Binding Parameters of [11C]MPC-6827, a Microtubule-Imaging PET Radiopharmaceutical in Rodents
Abstract
1. Introduction
2. Materials and Methods
2.1. Radiochemistry
2.2. Lipophilicity (LogP)
2.3. Serum Stability
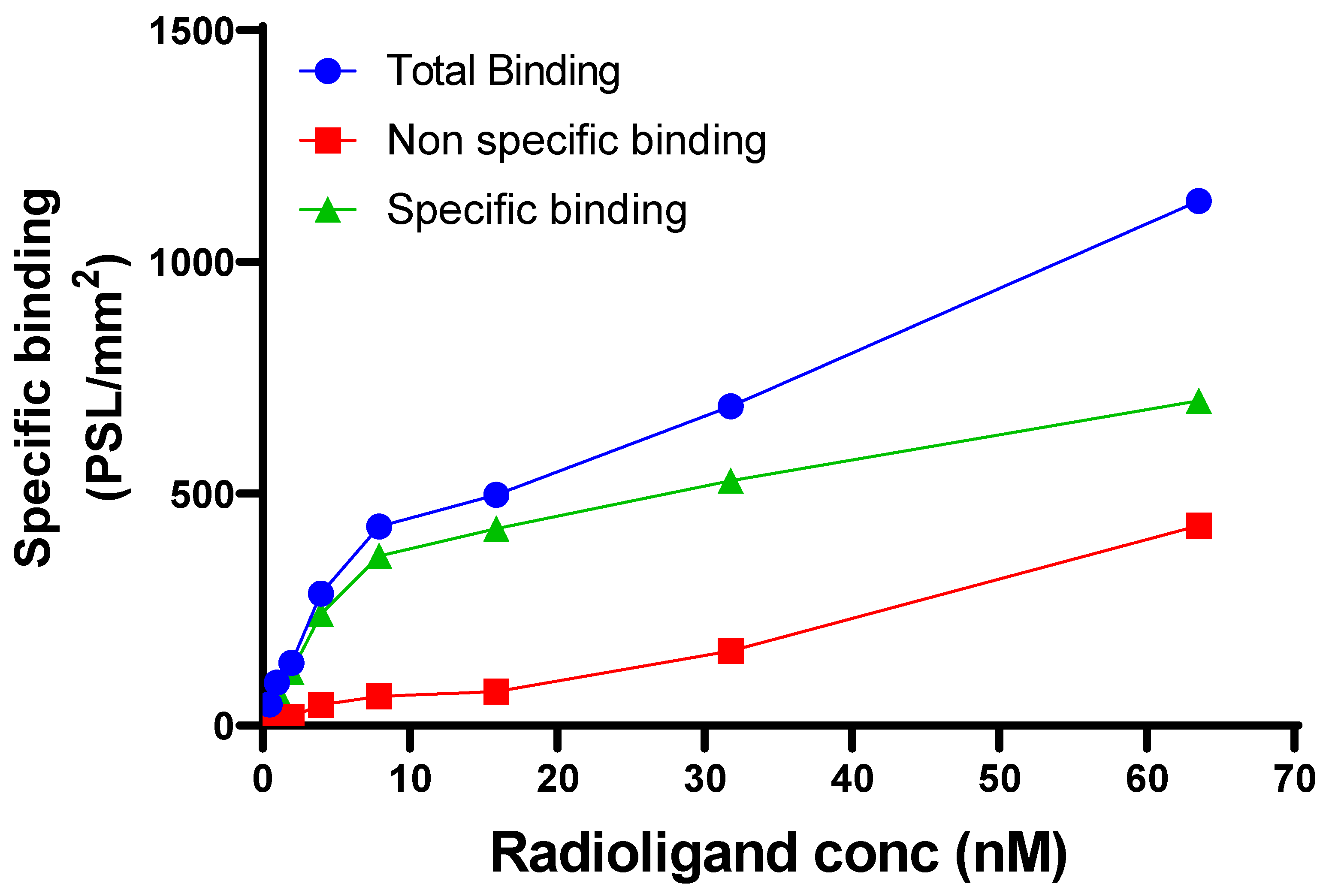
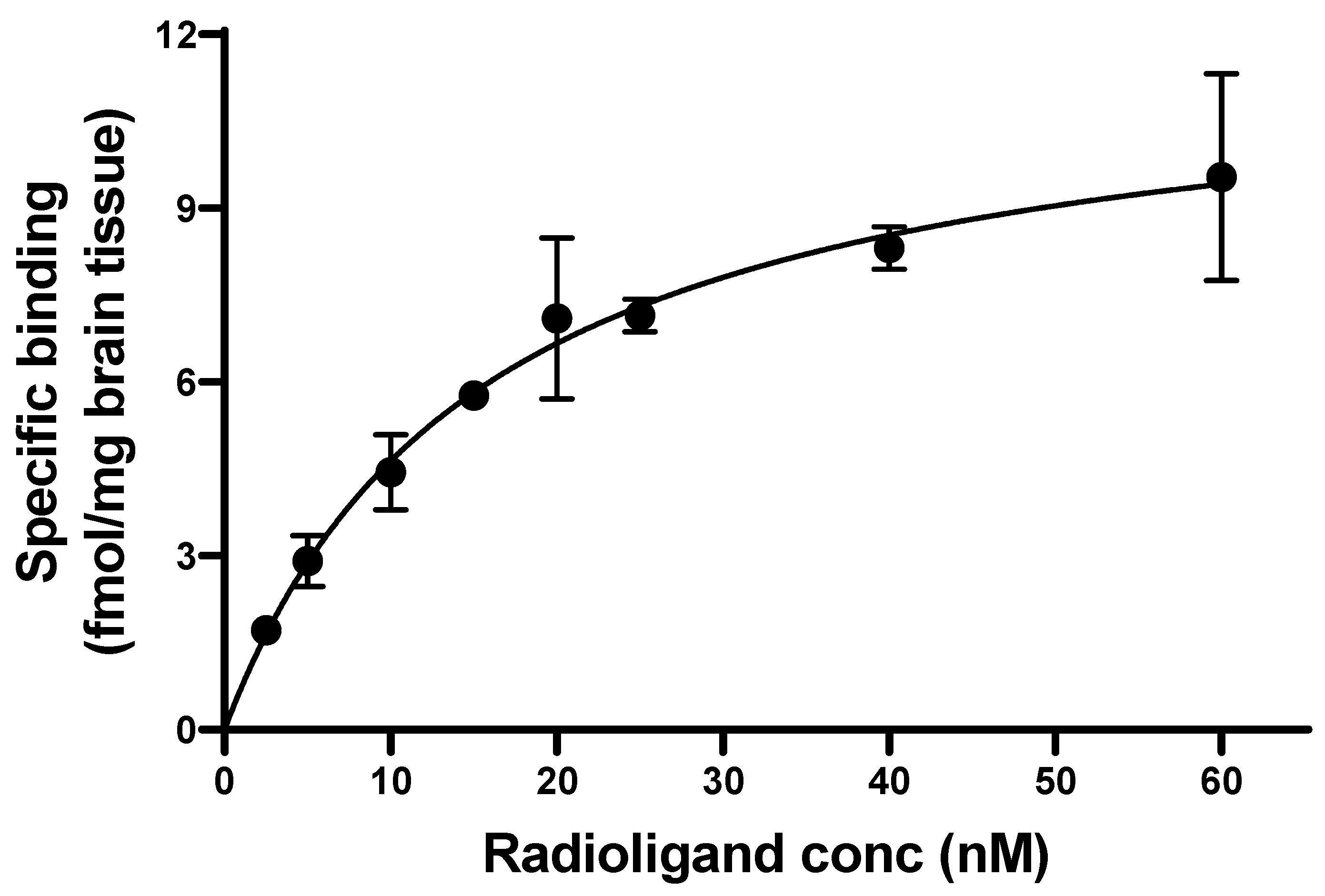
2.4. Binding Constants
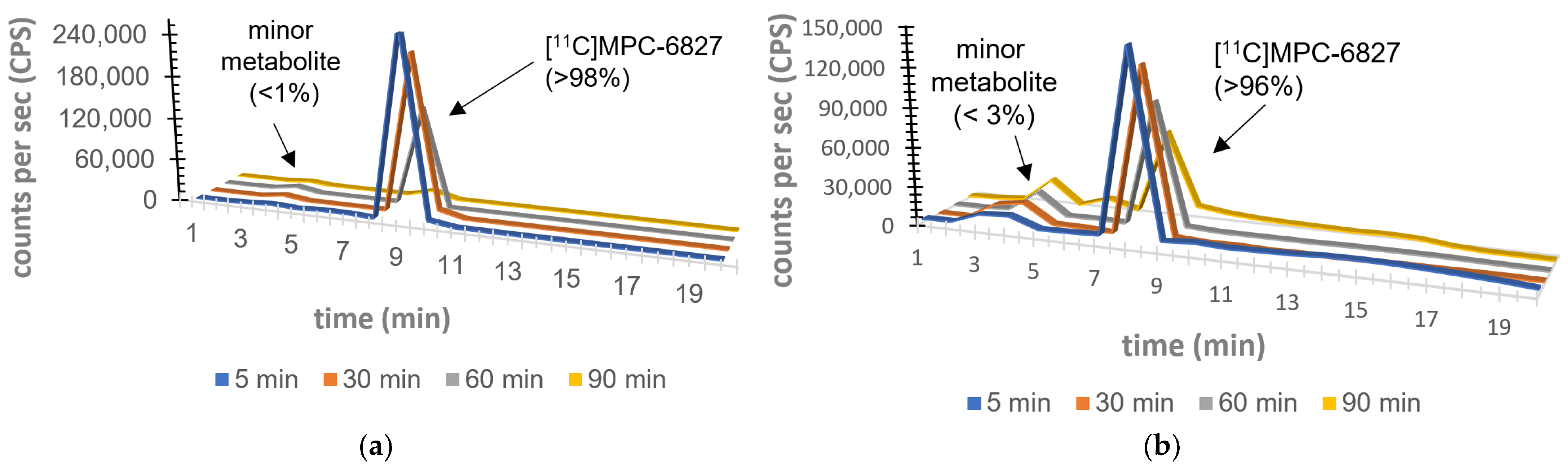
2.5. Plasma Metabolite Assays
2.6. Brain Metabolite Assays
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Ture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau: The Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The Role of Tau in Alzheimer’s Disease and Related Disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; Killick, R.; Lovestone, S. The GSK3 hypothesis of Alzheimer’s disease. J. Neurochem. 2008, 104, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Grundke-Iqbal, I. Pharmacological Approaches of Neurofibrillary Degeneration. Curr. Alzheimer Res. 2005, 2, 335–341. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X.; Adel, C.A.; Grundke-Iqbal, I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009, 118, 53–69. [Google Scholar] [CrossRef]
- Garcia, M.L.; Cleveland, D.W. Going new places using an old MAP: Tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001, 13, 41–48. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1739, 240–250. [Google Scholar] [CrossRef]
- Baird, F.J.; Bennett, C.L. Microtubule Defects and Neurodegeneration. J. Genet. Syndr. Gene Ther. 2013, 4, 203. [Google Scholar] [CrossRef]
- Franker, M.A.; Hoogenraad, C.C. Microtubule-based transport—Basic mechanisms, traffic rules and role in neurological pathogenesis. J. Cell Sci. 2013, 126, 2319–2329. [Google Scholar] [CrossRef]
- Hinckelmann, M.-V.; Zala, D.; Saudou, F. Releasing the brake: Restoring fast axonal transport in neurodegenerative disorders. Trends Cell Biol. 2013, 23, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Beharry, C.; Cohen, L.S.; Di, J.; Ibrahim, K.; Briffa-Mirabella, S.; Adel, C.A. Tau-induced neurodegeneration: Mechanisms and targets. Neurosci. Bull. 2014, 30, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Encalada, S.E.; Goldstein, L.S. Biophysical Challenges to Axonal Transport: Motor-Cargo Deficiencies and Neurodegeneration. Annu. Rev. Biophys. 2014, 43, 141–169. [Google Scholar] [CrossRef]
- Butner, K.A.; Kirschner, M.W. Tau protein binds to microtubules through a flexible array of distributed weak sites. J. Cell Biol. 1991, 115, 717–730. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal Hyperphosphorylation of Tau: Sites, Regulation, and Molecular Mechanism of Neurofibrillary Degeneration. J. Alzheimer’s Dis. 2013, 33 (Suppl. 1), S123–S139. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bloom, G.S. Cytoskeletal pathologies of Alzheimer disease. Cell Motil. Cytoskelet. 2009, 66, 635–649. [Google Scholar] [CrossRef]
- Dubey, J.; Ratnakaran, N.; Koushika, S.P. Neurodegeneration and microtubule dynamics: Death by a thousand cuts. Front. Cell. Neurosci. 2015, 9, 343. [Google Scholar] [CrossRef]
- Brunden, K.R.; Lee, V.M.; Smith, A.B., 3rd; Trojanowski, J.Q.; Ballatore, C. Altered microtubule dynamics in neurodegenerative disease: Therapeutic potential of microtubule-stabilizing drugs. Neurobiol. Dis. 2017, 105, 328–335. [Google Scholar] [CrossRef]
- Maschio, C.; Ni, R. Amyloid and Tau Positron Emission Tomography Imaging in Alzheimer’s Disease and Other Tauopathies. Front. Aging Neurosci. 2022, 14, 838034. [Google Scholar] [CrossRef]
- Chételat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Kasibhatla, S.; Baichwal, V.; Cai, S.X.; Roth, B.; Skvortsova, I.; Skvortsov, S.; Lukas, P.; English, N.M.; Sirisoma, N.; Drewe, J.; et al. MPC-6827: A Small-Molecule Inhibitor of Microtubule Formation That Is Not a Substrate for Multidrug Resistance Pumps. Cancer Res 2007, 67, 5865–5871. [Google Scholar] [CrossRef]
- Grossmann, K.F.; Colman, H.; Akerley, W.A.; Glantz, M.; Matsuoko, Y.; Beelen, A.P.; Yu, M.; De Groot, J.F.; Aiken, R.D.; Olsen, J.J.; et al. Phase I trial of verubulin (MPC-6827) plus carboplatin in patients with relapsed glioblastoma multiforme. J. Neuro-Oncol. 2012, 110, 257–264. [Google Scholar] [CrossRef]
- Mauck, K.; Demie, L.; Roman, O.; Fotheringham, L.; Middleton, S.; Mather, G. MPC-6827, a small molecule inhibitor of microtubule formation: Pharmacokinetics in Nu/+ mice, Sprague Dawley rats and beagle dogs following intravenous administration. Cancer Res. 2005, 65, 806. [Google Scholar]
- Chamberlain, M.C.; Grimm, S.; Phuphanich, S.; Recht, L.; Zhu, J.Z.; Kim, L.; Rosenfeld, S.; Fadul, C.E.; Brain Tumor Investigational Consortium. A phase 2 trial of verubulin for recurrent glioblastoma: A prospective study by the brain tumor investigational consortium (BTIC). J. Neuro-Oncology 2014, 118, 335–343. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.-J.; Priego, E.-M.; Bueno, O.; Martins, M.S.; Canela, M.-D.; Liekens, S. Blocking Blood Flow to Solid Tumors by Destabilizing Tubulin: An Approach to Targeting Tumor Growth. J. Med. Chem. 2016, 59, 8685–8711. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.D.; Sai, K.K.S.; Prabhakaran, J.; Oufkir, H.R.; Ramanathan, G.; Whitlow, C.T.; Dileep, H.; Mintz, A.; Mann, J.J. Radiosynthesis and in Vivo Evaluation of [11C]MPC-6827, the First Brain Penetrant Microtubule PET Ligand. J. Med. Chem. 2018, 61, 2118–2123. [Google Scholar] [CrossRef]
- Damuka, N.; Czoty, P.W.; Davis, A.T.; Nader, M.A.; Nader, S.H.; Craft, S.; Macauley, S.L.; Galbo, L.K.; Epperly, P.M.; Whitlow, C.T.; et al. PET Imaging of [11C]MPC-6827, a Microtubule-Based Radiotracer in Non-Human Primate Brains. Molecules 2020, 25, 2289. [Google Scholar] [CrossRef] [PubMed]
- Damuka, N.; Orr, M.; Czoty, P.W.; Weiner, J.L.; Martin, T.J.; Nader, M.A.; Bansode, A.H.; Pathirannahel, B.S.L.; Mintz, A.; Macauley, S.L.; et al. Effect of ethanol and cocaine on [11C]MPC-6827 uptake in SH-SY5Y cells. Mol. Biol. Rep. 2021, 48, 3871–3876. [Google Scholar] [CrossRef]
- Damuka, N.; Martin, T.J.; Bansode, A.H.; Krizan, I.; Martin, C.W.; Miller, M.; Whitlow, C.T.; Nader, M.A.; Sai, K.K.S. Initial Evaluations of the Microtubule-Based PET Radiotracer, [11C]MPC-6827 in a Rodent Model of Cocaine Abuse. Front. Med. 2022, 9, 817274. [Google Scholar] [CrossRef] [PubMed]
- Damuka, N.; Orr, M.E.; Bansode, A.H.; Krizan, I.; Miller, M.; Lee, J.; Macauley, S.L.; Whitlow, C.T.; Mintz, A.; Craft, S.; et al. Preliminary mechanistic insights of a brain-penetrant microtubule imaging PET ligand in a tau-knockout mouse model. EJNMMI Res. 2022, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Jin, L.; Garcia, A.; Da Silva, J.N.; Houle, S. An admonition when measuring the lipophilicity of radiotracers using counting techniques. Appl. Radiat. Isot. 2000, 54, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Padakanti, P.K.; Zhang, X.; Jin, H.; Cui, J.; Wang, R.; Li, J.; Flores, H.P.; Parsons, S.M.; Perlmutter, J.S.; Tu, Z. In Vitro and In Vivo Characterization of Two C-11-Labeled PET Tracers for Vesicular Acetylcholine Transporter. Mol. Imaging Biol. 2014, 16, 773–780. [Google Scholar] [CrossRef]
- Phelps, M.E.; Hoffman, E.J.; Mullani, N.A.; Ter-Pogossian, M.M. Application of annihilation coincidence detection to transaxial reconstruction tomography. J. Nucl. Med. 1975, 16, 210–224. [Google Scholar] [PubMed]
- Ter-Pogossian, M.M.; Phelps, M.E.; Hoffman, E.J.; Mullani, N.A. A Positron-Emission Transaxial Tomograph for Nuclear Imaging (PETT). Radiology 1975, 114, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.N. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef]
- Hulme, E.C.; Trevethick, M.A. Ligand binding assays at equilibrium: Validation and interpretation. Br. J. Pharmacol. 2010, 161, 1219–1237. [Google Scholar] [CrossRef]
- Eckelman, W.C.; Reba, R.C.; Gibson, R.E.; Rzeszotarski, W.J.; Vieras, F.; Mazaitis, J.K.; Francis, B. Receptor-binding radiotracers: A class of potential radiopharmaceuticals. J. Nucl. Med. 1979, 20, 350–357. [Google Scholar]
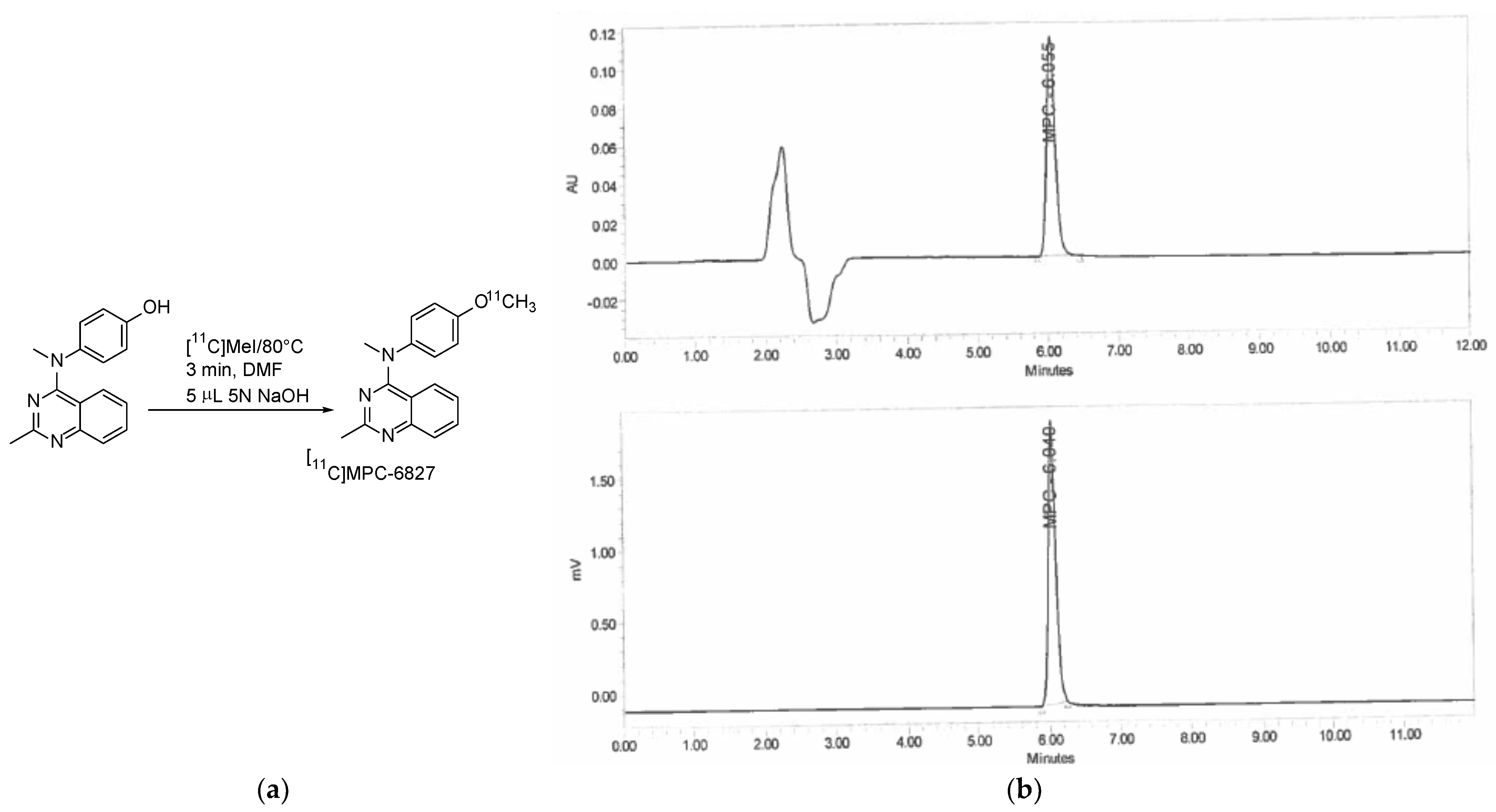
| Time Point | Radiochemical Purity |
|---|---|
| 15 min | 99.5% |
| 30 min | 99.3% |
| 60 min | 99.0% |
| 90 min | 99.0% |
| 120 min | 98.4% |
| 180 min | 97.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansode, A.H.; Bhoopal, B.; Gollapelli, K.K.; Damuka, N.; Krizan, I.; Miller, M.; Craft, S.; Mintz, A.; Solingapuram Sai, K.K. Binding Parameters of [11C]MPC-6827, a Microtubule-Imaging PET Radiopharmaceutical in Rodents. Pharmaceuticals 2023, 16, 495. https://doi.org/10.3390/ph16040495
Bansode AH, Bhoopal B, Gollapelli KK, Damuka N, Krizan I, Miller M, Craft S, Mintz A, Solingapuram Sai KK. Binding Parameters of [11C]MPC-6827, a Microtubule-Imaging PET Radiopharmaceutical in Rodents. Pharmaceuticals. 2023; 16(4):495. https://doi.org/10.3390/ph16040495
Chicago/Turabian StyleBansode, Avinash H., Bhuvanachandra Bhoopal, Krishna Kumar Gollapelli, Naresh Damuka, Ivan Krizan, Mack Miller, Suzanne Craft, Akiva Mintz, and Kiran Kumar Solingapuram Sai. 2023. "Binding Parameters of [11C]MPC-6827, a Microtubule-Imaging PET Radiopharmaceutical in Rodents" Pharmaceuticals 16, no. 4: 495. https://doi.org/10.3390/ph16040495
APA StyleBansode, A. H., Bhoopal, B., Gollapelli, K. K., Damuka, N., Krizan, I., Miller, M., Craft, S., Mintz, A., & Solingapuram Sai, K. K. (2023). Binding Parameters of [11C]MPC-6827, a Microtubule-Imaging PET Radiopharmaceutical in Rodents. Pharmaceuticals, 16(4), 495. https://doi.org/10.3390/ph16040495






