Spiroleiferthione A and Oleiferthione A: Two Unusual Isothiocyanate-Derived Thioketone Alkaloids from Moringa oleifera Lam. Seeds
Abstract
1. Introduction
2. Results
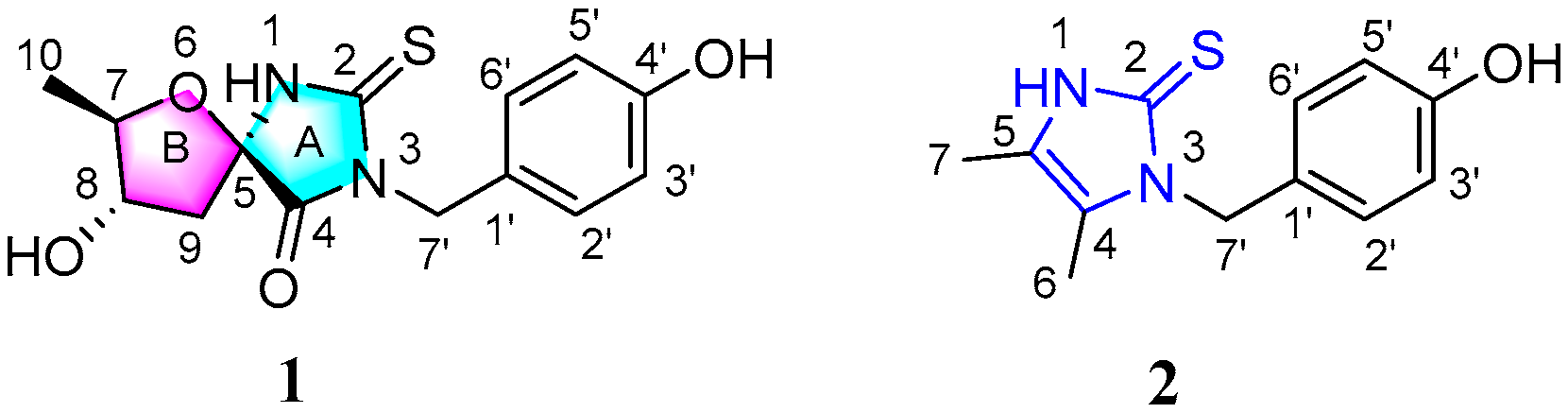
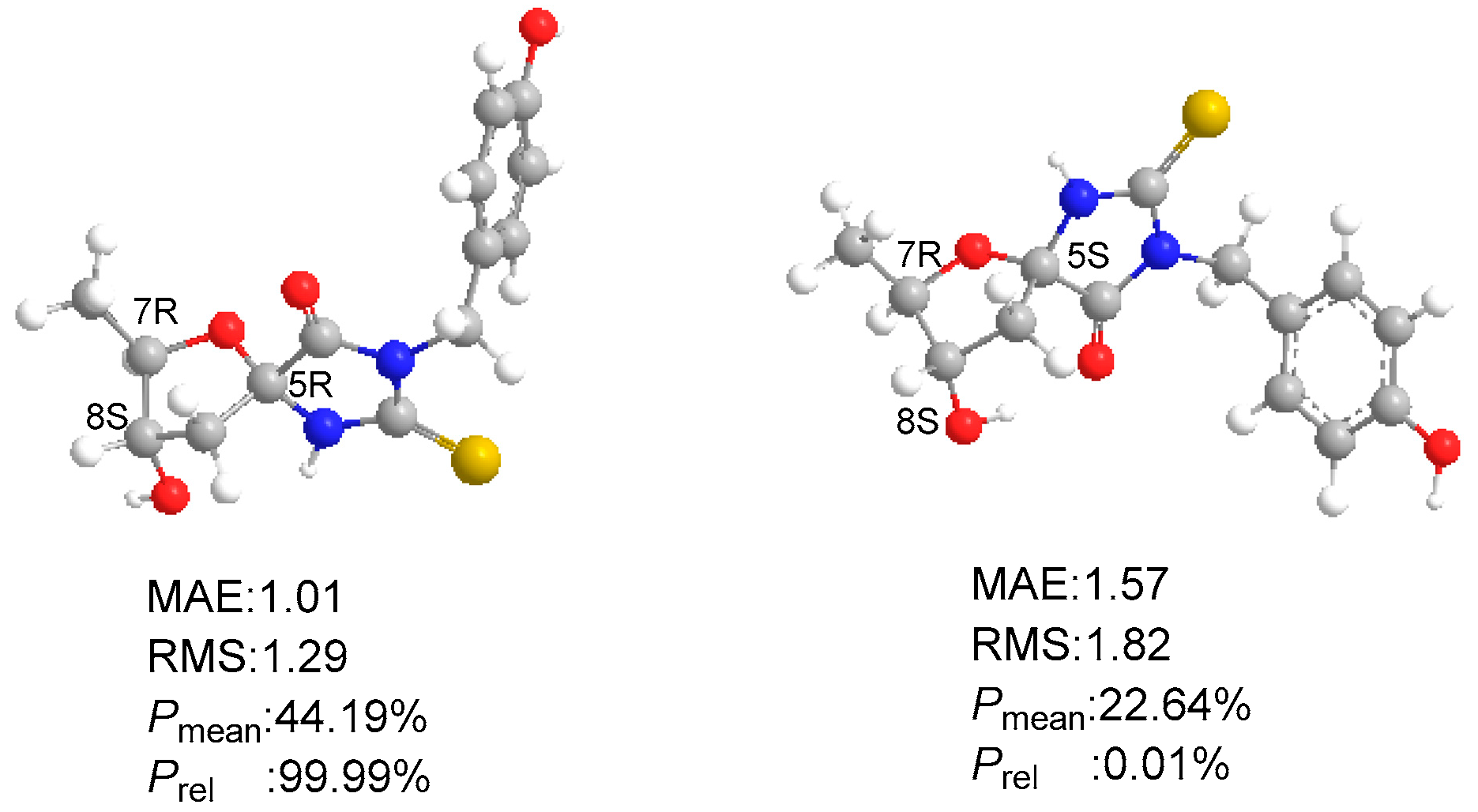
2.1. Structural Elucidation
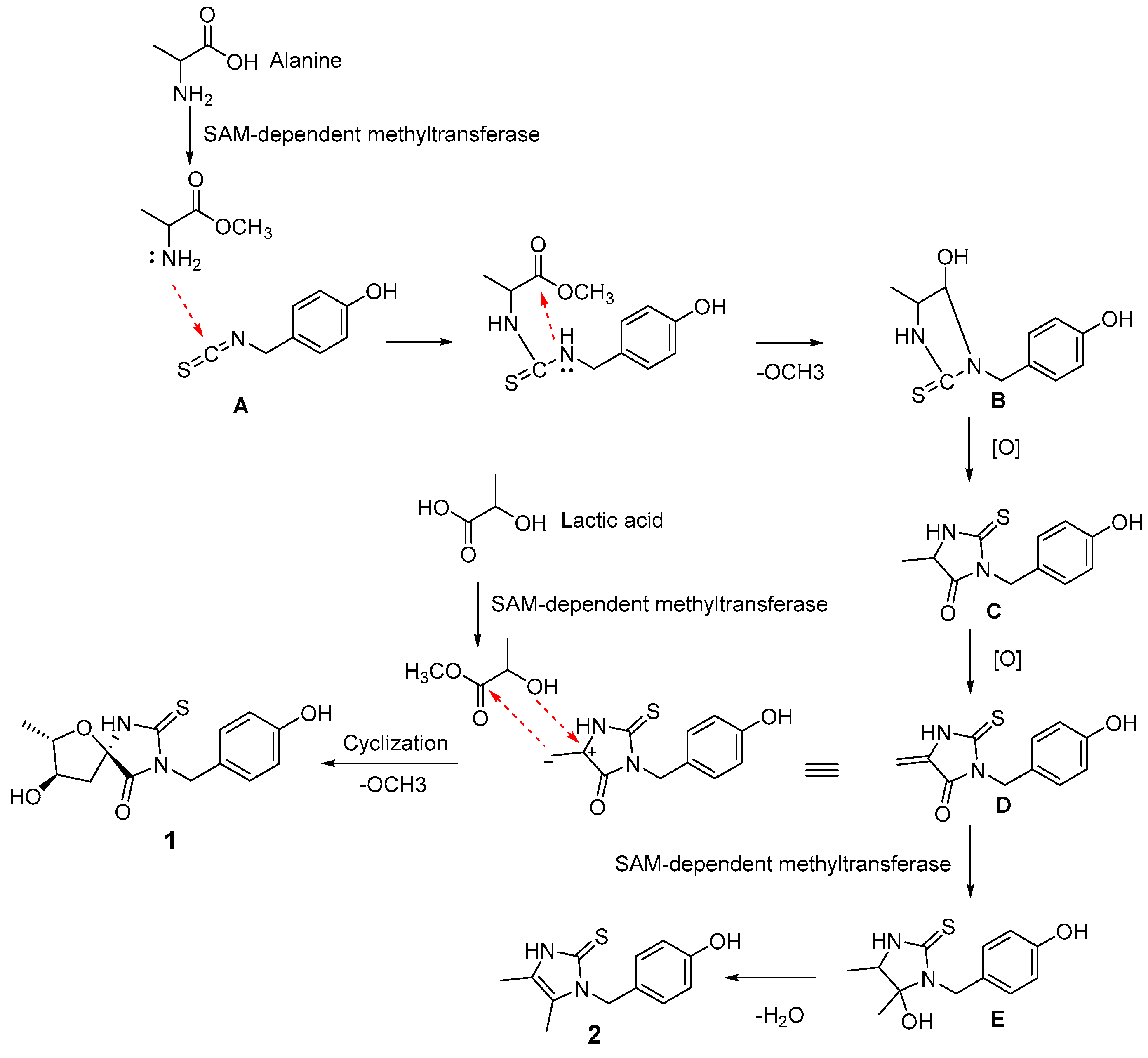
2.2. Proposed Biosynthetic Pathway of 1 and 2
2.3. Biological Activity Evaluation of 1 and 2
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Physicochemical Properties of Compounds 1 and 2
4.5. Antimicrobial Assay
4.6. Anti-Inflammatory Assay
4.7. Inhibitory Assay of High Glucose-Induced HRMC Proliferation
4.8. Statistical Analysis
4.9. Computational Section
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, S.; Kim, S.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, X.; Qiu, M. Progress on the Chemical Constituents Derived from Glucosinolates in Maca (Lepidium meyenii). Nat. Prod. Bioprospect. 2018, 8, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Khan, Z.; Kim, S.; Lee, K. Thiohydantoin and hydantoin derivatives from the roots of Armoracia rusticana and their neurotrophic and anti-neuroinflammatory activities. J. Nat. Prod. 2019, 82, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, J.; Yan, H.; Peng, X.; Zhang, L.; Qiu, M. Macathiohydantoin L, a novel thiohydantoin bearing a thioxohexahydroimidazo [1,5-a] pyridine moiety from Maca (Lepidium meyenii Walp.). Molecules 2021, 26, 4934. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ruan, J.; Guo, Y.; Ding, Z.; Yan, J.; Qu, L.; Zheng, C.; Zhang, Y.; Wang, T. Bioactive constituents study of Pugionium cornutum L. Gaertn on intestinal motility. Fitoterapia 2019, 138, 104291. [Google Scholar] [CrossRef]
- Geng, H.; Wang, X.; Liao, Y.; Qiu, S.; Fang, H.; Chen, X.; Wang, Y.; Zhou, M. Macathiohydantoins P–R, three new thiohydantoin derivatives from Maca (Lepidium meyenii). Phytochem. Lett. 2022, 51, 67–70. [Google Scholar] [CrossRef]
- Geng, H.; Yang, D.; Chen, X.; Wang, L.; Zhou, M.; Mei, W. Meyeniihydantoins A–C, three novel hydantoin derivatives from the roots of Lepidium meyenii Walp. Phytochem. Lett. 2018, 26, 208–211. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, R.; Liu, J.; Li, Z.; Zhou, L.; Qiu, M. Lepithiohydimerins A–D: Four pairs of neuroprotective thiohydantoin dimers bearing a disulfide bond from Maca (Lepidium meyenii Walp.). Chin. J. Chem. 2021, 39, 2738–2744. [Google Scholar] [CrossRef]
- Yu, M.; Qin, X.; Shao, L.; Peng, X.; Li, L.; Yang, H.; Qiu, M. Macahydantoins A and B, two new thiohydantoin derivatives from Maca (Lepidium meyenii): Structural elucidation and concise synthesis of macahydantoin A. Tetrahedron. Lett. 2017, 58, 1684–1686. [Google Scholar] [CrossRef]
- Yu, M.; Qin, X.; Peng, X.; Wang, X.; Tian, X.; Li, Z.; Qiu, M. Macathiohydantoins B–K, novel thiohydantoin derivatives from Lepidium meyenii. Tetrahedron 2017, 73, 4392–4397. [Google Scholar] [CrossRef]
- Guo, Q.; Lin, S.; Wang, Y.; Zhu, C.; Xu, C.; Shi, J. Gastrolatathioneine, an unusual ergothioneine derivative from an aqueous extract of ‘‘tian ma’’: A natural product co-produced by plant and symbiotic fungus. Chin. Chem. Lett. 2016, 27, 1577–1581. [Google Scholar] [CrossRef]
- Miyano, R.; Matsuo, H.; Mokudai, T.; Noguchi, Y.; Higo, M.; Nonaka, K.; Niwano, Y.; Sunazuka, T.; Shiomi, K.; Takahashi, Y.; et al. Trichothioneic acid, a new antioxidant compound produced by the fungal strain Trichoderma virens FKI-7573. J. Biosci. Bioeng. 2020, 129, 508–513. [Google Scholar] [CrossRef]
- Kimura, C.; Nukina, M.; Igarashi, K.; Sugawara, Y. β-hydroxyergothioneine, a new ergothioneine derivative from the mushroom Lyophyllum connatum, and its protective activity against carbon tetrachloride-induced injury in primary culture hepatocytes. Biosci. Biotechnol. Biochem. 2005, 69, 357–363. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakanishi, J.; Noguchi, Y.; Kitagawa, K.; Shigemura, K.; Sunazuka, T.; Takahashi, Y.; Ōmura, S.; Nakashima, T.; Nanaomycin, K. A new epithelial-mesenchymal transition inhibitor produced by the actinomycete “Streptomyces rosa subsp. notoensis” OS-3966. J. Biosci. Bioeng. 2020, 129, 291–295. [Google Scholar] [CrossRef]
- Dzuvor, C.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications—A critical review. Crit. Rev. Biotechnol. 2022, 42, 271–293. [Google Scholar] [CrossRef]
- Saini, R.; Sivanesan, I.; Keum, Y. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016, 6, 203. [Google Scholar] [CrossRef]
- Arora, S.; Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Liu, R.; Liu, J.; Huang, Q.; Liu, S.; Jiang, Y. Moringa oleifera Lam: A systematic review of its botany, ethnomedical uses, phytochemistry, pharmacology and toxicity. J. Pharm. Pharmacol. 2022, 74, 296–320. [Google Scholar] [CrossRef]
- Ghimire, S.; Subedi, L.; Acharya, N.; Gaire, B. Moringa oleifera: A tree of life as a promising medicinal plant for neurodegenerative diseases. J. Agric. Food Chem. 2021, 69, 14358–14371. [Google Scholar] [CrossRef]
- Stohs, S.; Hartman, M. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Li, J.; Huang, Q.; Liu, S.; He, J. Pyrrole-2-carbaldehydes with neuroprotective activities from Moringa oleifera seeds. Phytochemistry 2022, 204, 113451. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.; Huang, Q.; Liu, R.; Liu, J.; Zhang, F.; Liu, S.; Jiang, Y. Moringa oleifera Lam. seed extract protects kidney function in rats with diabetic nephropathy by increasing GSK-3β activity and activating the Nrf2/HO-1 pathway. Phytomedicine 2022, 95, 153856. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Wang, W. GIAO 13C NMR calculation with sorted training sets improves accuracy and reliability for structural assignation. J. Org. Chem. 2020, 85, 11350–11358. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.; Zalcmann, A.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Jha, S.; Silversides, J.; Boyle, R.; Stephen, J.; Archibald, S. Hydrogen bonded dimers vs. one-dimensional chains in 2-thiooxoimidazolidin-4-one (thiohydantoin) drug derivatives. CrystEngComm 2010, 12, 1730–1739. [Google Scholar] [CrossRef]
- Bassetto, M.; Ferla, S.; Pertusati, F.; Kandil, S.; Westwell, A.D.; Brancale, A.; Mcguigan, C. Design and synthesis of novel bicalutamide and enzalutamide derivatives as antiproliferative agents for the treatment of prostate cancer. Eur. J. Med. Chem. 2016, 118, 230–243. [Google Scholar] [CrossRef]
- Yu, H.; Ding, C.; Zhang, L.; Wei, X.; Cheng, G.; Liu, Y.; Zhang, R.; Luo, X. Alstoscholarisine K, an antimicrobial indole from gall-induced leaves of Alstonia scholaris. Org. Lett. 2021, 23, 5782–5786. [Google Scholar] [CrossRef]
- Barger, S.; Harmon, A. Microglial activation by alzhelmer amyloid precursor protein and modulation by apolipoprotein E. Nature 1997, 388, 878–881. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 22, 7169–7192. [Google Scholar] [CrossRef]
- Spicher, S.; Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. Int. Ed. Engl. 2020, 59, 15665–15673. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB an accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]




| no. | δH (J in Hz) a | δC a | δH (J in Hz) b | δCb |
|---|---|---|---|---|
| 2 | 184.3 | 182.6 | ||
| 4 | 174.3 | 172.9 | ||
| 5 | 92.5 | 90.7 | ||
| 7 | 4.13 m | 85.0 | 3.85 dq (6.0, 6.0) | 82.1 |
| 8 | 4.14 m | 76.8 | 3.83 ddd (7.2, 6.0, 6.0) | 75.4 |
| 9a | 2.53 dd (13.5, 5.5) | 42.0 | 2.41 dd (13.2, 6.0) | 41.2 |
| 9b | 2.06 dd (13.5, 5.0) | 1.95 dd (13.2, 7.2) | ||
| 10 | 1.22 d (6.0) | 19.2 | 1.09 d (6.0) | 18.8 |
| 1′ | 128.5 | 126.9 | ||
| 2′ | 7.22 d (8.5) | 130.9 | 7.04 d (8.4) | 129.7 |
| 3′ | 6.69 d (8.5) | 116.1 | 6.62 d (8.4) | 115.6 |
| 4′ | 158.1 | 157.3 | ||
| 5′ | 6.69 d (8.5) | 116.1 | 6.62 d (8.4) | 115.6 |
| 6′ | 7.22 d (8.5) | 130.9 | 7.04 d (8.4) | 129.7 |
| 7′a | 4.86 overlapped | 44.5 | 4.72 d (15.0) | 43.3 |
| 7′b | 4.61 d (15.0) |
| No. | δH (J in Hz) a | δC a |
|---|---|---|
| 2 | 159.3 | |
| 4 | 123.6 | |
| 5 | 121.5 | |
| 6 | 2.04 s | 8.8 |
| 7 | 1.93 s | 9.1 |
| 1′ | 128.8 | |
| 2′ | 7.09 d (8.5) | 129.6 |
| 3′ | 6.71 d (8.5) | 116.3 |
| 4′ | 158.1 | |
| 5′ | 6.71 d (8.5) | 116.3 |
| 6′ | 7.09 d (8.5) | 129.6 |
| 7′ | 5.19 s | 48.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Liu, R.; Huang, L.; Huang, Q.; Liu, M.; Liu, S.; Li, J. Spiroleiferthione A and Oleiferthione A: Two Unusual Isothiocyanate-Derived Thioketone Alkaloids from Moringa oleifera Lam. Seeds. Pharmaceuticals 2023, 16, 452. https://doi.org/10.3390/ph16030452
Jiang Y, Liu R, Huang L, Huang Q, Liu M, Liu S, Li J. Spiroleiferthione A and Oleiferthione A: Two Unusual Isothiocyanate-Derived Thioketone Alkaloids from Moringa oleifera Lam. Seeds. Pharmaceuticals. 2023; 16(3):452. https://doi.org/10.3390/ph16030452
Chicago/Turabian StyleJiang, Yueping, Rong Liu, Ling Huang, Qi Huang, Min Liu, Shao Liu, and Jing Li. 2023. "Spiroleiferthione A and Oleiferthione A: Two Unusual Isothiocyanate-Derived Thioketone Alkaloids from Moringa oleifera Lam. Seeds" Pharmaceuticals 16, no. 3: 452. https://doi.org/10.3390/ph16030452
APA StyleJiang, Y., Liu, R., Huang, L., Huang, Q., Liu, M., Liu, S., & Li, J. (2023). Spiroleiferthione A and Oleiferthione A: Two Unusual Isothiocyanate-Derived Thioketone Alkaloids from Moringa oleifera Lam. Seeds. Pharmaceuticals, 16(3), 452. https://doi.org/10.3390/ph16030452







