Efforts toward PET-Activatable Red-Shifted Silicon Rhodamines and Silicon Pyronine Dyes
Abstract
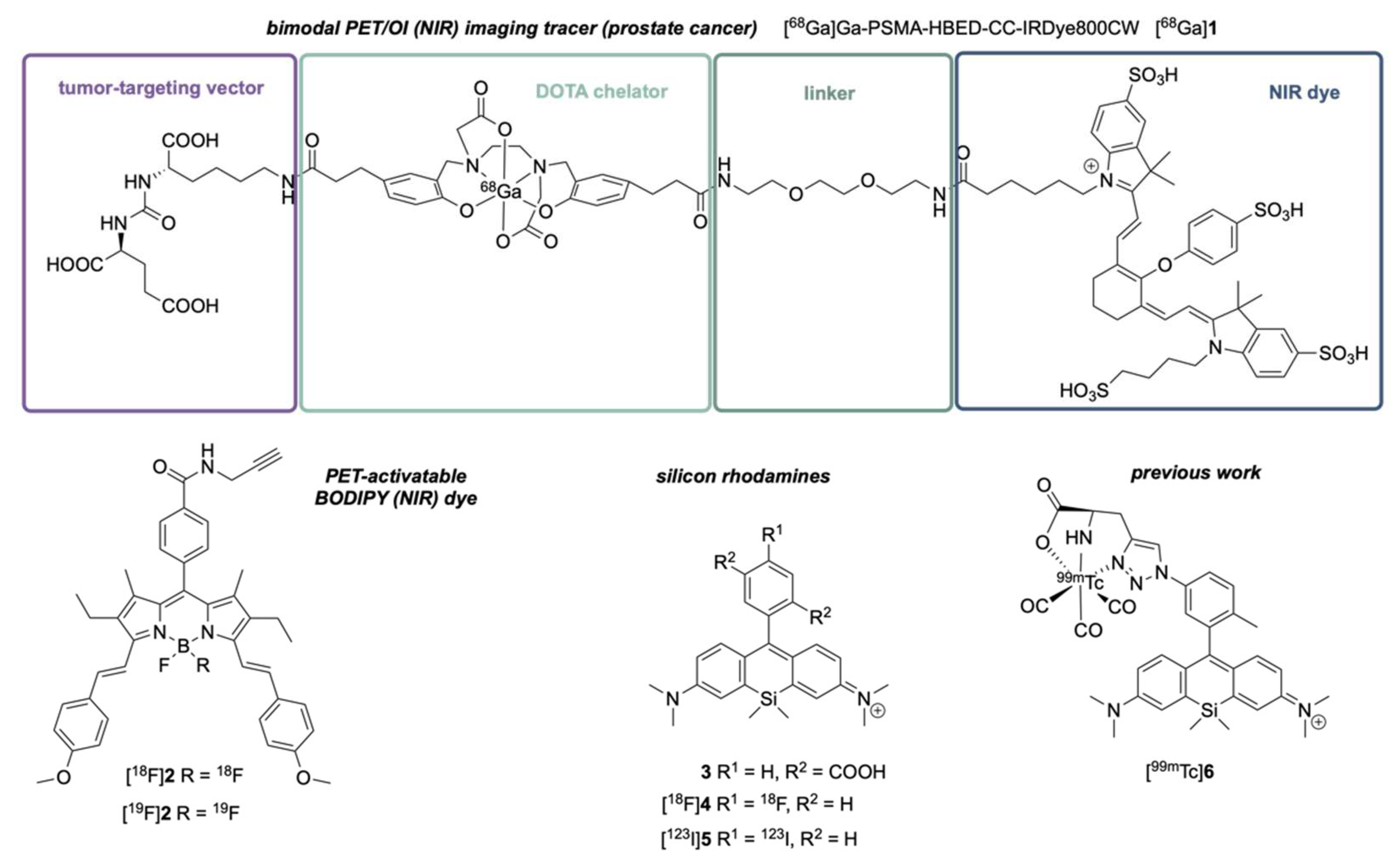
1. Introduction
2. Results
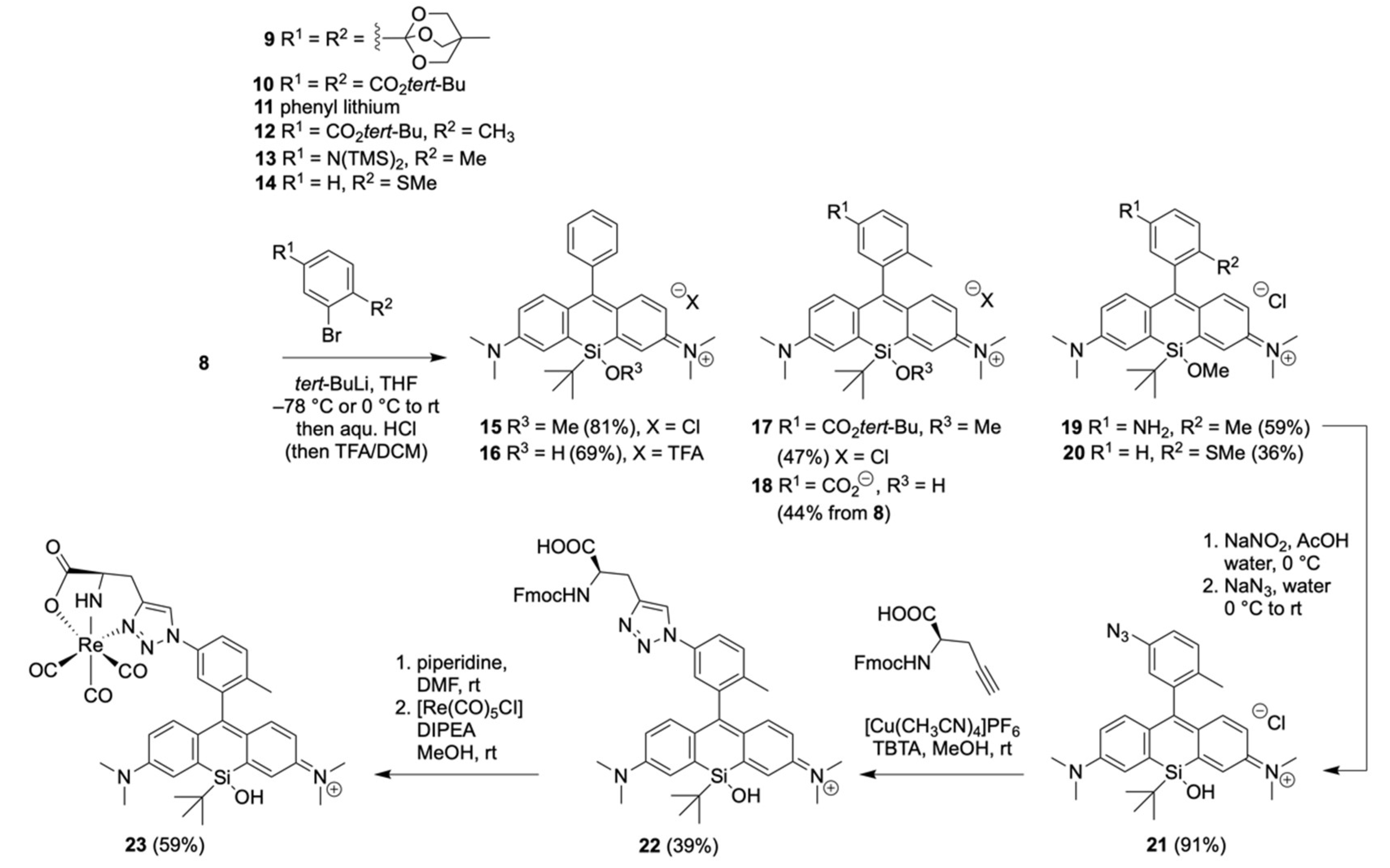
2.1. Synthesis of the Silicon Xanthone 8
2.2. Functionalization of Silicon Xanthone 8 to Silicon Rhodamines
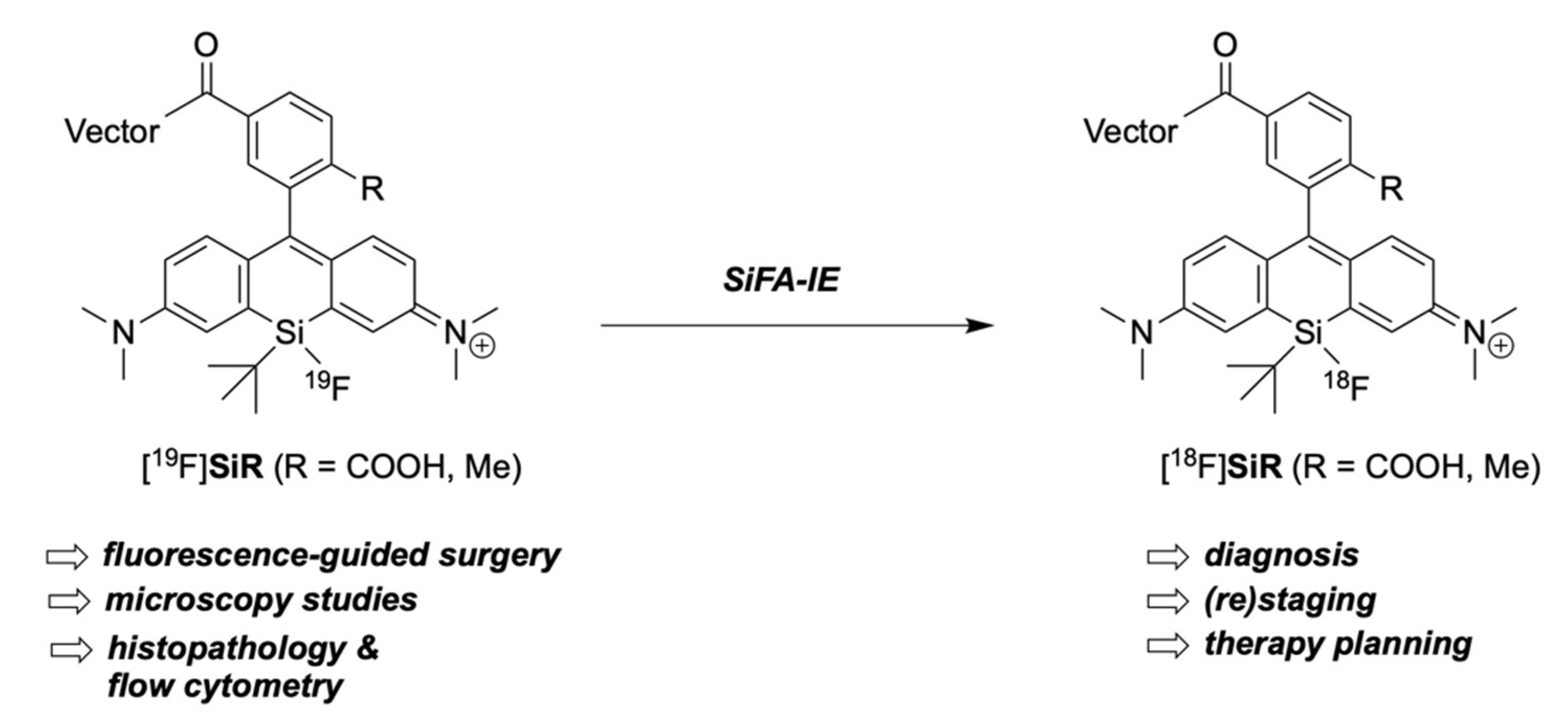
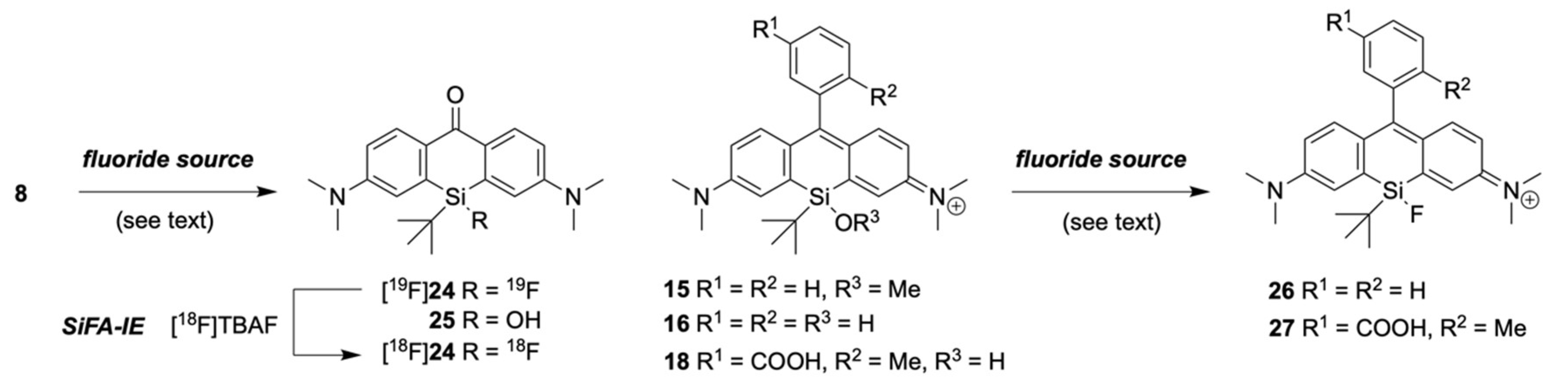
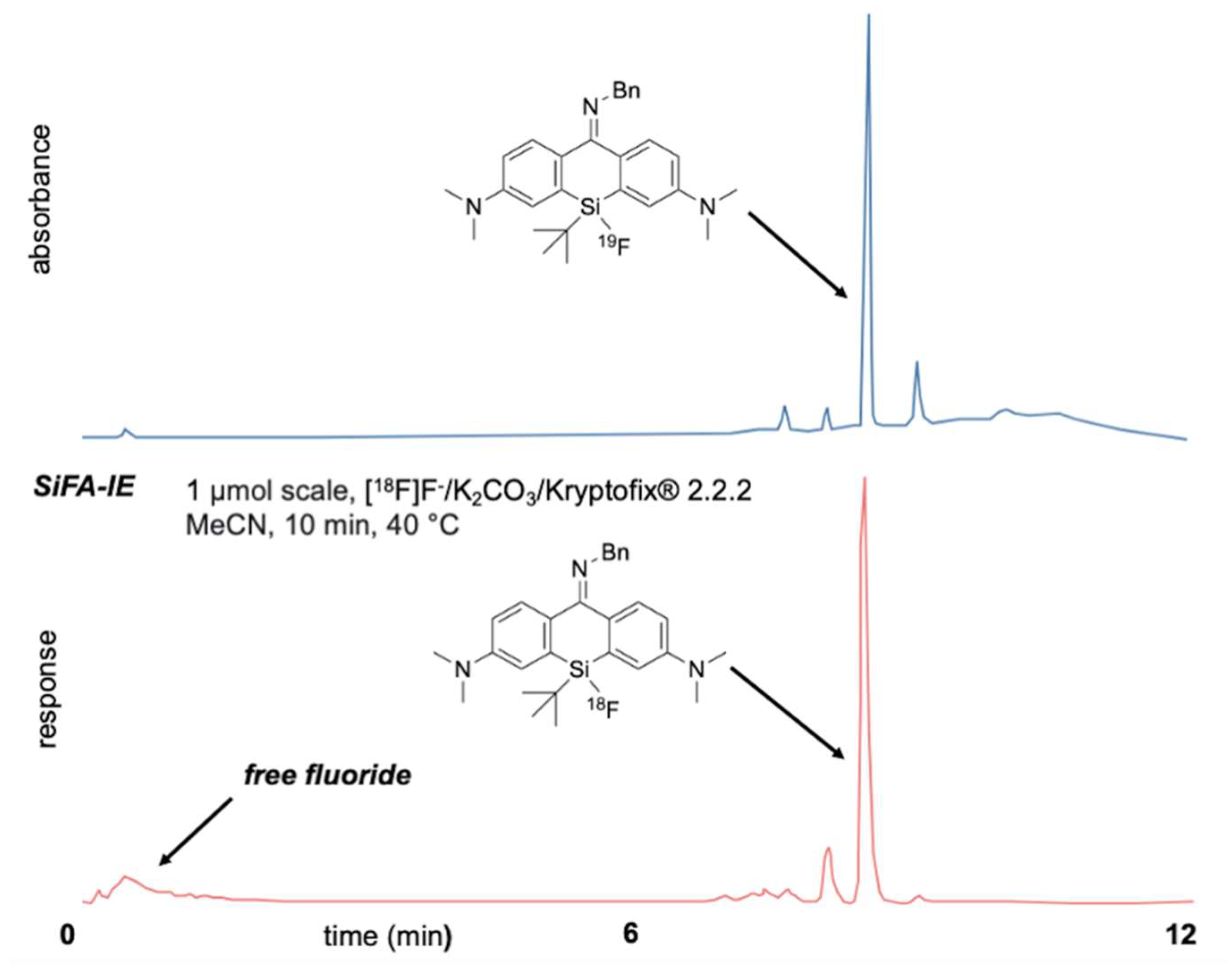
2.3. Fluorination of Silicon Rhodamines
2.4. Functionalization of Xanthone 8 and 24 to Silicon Pyronines
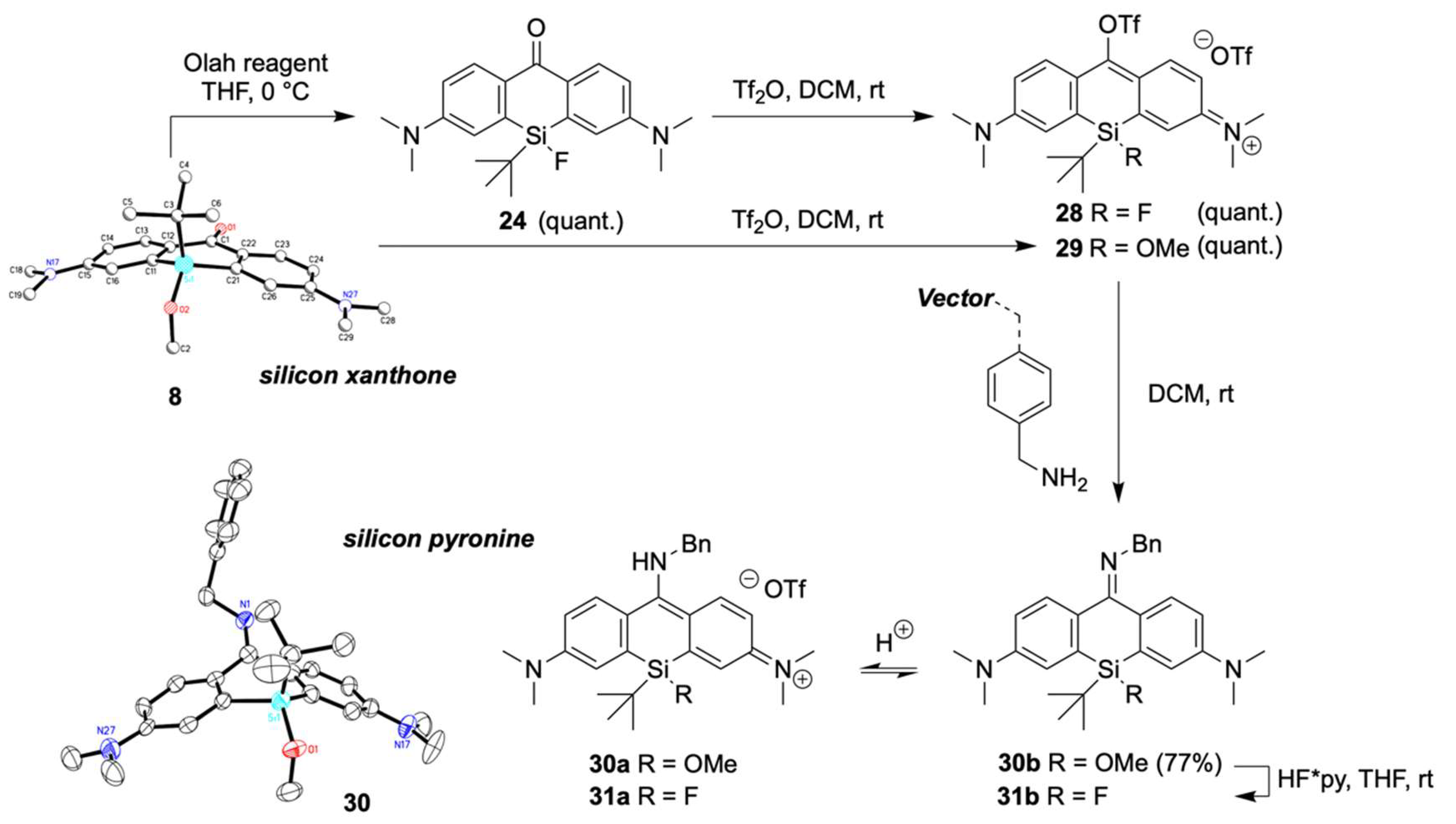
2.5. Fluorination of Silicon Pyronines
2.6. Optical Properties of Silicon Rhodamines and Pyronines
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Windisch, P.; Zwahlen, D.R.; Koerber, S.A.; Giesel, F.L.; Debus, J.; Haberkorn, U.; Adeberg, S. Clinical Results of Fibroblast Activation Protein (FAP) Specific PET and Implications for Radiotherapy Planning: Systematic Review. Cancers 2020, 12, 2629. [Google Scholar] [CrossRef] [PubMed]
- Strohl, M.P.; Ha, P.K.; Flavell, R.R.; Yom, S.S. PET/CT in Surgical Planning for Head and Neck Cancer. Semin. Nucl. Med. 2021, 51, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mieog, J.S.D.; Achterberg, F.B.; Zlitni, A.; Hutteman, M.; Burggraaf, J.; Swijnenburg, R.J.; Gioux, S.; Vahrmeijer, A.L. Fundamentals and developments in fluorescence-guided cancer surgery. Nat. Rev. Clin. Oncol. 2022, 19, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Ariztia, J.; Solmont, K.; Moise, N.P.; Specklin, S.; Heck, M.P.; Lamande-Langle, S.; Kuhnast, B. PET/Fluorescence Imaging: An Overview of the Chemical Strategies to Build Dual Imaging Tools. Bioconjug. Chem. 2022, 33, 24–52. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.C.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendorfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11 derived dual-labeled PSMA-inhibitors for preoperative PET imaging and precise fluorescence-guided surgery of prostate cancer. J. Nucl. Med. 2017, 60, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Eder, A.C.; Omrane, M.A.; Stadlbauer, S.; Roscher, M.; Khoder, W.Y.; Gratzke, C.; Kopka, K.; Eder, M.; Meyer, P.T.; Jilg, C.A.; et al. The PSMA-11-derived hybrid molecule PSMA-914 specifically identifies prostate cancer by preoperative PET/CT and intraoperative fluorescence imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2057–2058. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.H.; Miao, Z.; Cheng, Z.; Gambhir, S.S.; Cochran, J.R. A dual-labeled knottin peptide for PET and near-infrared fluorescence imaging of integrin expression in living subjects. Bioconjug. Chem. 2010, 21, 436–444. [Google Scholar] [CrossRef]
- Paulus, A.; Desai, P.; Carney, B.; Carlucci, G.; Reiner, T.; Brand, C.; Weber, W.A. Development of a clickable bimodal fluorescent/PET probe for in vivo imaging. EJNMMI Res. 2015, 5, 120. [Google Scholar] [CrossRef]
- Seibold, U.; Wängler, B.; Schirrmacher, R.; Wängler, C. Bimodal imaging probes for combined PET and OI: Recent developments and future directions for hybrid agent development. BioMed Res. Int. 2014, 2014, 153741. [Google Scholar] [CrossRef]
- Brand, C.; Abdel-Atti, D.; Zhang, Y.; Carlin, S.; Clardy, S.M.; Keliher, E.J.; Weber, W.A.; Lewis, J.S.; Reiner, T. In vivo imaging of GLP-1R with a targeted bimodal PET/fluorescence imaging agent. Bioconjug. Chem. 2014, 25, 1323–1330. [Google Scholar] [CrossRef]
- Li, Z.; Lin, T.P.; Liu, S.; Huang, C.W.; Hudnall, T.W.; Gabbai, F.P.; Conti, P.S. Rapid aqueous [18F]-labeling of a bodipy dye for positron emission tomography/fluorescence dual modality imaging. Chem. Commun. 2011, 47, 9324–9326. [Google Scholar] [CrossRef] [PubMed]
- Brizet, B.; Goncalves, V.; Bernhard, C.; Harvey, P.D.; Denat, F.; Goze, C. DMAP-BODIPY alkynes: A convenient tool for labeling biomolecules for bimodal PET-optical imaging. Chem. Eur. J. 2014, 20, 12933–12944. [Google Scholar] [CrossRef]
- Ikeno, T.; Nagano, T.; Hanaoka, K. Silicon-substituted Xanthene Dyes and Their Unique Photophysical Properties for Fluorescent Probes. Chem. Asian J. 2017, 12, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Kanagasundaram, T.; Laube, M.; Wodtke, J.; Kramer, C.S.; Stadlbauer, S.; Pietzsch, J.; Kopka, K. Radiolabeled Silicon-Rhodamines as Bimodal PET/SPECT-NIR Imaging Agents. Pharmaceuticals 2021, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kanagasundaram, T.; Kramer, C.S.; Boros, E.; Kopka, K. Rhenium and technetium-complexed silicon rhodamines as near-infrared imaging probes for bimodal SPECT- and optical imaging. Dalton Trans. 2020, 49, 7294–7298. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, R.; Bradtmoller, G.; Schirrmacher, E.; Thews, O.; Tillmanns, J.; Siessmeier, T.; Buchholz, H.G.; Bartenstein, P.; Wängler, B.; Niemeyer, C.M.; et al. 18F-labeling of peptides by means of an organosilicon-based fluoride acceptor. Angew. Chem. 2006, 45, 6047–6050. [Google Scholar] [CrossRef]
- Gower-Fry, L.; Kronemann, T.; Dorian, A.; Pu, Y.; Jaworski, C.; Wängler, C.; Bartenstein, P.; Beyer, L.; Lindner, S.; Jurkschat, K.; et al. Recent Advances in the Clinical Translation of Silicon Fluoride Acceptor (SiFA) 18F-Radiopharmaceuticals. Pharmaceuticals 2021, 14, 701. [Google Scholar] [CrossRef]
- Bernard-Gauthier, V.; Wängler, C.; Schirrmacher, E.; Kostikov, A.; Jurkschat, K.; Wängler, B.; Schirrmacher, R. 18F-labeled silicon-based fluoride acceptors: Potential opportunities for novel positron emitting radiopharmaceuticals. BioMed Res. Int. 2014, 2014, 454503. [Google Scholar] [CrossRef]
- Bernard-Gauthier, V.; Bailey, J.J.; Liu, Z.; Wängler, B.; Wängler, C.; Jurkschat, K.; Perrin, D.M.; Schirrmacher, R. From Unorthodox to Established: The Current Status of 18F-Trifluoroborate- and 18F-SiFA-Based Radiopharmaceuticals in PET Nuclear Imaging. Bioconjug. Chem. 2016, 27, 267–279. [Google Scholar] [CrossRef]
- Wängler, C.; Niedermoser, S.; Chin, J.; Orchowski, K.; Schirrmacher, E.; Jurkschat, K.; Iovkova-Berends, L.; Kostikov, A.P.; Schirrmacher, R.; Wängler, B. One-step 18F-labeling of peptides for positron emission tomography imaging using the SiFA methodology. Nat. Protoc. 2012, 7, 1946–1955. [Google Scholar] [CrossRef]
- Holzleitner, N.; Gunther, T.; Beck, R.; Lapa, C.; Wester, H.J. Introduction of a SiFA Moiety into the D-Glutamate Chain of DOTA-PP-F11N Results in Radiohybrid-Based CCK-2R-Targeted Compounds with Improved Pharmacokinetics In Vivo. Pharmaceuticals 2022, 15, 1467. [Google Scholar] [CrossRef] [PubMed]
- Niedermoser, S.; Chin, J.; Wängler, C.; Kostikov, A.; Bernard-Gauthier, V.; Vogler, N.; Soucy, J.P.; McEwan, A.J.; Schirrmacher, R.; Wängler, B. In Vivo Evaluation of F-SiFAlin-Modified TATE: A Potential Challenge for 68Ga-DOTATATE, the Clinical Gold Standard for Somatostatin Receptor Imaging with PET. J. Nucl. Med. 2015, 56, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Litau, S.; Niedermoser, S.; Vogler, N.; Roscher, M.; Schirrmacher, R.; Fricker, G.; Wängler, B.; Wängler, C. Next Generation of SiFAlin-Based TATE Derivatives for PET Imaging of SSTR-Positive Tumors: Influence of Molecular Design on In Vitro SSTR Binding and In Vivo Pharmacokinetics. Bioconjug. Chem. 2015, 26, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Di Carlo, D.; Schmidt, A.; Beck, R.; Eiber, M.; Schwaiger, M.; Wester, H.J. Radiohybrid Ligands: A Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA Inhibitors. J. Nucl. Med. 2020, 61, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hübner, R.; von Kiedrowski, V.; Fricker, G.; Schirrmacher, R.; Wängler, C.; Wängler, B. Design, Synthesis, In Vitro and In Vivo Evaluation of Heterobivalent SiFAlin-Modified Peptidic Radioligands Targeting Both Integrin αvβ3 and the MC1 Receptor-Suitable for the Specific Visualization of Melanomas? Pharmaceuticals 2021, 14, 547. [Google Scholar] [CrossRef]
- Narayanam, M.K.; Toutov, A.A.; Murphy, J.M. Rapid One-Step 18F-Labeling of Peptides via Heteroaromatic Silicon-Fluoride Acceptors. Org. Lett. 2020, 22, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hazari, P.P.; Schulz, J.; Vimont, D.; Chadha, N.; Allard, M.; Szlosek-Pinaud, M.; Fouquet, E.; Mishra, A.K. A new SiF-Dipropargyl glycerol scaffold as a versatile prosthetic group to design dimeric radioligands: Synthesis of the [18F]BMPPSiF tracer to image serotonin receptors. ChemMedChem 2014, 9, 337–349. [Google Scholar] [CrossRef]
- Otaru, S.; Imlimthan, S.; Sarparanta, M.; Helariutta, K.; Wahala, K.; Airaksinen, A.J. Evaluation of Organo [18F]Fluorosilicon Tetrazine as a Prosthetic Group for the Synthesis of PET Radiotracers. Molecules 2020, 25, 1208. [Google Scholar] [CrossRef]
- Rao, D.N.; Ji, X.; Miller, S.C. Silicon functionalization expands the repertoire of Si-rhodamine fluorescent probes. Chem. Sci. 2022, 13, 6081–6088. [Google Scholar] [CrossRef]
- Lukinavičius, G.; Umezawa, K.; Olivier, N.; Honigmann, A.; Yang, G.; Plass, T.; Mueller, V.; Reymond, L.; Correa, I.R., Jr.; Luo, Z.G.; et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013, 5, 132–139. [Google Scholar] [CrossRef]
- Bertozzi, C.R.; Shieh, P. Alkyne-Activated Fluorogenic Azide Compounds and Methods of Use Thereof. U.S. Patent 9,410,958, 9 August 2016. [Google Scholar]
- Kanagasundaram, T.; Timmermann, A.; Kramer, C.S.; Kopka, K. A new approach to silicon rhodamines by Suzuki-Miyaura coupling—Scope and limitations. Beilstein J. Org. Chem. 2019, 15, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Dolbier, W.R. Guide to Fluorine NMR for Organic Chemists, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Butkevich, A.N.; Lukinavičius, G.; D’Este, E.; Hell, S.W. Cell-permeant large Stokes shift dyes for transfection-free multicolor nanoscopy. J. Am. Chem. Soc. 2017, 139, 12378–12381. [Google Scholar] [CrossRef] [PubMed]
- Hymel, D.; Woydziak, Z.R.; Peterson, B.R. Detection of protein-protein interactions by proximity-driven S(N)Ar reactions of lysine-linked fluorophores. J. Am. Chem. Soc. 2014, 136, 5241–5244. [Google Scholar] [CrossRef]
- Zhou, X.; Lesiak, L.; Lai, R.; Beck, J.R.; Zhao, J.; Elowsky, C.G.; Li, H.; Stains, C.I. Chemoselective Alteration of Fluorophore Scaffolds as a Strategy for the Development of Ratiometric Chemodosimeters. Angew. Chem. 2017, 56, 4197–4200. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Sebej, P.; Solomek, T.; Klan, P. Small-molecule fluorophores with large Stokes shifts: 9-iminopyronin analogues as clickable tags. J. Org. Chem. 2015, 80, 1299–1311. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Evolution of group 14 rhodamines as platforms for near-infrared fluorescence probes utilizing photoinduced electron transfer. ACS Chem. Biol. 2011, 6, 600–608. [Google Scholar] [CrossRef]
- Ogawa, M.; Kosaka, N.; Choyke, P.L.; Kobayashi, H. H-type dimer formation of fluorophores: A mechanism for activatable, in vivo optical molecular imaging. ACS Chem. Biol. 2009, 4, 535–546. [Google Scholar] [CrossRef]
- Kim, S.; Fujitsuka, M.; Tohnai, N.; Tachikawa, T.; Hisaki, I.; Miyata, M.; Majima, T. The unprecedented J-aggregate formation of rhodamine moieties induced by 9-phenylanthracenyl substitution. Chem. Commun. 2015, 51, 11580–11583. [Google Scholar] [CrossRef]
- Kim, S.; Fujitsuka, M.; Miyata, M.; Majima, T. Excited-state dynamics of Si-rhodamine and its aggregates: Versatile fluorophores for NIR absorption. Phys. Chem. Chem. Phys. 2016, 18, 2097–2103. [Google Scholar] [CrossRef]
- Klar, T.A.; Jakobs, S.; Dyba, M.; Egner, A.; Hell, S.W. Fluorescence microscopy with diffraction resolution barrier broken by stimulated emission. Proc. Natl. Acad. Sci. USA 2000, 97, 8206–8210. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR chemical shifts of trace impurities: Common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Butkevich, A.N.; Mitronova, G.Y.; Sidenstein, S.C.; Klocke, J.L.; Kamin, D.; Meineke, D.N.; D’Este, E.; Kraemer, P.T.; Danzl, J.G.; Belov, V.N.; et al. Fluorescent rhodamines and fluorogenic carbopyronines for super-resolution STED microscopy in living cells. Angew. Chem. Int. Ed. 2016, 55, 3290–3294. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Siegrist, M.S.; Cullen, A.J.; Bertozzi, C.R. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 111, 5456. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Jin, L.; Garcia, A.; DaSilva, J.N.; Houle, S. An admonition when measuring the lipophilicity of radiotracers using counting techniques. Appl. Radiat. Isot. 2001, 54, 203–208. [Google Scholar] [CrossRef]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Sens, R.; Drexhage, K.H. Fluorescence quantum yield of oxazine and carbazine laser dyes. J. Lumin. 1981, 24, 709–712. [Google Scholar] [CrossRef]

 | |||||
| Compound | Solvent | λabs. (nm) | λem. (nm) | ϕfl | εmax (M−1 cm−1) |
| 15 | MeCN | 663 | 681 | 0.28 1 | 109,410 |
| 16 | PBS 2 | 659 | 685 | 0.14 1 | - |
| 18 | MeCN | 664 | 684 | 0.50 1 | - |
| 32 4 | PBS 2 | 646 | 660 | 0.31 4 | - |
| 33 5 | PBS 6 | 663 | 681 | 0.43 | 105,000 |
| 20 | MeCN | 672 | 696 | 0.55 1 | 89,124 |
| 34 7 | MeOH | 653 | - | - | 91,900 |
| PBS 2 | 651 | - | - | 77,300 | |
| 19 | MeOH | 664 | 679 | 0.074 1 | 67,750 |
| H2O 3 | 665 | 680 | 0.052 1 | 51,600 | |
| PBS 2 | 663 | 678 | 0.026 1 | 61,900 | |
| MeCN | 665 | 687 | 0.010 1 | 120,000 | |
| 35 7 | MeOH | 651 | 670 | 0.175 1 | 156,500 |
| H2O 3 | 651 | 670 | 0.103 1 | 123,700 | |
| PBS 2 | 651 | 671 | 0.116 1 | 99,000 | |
| 21 | MeOH | 663 | 680 | 0.076 1 | 73,900 |
| H2O 3 | 663 | 677 | 0.054 1 | 77,200 | |
| MeCN | 664 | 685 | 0.450 1 | 98,000 | |
| 36 7 | MeOH | 655 | 672 | 0.130 1 | 79,900 |
| H2O 3 | 653 | 671 | 0.098 1 | 73,890 | |
| PBS 2 | 655 | 672 | 0.127 1 | 79,900 | |
| 22 * | H2O 3 | 671 * | 693 * | - * | - * |
| 37 7 | MeOH | 654 | 672 | 0.135 1 | 63,900 |
| H2O 3 | 654 | 674 | 0.104 1 | 22,100 | |
| PBS 2 | 654 | 669 | 0.090 1 | 39,100 | |
| 23 * | PBS 2 | 671 * | 693 * | - * | - * |
| 30 | MeCN | 323 390 478 | 607 8 | n.d. | n.d. |
| 31 | MeCN | 323 (strong) 393 480 | 457 9 (weak) 461 10 (strong) 599 11 (medium) | n.d. | n.d. |
| 38 12 | MeCN | 317 462 | 607 13 | 0.58 | 21,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramer, C.S.; Kanagasundaram, T.; Matthias, J.; Kopka, K. Efforts toward PET-Activatable Red-Shifted Silicon Rhodamines and Silicon Pyronine Dyes. Pharmaceuticals 2023, 16, 401. https://doi.org/10.3390/ph16030401
Kramer CS, Kanagasundaram T, Matthias J, Kopka K. Efforts toward PET-Activatable Red-Shifted Silicon Rhodamines and Silicon Pyronine Dyes. Pharmaceuticals. 2023; 16(3):401. https://doi.org/10.3390/ph16030401
Chicago/Turabian StyleKramer, Carsten Sven, Thines Kanagasundaram, Jessica Matthias, and Klaus Kopka. 2023. "Efforts toward PET-Activatable Red-Shifted Silicon Rhodamines and Silicon Pyronine Dyes" Pharmaceuticals 16, no. 3: 401. https://doi.org/10.3390/ph16030401
APA StyleKramer, C. S., Kanagasundaram, T., Matthias, J., & Kopka, K. (2023). Efforts toward PET-Activatable Red-Shifted Silicon Rhodamines and Silicon Pyronine Dyes. Pharmaceuticals, 16(3), 401. https://doi.org/10.3390/ph16030401








