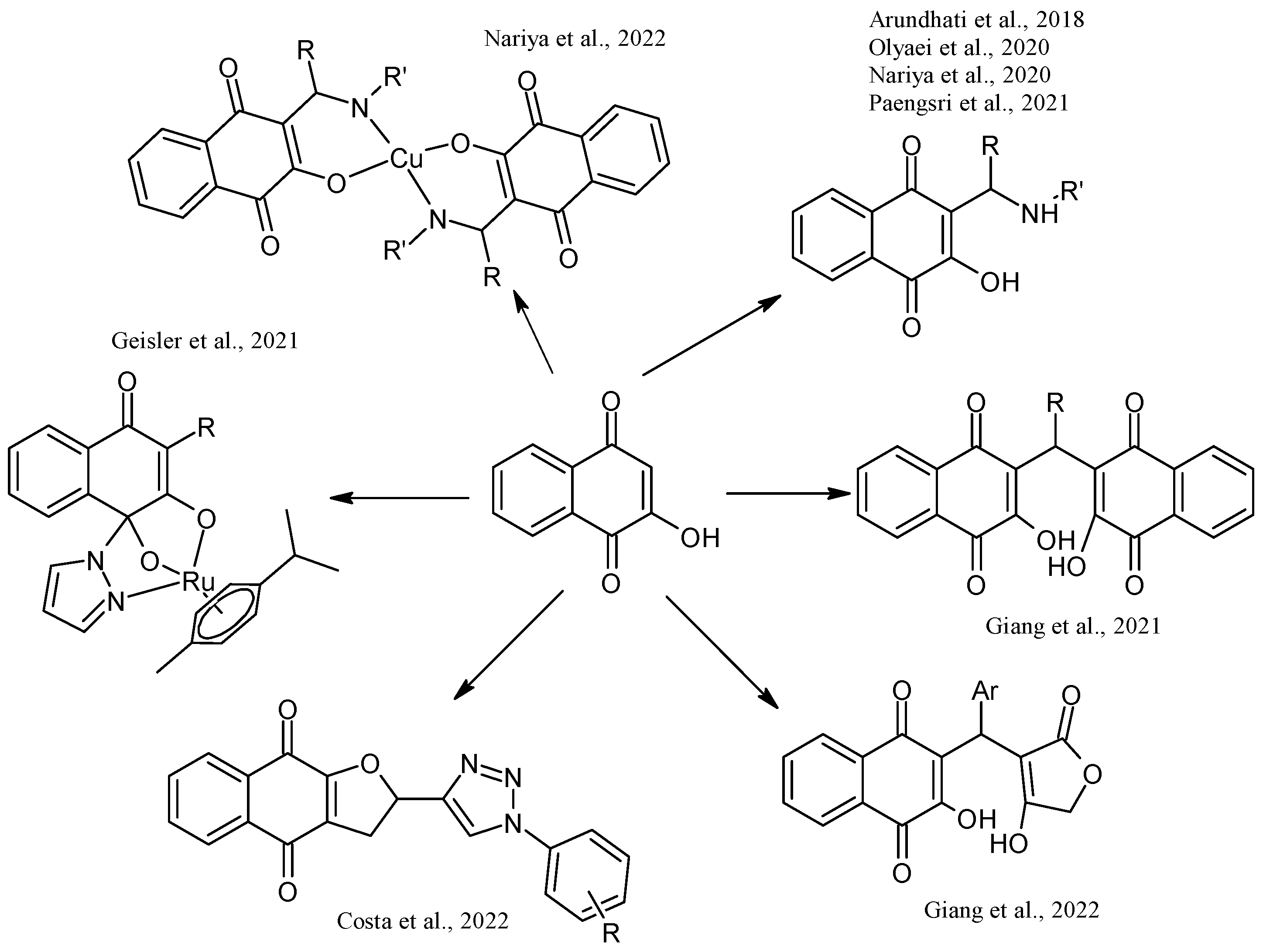
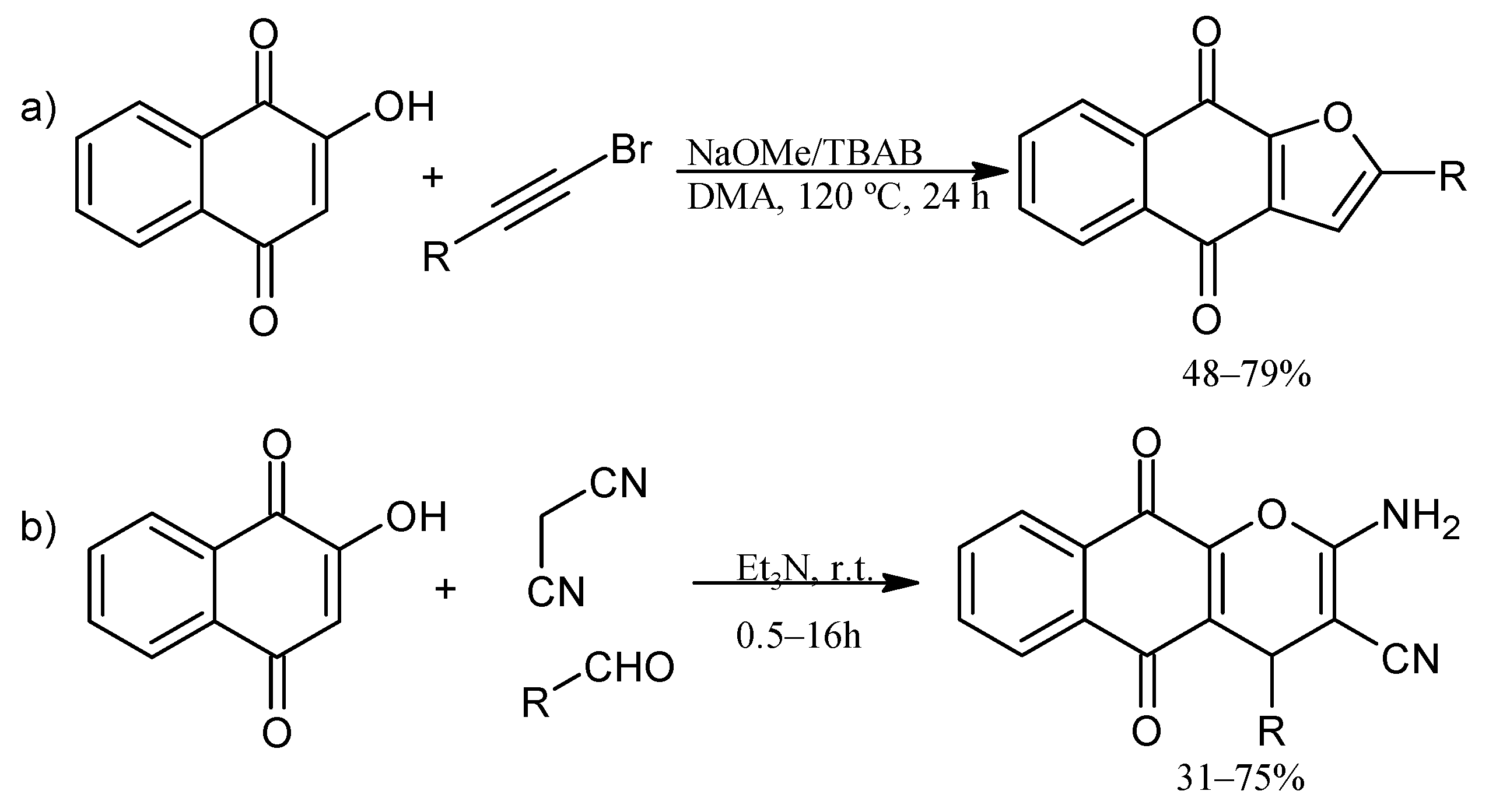
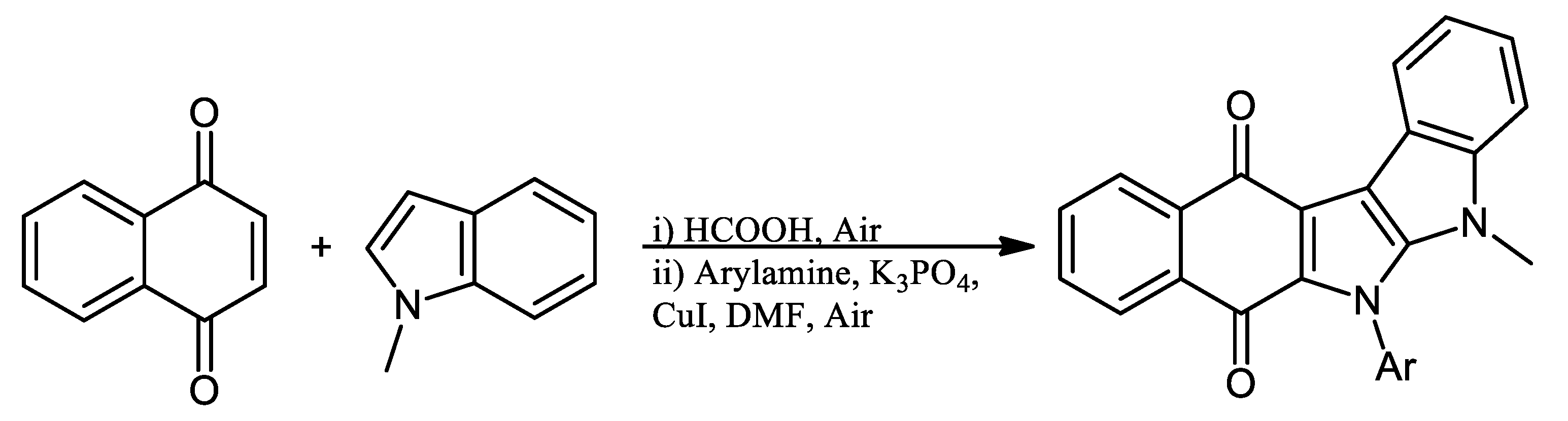
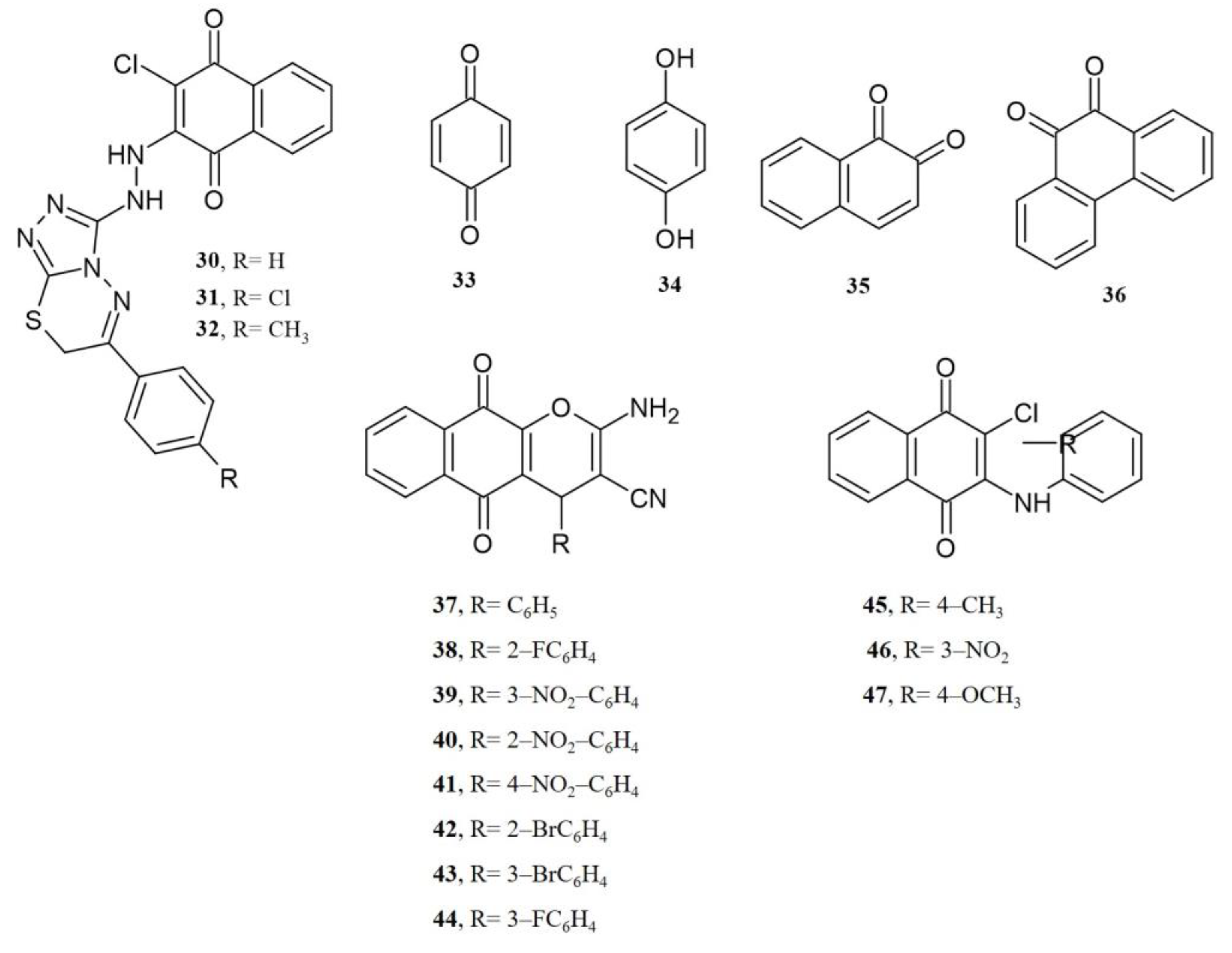
4. Biological Evaluations of Nitrogen NQ Derivatives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, S.F. Natural Products and Their Mimics as Targets of Opportunity for Discovery. J. Org. Chem. 2017, 82, 10757–10794. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Golan, Y.; Micek, S.T.; Shorr, A.F.; Restrepo, M.I. Appraising Contemporary Strategies to Combat Multidrug Resistant Gram-Negative Bacterial Infections-Proceedings and Data from the Gram-Negative Resistance Summit. Clin. Infect. Dis. 2011, 53, S33–S55. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.M.; Lanteri, C.A.; Oneil, M.T.; Johnson, J.D.; Gribble, G.W.; Trumpower, B.L. Design of Anti-Parasitic and Anti-Fungal Hydroxy-Naphthoquinones That Are Less Susceptible to Drug Resistance. Mol. Biochem. Parasitol. 2011, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Schindler, B.D.; Jacinto, P.L.; Patel, D.; Bains, K.; Seo, S.M.; Kaatz, G.W. Expression of Multidrug Resistance Efflux Pump Genes in Clinical and Environmental Isolates of Staphylococcus Aureus. Int. J. Antimicrob. Agents 2012, 40, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kempker, R.R.; Rabin, A.S.; Nikolaishvili, K.; Kalandadze, I.; Gogishvili, S.; Blumberg, H.M.; Vashakidze, S. Additional Drug Resistance in Mycobacterium Tuberculosis Isolates from Resected Cavities among Patients with Multidrug-Resistant or Extensively Drug-Resistant Pulmonary Tuberculosis. Clin. Infect. Dis. 2012, 54, e51–e54. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. In Dietary Phytochemicals and Microbes; Nature Publishing Group: Berlin, Germany, 2012; pp. 1–32. ISBN 9789400739260. [Google Scholar]
- López, L.; Leyva, E.; García de la Cruz, R. Las Naftoquinonas: Más Que Pigmentos Naturales. Rev. Mex. Ciencias Farm. 2011, 42, 311–346. [Google Scholar]
- López López, L.I.; Nery Flores, S.D.; Silva Belmares, S.Y.; Sáenz Galindo, A. Naphthoquinones: Biological Properties and Synthesis of Lawsone and Derivatives—A Structured Review. Vitae 2014, 21, 248–258. [Google Scholar] [CrossRef]
- Aminin, D.; Polonik, S. 1,4-Naphthoquinones: Some Biological Properties and Application. Chem. Pharm. Bull. 2020, 68, 46–57. [Google Scholar] [CrossRef]
- Leyva, E.; Loredo-Carrillo, S.E.; López, L.I. Catalytic, Ultrasonic, and Microwave-Assisted Synthesis of Naphthoquinone Derivatives by Intermolecular and Intramolecular N-Arylation Reactions. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Microwaves in Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 231–264. ISBN 9780128198483. [Google Scholar]
- Leyva, E.; Loredo-Carrillo, S.; López, L.; Escobedo-Avellaneda, E.; Navarro-Tovar, G. Importancia Química y Biológica de Naftoquinonas. Revisión Bibliográfica. Afinidad. J. Chem. Eng. Theor. Appl. Chem. 2017, 74, 36–50. [Google Scholar]
- Loredo Carrillo, S.E.; Leyva Ramos, E. Naftoquinonas: Metodologías de Síntesis y Propiedades Fotofísicas Obtención y Estudio de Derivados de Naftoquinonas; Publicia: Gibraltar, Gibraltar, 2015. [Google Scholar]
- Sebastián-Pérez, V.; Martínez de Iturrate, P.; Nácher-Vázquez, M.; Nóvoa, L.; Pérez, C.; Campillo, N.E.; Gil, C.; Rivas, L. Naphthoquinone as a New Chemical Scaffold for Leishmanicidal Inhibitors of Leishmania GSK-3. Biomedicines 2022, 10, 1136. [Google Scholar] [CrossRef]
- Kumagai, Y.; Shinkai, Y.; Miura, T.; Cho, A.K. The Chemical Biology of Naphthoquinones and Its Environmental Implications. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.M.G.; Sayeed, S.A.; Ashraf, S.; Naz, S.; Siddiqi, R.; Ali, R.; Ahmed Mesaik, M. A New Method for the Isolation and Purification of Lawsone from Lawsonia Inermis and Its ROS Inhibitory Activity. Pak. J. Bot. 2013, 45, 1431–1436. [Google Scholar]
- Yusuf, M.; Ahmad, A.; Shahid, M.; Khan, M.I.; Khan, S.A.; Manzoor, N.; Mohammad, F. Assessment of Colorimetric, Antibacterial and Antifungal Properties of Woollen Yarn Dyed with the Extract of the Leaves of Henna (Lawsonia inermis). J. Clean. Prod. 2012, 27, 42–50. [Google Scholar] [CrossRef]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on Medicinal Properties of Plumbagin and Its Analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Kiran Aithal, B.; Sunil Kumar, M.R.; Nageshwar Rao, B.; Udupa, N.; Satish Rao, B.S. Juglone, a Naphthoquinone from Walnut, Exerts Cytotoxic and Genotoxic Effects against Cultured Melanoma Tumor Cells. Cell Biol. Int. 2009, 33, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Mollazehi, F.; Shaterian, H.R. Design and Characterization of Dendrimer of MNPs as a Novel, Heterogeneous and Reusable Nanomagnetic Organometallic Catalyst for One-Pot Synthesis of Hydroxyl Naphthalene-1,4-Dione Derivatives under Solvent-Free Conditions. Appl. Organomet. Chem. 2018, 32, e4183. [Google Scholar] [CrossRef]
- Olyaei, A.; Sadeghpour, M.; Khalaj, M. Mannich Bases Derived from Lawsone and Their Metal Complexes: Synthetic Strategies and Biological Properties. RSC Adv. 2020, 10, 30265–30281. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Yang, J.; He, S.; Shi, Z.C.; Zhang, X.M.; Wang, J.Y. B(C6F5)3-Catalyzed C-C Coupling of 1,4-Naphthoquinones with the C-3 Position of Indole Derivatives in Water. ACS Omega 2019, 4, 21567–21577. [Google Scholar] [CrossRef]
- Gomes, M.P.; Correia, E.M.; Gomes, M.W.L.; dos Santos, C.C.C.; Barros, C.S.; de Abreu, F.V.; Antunes, L.S.; Ferreira, V.F.; Gonçalves, M.C.; de Resende, G.O.; et al. Antibacterial Profile in Vitro and in Vivo of New 1,4-Naphthoquinones Tethered to 1,2,3-1H-Triazoles Against the Planktonic Growth of Streptococcus Mutans. J. Braz. Chem. Soc. 2022, 33, 1028–1040. [Google Scholar] [CrossRef]
- López-López, L.I.; Rivera-Ávalos, E.; Villarreal-Reyes, C.; Martínez-Gutiérrez, F.; De Loera, D. Synthesis and Antimicrobial Evaluation of Amino Acid Naphthoquinone Derivatives as Potential Antibacterial Agents. Chemotherapy 2022, 67, 102–109. [Google Scholar] [CrossRef]
- Carneiro, P.F.; Pinto, M.D.C.F.R.; Coelho, T.S.; Cavalcanti, B.C.; Pessoa, C.; De Simone, C.A.; Nunes, I.K.C.; De Oliveira, N.M.; De Almeida, R.G.; Pinto, A.V.; et al. Quinonoid and Phenazine Compounds: Synthesis and Evaluation against H 37Rv, Rifampicin and Isoniazid-Resistance Strains of Mycobacterium Tuberculosis. Eur. J. Med. Chem. 2011, 46, 4521–4529. [Google Scholar] [CrossRef] [PubMed]
- Ríos, D.; Benites, J.; Torrejón, F.; Theoduloz, C.; Valderrama, J.A. Synthesis and in Vitro Antiproliferative Evaluation of 3-Acyl-2-Arylamino-1,4-Naphthoquinones. Med. Chem. Res. 2014, 23, 4149–4155. [Google Scholar] [CrossRef]
- Zheng, Z.; Touve, M.; Barnes, J.; Reich, N.; Zhang, L. Synthesis-Enabled Probing of Mitosene Structural Space Leads to Improved IC50 over Mitomycin C. Angew. Chem. Int. Ed. 2014, 53, 9302–9305. [Google Scholar] [CrossRef] [PubMed]
- Shvartsberg, M.S.; Kolodina, E.A.; Lebedeva, N.I.; Fedenok, L.G. Synthesis of Benz[f]Indole-4,9-Diones via Acetylenic Derivatives of 1,4-Naphthoquinone. Tetrahedron Lett. 2009, 50, 6769–6771. [Google Scholar] [CrossRef]
- Tapia, R.; Prieto, Y.; Pautet, F.; Domard, M.; Sarciron, M.-E.; Walchshofer, N.; Fillion, H. Synthesis and Antileishmanial Activity of Indoloquinones Containing a Fused Benzothiazole Ring. Eur. J. Org. Chem. 2002, 2002, 4005–4010. [Google Scholar] [CrossRef]
- Corey, E.; Czako, B.; Kürti, L. Molecules and Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-22749-7. [Google Scholar]
- Giuglio-Tonolo, A.G.; Curti, C.; Terme, T.; Vanelle, P. A Survey of Synthetic Routes and Antitumor Activities for Benzo[g]Quinoxaline-5,10-Diones. Molecules 2020, 25, 5922. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Pelageev, D.N.; Jakob, L.S.; Borisova, K.L.; Hauschild, J.; Busenbender, T.; Kaune, M.; Khmelevskaya, E.A.; Graefen, M.; Bokemeyer, C.; et al. Activity of New Synthetic (2-Chloroethylthio)-1,4-Naphthoqui-Nones in Prostate Cancer Cells. Pharmaceuticals 2021, 14, 949. [Google Scholar] [CrossRef]
- Flores, E.; Rivera-Avalos, E.; Rodríguez-Molina, B.; Frontana, C.; López, L.; de Loera, D. Study of Organic Radicals Generated upon Naphthoquinone-Hydantoins Reactions in Basic Aqueous Solution. In Chemistry Proceedings 2021; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2021; Volume 3, p. 58. [Google Scholar]
- Leyva, E.; López, L.I.; Loredo-Carrillo, S.E.; Rodríguez-Kessler, M.; Montes-Rojas, A. Synthesis, Spectral and Electrochemical Characterization of Novel 2-(Fluoroanilino)-1,4-Naphthoquinones. J. Fluor. Chem. 2011, 132, 94–101. [Google Scholar] [CrossRef]
- Lopez-Lopez, L.I.; Vaquera Garcia, J.J.; Saenz-Galindo, A.; Silva-Belmares, S.Y. Ultrasonic and Microwave Assisted Synthesis of Nitrogen-Containing Derivatives of Juglone as Potential Antibacterial Agents. Lett. Org. Chem. 2014, 11, 573–582. [Google Scholar] [CrossRef]
- Leyva, E.; López, L.I.; de la Cruz, R.F.G.; Espinosa-González, C.G. Synthesis and Studies of the Antifungal Activity of 2-Anilino-/2,3-Dianilino-/2-Phenoxy- and 2,3-Diphenoxy-1,4-Naphthoquinones. Res. Chem. Intermed. 2017, 43, 1813–1827. [Google Scholar] [CrossRef]
- Leyva, E.; Baines, K.M.; Espinosa-González, C.G.; Magaldi-Lara, D.A.; Loredo-Carrillo, S.E.; De Luna-Méndez, T.A.; López, L.I. 2-(Fluoro-) and 2-(Methoxyanilino)-1,4-Naphthoquinones. Synthesis and Mechanism and Effect of Fluorine Substitution on Redox Reactivity and NMR. J. Fluor. Chem. 2015, 180, 152–160. [Google Scholar] [CrossRef]
- Vega-Rodríguez, S.; Jiménez-Cataño, R.; Leyva, E.; Loredo-Carrillo, S.E. Intramolecular Hydrogen Bonds in Fluorinated, Methoxylated, or Unsubstituted 2-(Anilino)-1,4-Naphthoquinones. A Theoretical Study. J. Fluor. Chem. 2013, 145, 58–62. [Google Scholar] [CrossRef]
- Leyva, E.; Schmidtke Sobeck, S.J.; Loredo-Carrillo, S.E.; Magaldi-Lara, D.A. Spectral and Structural Characterization of 2-(Fluorophenylamino)- and 2-(Nitrophenylamino)-1,4-Naphthoquinone Derivatives. J. Mol. Struct. 2014, 1068, 1–7. [Google Scholar] [CrossRef]
- Leyva, E.; Cárdenas-Chaparro, A.; Loredo-Carrillo, S.E.; López, L.I.; Méndez-Sánchez, F.; Martínez-Richa, A. Ultrasound-Assisted Reaction of 1,4-Naphthoquinone with Anilines through an EDA Complex. Mol. Divers. 2018, 22, 281–290. [Google Scholar] [CrossRef]
- Cárdenas-Chaparro, A.; Leyva, E.; Loredo-Carrillo, S.E.; Carranza, V. Síntesis de Derivados de 2-Anilino-3-Cloro- 1,4-Naftoquinona Promovida Por Microondas y Ultrasonido. Afinidad. J. Chem. Eng. Theor. Appl. Chem. 2017, 74, 302–306. [Google Scholar]
- Razaque, R.; Raza, A.R.; Irshad, M.; Rubab, S.L.; Batool, S.; Nisar, B.; Akram, Z.; Akhtar, M.T.; Qadir, R.; Siddique, A.B.; et al. Synthesis and Evaluation of 2-Phenylamino-1,4-Naphthoquinones Derivatives as Potential Hypoglycaemic Agents. Braz. J. Biol. 2024, 84, e254234. [Google Scholar] [CrossRef]
- Pacheco, P.A.F.; Gonzaga, D.T.; Cirne-Santos, C.C.; Barros, C.S.; Gomes, M.W.L.; Gomes, R.S.P.; Gonçalves, M.C.; Ferreira, V.F.; Rabelo, V.W.; Abreu, P.A.; et al. Synthesis and Anti-Chikungunya Virus (CHIKV) Activity of Novel 1,4-Naphthoquinone Sulfonamide and Sulfonate Ester Derivatives. Artic. J. Braz. Chem. Soc. 2022, 33, 556–569. [Google Scholar] [CrossRef]
- Rivera-Ávalos, E.; De Loera, D.; Araujo-Huitrado, J.G.; Escalante-García, I.L.; Muñoz-Sánchez, M.A.; Hernández, H.; López, J.A.; López, L. Synthesis of Amino Acid-Naphthoquinones and in Vitro Studies on Cervical and Breast Cell Lines. Molecules 2019, 24, 4285. [Google Scholar] [CrossRef]
- Córdova-Rivas, S.; Araujo-Huitrado, J.G.; Rivera-Avalos, E.; Escalante-García, I.L.; Durón-Torres, S.M.; López-Hernández, Y.; Hernández-López, H.; López, L.; de Loera, D.; López, J.A. Differential Proliferation Effect of the Newly Synthesized Valine, Tyrosine and Tryptophan–Naphthoquinones in Immortal and Tumorigenic Cervical Cell Lines. Molecules 2020, 25, 2058. [Google Scholar] [CrossRef]
- Micheletti, G.; Boga, C.; Zalambani, C.; Farruggia, G.; Esposito, E.; Fiori, J.; Rizzardi, N.; Taddei, P.; Di Foggia, M.; Calonghi, N. Synthesis of Thia-Michael-Type Adducts between Naphthoquinones and N-Acetyl-L-Cysteine and Their Biological Activity. Molecules 2022, 27, 5645. [Google Scholar] [CrossRef]
- Giang, L.N.T.; Anh, D.T.T.; Phuong, H.T.; Thanh, N.H.; Giang, N.T.Q.; Anh, N.T.; Tuyen, N.V.; Kiem, P. Van DMAP-Catalyzed Efficient and Convenient Approach for the Synthesis of 3,3′-(Arylmethylene)Bis(2-Hydroxynaphthalene-1,4-Dione) Derivatives. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Giang, L.N.T.; Anh, D.T.T.; Thanh, N.H.; Phuong, H.T.; Anh, N.T.; Giang, N.T.Q.; Ha, V.T.T.; Tuyen, N. Van A Green and Efficient NH4OAc-Catalyzed Synthesis of 2-Hydroxy-3-(Arylmethyl)(4-Hydroxy-2-Oxo-2,5-Dihydrofuran-3-Yl)-1,4-Naphthoquinones. VNU J. Sci. Nat. Sci. Technol. 2022, 38, 42–47. [Google Scholar] [CrossRef]
- Costa, D.C.S.; Francisco, A.S.; Matuck, B.V.A.; Furtado, P.S.; de Oliveira, A.A.S.C.; Rabelo, V.W.H.; Sathler, P.C.; Abreu, P.A.; Ferreira, V.F.; da Silva, L.C.R.P.; et al. Diacetate Naphthoquinone Derivatives Tethered to 1,2,3-Triazoles: Synthesis and Cytotoxicity Evaluation in Caco-2 Cells. J. Braz. Chem. Soc. 2022, 33, 48–59. [Google Scholar] [CrossRef]
- Geisler, H.; Westermayr, J.; Cseh, K.; Wenisch, D.; Fuchs, V.; Harringer, S.; Plutzar, S.; Gajic, N.; Hejl, M.; Jakupec, M.A.; et al. Tridentate 3-Substituted Naphthoquinone Ruthenium Arene Complexes: Synthesis, Characterization, Aqueous Behavior, and Theoretical and Biological Studies. Inorg. Chem. 2021, 60, 9805–9819. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Xie, K.; Zhou, D.; Peng, J.; Fan, A.; Zhang, J.; Chen, C. Transition-Metal-Free One-Pot Synthesis of Naphthoquinonefuran Derivatives through Sequential Nucleophilic Substitution-Nucleophilic Addition Reaction. J. Org. Chem. 2020, 85, 9313–9320. [Google Scholar] [CrossRef]
- Arundhati, M.; Dipak, C.; Mithun, R. Synthesis and Antimalarial Activity of Lawsone Mannich Base Derivatives. Indian J. Pharm. Educ. Res. 2018, 52, 472–479. [Google Scholar] [CrossRef]
- Nariya, P.; Shukla, F.; Vyas, H.; Devkar, R.; Thakore, S. Synthesis and Characterization of Mannich Bases of Lawsone and Their Anticancer Activity. Synth. Commun. 2020, 50, 1724–1735. [Google Scholar] [CrossRef]
- Nariya, P.; Kumar, S.; Seshadri, S.; Patel, M.; Thakore, S. Novel Substituted Isoindolinones Derived from Lawsone: Synthesis, Characterization, Theoretical, Biological Activity and Docking Studies. J. Mol. Struct. 2022, 1267, 133601. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Jentzsch, J.; Shaikh, A.; Singh Shuveksh, P.; Koko, W.S.; Khan, T.A.; Ahmed, K.; Schobert, R.; Ersfeld, K.; Biersack, B. New Pyrano-4H-Benzo[g]Chromene-5,10-Diones with Antiparasitic and Antioxidant Activities. Chem. Biodivers. 2021, 18, e2000839. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen Thi, Q.G.; Nguyen Thi, T.H.; Thi, P.H.; Le-Nhat-Thuy, G.; Dang Thi, T.A.; Le-Quang, B.; Pham-The, H.; Van Nguyen, T. Synthesis and Biological Activity, and Molecular Modelling Studies of Potent Cytotoxic Podophyllotoxin-Naphthoquinone Compounds. RSC Adv. 2022, 12, 22004–22019. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Dang Thi, T.A.; Hoang Thi, P.; Le-Nhat-Thuy, G.; Nguyen Thi, Q.G.; Nguyen Tuan, A.; Le Thi, T.A.; Van Nguyen, T. A New Approach for the Synthesis of Novel Naphthoquinone Chalcone Hybrid Compounds. Tetrahedron Lett. 2021, 81, 153337. [Google Scholar] [CrossRef]
- Oramas-Royo, S.; López-Rojas, P.; Amesty, Á.; Gutiérrez, D.; Flores, N.; Martín-Rodríguez, P.; Fernández-Pérez, L.; Estévez-Braun, A. Synthesis and Antiplasmodial Activity of 1,2,3-Triazole-Naphthoquinone Conjugates. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Valença, W.O.; Baiju, T.V.; Brito, F.G.; Araujo, M.H.; Pessoa, C.; Cavalcanti, B.C.; de Simone, C.A.; Jacob, C.; Namboothiri, I.N.N.; da Silva Júnior, E.N. Synthesis of Quinone-Based N-Sulfonyl-1,2,3-Triazoles: Chemical Reactivity of Rh(II) Azavinyl Carbenes and Antitumor Activity. ChemistrySelect 2017, 2, 4301–4308. [Google Scholar] [CrossRef]
- Gholampour, M.; Ranjbar, S.; Edraki, N.; Mohabbati, M.; Firuzi, O.; Khoshneviszadeh, M. Click Chemistry-Assisted Synthesis of Novel Aminonaphthoquinone-1,2,3-Triazole Hybrids and Investigation of Their Cytotoxicity and Cancer Cell Cycle Alterations. Bioorg. Chem. 2019, 88, 102967. [Google Scholar] [CrossRef] [PubMed]
- Prasanna Kumari, S.; Philip Anthony, S.; Selva Ganesan, S. One-Pot Synthesis of Indole-Fused Nitrogen Heterocycles via the Direct C(Sp2)-H Functionalization of Naphthoquinones; Accessibility for Deep Red Emitting Materials. New J. Chem. 2022, 46, 16874–16879. [Google Scholar] [CrossRef]
- Maurya, H.K. Synthetic and biological utility of 2,3-dichloro-1,4-naphthoquinone: A review. Int. J. Res. GRANTHAALAYAH 2020, 7, 293–347. [Google Scholar] [CrossRef]
- Campora, M.; Canale, C.; Gatta, E.; Tasso, B.; Laurini, E.; Relini, A.; Pricl, S.; Catto, M.; Tonelli, M. Multitarget Biological Profiling of New Naphthoquinone and Anthraquinone-Based Derivatives for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Mahalapbutr, P.; Leechaisit, R.; Thongnum, A.; Todsaporn, D.; Prachayasittikul, V.; Rungrotmongkol, T.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V.; Pingaew, R. Discovery of Anilino-1,4-Naphthoquinones as Potent EGFR Tyrosine Kinase Inhibitors: Synthesis, Biological Evaluation, and Comprehensive Molecular Modeling. ACS Omega 2022, 7, 17881–17893. [Google Scholar] [CrossRef]
- Hsu, M.J.; Chen, H.K.; Lien, J.C.; Huang, Y.H.; Huang, S.W. Suppressing VEGF-A/VEGFR-2 Signaling Contributes to the Anti-Angiogenic Effects of PPE8, a Novel Naphthoquinone-Based Compound. Cells 2022, 11, 2114. [Google Scholar] [CrossRef]
- Shakh, Y.U.; Romanenko, I.; Slesarchuk, M.; Syngaevsky, V.; Kovalchuk, O.; Bolibrukh, K.; Karkhut, A.; Bolibrukh, L.; Gubytska, I.; Komarovska-Porokhnyavets, O.; et al. Synthesis and Antimicrobial Activity of 1,4-Naphthoquinones Derivatives with [1,2,4]-Triazole-3-Thione Substitution. Indian J. Pharm. Sci. 2017, 79, 650–654. [Google Scholar] [CrossRef]
- Polish, N.V.; Nesterkina, M.V.; Protunkevych, M.S.; Karkhut, A.I.; Marintsova, N.G.; Polovkovych, S.V.; Kravchenko, I.A.; Voskoboinik, O.Y.; Kovalenko, S.I.; Karpenko, O.V. Synthesis and Pharmacological Evaluation of Novel Naphthoquinone Derivatives Containing 1,2,4-Triazine and 1,2,4-Triazole Moieties. Vopr. Khimii i Khimicheskoi Tekhnologii 2021, 2021, 97–104. [Google Scholar] [CrossRef]
- Espinosa-Bustos, C.; Pérez, M.O.; Gonzalez-Gonzalez, A.; Zarate, A.M.; Rivera, G.; Belmont-Díaz, J.A.; Saavedra, E.; Cuellar, M.A.; Vázquez, K.; Salas, C.O. New Amino Naphthoquinone Derivatives as Anti-Trypanosoma Cruzi Agents Targeting Trypanothione Reductase. Pharmaceutics 2022, 14, 1121. [Google Scholar] [CrossRef] [PubMed]
- Abdassalam, A.F.S.H.; Deniz, N.G.; Sayil, C.; Ozyurek, M.; Yesil, E.A.; Salihoglu, H. Synthesis of New Regioisomers of 5-Nitro-1,4-Naphthoquinone, Evaluation of Antioxidant and Catalase Inhibition Activities. Acta Chim. Slov. 2022, 69, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Defant, A.; Mancini, I. Design, Synthesis and Cancer Cell Growth Inhibition Evaluation of New Aminoquinone Hybrid Molecules. Molecules 2019, 24, 2224. [Google Scholar] [CrossRef] [PubMed]
- Uysal, S.; Soyer, Z.; Saylam, M.; Tarikogullari, A.H.; Yilmaz, S.; Kirmizibayrak, P.B. Design, Synthesis and Biological Evaluation of Novel Naphthoquinone-4-Aminobenzensulfonamide/Carboxamide Derivatives as Proteasome Inhibitors. Eur. J. Med. Chem. 2021, 209. [Google Scholar] [CrossRef]
- Wang, W.; Chang, C.W.T.; Zhang, Q. 1,4-Naphthoquinone Analogs and Their Application as Antibacterial Agents. ChemistrySelect 2022, 7, e202203330. [Google Scholar] [CrossRef]
- Shields, H.J.; Traa, A.; Van Raamsdonk, J.M. Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies. Front. Cell Dev. Biol. 2021, 9, 181. [Google Scholar] [CrossRef]
- Gambhir, L.; Tyagi, G.; Bhardwaj, R.; Kapoor, N.; Sharma, G. Perturbation of Cellular Redox Status: Role of Nrf2, a Master Regulator of Cellular Redox; IntechOpen: London, UK, 2022; ISBN 978-1-83968-282-7. [Google Scholar]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.; Matic, M.; Djukic, M.; Avdovic, E. Redox Status, DNA and HSA Binding Study of Naturally Occurring Naphthoquinone Derivatives. EXCLI J. 2020, 19, 48–70. [Google Scholar] [CrossRef]
- Majiene, D.; Kuseliauskyte, J.; Stimbirys, A.; Jekabsone, A. Comparison of the Effect of Native 1,4-Naphthoquinones Plumbagin, Menadione, and Lawsone on Viability, Redox Status, and Mitochondrial Functions of C6 Glioblastoma Cells. Nutrients 2019, 11, 1294. [Google Scholar] [CrossRef]
- Goleva, T.N.; Lyamzaev, K.G.; Rogov, A.G.; Khailova, L.S.; Epremyan, K.K.; Shumakovich, G.P.; Domnina, L.V.; Ivanova, O.Y.; Marmiy, N.V.; Zinevich, T.V.; et al. Mitochondria-Targeted 1,4-Naphthoquinone (SkQN) Is a Powerful Prooxidant and Cytotoxic Agent. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148210. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Role of Bcl-2 Family Proteins in Apoptosis: Apoptosomes or Mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.H.; Shen, G.N.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Wang, J.R.; Feng, Y.C.; Li, J.Q.; Zhang, Y.; et al. Two Novel 1,4-Naphthoquinone Derivatives Induce Human Gastric Cancer Cell Apoptosis and Cell Cycle Arrest by Regulating Reactive Oxygen Species-Mediated MAPK/Akt/STAT3 Signaling Pathways. Mol. Med. Rep. 2019, 20, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Bustos, C.; Canales, C.; Ramírez, G.; Jaque, P.; Salas, C.O. Unveiling Interactions between DNA and Cytotoxic 2-Arylpiperidinyl-1,4-Naphthoquinone Derivatives: A Combined Electrochemical and Computational Study. Arab. J. Chem. 2020, 13, 2233–2244. [Google Scholar] [CrossRef]
- Kosiha, A.; Parthiban, C.; Ciattini, S.; Chelazzi, L.; Elango, K.P. Metal Complexes of Naphthoquinone Based Ligand: Synthesis, Characterization, Protein Binding, DNA Binding/Cleavage and Cytotoxicity Studies. J. Biomol. Struct. Dyn. 2018, 36, 4170–4181. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yu, B.; Friedrich, D.; Li, J.; Shen, H.; Krautscheid, H.; Huang, S.D.; Kim, M.H. Naphthoquinone-Derivative as a Synthetic Compound to Overcome the Antibiotic Resistance of Methicillin-Resistant S. Aureus. Commun. Biol. 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef]
- Wellington, K.W.; Nyoka, N.B.P.; McGaw, L.J. Investigation of the Antibacterial and Antifungal Activity of Thiolated Naphthoquinones. Drug Dev. Res. 2019, 80, 386–394. [Google Scholar] [CrossRef]
- Sánchez-Calvo, J.M.; Barbero, G.R.; Guerrero-Vásquez, G.; Durán, A.G.; Macías, M.; Rodríguez-Iglesias, M.A.; Molinillo, J.M.G.; Macías, F.A. Synthesis, Antibacterial and Antifungal Activities of Naphthoquinone Derivatives: A Structure–Activity Relationship Study. Med. Chem. Res. 2016, 25, 1274–1285. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Discovery of Juglone and Its Derivatives as Potent SARS-CoV-2 Main Proteinase Inhibitors. Eur. J. Med. Chem. 2021, 225, 113789. [Google Scholar] [CrossRef]
- Mohamady, S.; Gibriel, A.A.; Ahmed, M.S.; Hendy, M.S.; Naguib, B.H. Design and Novel Synthetic Approach Supported with Molecular Docking and Biological Evidence for Naphthoquinone-Hydrazinotriazolothiadiazine Analogs as Potential Anticancer Inhibiting Topoisomerase-IIB. Bioorg. Chem. 2020, 96, 103641. [Google Scholar] [CrossRef] [PubMed]
- Gurbani, D.; Kukshal, V.; Laubenthal, J.; Kumar, A.; Pandey, A.; Tripathi, S.; Arora, A.; Jain, S.K.; Ramachandran, R.; Anderson, D.; et al. Mechanism of Inhibition of the ATpase Domain of Human Topoisomerase IIα by 1,4-Benzoquinone, 1,2-Naphthoquinone, 1,4-Naphthoquinone, and 9,10-Phenanthroquinone. Toxicol. Sci. 2012, 126, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.A.S.; Nawaz, M.; Izzeldin, I.; Abubshait, H.A.; Alsadig, A.; Gomaa, M.S.; Abubshait, S.A.; Alsewdan, D. Molecular Docking and Anticancer Activity of Some Synthesized 1,4- Naphthoquinone Derivatives against Human Cancer Cell Line. J. Mol. Struct. 2023, 1275, 134702. [Google Scholar] [CrossRef]
- Paengsri, W.; Promsawan, N.; Baramee, A. Synthesis and Evaluation of 2-Hydroxy-1,4-Naphthoquinone Derivatives as Potent Antimalarial Agents. Chem. Pharm. Bull. 2021, 69, 253–257. [Google Scholar] [CrossRef]
- Kurban, S.; Deniz, N.G.; Sayil, C.; Ozyurek, M.; Guclu, K.; Stasevych, M.; Zvarych, V.; Komarovska-Porokhnyavet, O.; Novikov, V. Synthesis, Antimicrobial Properties, and Inhibition of Catalase Activity of 1,4-Naphtho-and Benzoquinone Derivatives Containing N-, S-, O-Substituted. Heteroat. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Yildirim, H.; Bayrak, N.; Tuyun, A.F.; Kara, E.M.; Çelik, B.Ö.; Gupta, G.K. 2,3-Disubstituted-1,4-Naphthoquinones Containing an Arylamine with Trifluoromethyl Group: Synthesis, Biological Evaluation, and Computational Study. RSC Adv. 2017, 7, 25753–25764. [Google Scholar] [CrossRef]
- Kacmaz, A.; Deniz, N.G.; Aydinli, S.G.; Sayil, C.; Onay-Ucar, E.; Mertoglu, E.; Arda, N. Synthesis and Antiproliferative Evaluation of Some 1,4-Naphthoquinone Derivatives against Human Cervical Cancer Cells. Open Chem. 2019, 17, 337–345. [Google Scholar] [CrossRef]
- Li, H.; Cai, M.; Cao, F.; Yu, D.; Yang, J.; Yu, W.; Chu, C.; Guan, X.; Qin, J.J.; Dong, J. S3I-201 Derivative Incorporating Naphthoquinone Unit as Effective STAT3 Inhibitors: Design, Synthesis and Anti-Gastric Cancer Evaluation. Bioorganic Med. Chem. 2022, 71, 116941. [Google Scholar] [CrossRef]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Millet, P.; Georges, A.J.; Lebibi, J. Synthesis and Antimalarial Activity in Vitro and in Vivo of a New Ferrocene-Chloroquine Analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef]
- Mahal, K.; Ahmad, A.; Schmitt, F.; Lockhauserbäumer, J.; Starz, K.; Pradhan, R.; Padhye, S.; Sarkar, F.H.; Koko, W.S.; Schobert, R.; et al. Improved Anticancer and Antiparasitic Activity of New Lawsone Mannich Bases. Eur. J. Med. Chem. 2017, 126, 421–431. [Google Scholar] [CrossRef]
- Al Nasr, I.; Jentzsch, J.; Winter, I.; Schobert, R.; Ersfeld, K.; Koko, W.S.; Mujawah, A.A.H.; Khan, T.A.; Biersack, B. Antiparasitic Activities of New Lawsone Mannich Bases. Arch. Pharm. 2019, 352, e1900128. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.V.; Leite, J.P.G.; Neves, A.P.; da Silva, G.B.; Vargas, M.D.; Paixão, I.C.N.P. Synthetic Aminomethylnaphthoquinones Inhibit the in Vitro Replication of Bovine Herpesvirus 5. Arch. Virol. 2014, 159, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Pires De Mello, C.P.; Sardoux, N.S.; Terra, L.; Amorim, L.C.; Vargas, M.D.; Da Silva, G.B.; Castro, H.C.; Giongo, V.A.; Madeira, L.F.; Paixão, I.C.N.P. Aminomethylnaphthoquinones and HSV-1: In Vitro and in Silico Evaluations of Potential Antivirals. Antivir. Ther. 2016, 21, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Giongo, V.; Falanga, A.; De Melo, C.P.P.; da Silva, G.B.; Bellavita, R.; De-simone, S.G.; Paixão, I.C.; Galdiero, S. Antiviral Potential of Naphthoquinones Derivatives Encapsulated within Liposomes. Molecules 2021, 26, 6440. [Google Scholar] [CrossRef]
- Santos, L.H.; Kronenberger, T.; Almeida, R.G.; Silva, E.B.; Rocha, R.E.O.; Oliveira, J.C.; Barreto, L.V.; Skinner, D.; Fajtová, P.; Giardini, M.A.; et al. Structure-Based Identification of Naphthoquinones and Derivatives as Novel Inhibitors of Main Protease Mproand Papain-like Protease PLproof SARS-CoV-2. J. Chem. Inf. Model. 2022, 62, 6553–6573. [Google Scholar] [CrossRef]
- Ahmad, A.; Mahal, K.; Padhye, S.; Sarkar, F.H.; Schobert, R.; Biersack, B. New Ferrocene Modified Lawsone Mannich Bases with Anti-Proliferative Activity against Tumor Cells. J. Saudi Chem. Soc. 2017, 21, 105–110. [Google Scholar] [CrossRef]
- Venturini Filho, E.; Antoniazi, M.K.; Ferreira, R.Q.; dos Santos, G.F.S.; Pessoa, C.; Guimarães, C.J.; Vieira Neto, J.B.; Silva, A.M.S.; Greco, S.J. A Green Multicomponent Domino Mannich-Michael Reaction to Synthesize Novel Naphthoquinone-Polyphenols with Antiproliferative and Antioxidant Activities. Eur. J. Org. Chem. 2022, 2022, e202200442. [Google Scholar] [CrossRef]
- Nguyen Thi, Q.G.; Le-Nhat-Thuy, G.; Dang Thi, T.A.; Hoang Thi, P.; Nguyen Tuan, A.; Nguyen Thi, T.H.; Nguyen, T.T.; Nguyen Ha, T.; Hoang Mai, H.; Nguyen, T. Van Synthesis of Novel Potent Cytotoxicy Podophyllotoxin-Naphthoquinone Compounds via Microwave-Assited Multicomponent Domino Reactions. Bioorganic Med. Chem. Lett. 2021, 37, 127841. [Google Scholar] [CrossRef]
- de Freitas, P.P.; Ribeiro, R.C.B.; dos Santos Guimarães, I.; Moreira, C.S.; Rocha, D.R.; de Carvalho da Silva, F.; Ferreira, V.F.; Gimba, E.R.P. (3,3’-Methylene)Bis-2-Hydroxy-1,4-Naphthoquinones Induce Cytotoxicity against DU145 and PC3 Cancer Cells by Inhibiting Cell Viability and Promoting Cell Cycle Arrest. Mol. Biol. Rep. 2021, 48, 3253–3263. [Google Scholar] [CrossRef]








| Biological Evaluations of NQs-Aniline | ||||
|---|---|---|---|---|
| Name | Chemical Structure | Potential Application | Evidence from Preclinical Assays | Ref |
| 2-Chloro-3-((2-(piperidin-1-yl)ethyl)amino)naphthalene-1,4-dione |  48 | Antibacterial | MIC of 31.2 µg mL−1 against S. aureus (209-P) and 15.6 µg mL−1 for M. luteum (B-917). | [91] |
| 2-((2-Hydroxypropyl)thio)-3-((3-(trifluoromethyl)phenyl)amino)naphthalene-1,4-dione | 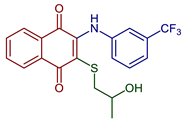 49 | Antibacterial | MIC of 2.44 µg m−1 L against S. epidermidis (9.8 µg mL−1 cefuroxime). | [92] |
| 2-(sec-Butylthio)-3-((3-(trifluoromethyl)phenyl)amino)naphthalene-1,4-dione | 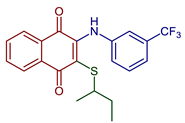 50 | Antibacterial | MIC of 4.88 µgmL−1 against S. epidermidis (9.8 µgmL−1 cefuroxime). | [92] |
| N-(4-((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)phenyl)-R-benzenesulfonamide derivatives |  51, R= 4-methylphenyl 52, R = 4-nitrophenyl | Antiviral | Anti-Chikungunya virus (CHIKV) activity, 51, CC50 of 281 ± 2.5 µM and 99 ± 4.3% inhibition CHIKV replication; 52, CC50 of 540 ± 3.7 µM and 98 ± 3.5% inhibition CHIKV replication. | [42] |
| 2-Chloro-3-((3-(2-methylpiperidin-1-yl)propyl)amino)naphthalene-1,4-dione | 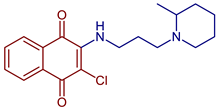 53 | Anticancer | IC50 of 12.82 µM for HeLa cell. | [93] |
| 2-Chloro-3-(phenyl(4-(phenylamino)phenyl)amino)naphthalene-1,4-dione | 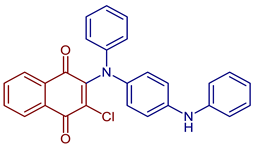 54 54 | Anticancer | IC50 of 16.71 µM for HeLa cell. | [93] |
| 2-Chloro-3-(methylphenylamino)naphthalene-1,4-dione | 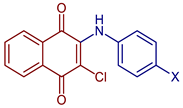 55, X=CH3 | Anticancer | In vitro IC50 (μg mL−1) of 4.30± 0.46 against MOLT-3 cell line and 10.68 ± 1.89 against MDA-MB231 cell line. Doxorubicin and etoposide were used as reference drugs. Compound 55, with an IC50 = 3.96 nM, could occupy the ATP-binding pocket of the target EGFR protein, similar to the pharmaceutical compound erlotinib EGFR inhibitor (IC50 = 16.17 nM). | [63] |
| 2-Chloro-3-(cyanophenylamino)naphthalene-1,4-dione | 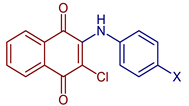 56, X=CN | Anticancer | IC50 (μg mL−1) of 1.75 ± 0.20 for MOLT-3 cell line. Doxorubicin and etoposide were used as reference drugs. | [63] |
| 2-Chloro-3-(hydroxyphenylamino)naphthalene-1,4-dione | 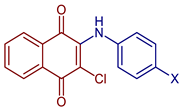 57, X=OH | Anticancer | IC50 (μg mL−1) of 8.21 ± 0.33 for HuCCA-1 cell line, and MDA-MB-231 cells. Doxorubicin and etoposide were used as reference drugs. | [63] |
| 2-((4-(4-((1,4-Dioxo-1,4-dihydronaphthalen-2-yl)amino)piperidine-1-carbonyl)-3-methylphenyl)amino)-2-oxoethyl 4-methylbenzenesulfonate | 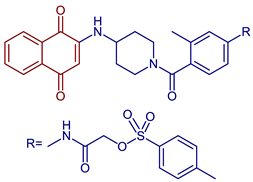 58 | Anticancer | Compound 58 inhibits in vitro clone formation, induces apoptosis, inhibits cell migration and the arrest cell cycle, and blocks the STAT3 signaling pathway of gastric cancer cell MGC803 at IC50 = 0.57 µM. 58, may be a promising STAT3 inhibitor for further developing potential anti-gastric cancer candidates. | [94] |
| 2-Chloro-3-((2,4-dimethoxyphenyl)amino)-5-nitronaphthalene-1,4-dione | 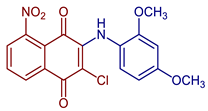 59 | Catalase inhibitors related to several diseases | 59, showed the strongest catalase enzyme inhibitory activity and highest antioxidant capacity with a 1.80 ± 0.06 CUPRAC-TEAC coefficient. | [68] |
| Biological evaluations of NQs-amino acids | ||||
| Name | Chemical Structure | Potential Application | Evidence from Preclinical Assays | Ref |
| 2-((1,4-Dioxo-1,4-dihydronaphthalen-2-yl)amino)acetic acid | 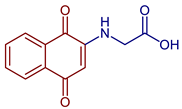 60 | Antibacterial | In vitro antibacterial analysis showed MIC (µg mL−1) of 7.8 against S. aureus ATCC 25923, 31.2 against E. coli ATCC 25922, E. faecalis ATCC 29212, and P. aeruginosa ATCC 27853. 72 presented high gastrointestinal absorption and good characteristics for oral bioavailability. | [31] |
| Anticancer | Inhibited ~80% of proliferation in SiHa cells and nearly 90% in MCF-7 cells. | [43] | ||
| 2-((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)acetic acid |  61 | Anticancer | Compound 61 showed proliferation inhibition of 90% in MCF-7 cells. | [43] |
| 2-((1,4-Dioxo-1,4-dihydronaphthalen-2-yl)amino)-3-phenylpropanoic acid | 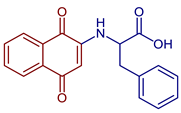 62 | Antibacterial | In vitro compound 62 showed MIC of 24.7 µg mL−1 against S. aureus ATCC 25923, E. coli ATCC 25922, E. faecalis ATCC 29212, and P. aeruginosa ATCC 27853. Isolated clinical strains showed MICs of 49.7 µg mL−1 against S. aureus and 24.7 µg mL−1 against E. coli by 62*. | [31] |
| 2-((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)-3-phenylpropanoic acid |  63 | Antibacterial | In vitro antibacterial analysis showed MIC of 24.7 µg mL−1 against S. aureus ATCC 25923, E. coli ATCC 25922, E. faecalis ATCC 29212, and P. aeruginosa ATCC 27853. Isolated clinical strains showed MICs of 49.7 µg mL−1 against S. aureus and 24.7 µg mL−1 against E. coli by 63*. | [31] |
| 2-((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)-3-(4-hydroxyphenyl)propanoic acid | 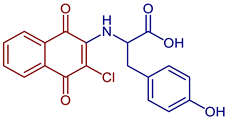 64 | Anticancer | In vitro potent proliferation inhibition in cervical tumorigenic cell lines, showing an IC50 (µM) of 6.83, 7.028, ~0.001577 for SiHa, CaLo, and C33-A line cells, respectively. | [44] |
| 4-Amino-2-((1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)-4-oxobutanoic acid | 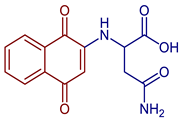 65 | Antibacterial | In vitro antibacterial analysis showed MIC of 24.7 µg mL−1 against S. aureus ATCC 25923, E. coli ATCC 25922, E. faecalis ATCC 29212, and P. aeruginosa ATCC 27853. Isolated clinical strains showed MICs of 49.7 µg mL−1 against S. aureus and 24.7 µg mL−1 against E. coli by compound 65*. | [31] |
| Anticancer | Inhibited ~80% of proliferation in SiHa cells. | [43] | ||
| 3-Amino-2-((3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)-3-oxopropanoic acid | 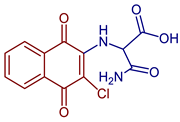 66 | Anticancer | Showed proliferation inhibition ~85% in MCF-7 cells. | [43] |
| 2-((3-Chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)-3-(1H-indol-2-yl)propanoic acid | 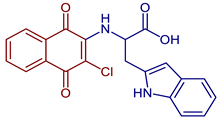 67 | Anticancer | In vitro potent proliferation inhibition in cervical tumorigenic cell lines, showing an IC50 (µM) of 28.8, 25.20, and 21.36 for SiHa, CaLo, and C33-A cells, respectively. | [44] |
| N-Acetyl-L-cysteine naphthoquinone derivatives | 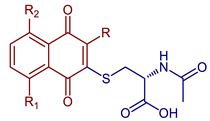 68, R=R1=R2=H 69, R=CH3, R1=R2=H 70, R=H, R1=OH, R2=H | Anticancer | Compounds 68, 69, and 70 showed potent cytostatic effects against HeLa, SH-SY5Y, SaOS2, and U2OS cancer cell lines with IC50 in the range of 0.50–1.81 µM. | [45] |
| Biological evaluations of NQs-Mannich bases | ||||
| Name | Chemical Structure | Potential Application | Evidence from Preclinical Assays | Ref |
| 2-((Heptylamino)methyl)-3-hydroxynaphthalene-1,4-dione |  71 | Antiparasitic | T. gondii (atovaquone-resistant) and P. falciparum (chloroquine-resistant) susceptible to compound 71. | [95] |
| 2-((Alkylamino)(pyridin-2-yl)methyl)-3-hydroxynaphthalene-1,4-dione | 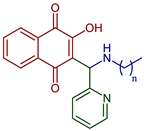 72, n=11 73, n=13 74, n=15 | Antiparasitic | Compounds 72–74 showed sub-micromolar anti-trypanosomal activity against T. brucei via deformation of the microtubule cytoskeleton. Moreover, N-hexadecyl compound 74 was highly active against locally isolated E. histolytica parasite samples exceeding the activity of metronidazole. | [96] |
| Anticancer | Compounds 72–74 exhibited strong and selective growth inhibitory activities in the low one-digit micromolar and sub-micromolar range against a panel of human cancer cell lines associated with ROS formation. | |||
| 2-Hydroxy-3-[(2-hydroxyphenyl)(hexylamino)methyl]naphthalene-1,4-dione hydrochloride 2-Hydroxy-3-[(2-hydroxyphenyl)(docecylamino)methyl]naphthalene-1,4-dione hydrochloride |  75, R=hexadecyl 76, R=dodecyl | Antiparasitic | EC50 of 3.60 for 75 and 1.56 µM for 76 against T. gondii tachyzoites. Compounds displayed some selectivity for the T. gondii parasite compared to nonmalignant Vero cells with selectivity index (SI) values of 2.38 for 75 and 3.12 for 76. Compound 75 exhibited EC50 of 10.2 and 3.62 µM for L. major promastigotes and amastigotes, respectively, and compound 76 showed EC50 of 5.57 and 4.16 µM for L. major promastigotes and amastigotes respectively (more efficacious that atovaquone). T. b. brucei was inhibited with an IC50 of 3.25 and 1.66 for 75 and 76, respectively. | [97] |
| 2-((Butylamino)methyl)-3-hydroxynaphthalene-1,4-dione | 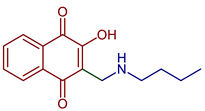 77 | Antiparasitic | Antimalarial activity in vitro against P. falciparum with IC50 of 0.77 μg mL−1 (P. falciparum K1, multidrug-resistant strain). | [90] |
| 2-(((4-Fluorophenyl)amino)(phenyl)methyl)-3-hydroxynaphthalene-1,4-dione |  78 | Antiparasitic | Compound 78 showed antimalarial activity with IC50 0.423 and 1.492 µg mL−1 for Chloroquine(CQ) –sensitive (3D-7) and CQ–resistant (RKL-2) strains of P. falciparum, respectively. | [51] |
| 2-Hydroxy-3-(((3-nitrophenyl)amino)(R-phenyl)methyl)naphthalene-1,4-dione derivatives | 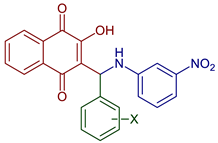 79, X=4NO2 80, X=2OH | Antiparasitic | Compound 79 showed antimalarial activity with IC50 0.475 and 1.391 µg mL−1 for CQ–sensitive (3D-7) and CQ–resistant (RKL-2) strains of P. falciparum, respectively. Compound 80 exhibited IC50 0.502 and 2.394 µg mL−1 for CQ–sensitive (3D-7) and CQ–resistant (RKL-2) strains of P. falciparum, respectively. | [51] |
| 2-Hydroxy-3-(R-phenyl(pyrrolidin-1-yl)methyl)naphthalene-1,4-dione |  81, R=H 82, R=2OH | Antiparasitic | Compound 81 showed antimalarial activity with IC50 0.412 and 2.212 µg mL−1 for CQ–sensitive (3D-7) and CQ–resistant (RKL-2) strains of P. falciparum, respectively. Compound 82 exhibited IC50 0.411 and 1.170 µg mL−1 for CQ–sensitive (3D-7) and CQ–resistant (RKL-2) strains of P. falciparum, respectively. | [51] |
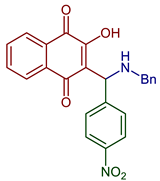 83 | Antiviral | Antiviral action of 83 against BoHV5 CC50 of 1867 ± 8.3 µM and EC50 3.8 ± 1.2 µM (Acyclovir: CC50 of 989 ± 2 µM and EC50 of 166 ± 2 µM). | [98] | |
| 2-((Butylamino)(2,4-dichlorophenyl)methyl)-3-hydroxynaphthalene-1,4-dione 2-((Benzylamino)(2,4-dichlorophenyl)methyl)-3-hydroxynaphthalene-1,4-dione |  84  85 | Antiviral | Compounds 84 and 85 affect the L-phase of the HSV-1 replicative cycle by gD protein expression inhibition. The nature of the substituent on the nitrogen atom, the conformation, and the LUMO distribution of benzyl portion versus n-butyl substituents modulates antiviral activity. Recently, antiviral activity with EC50 = 1.73 ± 0.08 µM for 84 and 0.56 ± 0.02 for 85 encapsulated in liposomes. | [99,100] |
| 3-(4-(((3-Bromo-1,4-dioxo-1,4-dihydronaphthalen-2-yl)amino)methyl)-1H-1,2,3-triazol-1-yl)-2,2-dimethyl-2,3-dihydronaphtho[1,2-b]furan-4,5-dione | 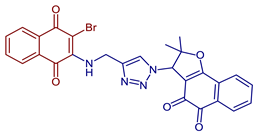 86 | Antiviral | Compound 86 at 10 µM showed 100% protease (Mpro)-SARS-CoV-2 inhibition action with IC50 = 1.9 ± 0.06 µM. | [101] |
| 2,3-Bis(phenylthio)naphthalene-1,4-dione | 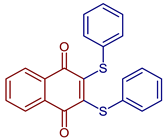 87 | Antiviral | Compound 87 at 10 µM showed 100% protease (Mpro)-SARS-CoV-2 inhibition action with IC50 = 0.63 ± 0.04 µM. | [101] |
| 2,3-Bis((4-methoxyphenyl)thio)-5-nitronaphthalene-1,4-dione | 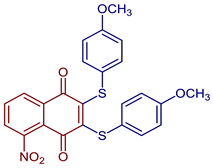 88 | Antiviral, SARS-CoV-2, Mpro inhibitors | Compound 88 at 10 µM showed 100% protease (Mpro)-SARS-CoV-2 inhibition action with IC50 = 0.41 ± 0.02 µM. | [101] |
| N-(5-Nitro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)acetamide |  89 | Antiviral, SARS-CoV-2, Mpro inhibitors | Compound 89 at 10 µM showed 100% protease (Mpro)-SARS-CoV-2 inhibition action with IC50 = 9 ± 1 µM. | [101] |
| Ferrocene 2-(amino(pyridin-2-yl)methyl)-3-hydroxynaphthalene-1,4-dione derivative | 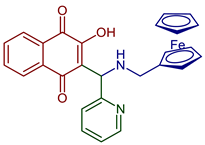 90 | Anticancer | Antiproliferative effects in the androgen-receptor negative PC-3 prostate and Pgp expressing KB-V1/Vb1 cervix carcinoma cell lines at sub-micromolar concentration. | [102] |
| 2-Hydroxy-3-((octylamino)(R-phenyl)methyl)naphthalene-1,4-dione | 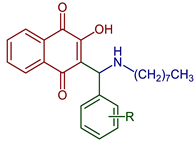 91, R: 2OH 92, R: OH, 5Br | Anticancer | Compounds 91 and 92 were active with IC50 of 11.68 and 1.64 µM against the HepG2 line cell, respectively. | [52] |
| 2-(2-Alkyl-3-oxo-2,3-dihydro-1H-isoindol-1-yl)-3-hydroxynaphthalene-1,4-dione derivatives | 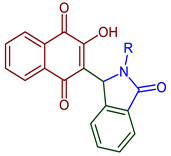 93, R=alkylamine | Anticancer | Isoindolinone derivatives enhanced cancer cell death and prevention of tumor growth by restoring serum SGOT and SGPT levels near to normal; docking studies revealed an association on promising liver cancer-associated Alpha-fetoprotein (AFP)]. | [53] |
| Naphthoquinone polyphenols derivatives |  94, 16 examples | Anticancer | Several polyphenols were tested on four cancer cell lines (HCT116, PC3, HL60, and SNB19), in which the best results showed antiproliferative activity with IC50 of 25.83 to 47.95 μM. Additionally, the antioxidant activity was determined using the CRAC assay. | [103] |
| 11-(5,6,7,8-Tetrahydronaphthalen-2-yl)-1H-R-benzo[g]cyclopenta[b]quinoline-1,5,10(4H,11H)-trione | 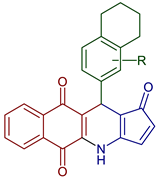 95: R=H, 96: 3Br, 97: 3NO2, 98: 3OMe | Anticancer | Podophyllotoxin-naphthoquinone derivatives. Compounds 95, 96, 97, and 98 displayed highly potent inhibitory activities with IC50 < 40 nM against HepG2 and SK-Lu-1 cell lines and showed lower toxicity for the non-cancerous Hek-293 cell line. | [104] |
| 3,3’-Methylene)Bis-2-hydroxy-1,4-naphthoquinones derivatives | 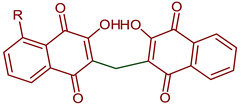 99: R=H 100: R=OH | Anticancer | Compounds 99 and 100 induced cytotoxicity against DU145 and PC3 cells. Promoted cell cycle arrest in G1/S and G2/M phases, increased Sub-G1 peak and inhibited cell viability. | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Tovar, G.; Vega-Rodríguez, S.; Leyva, E.; Loredo-Carrillo, S.; de Loera, D.; López-López, L.I. The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review. Pharmaceuticals 2023, 16, 496. https://doi.org/10.3390/ph16040496
Navarro-Tovar G, Vega-Rodríguez S, Leyva E, Loredo-Carrillo S, de Loera D, López-López LI. The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review. Pharmaceuticals. 2023; 16(4):496. https://doi.org/10.3390/ph16040496
Chicago/Turabian StyleNavarro-Tovar, Gabriela, Sarai Vega-Rodríguez, Elisa Leyva, Silvia Loredo-Carrillo, Denisse de Loera, and Lluvia Itzel López-López. 2023. "The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review" Pharmaceuticals 16, no. 4: 496. https://doi.org/10.3390/ph16040496
APA StyleNavarro-Tovar, G., Vega-Rodríguez, S., Leyva, E., Loredo-Carrillo, S., de Loera, D., & López-López, L. I. (2023). The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review. Pharmaceuticals, 16(4), 496. https://doi.org/10.3390/ph16040496








