Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing
Abstract
1. Introduction
2. Inflammation in NSCLC Initiation and Progression
3. Inflammatory Cytokines in NSCLC
4. Drugs and Molecules Targeting Inflammation in NSCLC
5. Natural Compounds Targeting Inflammation in NSCLC
6. Repurposing Drugs with Anti-Inflammation Properties and Their Delivery via Inhalation in NSCLC
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Key Statistics for Lung Cancer; American Cancer Society: Kennesaw, GA, USA, 2022. [Google Scholar]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Kalinke, L.; Thakrar, R.; Janes, S.M. The promises and challenges of early non-small cell lung cancer detection: Patient perceptions, low-dose CT screening, bronchoscopy and biomarkers. Mol. Oncol. 2021, 15, 2544–2564. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A.; et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chem. Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Mustachio, L.M.; Roszik, J. Current Targeted Therapies for the Fight against Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 374. [Google Scholar] [CrossRef]
- Schrank, Z.; Chhabra, G.; Lin, L.; Iderzorig, T.; Osude, C.; Khan, N.; Kuckovic, A.; Singh, S.; Miller, R.J.; Puri, N. Current Molecular-Targeted Therapies in NSCLC and Their Mechanism of Resistance. Cancers 2018, 10, 224. [Google Scholar] [CrossRef]
- Fukui, T.; Tachihara, M.; Nagano, T.; Kobayashi, K. Review of Therapeutic Strategies for Anaplastic Lymphoma Kinase-Rearranged Non-Small Cell Lung Cancer. Cancers 2022, 14, 1184. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Fabikan, H.; Illini, O.; Weinlinger, C.; Setinek, U.; Krenbek, D.; Prosch, H.; Rauter, M.; Schumacher, M.; Wöll, E.; et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals 2020, 13, 371. [Google Scholar] [CrossRef]
- Sui, H.; Ma, N.; Wang, Y.; Li, H.; Liu, X.; Su, Y.; Yang, J. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J. Immunol. Res. 2018, 2018, 6984948. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Nakano-Narusawa, Y.; Yokohira, M.; Yamakawa, K.; Ye, J.; Tanimoto, M.; Wu, L.; Mukai, Y.; Imaida, K.; Matsuda, Y. Relationship between Lung Carcinogenesis and Chronic Inflammation in Rodents. Cancers 2021, 13, 2910. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, H.; Wei, T.; Lin, A.; Sun, Y.; Luo, P.; Zhang, J. Single-Cell RNA Sequencing Reveals the Heterogeneity of Tumor-Associated Macrophage in Non-Small Cell Lung Cancer and Differences Between Sexes. Front. Immunol. 2021, 12, 756722. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Li, R. Tumor-Promoting Inflammation in Non-Small Cell Lung Cancer. UCLA. 2016. Available online: https://escholarship.org/uc/item/6972h04z (accessed on 5 January 2023).
- Berardi, R.; Santoni, M.; Rinaldi, S.; Bower, M.; Tiberi, M.; Morgese, F.; Caramanti, M.; Savini, A.; Ferrini, C.; Torniai, M.; et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Ann. Transl. Med. 2019, 7, 572. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ji, Y.; Yang, M. Prognostic value of pre-treatment advanced lung cancer inflammation index in non-small cell lung cancer: A meta-analysis. Biomarkers 2022, 27, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Begum, R.; Mohammad, A.; Tabassum, R.M.; Shaeri, N. The Role of Introns for the Development of Inflammation-Mediated Cancer Cell. In Inflammation in the 21st Century; Vijay, K., Aguilera, S.A., Shamsadin, A.S., Eds.; IntechOpen: Rijeka, Croatia, 2021; p. 9. [Google Scholar]
- Wislez, M.; Fujimoto, N.; Izzo, J.G.; Hanna, A.E.; Cody, D.D.; Langley, R.R.; Tang, H.; Burdick, M.D.; Sato, M.; Minna, J.D.; et al. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res. 2006, 66, 4198–4207. [Google Scholar] [CrossRef]
- De la Garza, M.M.; Cumpian, A.M.; Daliri, S.; Castro-Pando, S.; Umer, M.; Gong, L.; Khosravi, N.; Caetano, M.S.; Ramos-Castañeda, M.; Flores, A.G.; et al. COPD-Type lung inflammation promotes K-ras mutant lung cancer through epithelial HIF-1α mediated tumor angiogenesis and proliferation. Oncotarget 2018, 9, 32972–32983. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bian, Y.; Wang, Y.; Wang, Y.; Duan, X.; Han, Y.; Zhang, L.; Wang, F.; Gu, Z.; Qin, Z. HIF-1α is necessary for activation and tumour-promotion effect of cancer-associated fibroblasts in lung cancer. J. Cell. Mol. Med. 2021, 25, 5457–5469. [Google Scholar] [CrossRef] [PubMed]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Kastenmüller, W.; Kastenmüller, K.; Kurts, C.; Seder, R.A. Dendritic cell-targeted vaccines—Hope or hype? Nat. Rev. Immunol. 2014, 14, 705–711. [Google Scholar] [CrossRef]
- Goh, C.C.; Roggerson, K.M.; Lee, H.C.; Golden-Mason, L.; Rosen, H.R.; Hahn, Y.S. Hepatitis C Virus-Induced Myeloid-Derived Suppressor Cells Suppress NK Cell IFN-γ Production by Altering Cellular Metabolism via Arginase-1. J. Immunol. 2016, 196, 2283–2292. [Google Scholar] [CrossRef]
- Lv, M.; Wang, K.; Huang, X.-J. Myeloid-derived suppressor cells in hematological malignancies: Friends or foes. J. Hematol. Oncol. 2019, 12, 105. [Google Scholar] [CrossRef]
- Fallah, J.; Rini, B.I. HIF Inhibitors: Status of Current Clinical Development. Curr. Oncol. Rep. 2019, 21, 6. [Google Scholar] [CrossRef]
- Yuan, A.; Hsiao, Y.J.; Chen, H.Y.; Chen, H.W.; Ho, C.C.; Chen, Y.Y.; Liu, Y.C.; Hong, T.H.; Yu, S.L.; Chen, J.J.; et al. Opposite Effects of M1 and M2 Macrophage Subtypes on Lung Cancer Progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.H.S.; Asif, M.; Petersen, S.H.; Subramaniam, A.V.; Kono, K.; Majid, A.; Oon, C.E. Angiogenesis: Managing the Culprits behind Tumorigenesis and Metastasis. Medicina 2018, 54, 8. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Mutvei, A.P.; Chivukula, I.V.; Andersson, E.R.; Ramsköld, D.; Sandberg, R.; Lee, K.L.; Kronqvist, P.; Mamaeva, V.; Ostling, P.; et al. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene 2013, 32, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Bekaert, S.; Fillet, M.; Detry, B.; Pichavant, M.; Marée, R.; Noel, A.; Rocks, N.; Cataldo, D. Inflammation-Generated Extracellular Matrix Fragments Drive Lung Metastasis. Cancer Growth Metastasis 2017, 10, 5539. [Google Scholar] [CrossRef]
- El Rayes, T.; Catena, R.; Lee, S.; Stawowczyk, M.; Joshi, N.; Fischbach, C.; Powell, C.A.; Dannenberg, A.J.; Altorki, N.K.; Gao, D.; et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl. Acad. Sci. USA 2015, 112, 16000–16005. [Google Scholar] [CrossRef]
- Markowitz, G.J.; Havel, L.S.; Crowley, M.J.; Ban, Y.; Lee, S.B.; Thalappillil, J.S.; Narula, N.; Bhinder, B.; Elemento, O.; Wong, S.T.; et al. Immune reprogramming via PD-1 inhibition enhances early-stage lung cancer survival. JCI Insight 2018, 3, e96836. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3(+) T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Lee, I.K.; Song, H.; Kim, H.; Kim, I.S.; Tran, N.L.; Kim, S.H.; Oh, S.J.; Lee, J.M. RORα Regulates Cholesterol Metabolism of CD8(+) T Cells for Anticancer Immunity. Cancers 2020, 12, 1733. [Google Scholar] [CrossRef]
- Jin, C.; Lagoudas, G.K.; Zhao, C.; Bullman, S.; Bhutkar, A.; Hu, B.; Ameh, S.; Sandel, D.; Liang, X.S.; Mazzilli, S.; et al. Commensal Microbiota Promote Lung Cancer Development via γδ T Cells. Cell 2019, 176, 998–1013.e1016. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Hochreiter, B.; Schmid, J.A. Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks. Front. Cell Dev. Biol. 2022, 10, 859863. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yin, Z.; Yang, L.; Fan, J.; Xu, J.; Jin, Y.; Yu, J.; Zhang, D.; Yang, G. Smoking Induced Extracellular Vesicles Release and Their Distinct Properties in Non-Small Cell Lung Cancer. J. Cancer 2019, 10, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Fenoglio, S.; Gao, D.C.; Camiolo, M.; Stiles, B.; Lindsted, T.; Schlederer, M.; Johns, C.; Altorki, N.; Mittal, V.; et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 15535–15540. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Q.; Sai, B.; Zheng, L.; Xu, J.; Yin, N.; Feng, X.; Xiang, J. Ligand-independent EphB1 signaling mediates TGF-β-activated CDH2 and promotes lung cancer cell invasion and migration. J. Cancer 2020, 11, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Caetano, M.S.; Zhang, H.; Cumpian, A.M.; Gong, L.; Unver, N.; Ostrin, E.J.; Daliri, S.; Chang, S.H.; Ochoa, C.E.; Hanash, S.; et al. IL6 Blockade Reprograms the Lung Tumor Microenvironment to Limit the Development and Progression of K-ras-Mutant Lung Cancer. Cancer Res. 2016, 76, 3189–3199. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Zhou, J.; Li, L.; Shapiro, S.D.; Xiao, G. Interleukin-6 Prevents the Initiation but Enhances the Progression of Lung Cancer. Cancer Res. 2015, 75, 3209–3215. [Google Scholar] [CrossRef]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pretre, V.; Papadopoulos, D.; Regard, J.; Pelletier, M.; Woo, J. Interleukin-1 (IL-1) and the inflammasome in cancer. Cytokine 2022, 153, 155850. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases. Theranostics 2017, 7, 1543–1588. [Google Scholar] [CrossRef] [PubMed]
- Tekpli, X.; Landvik, N.E.; Anmarkud, K.H.; Skaug, V.; Haugen, A.; Zienolddiny, S. DNA methylation at promoter regions of interleukin 1B, interleukin 6, and interleukin 8 in non-small cell lung cancer. Cancer Immunol. Immunother. 2013, 62, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Carranza-Rua, O.; Alfaro, C.; Oñate, C.; Martín-Algarra, S.; Perez, G.; Landazuri, S.F.; Gonzalez, A.; Gross, S.; Rodriguez, I.; et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 2014, 20, 5697–5707. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J.; Yun, J.; Kim, K.H.; Kim, S.H.; Lee, S.C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; Lee, K.T.; et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J. Korean Med. Sci. 2012, 27, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
- Stathopoulos, G.T.; Kollintza, A.; Moschos, C.; Psallidas, I.; Sherrill, T.P.; Pitsinos, E.N.; Vassiliou, S.; Karatza, M.; Papiris, S.A.; Graf, D.; et al. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res. 2007, 67, 9825–9834. [Google Scholar] [CrossRef]
- Van Horssen, R.; Ten Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Kong, H.; Zeng, Y.; Hao, M.; Yu, T.; Peng, J.; Xu, Z.; Chen, J.; Shi, H. Monocyte chemotactic protein-1 expression as a prognosic biomarker in patients with solid tumor: A meta analysis. Int. J. Clin. Exp. Pathol. 2014, 7, 3876–3886. [Google Scholar] [PubMed]
- Mulholland, B.S.; Forwood, M.R.; Morrison, N.A. Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) Drives Activation of Bone Remodelling and Skeletal Metastasis. Curr. Osteoporos. Rep. 2019, 17, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef]
- Heim, L.; Yang, Z.; Tausche, P.; Hohenberger, K.; Chiriac, M.T.; Koelle, J.; Geppert, C.I.; Kachler, K.; Miksch, S.; Graser, A.; et al. IL-9 Producing Tumor-Infiltrating Lymphocytes and Treg Subsets Drive Immune Escape of Tumor Cells in Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 859738. [Google Scholar] [CrossRef] [PubMed]
- Bezel, P.; Valaperti, A.; Steiner, U.; Scholtze, D.; Wieser, S.; Vonow-Eisenring, M.; Widmer, A.; Kowalski, B.; Kohler, M.; Franzen, D.P. Evaluation of cytokines in the tumor microenvironment of lung cancer using bronchoalveolar lavage fluid analysis. Cancer Immunol. Immunother. 2021, 70, 1867–1876. [Google Scholar] [CrossRef]
- Ritzmann, F.; Jungnickel, C.; Vella, G.; Kamyschnikow, A.; Herr, C.; Li, D.; Menger, M.M.; Angenendt, A.; Hoth, M.; Lis, A.; et al. IL-17C-mediated innate inflammation decreases the response to PD-1 blockade in a model of Kras-driven lung cancer. Sci. Rep. 2019, 9, 10353. [Google Scholar] [CrossRef] [PubMed]
- Melese, E.S.; Franks, E.; Cederberg, R.A.; Harbourne, B.T.; Shi, R.; Wadsworth, B.J.; Collier, J.L.; Halvorsen, E.C.; Johnson, F.; Luu, J.; et al. CCL5 production in lung cancer cells leads to an altered immune microenvironment and promotes tumor development. Oncoimmunology 2022, 11, 2010905. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Wang, Y.; Qi, Y.; Yang, W.; Li, Y. Farnesoid X Receptor Agonists as Therapeutic Target for Cardiometabolic Diseases. Front. Pharmacol. 2020, 11, 1247. [Google Scholar] [CrossRef]
- Shi, J.; Yang, F.; Zhou, N.; Jiang, Y.; Zhao, Y.; Zhu, J.; Prelaj, A.; Malhotra, J.; Normanno, N.; Danese, E.; et al. Isochorismatase domain-containing protein 1 (ISOC1) participates in DNA damage repair and inflammation-related pathways to promote lung cancer development. Transl. Lung Cancer Res. 2021, 10, 1444–1456. [Google Scholar] [CrossRef]
- Serresi, M.; Siteur, B.; Hulsman, D.; Company, C.; Schmitt, M.J.; Lieftink, C.; Morris, B.; Cesaroni, M.; Proost, N.; Beijersbergen, R.L.; et al. Ezh2 inhibition in Kras-driven lung cancer amplifies inflammation and associated vulnerabilities. J. Exp. Med. 2018, 215, 3115–3135. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, C.; Wretham, N.; Osooly, M.; Milne, K.; Dash, T.; Thornton, S.; Tessier-Cloutier, B.; Sathiyaseelan, P.; Bortnik, S.; Go, N.E.; et al. Loss of Parkinson’s susceptibility gene LRRK2 promotes carcinogen-induced lung tumorigenesis. Sci. Rep. 2021, 11, 2097. [Google Scholar] [CrossRef]
- Takahashi, H.; Ogata, H.; Nishigaki, R.; Broide, D.H.; Karin, M. Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 2010, 17, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Wallings, R.L.; Tansey, M.G. LRRK2 regulation of immune-pathways and inflammatory disease. Biochem. Soc. Trans. 2019, 47, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Shutinoski, B.; Hakimi, M.; Harmsen, I.E.; Lunn, M.; Rocha, J.; Lengacher, N.; Zhou, Y.Y.; Khan, J.; Nguyen, A.; Hake-Volling, Q.; et al. Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner. Sci. Transl. Med. 2019, 11, eaas9292. [Google Scholar] [CrossRef] [PubMed]
- Wojewska, D.N.; Kortholt, A. LRRK2 Targeting Strategies as Potential Treatment of Parkinson’s Disease. Biomolecules 2021, 11, 1101. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Y.; Tang, M.; Luo, Z.; Wang, X. Knockdown of ISOC1 suppresses cell proliferation in pancreatic cancer in vitro. Oncol. Lett. 2019, 17, 4263–4270. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, L.; Wang, F.; Bai, H.; Li, J.; Li, M.; Hu, X.; Cao, J.; Wang, G. Knockdown of ISOC1 inhibits the proliferation and migration and induces the apoptosis of colon cancer cells through the AKT/GSK-3β pathway. Carcinogenesis 2020, 41, 1123–1133. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Dutta, P.; Sabri, N.; Li, J.; Li, W.X. Role of STAT3 in lung cancer. Jakstat 2014, 3, e999503. [Google Scholar] [CrossRef]
- Siveen, K.S.; Sikka, S.; Surana, R.; Dai, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Targeting the STAT3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Looyenga, B.D.; Hutchings, D.; Cherni, I.; Kingsley, C.; Weiss, G.J.; Mackeigan, J.P. STAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PLoS ONE 2012, 7, e30820. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Roy, A.; Saqib, U.; Rajpoot, S.; Srivastava, M.; Naim, A.; Liu, D.; Saluja, R.; Faisal, S.M.; Pan, Q.; et al. Repurposing Thioridazine (TDZ) as an anti-inflammatory agent. Sci. Rep. 2018, 8, 12471. [Google Scholar] [CrossRef]
- Ciarcia, R.; Damiano, S.; Montagnaro, S.; Pagnini, U.; Ruocco, A.; Caparrotti, G.; d’Angelo, D.; Boffo, S.; Morales, F.; Rizzolio, F.; et al. Combined effects of PI3K and SRC kinase inhibitors with imatinib on intracellular calcium levels, autophagy, and apoptosis in CML-PBL cells. Cell Cycle 2013, 12, 2839–2848. [Google Scholar] [CrossRef]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef]
- Bernal-Bello, D.; Jaenes-Barrios, B.; Morales-Ortega, A.; Ruiz-Giardin, J.M.; García-Bermúdez, V.; Frutos-Pérez, B.; Farfán-Sedano, A.I.; de Ancos-Aracil, C.; Bermejo, F.; García-Gil, M.; et al. Imatinib might constitute a treatment option for lung involvement in COVID-19. Autoimmun. Rev. 2020, 19, 102565. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Pentheroudakis, G.; Katsanos, K.; Pavlidis, N. Systemic treatment-induced gastrointestinal toxicity: Incidence, clinical presentation and management. Ann. Gastroenterol. 2012, 25, 106–118. [Google Scholar]
- Tsao, A.S.; Liu, S.; Fujimoto, J.; Wistuba, I.I.; Lee, J.J.; Marom, E.M.; Charnsangavej, C.; Fossella, F.V.; Tran, H.T.; Blumenschein, G.R.; et al. Phase II trials of imatinib mesylate and docetaxel in patients with metastatic non-small cell lung cancer and head and neck squamous cell carcinoma. J. Thorac. Oncol. 2011, 6, 2104–2111. [Google Scholar] [CrossRef]
- Brenner, D.R.; Fanidi, A.; Grankvist, K.; Muller, D.C.; Brennan, P.; Manjer, J.; Byrnes, G.; Hodge, A.; Severi, G.; Giles, G.G.; et al. Inflammatory Cytokines and Lung Cancer Risk in 3 Prospective Studies. Am. J. Epidemiol. 2017, 185, 86–95. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Hermine, O.; Tharaux, P.-L.; Resche-Rigon, M.; Porcher, R.; Ravaud, P.; Bureau, S.; Dougados, M.; Tibi, A.; Azoulay, E.; et al. Sarilumab in adults hospitalised with moderate-to-severe COVID-19 pneumonia (CORIMUNO-SARI-1): An open-label randomised controlled trial. Lancet Rheumatol. 2022, 4, e24–e32. [Google Scholar] [CrossRef]
- Salton, F.; Confalonieri, P.; Campisciano, G.; Cifaldi, R.; Rizzardi, C.; Generali, D.; Pozzan, R.; Tavano, S.; Bozzi, C.; Lapadula, G.; et al. Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med. 2022, 11, 2951. [Google Scholar] [CrossRef]
- Timpani, C.A.; Rybalka, E. Calming the (Cytokine) Storm: Dimethyl Fumarate as a Therapeutic Candidate for COVID-19. Pharmaceuticals 2020, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Moll, M.; Kuemmerle-Deschner, J.B. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clin. Immunol. 2013, 147, 242–275. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, M.P.; Prasad, V. Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br. J. Cancer 2022, 127, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Stabile, L.P.; Farooqui, M.; Kanterewicz, B.; Abberbock, S.; Kurland, B.F.; Diergaarde, B.; Siegfried, J.M. Preclinical Evidence for Combined Use of Aromatase Inhibitors and NSAIDs as Preventive Agents of Tobacco-Induced Lung Cancer. J. Thorac. Oncol. 2018, 13, 399–412. [Google Scholar] [CrossRef]
- Amin, F.; Fathi, F.; Reiner, Ž.; Banach, M.; Sahebkar, A. The role of statins in lung cancer. Arch. Med. Sci. 2022, 18, 141–152. [Google Scholar] [CrossRef]
- Coimbra, M.; Banciu, M.; Fens, M.H.; de Smet, L.; Cabaj, M.; Metselaar, J.M.; Storm, G.; Schiffelers, R.M. Liposomal pravastatin inhibits tumor growth by targeting cancer-related inflammation. J. Control. Release 2010, 148, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Raymakers, A.; Sin, D.D.; Sadatsafavi, M.; FitzGerald, J.M.; Marra, C.A.; Lynd, L.D. Statin use and lung cancer risk in chronic obstructive pulmonary disease patients: A population-based cohort study. Respir. Res. 2020, 21, 118. [Google Scholar] [CrossRef]
- Huwiler, A.; Zangemeister-Wittke, U. The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: Recent findings and new perspectives. Pharmacol. Ther. 2018, 185, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Gendron, D.R.; Lemay, A.M.; Lecours, P.B.; Perreault-Vallières, V.; Huppé, C.A.; Bossé, Y.; Blanchet, M.R.; Dion, G.; Marsolais, D. FTY720 promotes pulmonary fibrosis when administered during the remodelling phase following a bleomycin-induced lung injury. Pulm. Pharmacol. Ther. 2017, 44, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.; Traini, D.; Ammit, A.J.; Young, P.M.; Ong, H.X. Repurposing of statins via inhalation to treat lung inflammatory conditions. Adv. Drug Deliv. Rev. 2018, 133, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Harjai, K.; Chhibber, S. Thalidomide treatment modulates macrophage pro-inflammatory function and cytokine levels in Klebsiella pneumoniae B5055 induced pneumonia in BALB/c mice. Int. Immunopharmacol. 2010, 10, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, Y.; Lin, W.; Zhong, H.; Xu, K.; Qi, X. Roles of sphingosine-1-phosphate signaling in cancer. Cancer Cell Int. 2019, 19, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, F.; Shang, L. Advances in antitumor effects of NSAIDs. Cancer Manag. Res. 2018, 10, 4631–4640. [Google Scholar] [CrossRef]
- Malcova, H.; Strizova, Z.; Milota, T.; Striz, I.; Sediva, A.; Cebecauerova, D.; Horvath, R. IL-1 Inhibitors in the Treatment of Monogenic Periodic Fever Syndromes: From the Past to the Future Perspectives. Front. Immunol. 2020, 11, 619257. [Google Scholar] [CrossRef]
- Yang, Y.; Li, N.; Wang, T.M.; Di, L. Natural Products with Activity against Lung Cancer: A Review Focusing on the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10827. [Google Scholar] [CrossRef]
- Zareie, A.; Soleimani, D.; Askari, G.; Jamialahmadi, T.; Guest, P.C.; Bagherniya, M.; Sahebkar, A. Cinnamon: A Promising Natural Product Against COVID-19. Adv. Exp. Med. Biol. 2021, 1327, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Horng, C.T.; Lee, Y.L.; Chen, P.N.; Lin, C.Y.; Liao, C.Y.; Hsieh, Y.S.; Chu, S.C. Cinnamomum Cassia Extracts Suppress Human Lung Cancer Cells Invasion by Reducing u-PA/MMP Expression through the FAK to ERK Pathways. Int. J. Med. Sci. 2018, 15, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Baek, S.H. Combination Therapy with Cinnamaldehyde and Hyperthermia Induces Apoptosis of A549 Non-Small Cell Lung Carcinoma Cells via Regulation of Reactive Oxygen Species and Mitogen-Activated Protein Kinase Family. Int. J. Mol. Sci. 2020, 21, 6229. [Google Scholar] [CrossRef] [PubMed]
- Isago, H.; Mitani, A.; Kohno, S.; Nagoshi, S.; Ishimori, T.; Saito, M.; Tamiya, H.; Miyashita, N.; Ishii, T.; Matsuzaki, H.; et al. The Japanese Herbal (Kampo) Medicine Hochuekkito Attenuates Lung Inflammation in Lung Emphysema. Biol. Pharm. Bull. 2021, 44, 39–45. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L. Biological properties of andrographolide, an active ingredient of Andrographis Paniculata: A narrative review. Ann. Transl. Med. 2021, 9, 1186. [Google Scholar] [CrossRef]
- He, W.; Sun, J.; Zhang, Q.; Li, Y.; Fu, Y.; Zheng, Y.; Jiang, X. Andrographolide exerts anti-inflammatory effects in Mycobacterium tuberculosis-infected macrophages by regulating the Notch1/Akt/NF-κB axis. J. Leukoc. Biol. 2020, 108, 1747–1764. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.A.; Alarcón, P.; Quiroga, J.; Manosalva, C.; Hancke, J. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules 2020, 26, 5. [Google Scholar] [CrossRef]
- Tsai, H.R.; Yang, L.M.; Tsai, W.J.; Chiou, W.F. Andrographolide acts through inhibition of ERK1/2 and Akt phosphorylation to suppress chemotactic migration. Eur. J. Pharmacol. 2004, 498, 45–52. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Attar, R.; Sabitaliyevich, U.Y.; Alaaeddine, N.; de Sousa, D.P.; Xu, B.; Cho, W.C. The Prowess of Andrographolide as a Natural Weapon in the War against Cancer. Cancers 2020, 12, 2159. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, W.J.; Zhou, Y.H.; Chen, Z.M.; Liu, K. Andrographolide inhibits non-small cell lung cancer cell proliferation through the activation of the mitochondrial apoptosis pathway and by reprogramming host glucose metabolism. Ann. Transl. Med. 2021, 9, 1701. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; He, Z.; Chen, M.; Ding, Y.; Yao, Y.; Duan, Y.; Zixuan, L.; Qi, C.; Zheng, L.; et al. Andrographolide Suppresses the Growth and Metastasis of Luminal-Like Breast Cancer by Inhibiting the NF-κB/miR-21-5p/PDCD4 Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 643525. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Jia, L.; Zhang, J.W.; Wang, D.J.; Ren, Q.; Zhang, W. Andrographolide Against Lung Cancer-New Pharmacological Insights Based on High-Throughput Metabolomics Analysis Combined with Network Pharmacology. Front. Pharmacol. 2021, 12, 596652. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Xiang, G.; Yuwen, D.; Gao, J.; Guo, W.; Wu, X.; Wu, X.; Sun, Y.; Su, Y.; Shen, Y.; et al. Inhibition of autophagy by andrographolide resensitizes cisplatin-resistant non-small cell lung carcinoma cells via activation of the Akt/mTOR pathway. Toxicol. Appl. Pharmacol. 2016, 310, 78–86. [Google Scholar] [CrossRef]
- Surien, O.; Ghazali, A.R.; Masre, S.F. Chemopreventive effects of pterostilbene through p53 and cell cycle in mouse lung of squamous cell carcinoma model. Sci. Rep. 2021, 11, 14862. [Google Scholar] [CrossRef]
- Huang, W.C.; Chan, M.L.; Chen, M.J.; Tsai, T.H.; Chen, Y.J. Modulation of macrophage polarization and lung cancer cell stemness by MUC1 and development of a related small-molecule inhibitor pterostilbene. Oncotarget 2016, 7, 39363–39375. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Cherng, J.Y.; Yang, Y.H.; Lin, C.L.; Kuan, F.C.; Lin, Y.Y.; Lin, Y.S.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; et al. Danshen improves survival of patients with advanced lung cancer and targeting the relationship between macrophages and lung cancer cells. Oncotarget 2017, 8, 90925–90947. [Google Scholar] [CrossRef]
- Li, H.; Huang, N.; Zhu, W.; Wu, J.; Yang, X.; Teng, W.; Tian, J.; Fang, Z.; Luo, Y.; Chen, M.; et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer 2018, 18, 579. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Song, W.; Zhang, Y.; Dong, X.; Tan, M. Ginsenoside Rh2 Inhibits Migration of Lung Cancer Cells under Hypoxia via mir-491. Anticancer Agents Med. Chem. 2019, 19, 1633–1641. [Google Scholar] [CrossRef]
- Kloc, M.; Ghobrial, R.M.; Lipińska-Opałka, A.; Wawrzyniak, A.; Zdanowski, R.; Kalicki, B.; Kubiak, J.Z. Effects of vitamin D on macrophages and myeloid-derived suppressor cells (MDSCs) hyperinflammatory response in the lungs of COVID-19 patients. Cell. Immunol. 2021, 360, 104259. [Google Scholar] [CrossRef]
- Akutsu, T.; Kitamura, H.; Himeiwa, S.; Kitada, S.; Akasu, T.; Urashima, M. Vitamin D and Cancer Survival: Does Vitamin D Supplementation Improve the Survival of Patients with Cancer? Curr. Oncol. Rep. 2020, 22, 62. [Google Scholar] [CrossRef]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D Supplementation and Survival of Patients with Non-small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Y.; Zhang, Y.; Liu, Y.; Yu, Y.; Ma, M. Resveratrol Induces Autophagy and Apoptosis in Non-Small-Cell Lung Cancer Cells by Activating the NGFR-AMPK-mTOR Pathway. Nutrients 2022, 14, 2413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, Q.; Zhai, Y.; Lu, D.; Li, C.; Fu, Y.; Zheng, Z.; Song, Y.; Guo, J. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020, 111, 3802–3812. [Google Scholar] [CrossRef]
- Wang, G.; Mohammadtursun, N.; Lv, Y.; Zhang, H.; Sun, J.; Dong, J. Baicalin Exerts Anti-Airway Inflammation and Anti-Remodelling Effects in Severe Stage Rat Model of Chronic Obstructive Pulmonary Disease. Evid. Based Complement. Alternat. Med. 2018, 2018, 7591348. [Google Scholar] [CrossRef]
- Xu, T.; Ge, X.; Lu, C.; Dai, W.; Chen, H.; Xiao, Z.; Wu, L.; Liang, G.; Ying, S.; Zhang, Y.; et al. Baicalein attenuates OVA-induced allergic airway inflammation through the inhibition of the NF-κB signaling pathway. Aging 2019, 11, 9310–9327. [Google Scholar] [CrossRef] [PubMed]
- Kourakis, S.; Timpani, C.A.; de Haan, J.B.; Gueven, N.; Fischer, D.; Rybalka, E. Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility? Pharmaceuticals 2020, 13, 306. [Google Scholar] [CrossRef]
- Cattani-Cavalieri, I.; da Maia Valença, H.; Moraes, J.A.; Brito-Gitirana, L.; Romana-Souza, B.; Schmidt, M.; Valença, S.S. Dimethyl Fumarate Attenuates Lung Inflammation and Oxidative Stress Induced by Chronic Exposure to Diesel Exhaust Particles in Mice. Int. J. Mol. Sci. 2020, 21, 9658. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Makvandi, P.; Zarrabi, A.; Farkhondeh, T.; Samarghandian, S. Versatile role of curcumin and its derivatives in lung cancer therapy. J. Cell. Physiol. 2020, 235, 9241–9268. [Google Scholar] [CrossRef]
- Tsai, J.R.; Liu, P.L.; Chen, Y.H.; Chou, S.H.; Cheng, Y.J.; Hwang, J.J.; Chong, I.W. Curcumin Inhibits Non-Small Cell Lung Cancer Cells Metastasis through the Adiponectin/NF-κb/MMPs Signaling Pathway. PLoS ONE 2015, 10, e0144462. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, S.C.; Chen, I.C.; Chiu, F.Y.; Liu, P.L.; Huang, C.H.; Huang, K.H.; Fang, S.H.; Cheng, W.C.; Huang, S.P.; et al. Zerumbone Suppresses the LPS-Induced Inflammatory Response and Represses Activation of the NLRP3 Inflammasome in Macrophages. Front. Pharmacol. 2021, 12, 652860. [Google Scholar] [CrossRef]
- Radaei, Z.; Zamani, A.; Najafi, R.; Saidijam, M.; Jalilian, F.A.; Ezati, R.; Solgi, G.; Amini, R. Promising Effects of Zerumbone on the Regulation of Tumor-promoting Cytokines Induced by TNF-α-activated Fibroblasts. Curr. Med. Sci. 2020, 40, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Eroglu, A. The Promising Effects of Astaxanthin on Lung Diseases. Adv. Nutr. 2021, 12, 850–864. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, J.; Liu, T.; Jiao, G.; Li, C.; Hu, B. Astaxanthin inhibits proliferation and promotes apoptosis of A549 lung cancer cells via blocking JAK1/STAT3 pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016, 32, 784–788. [Google Scholar]
- Akduman, H.; Tayman, C.; Çakir, U.; Çakir, E.; Dilli, D.; Türkmenoğlu, T.T.; Gönel, A. Astaxanthin Prevents Lung Injury Due to Hyperoxia and Inflammation. Comb. Chem. High Throughput Screen. 2021, 24, 1243–1250. [Google Scholar] [CrossRef]
- You, W.; Li, L.; Sun, D.; Liu, X.; Xia, Z.; Xue, S.; Chen, B.; Qin, H.; Ai, J.; Jiang, H. Farnesoid X Receptor Constructs an Immunosuppressive Microenvironment and Sensitizes FXR(high)PD-L1(low) NSCLC to Anti-PD-1 Immunotherapy. Cancer Immunol. Res. 2019, 7, 990–1000. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alrumaihi, F.; Alsahli, M.A.; Alhommrani, M.F.; Khan, A.; Rahmani, A.H. Curcumin, an Active Constituent of Turmeric Spice: Implication in the Prevention of Lung Injury Induced by Benzo(a) Pyrene (BaP) in Rats. Molecules 2020, 25, 724. [Google Scholar] [CrossRef]
- Chai, Y.S.; Chen, Y.Q.; Lin, S.H.; Xie, K.; Wang, C.J.; Yang, Y.Z.; Xu, F. Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice. Biomed. Pharmacother. 2020, 125, 109946. [Google Scholar] [CrossRef]
- Hasan, M.; Paul, N.C.; Paul, S.K.; Saikat, A.S.M.; Akter, H.; Mandal, M.; Lee, S.S. Natural Product-Based Potential Therapeutic Interventions of Pulmonary Fibrosis. Molecules 2022, 27, 1481. [Google Scholar] [CrossRef]
- Mehta, P.P.; Dhapte-Pawar, V.S. Repurposing drug molecules for new pulmonary therapeutic interventions. Drug Deliv. Transl. Res. 2021, 11, 1829–1848. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.; Cho, W.C. Drug Repurposing in Cancer Therapy: Approached and Applications; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Talevi, A.; Bellera, C.L. Challenges and opportunities with drug repurposing: Finding strategies to find alternative uses of therapeutics. Expert Opin. Drug Discov. 2020, 15, 397–401. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Claus, S.; Weiler, C.; Schiewe, J.; Friess, W. How can we bring high drug doses to the lung? Eur. J. Pharm. Biopharm. 2014, 86, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.S.; Byron, P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Anzar, N.; Mirza, M.A.; Anwer, K.; Khuroo, T.; Alshetaili, A.S.; Alshahrani, S.M.; Meena, J.; Hasan, N.; Talegaonkar, S.; Panda, A.K.; et al. Preparation, evaluation and pharmacokinetic studies of spray dried PLGA polymeric submicron particles of simvastatin for the effective treatment of breast cancer. J. Mol. Liq. 2018, 249, 609–616. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Ghadiri, M.; Leong, C.R.; Young, P.M.; Traini, D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv. Drug Deliv. Rev. 2018, 133, 107–130. [Google Scholar] [CrossRef]
- Kumbhar, P.; Manjappa, A.; Shah, R.; Jha, N.K.; Singh, S.K.; Dua, K.; Disouza, J.; Patravale, V. Inhalation delivery of repurposed drugs for lung cancer: Approaches, benefits and challenges. J. Control. Release 2022, 341, 1–15. [Google Scholar] [CrossRef]
- Guagliardo, R.; Pérez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Fahad, S.H.B.; Nesamony, J. Mechanism and Ways of Pulmonary Drug Administration. In Handbook of Lung Targeted Drug Delivery Systems; Pathak, N.I.Y., Ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- He, Y.; Liang, Y.; Han, R.; Lu, W.L.; Mak, J.C.W.; Zheng, Y. Rational particle design to overcome pulmonary barriers for obstructive lung diseases therapy. J. Control. Release 2019, 314, 48–61. [Google Scholar] [CrossRef]
- Bäckman, P.; Arora, S.; Couet, W.; Forbes, B.; de Kruijf, W.; Paudel, A. Advances in experimental and mechanistic computational models to understand pulmonary exposure to inhaled drugs. Eur. J. Pharm. Sci. 2018, 113, 41–52. [Google Scholar] [CrossRef]
- Zellnitz, S.; Zellnitz, L.; Müller, M.T.; Meindl, C.; Schröttner, H.; Fröhlich, E. Impact of drug particle shape on permeability and cellular uptake in the lung. Eur. J. Pharm. Sci. 2019, 139, 105065. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Sivakumar, A. Biomedical application of microemulsion delivery systems: A review. J. Res. Pharm. 2022, 26, 1052–1064. [Google Scholar] [CrossRef]
- İsar, S.; Akbaba, H.; Akbaba, G.E.; Baspinar, Y. Development and characterization of cationic nanoemulsions as non-viral vectors for plasmid DNA delivery. J. Res. Pharm. 2020, 24, 952–960. [Google Scholar] [CrossRef]
- Gbian, D.L.; Omri, A. Lipid-Based Drug Delivery Systems for Diseases Managements. Biomedicines 2022, 10, 2137. [Google Scholar] [CrossRef]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in liposome technology: Preparation techniques and applications in food, functional foods, and bioactive delivery: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef]
- Loh, J.S.; Tan, L.K.S.; Lee, W.L.; Ming, L.C.; How, C.W.; Foo, J.B.; Kifli, N.; Goh, B.H.; Ong, Y.S. Do Lipid-based Nanoparticles Hold Promise for Advancing the Clinical Translation of Anticancer Alkaloids? Cancers 2021, 13, 5346. [Google Scholar] [CrossRef] [PubMed]
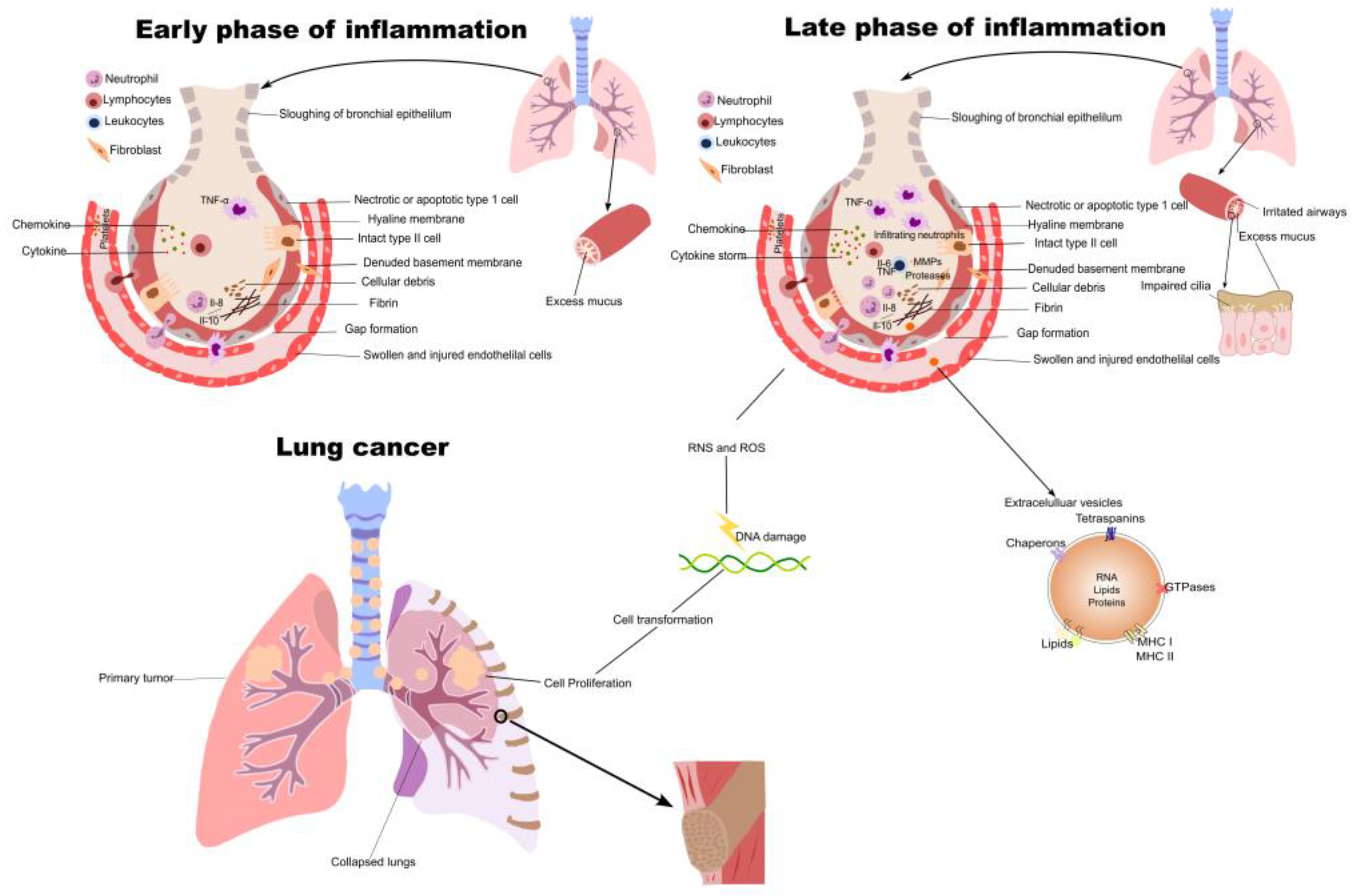
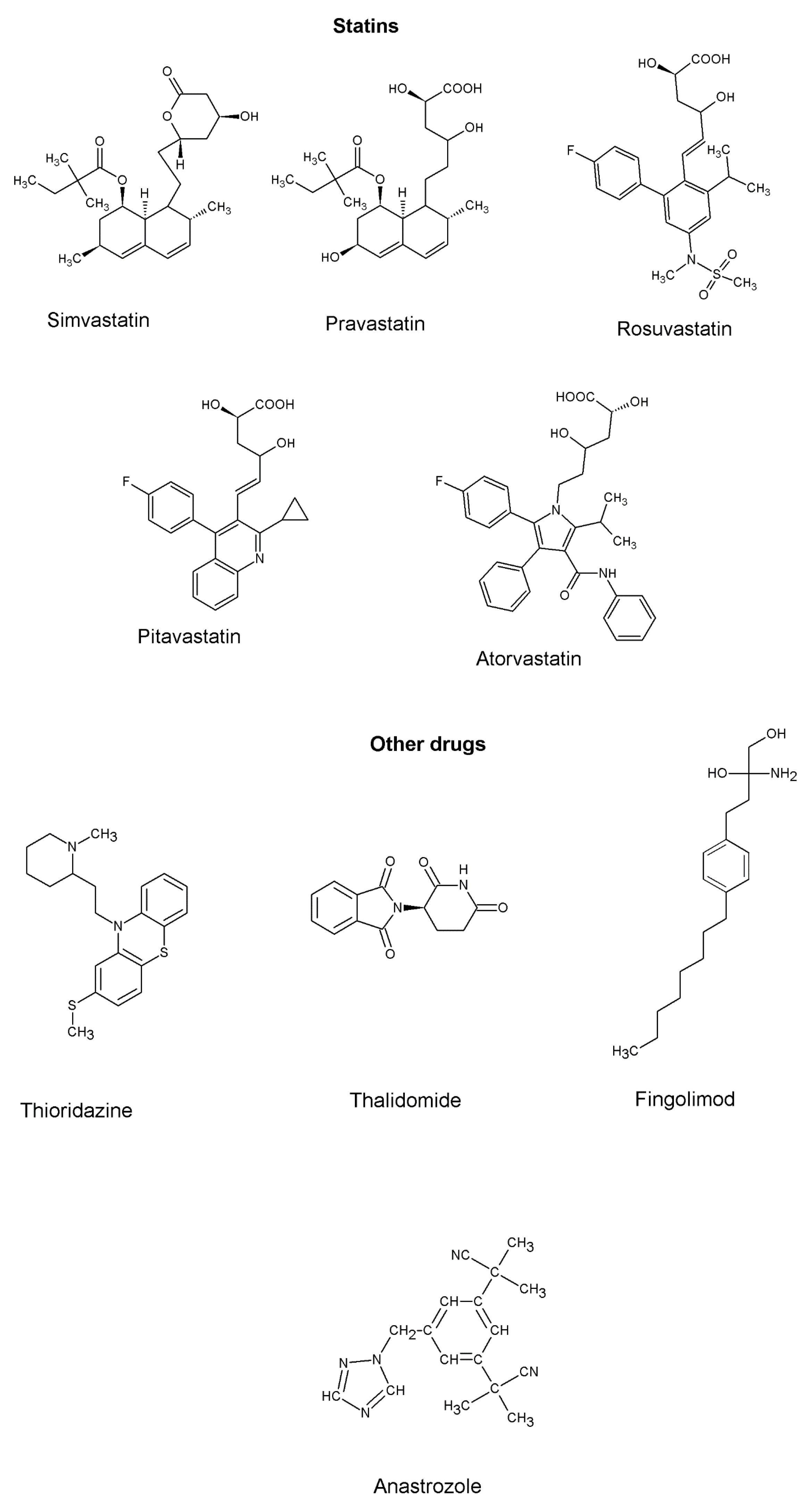


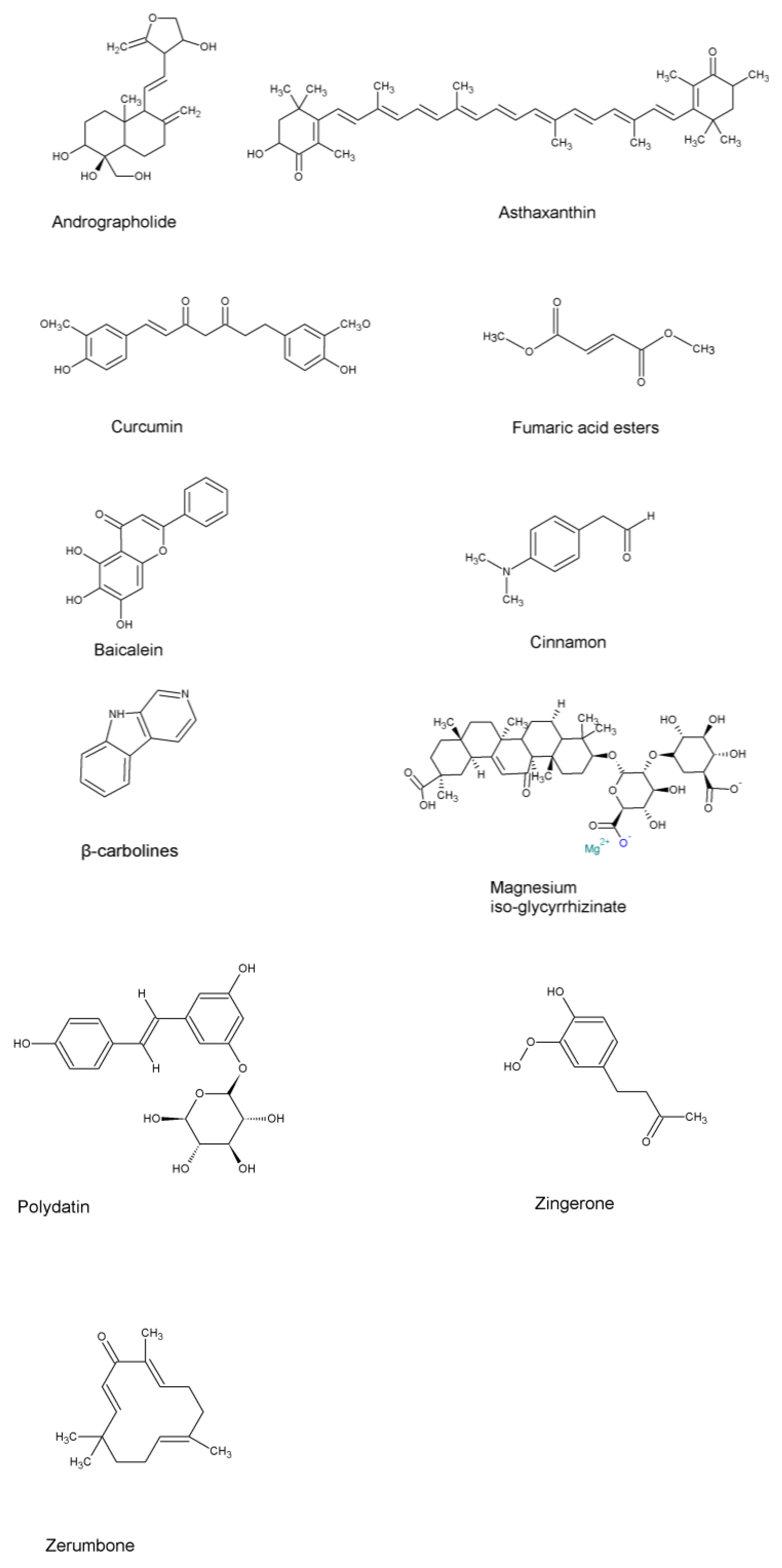
| Targets | Role in Lung Cancer Inflammation | References |
|---|---|---|
| Inflammatory cells | ||
| Neutrophils | Release proteases which degrade Tsp-1 and promotes tumor metastasis | [40] |
| Macrophages | Generate reactive oxygen and nitrogen intermediates, which induces DNA damage in proliferating cells, leading to neoplastic transformation | [15,21,33] |
| Myeloid derived suppressor cells (MDSC) | Degrade L-arginine, produce ROS, and secrete anti-inflammatory cytokines, such as IL-10 and TGF-β, to suppress the activity of other immune cells | [29,30] |
| Gamma-delta (γδ) T cells | Produce and release IL-17 and other effector molecules, which promotes inflammation and tumor proliferation | [44] |
| Fibroblasts | Produce and release inflammatory cytokines, such as MCP-1 and IL-6, in the tumor microenvironment | [37] |
| Inflammatory cytokines | ||
| IL-1β | Increased IL-1β expression is linked to aggressive tumor biology and tumor invasiveness | [66] |
| IL-4 | ||
| IL-6 | Produced by macrophages, T-lymphocytes, B-lymphocytes, and monocytes and promotes tumor cell proliferation, angiogenesis invasion, and metastasis | [67] |
| IL-8 | Produced by endothelial cells, epithelial cells, and fibroblasts to promote angiogenesis, proliferation, and cancer cell invasion | [67] |
| IL-9 | Through its effects on tumor-infiltrating T cells and tumor cell survival, promotes immune escape of lung tumor cells | [68] |
| IL-13 | IL-13 has been linked to lung cancer metastasis and progression | [69] |
| IL-17C | Promotes tumorigenesis in Kras-driven lung cancer by inducing inflammation | [70] |
| CCL5 | CCL5 production changes the immune microenvironment and encourages tumor growth | [71] |
| HIF-1α | Key mediator of adaptation to hypoxic condition and promotes tumorigenesis via inflammation | [25] |
| TNF-α | Tumor necrosis factor-alpha (TNF-α) controls inflammation and tumor development in non-small cell lung cancer (NSCLC) | [72] |
| Inflammatory gene expressions | ||
| ISOC1 | Participates in DNA damage repair and inflammation to promote lung cancer development | [73] |
| Ezh2 | Ezh2 inhibition amplifies inflammation in Kras-driven lung cancer | [74] |
| LRRK2 | Loss of LRRK2 promotes tumor initiation and size (tumorigenesis) | [75] |
| Signaling Proteins | ||
| NF-ĸB | Promotes tumor formation by inducing inflammation | [76] |
| JAK/STAT3 | [36] | |
| JNK1 | [76] | |
| Drug | Mechanism of Action | Initial Purpose | Performance Remarks | Reference |
|---|---|---|---|---|
Statins
| Inhibits 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase | To treat hypercholesterolemia | In vivo—Atorvastatin showed better anti-inflammatory properties than simvastatin | [108] |
| Thioridazine | Inhibits IκBα protein degradation, NF-ĸB activation | Anti-psychotic drug against schizophrenia | In vivo—potent anti-inflammatory target specific drug | [87] |
| Thalidomide | Inhibits the production of pro-inflammatory cytokines (TNF-α, IL-1α) | To treat morning sickness in pregnant women | In vivo—significant reduction in pro-inflammatory cytokines in pneumonia-induced acute lung inflammation | [109] |
| Fingolimod (FTY720) | Inhibits SphK/S1P signaling and S1PR3 in lung cancer metastasis | To treat multiple sclerosis | [110] | |
| Anastrozole | In combination with non-steroidal anti-inflammatory drug (Aspirin) to reduce circulating Beta-estradiol, pro-inflammatory cytokines, and macrophages recruitment in a tobacco induced lung cancer model | Hormone therapy | In vivo—downregulation of SOX-2 expression in the lungs | [102] |
NSAIDS
|
| To treat inflammation, antipyretic, analgesics | [111] | |
Tyrosine Kinase Inhibitor
| Inhibits LPS-induced production of TNF-a, IL-6, and IL-8, via inhibition of nuclear factor kappa B (NF-ĸB) | To treat leukemias characterized by the presence of the Philadelphia chromosome. Recently, it has been proposed to treat inflammation linked to COVID-19 infection | Significant decrease of NF-ĸB in chronic myelogenous leukemia patients | [88,90] |
H1 histamine antagonist
| Reduces NF-ĸB activity and TLR4 expression | To treat allergy symptoms | [89] | |
Phosphodiesterase Inhibitor
| Inhibit NF-ĸB by preventing nuclear translocation | To treat asthma and stroke | [89] | |
Interleukin-6 inhibitors
| Monocolonal antibodies inhibit IL-6 receptor and IL-6 | Treatment of inflammatory diseases such as rheumatoid arthritis and COVID-19 infection | [95] | |
Interleukin-1 inhibitors
| Inhibits IL-1 directly or binds to IL-1 receptor | [112] | ||
HIF-1α inhibitors
| Inhibits HIF-1α by either inhibiting its production, promoting the degradation, interfering the signaling pathway, or direct binding | [31] |
| Trial Number | Phase | Status | Estimated Completion Date | Treatment |
|---|---|---|---|---|
| NCT04648033 | 1 | Recruiting | December 2027 | Vancomycin + Stereotactic Body Radiation Therapy |
| NCT04905316 | 2 | Recruiting | May 2024 | Canakinumab + Durvalumab + Radiation therapy + Chemotherapy |
| NCT04382300 | 2 | Recruiting | April 2023 | Pyrotinib + thalidomide |
| NCT02779751 | 1 | Active, not recruiting | September 2023 | Abemaciclib + Pembrolizumab + Anastrozole |
| NCT04184921 | - | Active, not recruiting | December 2023 | Aspirin + Osimertinib |
| NCT00408460 | 2 | Completed | April 2017 | Imatinib Mesylate + paclitaxel |
| NCT05704634 | 1 | Not yet recruiting | January 2028 | Cemiplimab + Sarilumab |
| NCT04691817 | 2 | Not yet recruiting | April-2026 | Atezolizumab + Tocilizumab |
| NCT03337698 | 1 and 2 | Recruiting | August 2025 | Atezolizumab + Cobimetinib + RO6958688 + Docetaxel + CPI-444 + Pemetrexed + Carboplatin + Gemcitabine + Linagliptin + Tocilizumab + Ipatasertib + Bevacizumab + Sacituzumab Govitecan + Radiation + Evolocumab |
| NCT02638090 | 1 and 2 | Active, not recruiting | January 2024 | Vorinostat + Pembrolizumab |
| NCT01380769 | 2 | Completed | February 2014 | CRLX101 |
| NCT05636592 | 1 | Recruiting | December 2027 | Statins + PD-1/PD-L1 inhibitors |
| NCT05445791 | 3 | Recruiting | July 2025 | Metformin hydrochloride |
| Compound | Mechanism of Action | Initial Purpose | Performance Remarks | Reference |
|---|---|---|---|---|
| Andrographolide | Inhibition of NF-ĸB | Treatment of upper airway disorders | [122,124,125] | |
| Asthaxanthin | Regulating the nuclear factor erythroid 2-related factor/heme oxygenase-1 pathway, NF-ĸB signaling, MAPK signaling, JAK-STAT 3 signaling, Pi3-kinase/Akt pathway, and modulating immune response | Dietary supplement | [145,146,147] | |
| Curcumin | Inhibition of NF-ĸB | Dietary supplement | [149,150] | |
| Fumaric Acid Esters | Alters leukocyte, keratinocyte, and/or endothelial functions | To treat psoriasis and multiple sclerosis | [139] | |
| Baicalein | Inhibits metastasis (exact mechanism of action yet to be confirmed) | [136] | ||
| Kampo medicine, Hochuekkito, TJ-41 | Inhibited influenza A virus replication by IFN-α upregulation | To treat infectious disease, possesses virological activity | In vivo and in vitro study shows positive results | [117] |
| Cinnamon (cinnamaldehyde, cinnamic acid, 2-hydroxycinnamaldehyde, 2-methoxycinnamaldehyde, and eugenol) | Suppressed nitric oxide (NO), IL-6, TNF-α, and IL-1β production. Production and blocking of nuclear factor-ĸB (NF-ĸB) and mitogen-activated protein kinase (MAPK) | Has immunomodulator, antiseptic and antiviral properties | [114] | |
| β-carbolines | Inhibits NF-κB/p65 and EMT transition | To treat altitude sickness and possess anti-inflammatory properties | [151] | |
| Magnesium isoglycyrrhizinate (MgIG) | Inhibits fibroblast differentiation via the p38MAPK/Nox4/Akt pathway | Respiratory disorders, hyperdipsia, epilepsy, fever | [151] | |
| Polydatin (PD) | NLRP3 inflammasome and NF-κB pathway | Used to reduce symptoms of menopause, digestive system | [151] | |
| Zingerone (vanillylacetone) | Inhibiting NF-κB and MAPKs | To treat infections, nausea, bronchitis, dysentery, heartburn, cough, flatulence, diarrhea, loss of appetite | [151] | |
| Zerumbone | Inhibits TNF-α or LPS-induced production inflammatory cytokines via inhibition of NF-ĸB | To treat fever, sprains, asthma, torment, severe sprains, toothache, allergies, wounds, and stomachache | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajasegaran, T.; How, C.W.; Saud, A.; Ali, A.; Lim, J.C.W. Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing. Pharmaceuticals 2023, 16, 451. https://doi.org/10.3390/ph16030451
Rajasegaran T, How CW, Saud A, Ali A, Lim JCW. Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing. Pharmaceuticals. 2023; 16(3):451. https://doi.org/10.3390/ph16030451
Chicago/Turabian StyleRajasegaran, Thiviyadarshini, Chee Wun How, Anoosha Saud, Azhar Ali, and Jonathan Chee Woei Lim. 2023. "Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing" Pharmaceuticals 16, no. 3: 451. https://doi.org/10.3390/ph16030451
APA StyleRajasegaran, T., How, C. W., Saud, A., Ali, A., & Lim, J. C. W. (2023). Targeting Inflammation in Non-Small Cell Lung Cancer through Drug Repurposing. Pharmaceuticals, 16(3), 451. https://doi.org/10.3390/ph16030451







