Interactions of Antibacterial Naphthoquinones with Mesoporous Silica Surfaces: A Physicochemical and Theoretical Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Silica Particle Characterization
2.3. NQs Adsorption Measurements
2.4. Spectroscopic Characterization of SBA-15 and NQs
2.5. Theoretical Approach
3. Results
3.1. TEM Images of SBA-15 Particles
3.2. FT–IR of NQ, 2NQ, and 5NQ onto SBA-15 Particles
3.3. Adsorption Isotherm Models of NQ Derivatives on SBA-15 Particles
3.3.1. Langmuir Isotherm Model
3.3.2. Freundlich Isotherm Model
3.3.3. Temkin Isotherm Model
3.4. Theoretical Evaluation of the Adsorption Mechanism of NQ Derivatives on SBA-15
3.4.1. Molecular Model Optimization Calculation
3.4.2. Calculation of the Interaction Energy (ΔE)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naimi, T.; Ringwald, P.; Besser, R.; Thompson, S.; Bell, D. Antimicrobial resistance. Emerg. Infect. Dis. 2001, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.K.Y.; Tan, S.Y.Y.; Tang, S.Q.; Thien, V.K.; Chan, E.W.L. Synergistic antibacterial activity between 1,4-naphthoquinone and β-lactam antibiotics against methicillin-resistant staphylococcus aureus. Microb. Drug Resist. 2021, 27, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Yu, B.; Friedrich, D.; Li, J.; Shen, H.; Krautscheid, H.; Huang, S.D.; Kim, M.H. Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant S. aureus. Commun. Biol. 2020, 3, 529. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.H.; Han, M.K.; Yoo, D.J. Synthesis and antimicrobial evaluation of 1,4-naphthoquinone derivatives as potential antibacterial agents. ChemistryOpen 2019, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ibis, C.; Ozsoy-Gunes, Z.; Tuyun, A.F.; Ayla, S.S.; Bahar, H.; Stasevych, M.V.; Musyanovych, R.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis, antibacterial and antifungal evaluation of thio- or piperazinyl-substituted 1,4-naphthoquinone derivatives. J. Sulfur Chem. 2016, 37, 477–487. [Google Scholar] [CrossRef]
- Estolano-Cobián, A.; Noriega-Iribe, E.; Díaz-Rubio, L.; Padrón, J.M.; Brito-Perea, M.; Cornejo-Bravo, J.M.; Chávez, D.; Rivera, R.R.; Quintana-Melgoza, J.M.; Cruz-Reyes, J.; et al. Antioxidant, antiproliferative, and acetylcholinesterase inhibition activity of amino alcohol derivatives from 1,4-naphthoquinone. Med. Chem. Res. 2020, 29, 1986–1999. [Google Scholar] [CrossRef]
- Goleva, T.N.; Lyamzaev, K.G.; Rogov, A.G.; Khailova, L.S.; Epremyan, K.K.; Shumakovich, G.P.; Domnina, L.V.; Ivanova, O.Y.; Marmiy, N.V.; Zinevich, T.V.; et al. Mitochondria-targeted 1,4-naphthoquinone (SkQN) is a powerful prooxidant and cytotoxic agent. Biochim. Biophys. Acta—Bioenerg. 2020, 1861, 148210. [Google Scholar] [CrossRef]
- McCall, R.; Miles, M.; Lascuna, P.; Burney, B.; Patel, Z.; Sidoran, K.J.; Sittaramane, V.; Kocerha, J.; Grossie, D.A.; Sessler, J.L.; et al. Dual targeting of the cancer antioxidant network with 1,4-naphthoquinone fused gold(i) N-heterocyclic carbene complexes. Chem. Sci. 2017, 8, 5918–5929. [Google Scholar] [CrossRef]
- Clementino-Neto, J.; da Silva, J.K.S.; de Melo Bastos Cavalcante, C.; da Silva-Júnior, P.F.; David, C.C.; de Araújo, M.V.; Mendes, C.B.; de Queiroz, A.C.; da Silva, E.C.O.; de Souza, S.T.; et al. In vitro antitumor activity of dialkylamine-1,4-naphthoquinones toward human glioblastoma multiforme Cells. New J. Chem. 2022, 46, 4587–4602. [Google Scholar] [CrossRef]
- Shen, X.B.; Wang, Y.; Han, X.Z.; Sheng, L.Q.; Wu, F.F.; Liu, X. Design, synthesis and anticancer activity of naphthoquinone derivatives. J. Enzym. Inhib. Med. Chem. 2020, 35, 773–785. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Rhodes, D. Biosynthesis and molecular actions of specialized 1,4-naphthoquinone natural products produced by horticultural plants. Hortic. Res. 2016, 3, 16046. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone analogues: Potent antibacterial agents and mode of action evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef]
- Pereyra, C.E.; Dantas, R.F.; Ferreira, S.B.; Gomes, L.P.; Silva, F.P. The diverse mechanisms and anticancer potential of naphthoquinones. Cancer Cell Int. 2019, 19, 207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.H.; Piao, X.J.; Shen, G.N.; Meng, L.Q.; Zhang, Y.; Wang, J.R.; Li, J.Q.; Wang, H.; Xu, W.T.; et al. Novel 1,4-naphthoquinone derivatives induce reactive oxygen species-mediated apoptosis in liver cancer cells. Mol. Med. Rep. 2019, 19, 1654–1664. [Google Scholar] [CrossRef]
- Ishihara, Y.; Ishii, S.; Sakai, Y.; Yamamura, N.; Onishi, Y.; Shimamoto, N. Crucial role of cytochrome p450 in hepatotoxicity induced by 2,3-dimethoxy-1,4-naphthoquinone in rats. J. Appl. Toxicol. 2011, 31, 173–178. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Alkahtani, S.; Alarifi, S.; Aljarba, N.H.; Alghamdi, H.A.; Alkahtane, A.A. Mesoporous SBA-15 silica–loaded nano-formulation of quercetin: A probable radio-sensitizer for lung carcinoma. Dose-Response 2022, 20. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-property relationship for different mesoporous silica nanoparticles and its drug delivery applications: A review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef]
- Žid, L.; Zeleňák, V.; Almáši, M.; Zeleňáková, A.; Szücsová, J.; Bednarčík, J.; Šuleková, M.; Hudák, A.; Váhovská, L. Mesoporous silica as a drug delivery system for naproxen: Influence of surface functionalization. Molecules 2020, 25, 4722. [Google Scholar] [CrossRef]
- Gkiliopoulos, D.; Tsamesidis, I.; Theocharidou, A.; Pouroutzidou, G.K.; Christodoulou, E.; Stalika, E.; Xanthopoulos, K.; Bikiaris, D.; Triantafyllidis, K.; Kontonasaki, E. SBA-15 mesoporous silica as delivery vehicle for RhBMP-2 bone morphogenic protein for dental applications. Nanomaterials 2022, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.E.; Lee, J.H.; Jeong, J.H.; Kim, J. A biodegradation study of SBA-15 microparticles in simulated body fluid and in vivo. Langmuir 2015, 31, 6457–6462. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric sufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Fathi Vavsari, V.; Mohammadi Ziarani, G.; Badiei, A. The role of SBA-15 in drug delivery. RSC Adv. 2015, 5, 91686–91707. [Google Scholar] [CrossRef]
- Alazzawi, H.F.; Salih, I.K.; Albayati, T.M. Drug delivery of amoxicillin molecule as a suggested treatment for COVID-19 implementing functionalized mesoporous SBA-15 with aminopropyl groups. Drug Deliv. 2021, 28, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tovar, G.; Palestino, G.; Rosales-Mendoza, S. An overview on the role of silica-based materials in vaccine development. Expert Rev. Vaccines 2016, 15, 1449–1462. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Bordallo, H.N.; Bordenalli, M.A.; Akamatsu, M.A.; Trezena, A.G.; Tino-De-Franco, M.; Sant’Anna, O.A.; da Silva Martins, T.; de Souza Lopes, J.L.; de Abreu Fantini, M.C.; et al. Assessing the efficiency of SBA-15 as a nanocarrier for diphtheria anatoxin. Microporous Mesoporous Mater. 2021, 312, 110763. [Google Scholar] [CrossRef]
- Rathinavel, S.; Ekambaram, S.; Korrapati, P.S.; Sangeetha, D. Design and fabrication of electrospun SBA-15-incorporated PVA with curcumin: A biomimetic nanoscaffold for skin tissue engineering. Biomed. Mater. 2020, 15, 035009. [Google Scholar] [CrossRef]
- Li, K.; Sun, H.; Sui, H.; Zhang, Y.; Liang, H.; Wu, X.; Zhao, Q. Composite mesoporous silica nanoparticle/chitosan nanofibers for bone tissue engineering. RSC Adv. 2015, 5, 17541–17549. [Google Scholar] [CrossRef]
- Ugliengo, P.; Sodupe, M.; Musso, F.; Bush, I.J.; Orlando, R.; Dovesi, R. Realistic models of hydroxylated amorphous silica surfaces and MCM- 41 mesoporous material simulated by large-scale periodic B3LYP calculations. Adv. Mater. 2008, 20, 4579–4583. [Google Scholar] [CrossRef]
- Tandon, H.; Chakraborty, T.; Suhag, V. A brief review on importance of DFT in drug design. Res. Med. Eng. Sci. 2019, 7, 791–795. [Google Scholar] [CrossRef]
- Makkar, P.; Ghosh, N.N. A review on the use of DFT for the prediction of the properties of nanomaterials. RSC Adv. 2021, 11, 27897–27924. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in molecules. Acc. Chem. Res. 1985, 18, 9–15. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Pereira, F.S.; de Araújo, R.C.M.U.; Ramos, M.N. The hydrogen bond strength: New proposals to evaluate the intermolecular interaction using DFT calculations and the AIM theory. Chem. Phys. Lett. 2006, 427, 181–184. [Google Scholar] [CrossRef]
- Najafi, M.; Morsali, A.; Bozorgmehr, M.R. DFT study of SiO2 nanoparticles as a drug delivery system: Structural and mechanistic aspects. Struct. Chem. 2019, 30, 715–726. [Google Scholar] [CrossRef]
- Grau, E.N.; Román, G.; Compañy, A.D.; Brizuela, G.; Juan, A.; Simonetti, S. Surface modification vs sorption strength: Study of nedaplatin drug supported on silica. Appl. Surf. Sci. 2019, 465, 693–699. [Google Scholar] [CrossRef]
- Noseda Grau, E.; Román, G.; Juan, J.; Compañy, A.D.; Simonetti, S. Advance on adsorption of amino-functionalized silica nanocarrier for the delivery of therapeutic ampicillin as drug model. Inorg. Chem. Commun. 2021, 123, 108346. [Google Scholar] [CrossRef]
- Luo, S.; Hao, J.; Gao, Y.; Liu, D.; Cai, Q.; Yang, X. Pore size effect on adsorption and release of metoprolol tartrate in mesoporous silica: Experimental and molecular simulation studies. Mater. Sci. Eng. C 2019, 100, 789–797. [Google Scholar] [CrossRef]
- Rafati, A.A.; Ebadi, A.; Bavafa, S.; Nowroozi, A. Kinetic study, structural analysis and computational investigation of novel xerogel based on drug-PEG/SiO2 for controlled release of enrofloxacin. J. Mol. Liq. 2018, 266, 733–742. [Google Scholar] [CrossRef]
- Masoumi, M.; Jahanshahi, M.; Ahangari, M.G.; Darzi, G.N. Density functional theory study on the interaction of chitosan monomer with TiO2, SiO2 and carbon nanotubes. Mater. Chem. Phys. 2020, 255, 123576. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–41. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The high score suite. In Powder Diffraction; Cambridge University Press: Cambridge, UK, 2014; Volume 29, pp. S13–S18. [Google Scholar]
- Dabrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 132–224. [Google Scholar] [CrossRef] [PubMed]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Chaker, M. Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J. Hazard. Mater. 2008, 153, 207–212. [Google Scholar] [CrossRef]
- El-khaiary, M.I. Least-squares regression of adsorption equilibrium data: Comparing the options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef]
- Dennington, R.D., II; Keith, T.A.; Milan, J.M. Gauss View 5.0 2008; Gaussian, Inc.: Wallingford, CT, USA, 2008. [Google Scholar]
- López, L.I.; Leyva, E.; de la Cruz, R.F.G. Naphthoquinones: More than natural pigments. Rev. Mex. Ciencias Farm. 2011, 42, 6–17. [Google Scholar]
- Dera, P.; Lazarz, J.D.; Prakapenka, V.B.; Barkley, M.; Downs, R.T. New insights into the high-pressure polymorphism of SiO2 cristobalite. Phys. Chem. Miner. 2011, 38, 517–529. [Google Scholar] [CrossRef]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The atomic simulation environment—A python library for working with atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Spitznagel, G.W.; Clark, T.; Chandrasekhar, J.; Schleyer, P.V.R. Stabilization of methyl anions by first-row substituents. The superiority of diffuse function-augmented basis sets for anion calculations. J. Comput. Chem. 1982, 3, 363–371. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09. Wallingford CT 2009, 121, 150–166. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Niculescu, V.C.; Paun, G.; Parvulescu, V. New organometallic complex supported on mesoporous silica and its enzymes activity inhibition properties. Appl. Organomet. Chem. 2018, 32, e4590. [Google Scholar] [CrossRef]
- Tahiri, N.; Khouchaf, L.; Elaatmani, M.; Louarn, G.; Zegzouti, A.; Daoud, M. Study of the thermal treatment of SiO2 aggregate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 62, p. 012002. [Google Scholar]
- Scherrer, P. Bestimmung der inneren struktur und der größe von kolloidteilchen mittels röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Patterson, A.L. The scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Fiorilli, S.; Onida, B.; Bonelli, B.; Garrone, E. In situ infrared study of SBA-15 functionalized with carboxylic groups incorporated by a co-condensation route. J. Phys. Chem. B 2005, 109, 16725–16729. [Google Scholar] [CrossRef]
- Esperanza Adrover, M.; Pedernera, M.; Bonne, M.; Lebeau, B.; Bucalá, V.; Gallo, L. Synthesis and characterization of mesoporous SBA-15 and SBA-16 as carriers to improve albendazole dissolution rate. Saudi Pharm. J. 2020, 28, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Yushin, N.; Humelnicu, D.; Grozdov, D.; Ignat, M.; Demcak, S.; Humelnicu, I. Sorption of Ce(Iii) by silica Sba-15 and titanosilicate Ets-10 from aqueous solution. Water 2021, 13, 3263. [Google Scholar] [CrossRef]
- Aranda-López, Y.; López-López, L.; Castro, K.E.N.; Ponce-Regalado, M.D.; Becerril-Villanueva, L.E.; Girón-Pérez, M.I.; Del Río-Araiza, V.H.; Morales-Montor, J. Cysticidal effect of a pure naphthoquinone on taenia crassiceps cysticerci. Parasitol. Res. 2021, 120, 3783–3794. [Google Scholar] [CrossRef]
- Rivera-Ávalos, E.; de Loera Carrera, D.; Araujo-Huitrado, J.G.; Escalante-García, I.L.; Muñoz-Sánchez, M.A.; Hernández, H.; López, J.A.; López López, L. Synthesis of amino acid–naphthoquinones and in vitro studies on cervical and breast cell lines. Molecules 2019, 24, 4285. [Google Scholar] [CrossRef]
- Silveira, G.Q.; Ronconi, C.M.; Vargas, M.D.; Gil, R.A.S.S.; Magalhães, A. Modified silica nanoparticles with an aminonaphthoquinone. J. Braz. Chem. Soc. 2011, 22, 961–967. [Google Scholar] [CrossRef][Green Version]
- Sun, D.; Kang, S.; Liu, C.; Lu, Q.; Cui, L.; Hu, B. Effect of zeta potential and particle size on the stability of SiO2 nanospheres as carrier for ultrasound imaging contrast agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Wang, P.; Keller, A.A. Natural and engineered nano and colloidal transport: Role of zeta potential in prediction of particle deposition. Langmuir 2009, 25, 6856–6862. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Everett, D.H.; Fairbridge, C.; Haynes, M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Guidelines for the characterization of porous solids. Stud. Surf. Sci. Catal. 1994, 87, 1–9. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Norsuraya, S.; Fazlena, H.; Norhasyimi, R. Sugarcane bagasse as a renewable source of silica to synthesize santa barbara amorphous-15 (SBA-15). Procedia Eng. 2016, 148, 839–846. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Zienkiewicz-Strzałka, M.; Skrzypczyńska, K.; Świątkowski, A.; Kuśmierek, K. Evaluation of the SBA-15 materials ability to accumulation of 4-chlorophenol on carbon paste electrode. Adsorption 2016, 22, 801–812. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. Liquids. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Frankl. Inst. 1917, 183, 102–105. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, J.; Dai, G.; Wu, J.; Yan, H. Adsorption characteristics of Pb(II) from aqueous solution onto a natural biosorbent, fallen Cinnamomum camphora leaves. Desalination 2010, 262, 174–182. [Google Scholar] [CrossRef]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Yao, Y.; Bing, H.; Feifei, X.; Xiaofeng, C. Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem. Eng. J. 2011, 170, 82–89. [Google Scholar] [CrossRef]
- Albayati, T.M.; Jassam, A.A.A. Synthesis and characterization of mesoporous materials as a carrier and release of prednisolone in drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 53, 101176. [Google Scholar] [CrossRef]
- Alkafajy, A.M.; Albayati, T.M. High performance of magnetic mesoporous modification for loading and release of meloxicam in drug delivery implementation. Mater. Today Commun. 2020, 23, 100890. [Google Scholar] [CrossRef]
- Abniki, M.; Azizi, Z.; Panahi, H.A. Design of 3-aminophenol-grafted polymer-modified zinc sulphide nanoparticles as drug delivery system. IET Nanobiotechnol. 2021, 15, 664–673. [Google Scholar] [CrossRef]
- Armendáriz-Vidales, G.; Martínez-González, E.; Cuevas-Fernández, H.J.; Fernández-Campos, D.O.; Burgos-Castillo, R.C.; Frontana, C. The stabilizing role of intramolecular hydrogen bonding in disubstituted hydroxy-quinones. Electrochim. Acta 2013, 110, 628–638. [Google Scholar] [CrossRef]
- Vega-Rodríguez, S.; Jiménez-Cataño, R.; Leyva, E.; Loredo-Carrillo, S.E. Intramolecular hydrogen bonds in fluorinated, methoxylated, or unsubstituted 2-(anilino)-1,4-naphthoquinones. A theoretical study. J. Fluor. Chem. 2013, 145, 58–62. [Google Scholar] [CrossRef]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Mayer, I.; Salvador, P. Overlap populations, bond orders and valences for “fuzzy” atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Bridgeman, A.J.; Cavigliasso, G.; Ireland, L.R.; Rothery, J. The mayer bond order as a tool in inorganic chemistry. J. Chem. Soc. Dalton Trans. 2001, 2095–2108. [Google Scholar] [CrossRef]
- Emamian, S.; Lu, T.; Kruse, H.; Emamian, H. Exploring nature and predicting strength of hydrogen bonds: A correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem. 2019, 40, 2868–2881. [Google Scholar] [CrossRef] [PubMed]
- Buemi, G.; Zuccarello, F. DFT study of the intramolecular hydrogen bonds in the amino and nitro-derivatives of malonaldehyde. Chem. Phys. 2004, 306, 115–129. [Google Scholar] [CrossRef]








| Sample | Crystallite Size | Hydrodynamic Diameter | Z Potential | Surface Area | Pore Volume | Pore Width |
|---|---|---|---|---|---|---|
| (Å) | (nm) | (mV) | (m2 g−1) | (cm3 g−1) | (nm) | |
| SBA-15 | 911.23 | 618 | −19.40 | 597.25 | 0.936 | 6.17 |
| NQ@SBA-15 | 669.33 | 2301 | −25.17 | 434.88 | 1.15 | 8.57 |
| 2NQ@SBA-15 | 814.66 | 667 | −28.40 | 441.70 | 1.16 | 8.70 |
| 5NQ@SBA-15 | 743.52 | 3576 | −22.97 | 438.99 | 1.14 | 8.84 |
| Langmuir | Freundlich | Temkin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q0 | L | R2 | KF | N | R2 | AT | BT | R2 | |
| (mg/g) | (dm3/mg) | (mg/g) | (L/g) | ||||||
| NQ @ SBA-15 | 3.9746 | 0.0390 | 0.9576 | 6.3010 | 0.1394 | 0.9592 | 0.2251 | 175.6193 | 0.4083 |
| 2NQ @ SBA-15 | 51,590.5 | 0.0009 | 0.9836 | 32.2276 | 0.9071 | 0.9847 | 0.0767 | 1.0405 | 0.8289 |
| 5NQ @ SBA-15 | 3417.4 | 0.0024 | 0.9719 | 1.8176 | 0.7174 | 0.9797 | 2.0352 | 0.0281 | 0.9071 |
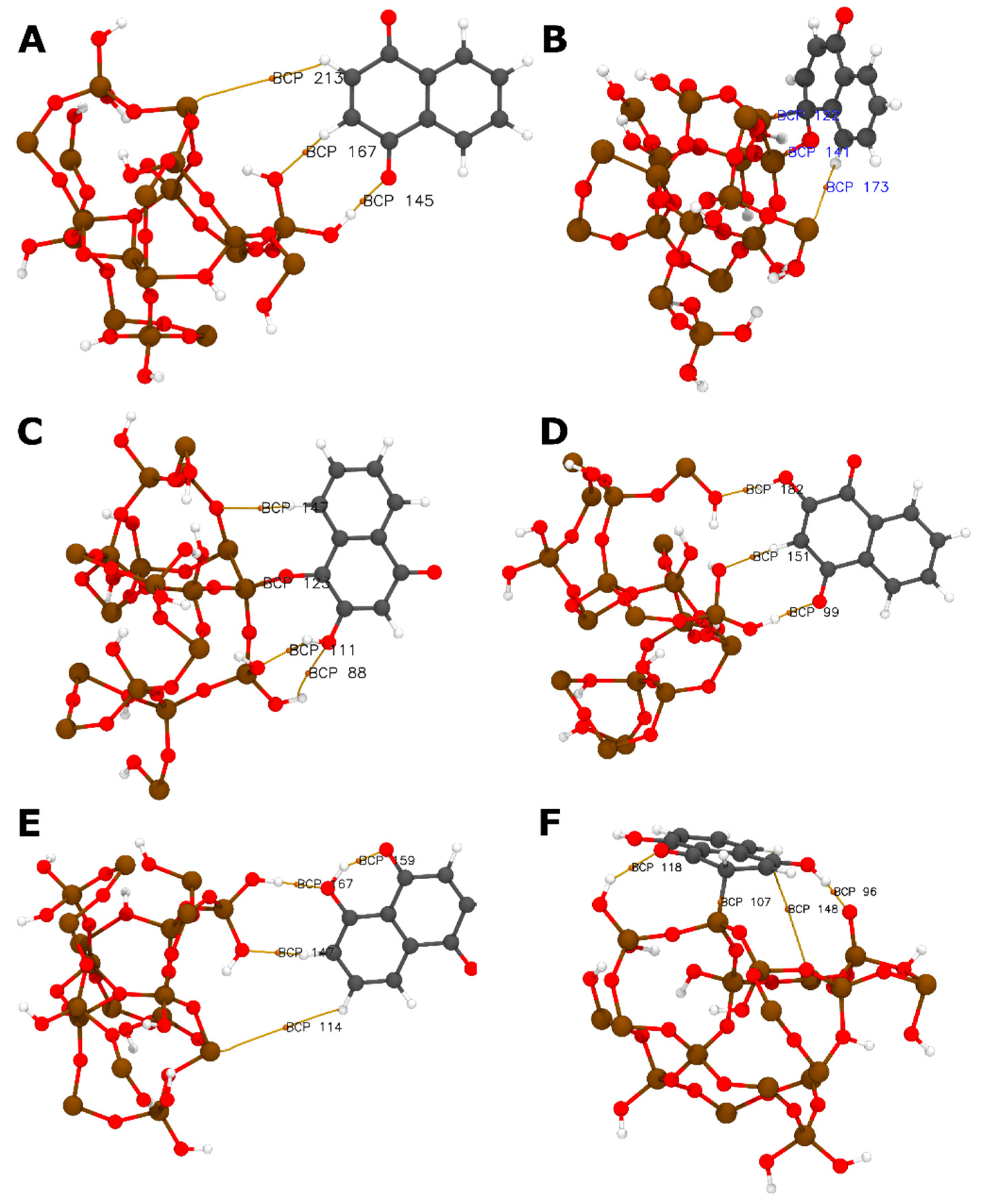
| H-Bond | LH | BCP | ρrBCP | BE | |
|---|---|---|---|---|---|
| Donnor | Acceptor | (Å) | (Ha) | kcal mol−1 | |
| NQ@SiO2 (H) | (ΔE = −10.74 kcal mol−1) | ||||
| SiO2−O35 | H76−NQ | 2.23 | 145 | 0.0149 | −2.57 |
| NQ−O74 | H53−SiO2 | 1.80 | 167 | 0.0326 | −6.53 |
| NQ@SiO2 (V) | (ΔE = −96.08 kcal mol−1) | ||||
| SiO2−Si15 | H80−NQ | 3.42 | 173 | 0.0034 | −0.16 |
| 2NQ@SiO2 (CH) | (ΔE = −18.99 kcal mol−1) | ||||
| SiO2−O49 | H80−2NQ | 3.27 | 147 | 0.0307 | −6.11 |
| 2NQ−O76 | H53−SiO2 | 2.52 | 88 | 0.0083 | −1.10 |
| SiO2−O35 | H81−2NQ | 2.04 | 111 | 0.0219 | −4.14 |
| 2NQ@SiO2 (OH) | (ΔE = −16.06 kcal mol−1) | ||||
| SiO2−O16 | H81−2NQ | 1.83 | 182 | 0.0320 | −6.40 |
| SiO2−O14 | H75−2NQ | 2.33 | 151 | 0.0130 | −2.15 |
| 2NQ−O73 | H59−SiO2 | 1.77 | 99 | 0.0355 | −7.18 |
| 5NQ@SiO2 (CH) | (ΔE = −8.95 kcal mol−1) | ||||
| 5NQ−O75 | H81−5NQ | 1.66 | 159 | 0.0492 | −10.24 |
| 5NQ−O80 | H53−SiO2 | 1.86 | 167 | 0.0276 | −5.41 |
| SiO2−O35 | H77−5NQ | 2.27 | 147 | 0.0097 | −1.42 |
| 5NQ@SiO2 (OH) | (ΔE = −88.92 kcal mol−1) | ||||
| SiO2−O35 | H55−5NQ | 1.56 | 96 | 0.0648 | −13.70 |
| 5NQ−O73 | H59−SiO2 | 2.12 | 118 | 0.0132 | −2.20 |
| Bond | LC | MBO | FBO | |
|---|---|---|---|---|
| (Å) | ||||
| NQ@SiO2 (V) | (ΔE = −96.08 kcal mol−1) | |||
| SiO2−Si48 | C69−NQ | 2.01 | 0.6365 | 0.7950 |
| NQ−O74 | Si36−SiO2 | 1.69 | 0.1579 | 1.3143 |
| 2NQ@SiO2 (C.H.) | (ΔE = −18.99 kcal mol−1) | |||
| NQ−O74 | Si36−SiO2 | 1.73 | 0.9474 | 1.1935 |
| 5NQ@SiO2 (O.H.) | (ΔE = −88.92 kcal mol−1) | |||
| SiO2−O11 | C72−NQ | 1.89 | 0.7912 | 0.9522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corpus-Mendoza, C.I.; de Loera, D.; López-López, L.I.; Acosta, B.; Vega-Rodríguez, S.; Navarro-Tovar, G. Interactions of Antibacterial Naphthoquinones with Mesoporous Silica Surfaces: A Physicochemical and Theoretical Approach. Pharmaceuticals 2022, 15, 1464. https://doi.org/10.3390/ph15121464
Corpus-Mendoza CI, de Loera D, López-López LI, Acosta B, Vega-Rodríguez S, Navarro-Tovar G. Interactions of Antibacterial Naphthoquinones with Mesoporous Silica Surfaces: A Physicochemical and Theoretical Approach. Pharmaceuticals. 2022; 15(12):1464. https://doi.org/10.3390/ph15121464
Chicago/Turabian StyleCorpus-Mendoza, César Iván, Denisse de Loera, Lluvia Itzel López-López, Brenda Acosta, Sarai Vega-Rodríguez, and Gabriela Navarro-Tovar. 2022. "Interactions of Antibacterial Naphthoquinones with Mesoporous Silica Surfaces: A Physicochemical and Theoretical Approach" Pharmaceuticals 15, no. 12: 1464. https://doi.org/10.3390/ph15121464
APA StyleCorpus-Mendoza, C. I., de Loera, D., López-López, L. I., Acosta, B., Vega-Rodríguez, S., & Navarro-Tovar, G. (2022). Interactions of Antibacterial Naphthoquinones with Mesoporous Silica Surfaces: A Physicochemical and Theoretical Approach. Pharmaceuticals, 15(12), 1464. https://doi.org/10.3390/ph15121464








