Abstract
Non-small cell lung cancer (NSCLC) is the most prevalent type of lung cancer, which is the leading cause of cancer-related deaths worldwide. Over the past decades, tumour angiogenesis has been intensely studied in the treatment of NSCLC due to its fundamental role in cancer progression. Several anti-angiogenic drugs, such as recombinant endostatin (RE), have been evaluated in several preclinical and clinical trials, with mixed and often disappointing results. However, there is currently an emerging interest in RE due to its ability to create a vascular normalization window, which could further improve treatment efficacy of the standard NSCLC treatment. This review provides an overview of preclinical and clinical studies that combined RE and radiotherapy for NSCLC treatment. Furthermore, it highlights the ongoing challenges that have to be overcome in order to maximize the benefit; as well as the potential advantage of combinations with particle therapy and immunotherapy, which are rapidly gaining momentum in the treatment landscape of NSCLC. Different angiogenic and immunosuppressive effects are observed between particle therapy and conventional X-ray radiotherapy. The combination of RE, particle therapy and immunotherapy presents a promising future therapeutic triad for NSCLC.
1. Introduction
1.1. Treatment Landscape of Non-Small Cell Lung Cancer
Globally, lung cancer is the second most commonly diagnosed cancer and leading cause of cancer related deaths [1]. Non-small cell lung cancer (NSCLC) is the most prevalent type of lung cancer making up about 85% of all lung cancer cases [2]. Based on histological features, NSCLC can be further subdivided into squamous cell carcinoma, large cell carcinoma, and lung adenocarcinomas [3]. The five year survival rate for patients with localized NSCLC is 64%, however, most patients already present advanced disease with local progression and metastasis at first diagnosis, leading to an overall five year survival rate of approximately 15% for all NSCLC stages [4,5,6]. Depending on the stage, histology, genetic alterations, and the general condition of the patient, the standard treatment of NSCLC usually includes surgery, chemotherapy, radiotherapy (RT), targeted therapy or immunotherapy, either alone or in a combined treatment regimen [7]. The latest advances in systemic treatment have been driven primarily by the development of molecularly targeted therapeutics, immune checkpoint inhibitors, and anti-angiogenic agents. Tyrosine kinase inhibitors are now approved to treat several subtypes of NSCLC [7]. Chemotherapy for NSCLC consists of a combination of platinum-based drugs and cytotoxic drugs like paclitaxel [7]. A well-known example of an anti-angiogenic drug that reached the clinic for NSCLC patients is an antibody against the vascular endothelial growth factor (VEGF)-A, bevacizumab. It has been approved as a first-line treatment for advanced-stage patients in combination with platinum-based chemotherapy [8,9]. Until today, extensive research has been performed on anti-angiogenic drug candidates for the treatment of NSCLC patients. This review will focus specifically on Endostatin, a promising drug for NSCLC in combination strategies. However, this narrative review will also clarify that more work is needed to identify predictive biomarkers and main angiogenic role players in order to optimise angiogenic therapy, particularly in the context of combination treatments for NSCLC.
1.2. The Role of Tumour Angiogenesis in NSCLC
During the early stages or dormant phases of cancer development, tumours can exist without blood supply for extended periods of time (months to years) [10]. However, the absence of tumour vasculature becomes a critical determinant during tumour progression. Adequate delivery of nutrients and oxygen are a significant requirement to meet the metabolic demands of a growing tumour [11]. In addition, as the neoplasm enlarges, apoptotic and necrotic zones are created. This leads to the presence of hypoxia in the growing tumour mass, which is the vital initial stimulus for tumour vascularisation and the initiation of angiogenesis [12]. While angiogenesis is a tightly regulated process under normal physiologic conditions, the vascular network created in tumour angiogenesis is markedly disordered and dysfunctional, resulting in increased vascular permeability [13].
The tumour angiogenesis process requires an imbalance between the different major pro- and anti-angiogenic factors, which have been extensively studied and defined over the past decades [14]. This imbalance is also coined as the “angiogenic switch” in tumours and is characterized by the promotion of a pro-angiogenic milieu [15,16,17]. The key signalling process in the development of the tumour vasculature is the hypoxia-induced stimulation of Hypoxia Inducible Factor 1 alpha (HIF 1-α), resulting in the suppression of anti-angiogenic factors, such as trombospondin-1 and angiostatin, whilst simultaneously promoting the production of Vascular Endothelial Growth Factors (VEGF) and basic fibroblast growth factor (bFGF) [18]. The latter are known as positive regulators of angiogenesis, promoting vascular permeability and endothelial cell proliferation, respectively [19]. The VEGF family comprises 5 VEGF glycoproteins, namely VEGF-A to D, and placental growth factor 1 and 2 (PIGF-1 and -2) [20]. They exert their biological action by activating tyrosine kinase receptors and VEGF receptors (VEGFR) 1 and 2 present on vascular endothelial cells [15]. Together with VEGFs, angiopoietins co-regulate angiogenesis. In particular, Ang-1 and Ang-2 bind to Tie-2 receptors to control blood vessel stabilization signals [21]. Alternatively, in the event of their blockade, the roles of VEGFs and angiopoietins can be assumed by bFGF. The FGF family interacts with four main FGF receptors (FGFRs), and the FGF ligands are involved in promoting proliferation, survival, migration, proteinase production, and the expression of specific integrins in vascular endothelial cells [22]. It is important to note that FGFs have been reported to promote angiogenesis independently from VEGF and potent angiogenic activity has been identified for FGF-2 [19,22]. Therefore, the signalling pathways of FGF/FGFR as well as the platelet-derived growth factor (PDGF) and PDGF receptor (PDGFR), could provide potential escape mechanisms from anti-VEGF/VEGFR therapy that could facilitate resumption of tumour growth [23]. Undeniably, complex and overlapping signalling cascades govern tumour angiogenesis and cause resistance to anti-angiogenic therapy [24,25,26,27,28,29]. In addition, once the tumour angiogenesis process has been activated, tumour metastasis can also occur via these newly formed vessels [4].
In NSCLC specifically, HIF-1α and HIF-2α are commonly overexpressed, correlating with poorer survival and increased microvascular density (MVD), respectively [30,31]. Work by Lin and colleagues provided evidence that high HIF-1α expression is an independent prognostic factor of small cell lung cancer (SCLC) with a 39.2 fold risk of mortality [32]. Tumour angiogenesis has also been identified as a crucial prognostic factor in advanced NSCLC. For example, the level of angiogenesis (high MVD) was associated with shorter survival of patients with stage IIIA NSCLC [32]. The use of MVD as a prognostic factor remains a controversial subject in NSCLC, mainly due to the large variation in different histological studies regarding tumour growth patterns and different definitions of angiogenic profiles and prognosis [33]. Although the traditional angiogenic processes govern tumorigenesis in NSCLC, it also exhibits unique features, such as vessel co-option (VCO) [34]. In this instance, the tumour growth also relies on its invasion of the host tissue. In fact, it was possible to classify NSCLC according to its morphological features, based on the biological characteristics of the tumour-lung interface [35]. This classification of Sardari et al. distinguishes three distinct NSCLC growth patterns: (a) a destructive growth pattern, which entails a traditional angiogenic growth pattern; (b) the papillary growth pattern that involves the preservation of the alveolar structure of the lung parenchyma at the interface with co-option of alveolar blood vessels with stromal stalk formation and subsequent angiogenesis. Finally, there is (c) the alveolar growth pattern; wherein the alveolar structures of the lung parenchyma are preserved, and co-option of septal blood vessels occurs without evidence of new stroma formation at the interface [35]. Recently, Cuypers and co-authors described VCO in great detail and identified it as a new placeholder in the assessment of tumour vasculature [36]. However, compared to traditional tumour angiogenesis, the molecular events governing VCO remain largely understudied. Consequently, no treatment strategies exist to inhibit VCO. New role players in the angiogenic progression of NSCLC have been described, such as sex dependency and specific non-coding RNAs [32,37]. It is clear that the angiogenic landscape in NSCLC is multifaceted, and various efforts in mechanistic investigation and treatment strategy remain necessary.
1.3. The Potential of Anti-Angiogenic Drugs in Combination Treatments for NSCLC
Since tumour angiogenesis was first proposed in the pioneering work of Folkman in 1971 by the discovery of the first angiogenic factors, anti-angiogenic drugs have been earmarked as a promising cancer strategy [38]. With the more precise classification of angiogenic processes and signalling pathways, targeted therapies evolved with the aim to block tumour angiogenesis [39]. They would inhibit tumour blood vessel production, leaving cancer cells in starvation by blocking the supply of oxygen and nutrients, which could subsequently lead to the creation of hypoxic areas in the tumour [40,41]. The presence of hypoxia is particularly important in the context of RT, where it can reduce the radiosensitivity of the tumour cells. Therefore, it sounds counterintuitive to inhibit tumour angiogenesis to improve the efficacy of RT to obtain tumour control [42]. However, the rationale lies in the use of anti-angiogenic therapies to ‘normalize’ the tumour vasculature by pruning the immature and inefficient blood vessels [13]. This could eventually lead to a normalized vasculature, which is more conducive to the delivery of targeted drugs as well as a net increase in tumour oxygen concentration, which can increase the effectiveness of RT. The latter will be discussed in Section 3 of this review. Besides, the concept of vascular normalization is also receiving growing attention in combination with immunotherapy, with or without the addition of RT [43,44,45,46,47].
This is particularly important for NSCLC, known as a highly vascularized tumour, where histological evidence of enhanced angiogenesis has been associated with poor prognosis [48,49]. As a result, anti-angiogenic therapy for NSCLC was already tested at the beginning of this century [8]. Unfortunately, only marginal benefit was observed in early clinical trials with vessel-inhibiting therapies for advanced NSCLC, where the focus was mainly on the repression of vessel sprouting by inhibition of VEGF signalling [50]. Multiple trials also explored the combination of bevacizumab with tyrosine kinase inhibitors (TKIs), but the optimal sequence for administration of these drugs remains to be determined [51]. Other anti-angiogenic agents, such as sunitinib, sorafenib, and vandetanib, have proven to be unsuccessful in clinical trials, while two new anti-angiogenic agents (ramucirumab and nintedanib) produced a significant survival benefit in a second-line setting [9]. The limited clinical efficacy is most likely attributable to alternative processes in the tumour microenvironment (TME) which are resistant to traditional angiogenesis inhibitors. Specific sub-groups of NSCLC with non-angiogenic patterns have been described, where tumours seem to co-opt the existing blood and lymphatic vessels via a process called VCO (as described in Section 1.2), rather than inducing angiogenesis [34,52]. Another non-angiogenic mechanism is termed vasculogenic mimicry and is based on the self-organizing ability of cancer cells into vascular-like structures, allowing them to obtain nutrients and oxygen autonomously [53]. These alternative non-angiogenic processes in NSCLC progression lead to resistance to VEGF-inhibitors and contribute to therapy failure [54]. Furthermore, it is unlikely that the inhibition and control of the tumour vasculature as a stand-alone therapy will cure a cancer, but it has the potential to limit its growth and spread. More importantly, it can also potentiate the effect of direct anti-tumour therapies, such as standard chemotherapy and RT [14].
In this review, we will focus on a drug that was inspired by one of the first anti-angiogenic factors to be discovered, namely endostatin, produced by murine haemangioendothelioma cells in Folkman’s laboratory [38]. It is one of the most effective endogenous inhibitors of angiogenesis, proven to be a promising tool to inhibit at least 65 different tumour types [55,56,57]. As soon as recombinant endostatin (Endostar®, further referred to as RE) was generated in a stable and soluble form that was cost-effective to produce, the drug was subsequently tested for the treatment of many cancer types [58]. RE was found to be more stable with a longer half-life than bevacizumab and inhibited tumour vascular growth through multiple targets [59]. Extensive research has been performed on the chemo- and radiosensitising effects of endostatin [58,60,61,62,63,64,65]. This led to its approval by the State Food and Drug Administration (FDA) of China in 2005 for the treatment of NSCLC [58]. More recently, it has also been approved by the USA FDA for the first or second line treatment of NSCLC [66]. This review will focus on the current status of endostatin/RE for the treatment of NSCLC, with a focus on the combined use with RT to overcome treatment resistance.
2. Endostatin and Its Mechanism of Action
Endostatin is a C-terminal fragment cleaved from the NC1 domain of Collagen XVIII via the proteolytic activity of proteinases like elastase, procathepsin L, and matrix metalloproteinases (MMPs) [67]. Several animal studies demonstrated that endostatin had the ability to suppress neovascularization, resulting in growth inhibition in several murine and human tumours [68,69]. Endostatin and RE are known as broad-spectrum angiogenesis inhibitors, which interfere mainly with the pro-angiogenic function of VEGFs and FGFs. Nucleolin, a cell surface phosphoprotein constituent, also acts as a receptor for endostatin. Upon binding to RE and its heparin-binding site, nucleolin is internalized and translocated to the cell nucleus, shuttling RE along with it (Figure 1) [70]. The abundance of nucleolin receptors on endothelial cell surfaces gives RE the ability to stall the migratory action of endothelial cells [71]. In addition to the inhibition of endothelial cell migration, RE also inhibits proliferation, induces cell cycle arrest, and stimulates endothelial cell death by apoptosis [72,73]. Furthermore, the interaction of RE with endothelial cells results in the activation of a variety of downstream effects, such as the inhibition of the Wnt/β-catenin pathway and an actin reorganization in endothelial cells [74]. However, despite the growing number of clinical trials, the mechanism of action of RE has proven to be more complex than initially surmised. In addition to its binding efficiency with nucleolin, endostatin interferes with several processes and is also known to interact with various other receptors, such as VEGFR-2 and -3, glypican 1 and 4 and integrin v5 and α5 receptors. The interaction between RE and integrins is also related to the disruption of cell migration by outcompeting the binding of the pro-angiogenic ligand fibronectin to α5β1 integrin, which would promote cell migration [75]. RE suppresses the VEGF-induced tyrosine phosphorylation of kinase insert domain containing receptor/fetal liver kinase 1 (KDR/Flk-1/VEGFR-2) as well as the overall VEGFR-2 expression and the activation of extracellular signal related kinase (ERK), p38 mitogen-activated protein kinase (p38 MAPK), and Protein kinase B (AKT) in human umbilical vein endothelial cells (HUVECs) (Figure 1) [76]. It is also suggested that both heparan sulphate (HS) and α5β1 integrin need to be present for the localization of endostatin in endothelial cell lipid rafts [77]. The association between HS and integrin was proven to lead to the inhibition of focal adhesion kinase c-Raf-MAPK pathway, showing similar downstream suppression effects to VEGF-A binding to endostatin, ultimately leading to the inhibition of endothelial migration [75].
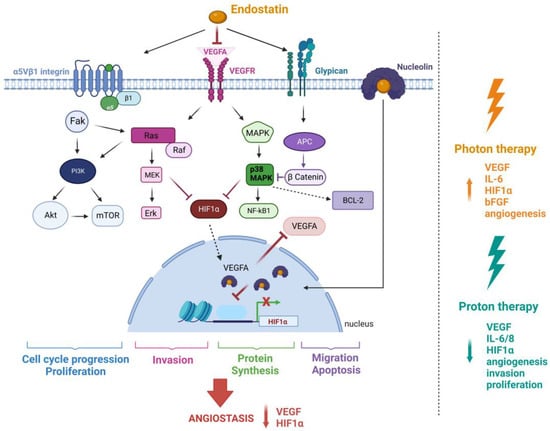
Figure 1.
The The mechanism of action of RE and the effect of photon-based radiotherapy and proton therapy (PT) on tumour angiogenesis. RE inhibits VEGF-A binding to VEGFR-1/2 resulting in the inhibited activation of kinase/c-Raf/MEK1/2/p38/ERK1 MAPK pathway. It exerts dual suppression on the PI3K-AKTpathway by binding to the receptor α5β1 integrin. RE’s low affinity glypican binding leads to Wnt pathway signalling disruption and β-catenin degradation. The association of RE with nucleolin and subsequent nuclear translocation inhibits the transcription of HIF-1α resulting in decreased VEGF-A production. Endostatin also increases apoptosis in endothelial cells by the downregulation of the anti-apoptotic protein Bcl-2. Different cellular mechanisms corresponding to various signalling pathways are consequently inhibited. Synergy with radiotherapy is dependent on the radiation quality. Photons are known to promote angiogenesis and cause an increase in the expression of pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), Interleukin 6 (IL-6), Hypoxia inducible factor 1 alpha (HIF-1α), and basic fibroblast growth factor (bFGF). In contrast, proton irradiation significantly downregulates some of the same and other pro-angiogenic factors, resulting in the inhibition of tumour angiogenesis. Figure was created with BioRender.
Molecular studies have reported that RE can induce the attenuation of focal adhesions, a functional protein complex that links the actin cytoskeleton of the endothelial cells to the underlying basal membrane in human dermal microvascular endothelial cells [78]. In contrast, RE increased the number of focal adhesions in bovine capillary endothelial cells. These increases in focal adhesions were sustained with the administration of FGF-2. In contrast, RE has also been proven to disrupt cytoskeletal arrangement in addition to cell-cell matrix interactions [79].
RE also possesses ATPase activity, which led to the development of an engineered form of endostatin, which exhibits much higher ATPase activity than the wildtype one [75]. It was shown that the ATPase activity of RE is required to inhibit the action of tumour-associated macrophages (TAM) [80]. This is an intriguing finding since TAMs can enhance tumour angiogenesis, immunosuppression, tumour cell invasion and metastasis. Furthermore, their association with angiogenesis and lymphangiogenesis, contributes to the progression of NSCLC [16,81].
Collectively, these studies fall under a large blanket of experimental findings that show RE’s effect on the vascular endothelium and successfully served as a validation of the original work by O’Reilly and colleagues [38]. Importantly, anti-angiogenic drugs like RE target rapidly proliferating tumour-associated endothelial cells rather than relatively dormant endothelial cells in healthy tissue, making them less toxic than chemotherapeutic agents [82].
RE Re-Imagined
The formulation of RE, a protein drug, is coupled with limitations such as poor bioavailability, insoluble and unstable nature, and high production cost. Therefore, several attempts were made to improve RE structurally, that include PEGylation of its N-terminus, the addition of an RGD (Arg-Gly-Asp) sequence that is present in integrin ligands, fusing endostatin to the Fc region of IgG, or the addition of Zinc [83,84,85,86]. An alternative to RE is the introduction of human endostatin cDNA via viral and non-viral vectors [87,88]. In a phase I dose-escalation clinical trial for multiple cancer types, the intra-tumoural injections of an adenovirus containing the human endostatin gene resulted in a decrease in bFGF expression levels and angiogenic serum markers [87].
RE-loaded nanoparticles have also shown anti-angiogenic effects in vivo; for example, a folic acid-decorated chitosan nanoparticle successfully targeting squamous cell carcinoma (SCC) [89,90,91]. VEGFR-2 was successfully targeted in the blood brain barrier by Lu et al., using a dual receptor peptide functionalized polyethyleneimine nanocomplex for secretory RE delivery to malignant glioma [92]. Gold nanoparticles (rHES-AuNPs) were utilized by Li and colleagues to normalize vasculature in NSCLC and improve chemotherapy in mice bearing H22 tumours [93]. Interesting work has also been performed with RE liposomal formulations. Liposomal encapsulation of RE resulted in increased stability and half-life of the peptide as well as gradual release of the peptide. [94]. RE loading into liposomes has also been used in a nanoformulation that was used as a mode of gene therapy with strong anti-tumour effects in vivo [95]. Nanoformulations of RE have also been studied in combination with RT [96]. Using hyaluronic acid-tyramine as a carrier, an ES-loaded hydrogel drug (ES/HA-Tyr) was synthesized for local injection [96]. The ES/HA-Tyr formulation increased local drug concentration, decreased blood drug concentration, and caused less systemic toxicity in an in vivo study design. Additionally, ES/HA-Tyr effectively reduced tumour microvessel density, increased tumour pericyte coverage, decreased tumour hypoxia, and increased RT response [96]. Although the pre-clinical work in nanomedicine was promising, the clinical translation of nano-incorporated drug formulations has been hindered by the lack of patient overall survival improvement, suboptimal nanoparticle biodistribution, and safety concerns [97].
3. Radiotherapy and Anti-Angiogenic Therapy: A Dilemma
As previously mentioned, the administration of anti-angiogenic therapy as a radiosensitizer seems paradoxical. Blocking the formation of blood vessels could enhance hypoxia within the tumour and contribute to increased radioresistance (Figure 2) [98,99]. This principle is based on the oxygen effect, one of the pillars of the “five Rs” of radiation biology, which form the basis of RT [100]. When low linear energy transfer (LET) radiation is used, such as high-energy (MV) X-rays, the presence of oxygen will “fixate” the DNA radicals, which are a result of the reaction between the DNA and the hydroxyl radicals produced in the surrounding water [101]. The excessive reduction of vessels by anti-angiogenesis therapy has been shown to cause additional intra-tumoural hypoxia resulting in pathological angiogenesis, inflammation, increased migration, and additional sequelae [102]. However, the theory of vascular normalization, initially proposed by Jain et al. [103], is based on the hypothesis that anti-angiogenic treatment can revert the structurally and functionally abnormal tumour vasculature toward its normal state (Figure 2) [104]. If treated with the appropriate treatment dose, this results in the conversion from a chaotic to a more ‘normal’ vascular network in the tumour. In short, this leads to reduced vascular permeability and interstitial fluid pressure, improved blood flow and increased tumour perfusion, and consequently, a reduction of tumour hypoxia. This effect can enhance the systemic delivery of cytotoxic drugs, immunotherapy, and improve tumour radiosensitivity [102,105] The concept was elegantly demonstrated by Lee and colleagues for RT, where an enhanced tumour response was observed by combining radiation with an anti-VEGF monoclonal antibody [106]. Tumour growth inhibition was accompanied with a significant reduction in tumour vasculature density, a decrease in interstitial fluid pressure, and an increase in partial oxygen tension. The most complex part is defining the “normalization window” since vessel normalization is transient and hard to capture, despite its recognisable pattern (e.g., vascular structure plasticity and changes to the tumour microenvironment) [13,107,108]. Vascular normalization occurs very quickly in both human and murine models, often within a day and it lasts approximately 1 week to a couple of months in humans [109,110]. These effects are also limited spatially and temporally and differ for diverse types of cancers. Predictive detection of microvessel architectural parameters could be based on Magnetic Resonance Imaging (MRI), Vessel Architectural Imaging (VAI), Microvascular Density (MVD), or Positron Emission Tomography (PET) [108,110].
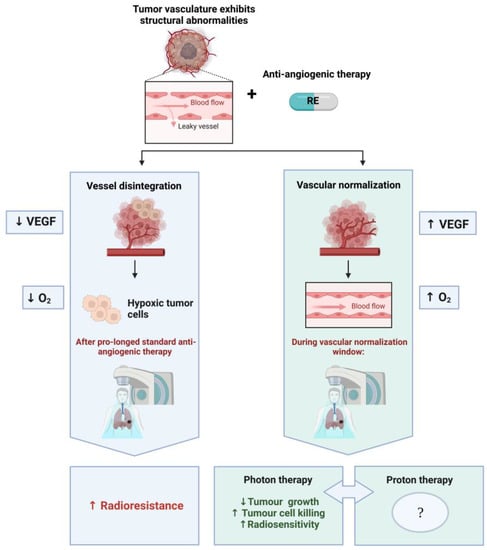
Figure 2.
The paradoxal combination of RE and RT. The left-hand side (blue) illustrates the initial approach, where anti-angiogenic therapy blocks the formation of blood vessels and enhances hypoxia within the tumour, due to the reduction of blood vessels, resulting in increased radioresistance during RT. If one administers RE at a well-defined dose, a normalization window will appear at a given time (variable timing depending on tumour type, administration route, and dose). In this case, the RT can benefit from vascular normalization (right column—green), which induces the conversion of a chaotic to a more normal vascular network in the tumour and results in an enhancement of tumour oxygenation and radiosensitivity. This contributes to increased tumour control and tumour cell killing in experimental studies with X-ray (photon)-based RT. No preclinical or clinical studies are currently available on the combination of RE with proton therapy, which calls for further investigation to decipher the combined effects. Figure was created with BioRender.
In addition to the dilemma created by the normalization window and the optimal timing between drug administration and fractionated RT, anti-angiogenics have not been investigated in combination with particle therapy. This is a rapidly growing field within RT, where particles such as protons and carbon-ions are used which present physical and radiobiological advantages over conventional high-energy X-rays to treat cancer [111,112,113]. Furthermore, contrasting radiobiology reports exist on the tissue-level effects of X-ray based RT versus particle therapy, which could significantly impact disease progression, such as angiogenesis [114,115,116]. A growing number of studies highlights the differences between X-rays and particle beams on pro- and anti-angiogenic effects (Figure 1). Kamlah and collaborators explored the angiogenic effects of carbon ions and X-rays in A459 tumour bearing BALB/c nu/nu mice [117]. The A549 cells were irradiated with both radiation types and injected in the mice to generate a plug, allowing the quantification of blood vessel formation. A significant increase in blood vessel density was observed after X-ray irradiation (6 Gy), but not with carbon ions (biological equivalent dose, 2 Gy) [117]. Takahashi and colleagues reported the inhibition of endothelial cell migration and invasion at sublethal doses of carbon ions, while sublethal X-rays promoted endothelial cell migration and progression of capillary-like tube structures, even after doses as high as 16 Gy [118]. High-energy proton irradiation inhibits multiple angiogenesis-associated processes, including invasion and endothelial cell proliferation [116]. Dose-dependent suppression of angiogenic signalling was demonstrated in both cancerous and non-transformed cells. Additionally, the downregulation of VEGF-A, interleukin 6 and 8 (IL-6, IL-8), and HIF-1α was reported [116]. In contrast, Girdhani reported the upregulation of anti-angiogenic genes like VEGF-A, IL-6, and HIF1α after endothelial cell exposure to high-LET 56Fe ion radiation [119]. The radiation quality that is used for therapy will have to be considered in targeting tumour angiogenesis, as well as in the successful incorporation of anti-angiogenics. The lessons learned from X-ray-based RT may not guarantee similar outcomes when incorporated into particle therapy.
3.1. RE and Vascular Normalization
The current status and study progress on the normalization window of RE was comprehensively reviewed by He et al. [120]. RE’s effectiveness as an anti-tumour drug has been correlated to its ability to restore vascular normalization and reduce HIF-1α expression and its related signalling pathways [58,76,121]. This observation has led to investigations into RE’s efficacy as a vascular normalizer in various lung cancer models [122,123,124]. Transient vascular normalization occurred in A549 lung adenocarcinoma murine models between 7–10 days after RE administration. Within this same period, an increase in activated circulating endothelial cells, decreases in intra-tumour hypoxia, vessel permeability, and microvascular density were reported [122]. Furthermore, the maximal anti-tumour effects of cisplatin were observed on day 5–9 after the initial administration of RE, improving the synergistic efficacy of RE and cisplatin. In a murine xenograft model of lung cancer, tumour vessels normalized and matured on day 6 of RE-therapy. In addition, the amount of M2-like TAMs in the tumours decreased, whereas the number of M1-like TAMs increased during vascular normalization [123]. An RE-induced decrease in tumour hypoxia after 5 days of RE administration has also been reported in NSCLC patients [124]. Hypoxic alleviation was shown on day 5, both clinically (in patients) and in Lewis lung carcinoma (LLC) models. In mice, the most significant growth delay was observed when RT was given on day 5, which was superior to single therapy with RE, RT or when RT was given 1 day before or after RE [125].
RE’s vascular normalization capability has also been exhibited in other cancer models. In colon and nasopharyngeal carcinoma-bearing mice, RE treatment inhibited vascular endothelial growth and increased pericyte coverage, which led to tumour vascular normalization [126,127]. A normalization window appeared by day 5 to 7, resulting in improved anti-tumour effects of RT [127]. RE also improved the anti-tumour effects of Programmed death ligand 1 (PDL-1) inhibitors in a colon carcinoma model. Furthermore, after 27 days, the effect of RE alone was reported to cause significantly lowered levels of VEGF and transforming growth factor β (TGF-β) [128].
3.2. Summary of Preclinical Results on RE Combined with RT
Interestingly, there are less preclinical studies compared to clinical evaluations of native RE in combination with RT. Table 1 and Table 2 summarize multiple studies that investigated the combined effect for different cancer types in vitro (cells) and in vivo (animal models). The most notable in vitro effects caused by RE and RT were cell cycle disruptions [129,130], enhanced cellular radiosensitivity resulting in changes in proliferation, invasion, and migration [130,131,132]. In general, a combination therapy seems to inhibit tumour cell growth and improve the effects of photon-based RT (incl. high-energy X-rays and 60Co γ-rays).
Tumour growth inhibition was often noted in in vivo models (Table 2). Normalized vasculature was observed when RE was combined with photon-based RT, as well as successful tumour regression attributable to improved hypoxic conditions [107,119,126]. Ling and co-workers treated RE gene-transfected lung adenocarcinoma (A549) cells with RT, which synergistically inhibited neovascularization and tumour growth [133]. RE gene-transfected B16 melanoma bearing mice showed marked reductions in intra-tumoural vascularization upon combination with X-rays [134]. At a selected dose of 15 Gy, RE incorporated with 137Cs γ-rays showed pronounced tumour growth in mice bearing A431 cell epidermoid tumour xenografts. RE had little to no effect on tumour cell apoptosis over time, while IR alone significantly increased tumour cell apoptosis. RE combined with RT, however, increased tumour cell apoptosis by a factor of two and blocked tumour revascularization [135]. Finally, in both in vitro and in vivo analyses, VEGF/ VEGFR pathway signalling was implicated in the efficacy of the RE and RT combination [132,136]. By examining the VEGFR-2 high-expressing cell line Calu-1 and VEGFR-2 low-expressing cell line A549, Liu et al. showed that RE and RT induced apoptosis and enhanced radiosensitivity in Calu-1 cells, while a limited effect was observed in A549 lung adenocarcinoma cells [132].
As illustrated in Table 1 and Table 2, RE in combination with RT has also be evaluated in preclinical settings for several non-pulmonary cancer types, such as breast, oesophageal, hepatocellular, colorectal and nasopharyngeal carcinoma [127,131,137,138,139,140,141,142,143]. The two in vitro studies on breast cancer cell lines of Aydemir and co-workers confirmed that RE potentiated the anti-tumour effect of RT [138,139]. In their first study, RT alone inhibited the growth of 4T1 (30.81%) and 4THMpc (39.64%) cells, while the addition of RE enhanced the growth inhibition to 83% in 4T1 and 80% in 4THMpc cells [138]. For oesophageal cancer, the in vitro study did not show an enhanced level of apoptosis on the Eca-109 and TE13 cell lines when RE was combined with RT [131]. However, RE combined with radiotherapy significantly inhibited the proliferation, migration, invasion, and vascular mimicry of human oesophageal cancer cells in a dose-dependent manner. The latter showed that the combination of RE with RT has the potential to significantly change the microenvironment of oesophageal carcinoma. In parallel, two in vivo studies illustrated that RE improved the radioresponse in oesophageal xenograft mouse model [139,144]. Both studies showed a reduction in MVD on histological tumour sections and a delay in tumour growth in the treatment groups with RE and RT, compared to RT alone. Both studies did not clearly define the appearance of the vascular normalization window, but Zhu and co-workers reported an improvement of tumour hypoxia 12 days after the start of the RE treatment [141]. In a hepatocellular carcinoma (HCC) bearing mouse model, RT alone increased the expression of VEGF (38.7 ± 5.8), while combination therapy with RE reduced VEGF (15.0 ± 1.8) expression as well as the MVD [142]. The combination of RE and RT showed the highest levels of tumour growth inhibition, while RE alone did not always lead to a higher inhibition effect. The latter study did not specifically determine the normalization window, but administered RE 7 days before the RT, followed by continuous RE treatment after RT to obtain the synergic therapeutic effect. Based on the different preclinical studies, it is clear that more studies are needed to clearly investigate the influence of different RE concentrations and the timing of the vascular normalization window. The administration of RT within this window is critical to obtain a maximal therapeutic effect.

Table 1.
Summary of in vitro studies on RE combined with radiotherapy.
Table 1.
Summary of in vitro studies on RE combined with radiotherapy.
| Cancer Type Cell Lines | Endostatin Type Dose | Main Result | RT Type Dose | Year | Reference |
|---|---|---|---|---|---|
| Breast Cancer 4T1 or 4MTMHpc | RE (murine) 0.5, 1, 2, 4 and 8 µg/mL | Inhibits the in vitro growth and potentiates the anti-tumour effects of RT via alteration of the amount of substance P | 60Co γ-rays 45 Gy | 2011 | [138] |
| Human Pulmonary Adenocarcinoma A549 | RE 300 mg/L normoxia; 400 mg/L hypoxia | Radiosensitizing effect under hypoxia, but not under normoxia. RE enhanced radiosensitivity through G2/M arrest | 6 MV X-ray 2 Gy | 2012 | [129] |
| Human ESCC Eca109 and TE3 | RE 25, 50, 100, 200, 400, 600, and 800 µg/mL | Combined treatment inhibited migration, invasion, and vasculogenic mimicry formation, but did not enhance radiosensitivity | 6 MV X-ray 2, 4, 6 or 8 Gy | 2016 | [131] |
| NSCLC Calu-1, A549, 95D, NCI-H292, NCI-H1299 | RE 0, 200, 500, 1000, 2000, and 2500 µg/mL. IC20 of Calu-1 cells: 296.5 μg/mL | Induces apoptosis and enhances radiosensitivity of the VEGFR-2 high-expressing cell line Calu-1, but it has a limited effect on the VEGFR-2 low- expressing cell line A549 | not stated 2, 4, 6 and 8 Gy | 2016 | [132] |
| Breast Cancer 4T1 or 4MTMHpc | RE 0.5, 1, 2, 4 and 8 µg/mL: 4 µg/mL-most cytotoxic | Increase in ADAM10 enzyme activity (4T1 or 4MTMHpc cell line, respectively): RT (55%) vs. RE + RT (74.5%) RE (43.3%) vs. RT (70.9%) vs. RE + RT (72.5%) | 60Co γ-rays 45 Gy | 2016 | [139] |
| Human lung squamous carcinoma H-520 | RE 200 µg/mL | RE significantly enhanced the radiosensitivity by inhibition of cellular proliferation, promotion of cell apoptosis and redistribution of cell cycle, possibly via deactivation of the Akt pathway | 60Co γ-rays 1, 2, 4, 6, 8 and 10 Gy | 2010 | [130] |
ESCC = oesophageal squamous cell carcinoma; NSCLC = Non small cell lung cancer; RE = Recombinant Endostatin; RT = Radiotherapy.

Table 2.
Endostatin combined with radiotherapy in vivo mouse models.
Table 2.
Endostatin combined with radiotherapy in vivo mouse models.
| Cancer Type | E/RE Dose | Main Result | RT Type Dose | Year | Reference |
|---|---|---|---|---|---|
| LLC | RE 15 mg/kg | Can promote the normalization of tumour blood vessels and increase the anti-tumour immune-related immune cells infiltrating the tumour post RT | Varian Clinac 600C (energy not specified, 6–10 MV X-rays) 10 Gy | 2020 | [125] |
| EC | E 50 mg/kg | Enhanced the anti-tumour effects of RT and prolonged disease-free survival | Cs137 γ-rays Dose rate 6 Gy/min (dose not specified) | 2007 | [135] |
| ESCC | RE 2.5, 5 and 10 mg/kg | RE promotes the efficacy of RT on esophageal cancer, which may be partly realized by inhibiting the activity of VEGF related signal pathways | 6 MV X-ray 10 Gy | 2016 | [140] |
| NSCLC | RE 0.75 mg/mL for 7 days | RT + weekly RE showed synergistic effects, produced by: RE’s stability, RE’s improvement of tumour hypoxia resulting in increased sensitivity to RT and RE’s inhibition of RT-induced tumour angiogenesis | 6 MV X-ray 10 Gy | 2011 | [144] |
| ESCC | RE 15 mg/kg | RE + RT was more effective at delaying tumour growth than single therapy | RS2000 X-ray irradiator (kV range) 2, 4, 6 or 8 Gy | 2015 | [141] |
| LLC | RE 0, 2.5, 5, 10, and 20 mg/kg | RT + Endo + CP673451 treatment markedly inhibited tumour growth with no improvement in the overall survival and significantly reduced the tumour MVD | Varian Clinac 600C (6–10 MV X-rays) 12 Gy | 2018 | [145] |
| HCC | RE 2, 4, 8, 16, and 32 mg/kg | Combination therapy regulated the expression of genes controlling angiogenesis and cell adhesion. Synergistic effect of RE + RT against HCC in vivo and in vitro | 6 MeV electron beam 10 Gy | 2017 | [142] |
| NPC | RE 20 mg/kg/d | RE normalized tumour vasculature, which alleviated hypoxia and caused significant radiosensitization in human NPC | 160 kV X-ray 6 Gy | 2012 | [127] |
| HNSSC | Endostatin 2.5 mg/kg/day | Endostatin + RT produced an increase in cow pulmonary artery endothelial apoptosis compared with either treatment alone | not stated 15 Gy/day | 2000 | [146] |
| Colorectal cancer | RE 20 mg/kg | The tumour growth inhibition rate in the RT + RE treatment group > single therapy groups | 6 MV X-ray 6 Gy | 2017 | [143] |
| NPC | RE 20 mg/kg | The tumour inhibition rates of RE, RT and RE + RT were 27.12, 60.45 and 86.11%, respectively. Tumour VEGF levels in the RE + RT group < RT only and control groups | 5 MV X-ray 20 Gy | 2012 | [136] |
| NPC/ ung adenocarcinoma | RE 20 mg/kg | RE sensitized anti-tumour/anti-angiogenic RT effects by increasing apoptosis of the endothelial and tumour cells, decreasing hypoxia, and changing proangiogenic factors | 6 MV X-rays 6 Gy per day to 30 Gy, once a day for 1 week | 2009 | [147] |
EC = epidermoid carcinoma; SSC = squamous-cell carcinoma; ESCC = oesophageal squamous cell carcinoma; LLC = Lewis lung carcinoma; HCC = Hepatocellular carcinoma; HNSCC = head and neck squamous cell carcinoma; NPC= Human nasopharyngeal carcinoma; NSCLC = Non-small cell lung cancer; RE = Recombinant Endostatin; RT = Radiotherapy; MVD=Microvessel Density.
3.3. Current Status of Clinical Trials in NSCLC Patients Investigating Radiotherapy Combined with RE
Numerous clinical trials and meta-analyses have demonstrated a significant survival benefit with an acceptable safety profile when treating late-stage NSCLC patients with RE, including synergistic effects with concurrent chemoradiotherapy (CCRT), such as vinorelbine, platinum-based chemotherapy, docetaxel, and etoposide. These improvements were also seen in patients resistant to previous chemotherapy or patients with complete surgical resection [61,137,148,149,150,151,152,153,154,155,156]. Table 3 and the Supplementary material Table S1 provide a selected overview of clinical trials investigating RE combined with chemotherapy alone or with RT/CCRT, respectively. Already in 2005, RE was approved by CFDA in combination with vinorelbine/cisplatin for patients with advanced NSCLC [148]. The benefits of RE therapy have also been shown in NSCLC patients with bone metastasis [124,157]. Despite mostly positive findings, a few trials could not confirm a significant prolongation of the progression-free survival (PFS) and overall survival (OS) for RE combined with chemotherapy. In addition, no PFS benefit was shown in a multi-center phase II study in which 126 previously untreated advanced-stage NSCLC patients were enrolled and randomized to receive RE plus paclitaxel/carboplatin or paclitaxel/carboplatin alone [158].
RE combined with photon-based RT has also been studied extensively to validate its function as a hypoxic tumour radiosensitizer (Table 3). Multiple trials demonstrated a good short-term survival and response in non-resectable stage III NSCLC [124,159]. Interestingly, vascular normalization appeared approximately 1 week after administration of RE, opening an ideal time window for RT [160]. Recently, Yuan et al. clarified via a meta-analysis that the benefits of the addition of RE to CCRT in NSCLC are associated with a significantly higher ORR (objective response rate), disease control rate (DCR), and survival rate compared to CCRT, with similar incidences of main adverse events [149]. The pooled analysis of Zhang et al. concluded that RT combined with endostatin may be a promising strategy for locally advanced NSCLC patients with poor performance status who cannot tolerate chemotherapy [66]. However, the phase II study on RE in combination with paclitaxel, carboplatin, and RT in patients with unresectable NSCLC did not meet its goal without inducing unacceptable toxicity [161]. Continuous intravenous RE in combination with concurrent etoposide/cisplatin and RT resulted in a preferable OS, promising 2-year PFS with tolerable toxicities but did not prolong median PFS (HELPER study 2019) [159]. Interestingly, RE delivered by continuous intravenous pumping with CCRT may be a better option than intravenous injection in terms of potential survival and safety [162,163]. Recently, RE combined with whole-brain RT showed better survival and improved cerebral perfusion parameters in NSCLC patients with brain metastasis [164]. However, it should be noted that the number of patients in every single trial is too limited to achieve a definite conclusion.
Next to CCRT, immune checkpoint inhibitors (ICIs) and anti-angiogenic drugs are gaining momentum as a promising combined treatment strategy for NSCLC. Nivolumab, atezolizumab, and pembrolizumab have been approved as second-line treatments for advanced NSCLC [60]. Recently, the first study investigating the combination of RE with nivolumab showed a favourable efficacy and safety profile [60].

Table 3.
A selection of clinical trials on RE combined with RT in NSCLC.
Table 3.
A selection of clinical trials on RE combined with RT in NSCLC.
| Cancer Type | Phase | E/RE Dose | Year | n | Combined Therapy | Overall Result | Reference |
|---|---|---|---|---|---|---|---|
| NSCLC | Pro cohort | RE 15 mg/day | 2012 | 25 | RT | (+) short term therapeutic effects and local control rates. no severe adverse effects (-) no improvement of 1/3 year OS | [160] |
| NSCLC | n.s. | RE 15mg/day for 10 days | 2013 | RT | (+) decreased hypoxia | [124] | |
| BM of NSCLC | II | RE 7.5 mg/m2/day | 2014 | RT | (+) can relieve brain oedema | [165] NCT01410370 | |
| Stage III NSCLC | SA pro II | RE 7.5 mg/m2/day for 7 days at week 1, 3, 5 and 7 | 2015 | 48 | RT/DOC and CIS | (+) promising survival and local control rates | [166] NCT01218594 |
| Stage IIIA/B NSCLC | SA pro II | E 7.5 mg/m2 on day 1–14, every 3 weeks | 2016 | 19 | RT/TC | (-) did not meet the goal per study design with unacceptable toxicity | [161] NCT01158144 |
| Stage IIIA/B NSCLC | SA retro | RE 7.5 mg/m2/day for 7 days at week 1, 3, 5 and 7 | 2020 | CCRT | Inflammation-based factors as biomarker | [167] | |
| Stage III NSCLC | SA pro II | RE 7.5 mg/m2/day, 14 days/cycle | 2019 | 67 | RT/ ETO-CIS | (-) did not prolong median PFS (+) preferable OS, promising 2-year PFS with tolerable toxicities | [159] HELPER study NCT01733589 |
| Stage III NSCLC | II | RE 7.5 mg/m2/day for seven day | 2020 | 48 | IV RE + RT/DOC/ CIS vs. CIV RE + RT/ ETO-CIS | CIV > IV | [162] |
| Local aLSCC | retro | RE 7.5 mg/m2/day for 14 days (every 3 weeks) | 2020 | 94 | RT/NP | Lipoprotein (a) as biomarker | [168] |
| Stage III NSCLC* | IV | RE 7.5 mg/m2/day, 14 days/cycle | / | / | Durvalumab/ reduced-dose CCRT (50 Gy) | Not yet recruiting | NCT04613284 |
| Stage III NSCLC | Multi-centre, prospective real-world study | RE n.s. | / | / | CCRT | Not yet recruiting | NCT04161352 |
(aNSCLC) advanced non-small cell lung cancer, (aLCC) advanced lung squamous carcinoma, (BM) brain metastasis, (CCRT) concurrent chemoradiotherapy, (CIS) Cisplatin, (CIV) Continuous intravenous pumping, (DOC) Docetaxel, (E) Endostatin, (ETO-CIS) etoposide-cisplatin, (IV) intravenous injection, (n) number of study participants, (n.s.) not specified, (NP) vinorelbine and cisplatin, (pro) prospective, (RE) Recombinant endostatin or endostar, (retro) retrospective, (SA) Single arm, (TC) paclitaxel-carboplatin, (*) who cannot tolerate 60 Gy RT.
4. Discussion and Concluding Remarks
Targeting angiogenesis as a tumour treatment strategy is undoubtedly complex, since pathological angiogenesis causes numerous changes in the TME. In addition, potential elevated toxicity in chemotherapeutic-anti-angiogenic combinations, and the impact of the normalization window and resulting oxygenation status on RT are still a topic of debate. To assess the ability of an additional variable to potentiate the effect of a treatment, it is of critical importance to first assess the impact of each individual treatment alone and understand the underlying biological mechanisms. To that end, photon-based RT as a standalone target against tumour angiogenesis has been shown to cause angiogenic stimulation when compared to proton therapy (PT) (Figure 1). There is an opportunity to take advantage of the vascular normalization window of 5–7 days upon RE administration, but the combination of RE with PT may cause a synergistic angiogenic suppression and the effect of such a combined treatment has not been assessed so far. Furthermore, an argument could also be made on the necessity to administer RE or to lower the dosage, since PT already boosts anti-angiogenic pathways. In addition, high-LET radiation, such as carbon ions, is less dependent on the oxygen effect to kill tumour cells, which questions the benefit of concurrent anti-angiogenic therapy [115,169].
NSCLC is known as a radioresistant cancer due to the presence of cancer stem cells, an epithelial-mesenchymal transition, and its high proportion of hypoxic cell populations [6,170]. RE has already been shown to act as a radiosensitizer in several cancer types (Table 2) and this led to several clinical trials (Table 3 and Supplementary Table S1). These clinical trials confirmed synergism when RE was combined with CCRT without causing major toxicities. However, more randomized controlled trials are needed to confirm long-term survival benefits [149]. In addition, while the majority of clinical trials apply similar RE dosages (Table 3 and Supplementary Table S1), the administration routes and timing varies, as well as the total length of the combined treatment cycles. It was out of the scope of the current work to compare administration routes and clinical details regarding patient selection and treatment evaluation, but it is important to keep into consideration that the dosage and timing of RE administration requires careful attention in both clinical and preclinical studies. Multiple short-term doses of anti-VEGF therapy could be required to generate a true long-term benefit and tumour regression. Unfortunately, prolonged exposure to anti-angiogenics results in increased hypoxia and systemic toxicity, compounding the dilemma that exists around the incorporation of anti-angiogenics with RT. Re-increased hypoxia and prolonged VEGF suppression, causing further local increases in hypoxia, are also coupled with pH shifts in the TME and consequent acidosis [171]. Furthermore, anti-angiogenic therapy has been shown to promote tumour metastasis. Due to hypoxic conditions, a pressure mechanism is generated that causes selectivity for tumour cells that harbour increased aggressiveness and lower sensitivity to anti-angiogenic therapy [172]. Work by Yang and co-workers also exhibited that anti-VEGF cessation-associated regrowth and remodelling of hepatic vasculatures provided a structural basis for cancer metastasis [173]. Additionally, a double-edged sword scenario appears when trying to resolve these complications with shortened anti-angiogenic treatment periods, as a relapse in pathological angiogenesis has been observed in multiple diseases after shorter treatment with anti-angiogenics or during drug holidays [174,175]. These preliminary conclusions provide reasons to motivate the use of RE in combination with particle therapy in future assessments. The inverted depth-dose profile of carbon-ion and protons allows dose sparing of organs at risk of co-irradiation, maximum dose deposition within the tumour, and the potential of dose escalation [176]. Several studies have also revealed previously unrecognized biological advantages of proton therapy (PT) specifically [177]. Moreover, the lower integral dose of PT and its dose sparing properties, have been found to reduce the exposure of circulating lymphocytes and the immune organs at risk compared to photon-based RT [178]. Preliminary findings of a study in NSCLC with underlying idiopathic pulmonary fibrosis showed a trend of non-statistically significant better OS compared to X-rays for patients treated with PT [179]. However, a randomized phase III clinical trial of intensity modulated photon therapy versus passive scattering proton therapy of locally advanced NSCLC, reported no benefit in the primary endpoints (grade 3 pneumonitis and local failure) after PT [180]. Furthermore, it is also important to note the difficulties in treating lung cancer with PT, largely due to the impact of highly heterogeneous tissues in the proton path on the proton dose distributions and respiratory motion during irradiations [181,182]. The higher RBE at the distal edge of the beam could potentially be problematic if this region is deposited in an organ at risk such as the heart. When one applies generous margins to circumvent the problem of tumour motion and tissue heterogeneity, this might counteract the dosimetric advantage and cause more normal tissue injury [178]. This adds an additional layer of complexity in the incorporation of proton therapy with RE seeing as microvascular heterogeneity is another important variable to consider due to its abundance in lung tissue [183], and its direct influence on the efficacy of anti-angiogenic therapy. However, the use of intensity modulated PT in combination with real-time volumetric image guidance, management of organ and tumour motion and accurate models which incorporate set-up uncertainties will assist to solve this problem [182].
Anti-VEGF therapy supresses neovascularization efficiently, whilst mature blood vessels are not as affected. This has been postulated to be attributable to a loss in dependence on growth factor signalling by mature vessels and potential anti-VEGF-A/VEGFR pathway therapy resistance as a consequence [108]. It is clear that the potential for improved therapeutic outcomes by combining RE and PT for the treatment of NSCLC is an avenue worth exploring, particularly in combination with immunotherapy. However, this will not only require a better understanding of the effects of PT on angiogenesis pathways, but also on the immunomodulatory effect of particles, such as protons and carbon ions. Immune checkpoint modulators such as anti-PD1 or anti-PDL1 agents are considered to be a breakthrough in the treatment of NSCLC [184]. The view that RT, and particularly particle therapy, can provoke a systemic immune response, provides a strong rationale for the combination with immunotherapy [185]. In this context, anti-angiogenic drugs, such as RE, could potentiate immunotherapy through vascular normalization and optimizing the tumour immune microenvironment. This rationale is currently accepted as a valid therapeutic strategy that can enhance cancer immunity, where the addition of RT could further expand the treatment landscape of NSCLC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph16020219/s1, Table S1: Current status of clinical trials on endostatin combined with chemotherapy in NSCLC. References [186,187,188,189,190,191,192,193,194,195,196] are cited in the supplementary materials.
Author Contributions
C.C. and J.B. contributed equally to the work in this paper. Conceptualization C.C., J.B. and C.V. validation, J.B., C.V., A.G., A.B. and H.S.; formal analysis, C.C., J.B. and C.V.; investigation, C.C., J.B. and C.V.; data curation, J.B. and C.C.; writing—original draft preparation, C.C. and J.B.; writing—review and editing, C.C., J.B., C.V., A.B., A.G. and H.S.; visualization, C.C. and J.B.; supervision, J.B., C.V., A.G. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
The National Research Foundation of South Africa supported this work by providing postgraduate funding support under the Scarce Skills and Innovation Program for PhD-student C.C (grant number: 119944). The publication is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—491382106, and by the Open Access Publishing Fund of GSI Helmholtzzentrum fuer Schwerionenforschung.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Society of Clinical Oncology, I. Lung Cancer- Non Small-Cell: Statistics. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 24 November 2022).
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward Personalized Treatment Approaches for Non-Small-Cell Lung Cancer Meina. Nat. Med. 2021, 27. [Google Scholar] [CrossRef]
- Houston, K.A.; Henley, S.J.; Li, J.; White, M.C.; Richards, T.B. Patterns in Lung Cancer Incidence Rates and Trends by Histologic Type in the United States, 2004–2009. Lung Cancer 2014, 86, 22–28. [Google Scholar] [CrossRef]
- American Cancer Society, Lung Cancer Early Detection, Diagnosis, and Staging. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8661.00.pdf (accessed on 24 November 2022).
- Qiang, H.; Chang, Q.; Xu, J.; Qian, J.; Zhang, Y.; Lei, Y.; Han, B. New Advances in Antiangiogenic Combination Therapeutic Strategies for Advanced Non - Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 631–645. [Google Scholar] [CrossRef]
- Hong, Y.; Park, S.; Lee, M.K. The Prognosis of Non-Small Cell Lung Cancer Patients According to Endobronchial Metastatic Lesion. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-Carboplatin Alone or with Bevacizumab for Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef]
- Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, M.C.; Daniele, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. [Google Scholar] [CrossRef]
- Yadav, L.; Puri, N.; Rastogi, V.; Satpute, P.; Sharma, V. Tumour Angiogenesis and Angiogenic Inhibitors: A Review. J. Clin. Diagnostic Res. 2015, 9, XE01–XE05. [Google Scholar] [CrossRef]
- Baeriswyl, V.; Christofori, G. The Angiogenic Switch in Carcinogenesis. Semin. Cancer Biol. 2009, 19, 329–337. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing Tumor Vasculature with Anti-Angiogenic Therapy: A New Paradigm for Combination Therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Bishop-Bailey, D. Tumour Vascularisation: A Druggable Target. Curr. Opin. Pharmacol. 2009, 9, 96–101. [Google Scholar] [CrossRef]
- Poto, R.; Cristinziano, L.; Modestino, L.; de Paulis, A.; Marone, G.; Loffredo, S.; Galdiero, M.R.; Varricchi, G. Neutrophil Extracellular Traps, Angiogenesis and Cancer. Biomedicines 2022, 10, 431. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.W.; Ylaya, K.; Chung, E.J.; Kitano, H.; Perry, C.; Hanaoka, J.; Fukuoka, J.; Chung, J.Y.; Hewitt, S.M. Tumor-Associated Macrophage, Angiogenesis and Lymphangiogenesis Markers Predict Prognosis of Non-Small Cell Lung Cancer Patients. J. Transl. Med. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Sammarco, G.; Varricchi, G.; Ferraro, V.; Ammendola, M.; De Fazio, M.; Altomare, D.F.; Luposella, M.; Maltese, L.; Currò, G.; Marone, G.; et al. Mast Cells, Angiogenesis and Lymphangiogenesis in Human Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 2106. [Google Scholar] [CrossRef]
- Loizzi, V.; Del Vecchio, V.; Giulio, G.; De Liso, M.; Kardashi, A.; Naglieri, E.; Resta, L.; Cicinelli, E.; Cormio, G. Biological Pathways Involved in Tumor Angiogenesis and Bevacizumab Based Anti-Angiogenic Therapy with Special References to Ovarian Cancer. Int. J. Mol. Sci. 2017, 18, 1967. [Google Scholar] [CrossRef]
- Sakurai, T.; Kudo, M. Signaling Pathways Governing Tumor Angiogenesis. Oncology 2011, 81 (Suppl. 1), 24–29. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The Biology of VEGF and Its Receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Huang, Z.; Bao, S.D. Roles of Main Pro- and Anti-Angiogenic Factors in Tumor Angiogenesis. World J. Gastroenterol. 2004, 10, 463–470. [Google Scholar] [CrossRef]
- Jia, T.; Jacquet, T.; Dalonneau, F.; Coudert, P.; Vaganay, E.; Exbrayat-Héritier, C.; Vollaire, J.; Josserand, V.; Ruggiero, F.; Coll, J.-L.; et al. FGF-2 Promotes Angiogenesis through a SRSF1/SRSF3/SRPK1-Dependent Axis That Controls VEGFR1 Splicing in Endothelial Cells. BMC Biol. 2021, 19, 173. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting Signalling Pathways in Tumour Angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF Function in Angiogenesis: Signalling Pathways, Biological Responses and Therapeutic Inhibition. Trends Pharmacol. Sci. 2001, 22, 201–207. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Bicknell, R. New Molecular Pathways in Angiogenesis. Br. J. Cancer 2003, 89, 228–231. [Google Scholar] [CrossRef]
- Farzaneh, Z.; Vosough, M.; Agarwal, T.; Farzaneh, M. Critical Signaling Pathways Governing Hepatocellular Carcinoma Behavior; Small Molecule-Based Approaches. Cancer Cell Int. 2021, 21. [Google Scholar] [CrossRef]
- Akil, A.; Gutiérrez-García, A.K.; Guenter, R.; Rose, J.B.; Beck, A.W.; Chen, H.; Ren, B. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front. Cell Dev. Biol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Oliveira, R.H.M.; Zhao, C.; Popel, A.S. Systems Biology of Angiogenesis Signaling: Computational Models and Omics. WIREs Mech. Dis. 2021, 1–37. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.I.; Sivridis, E.; Turley, H.; Talks, K.; Pezzella, F.; Gatter, K.C.; Harris, A.L. Relation of Hypoxia Inducible Factor 1 Alpha and 2 Alpha in Operable Non-Small Cell Lung Cancer to Angiogenic/Molecular Profile of Tumours and Survival. Br. J. Cancer 2001, 85, 881–890. [Google Scholar] [CrossRef]
- Jackson, A.L.; Zhou, B.; Kim, W.Y. HIF, Hypoxia and the Role of Angiogenesis in Non-Small Cell Lung Cancer. Expert Opin. Ther. Targets 2010, 14, 1047–1057. [Google Scholar] [CrossRef]
- Lin, C.; Liu, T.; Lee, M.; Yang, S.; Tsao, T.C. Independent Prognostic Value of Hypoxia-Inducible Factor 1-Alpha Expression in Small Cell Lung Cancer. Int. J. Med. Sci. 2017, 14, 785–790. [Google Scholar] [CrossRef]
- Sardari Nia, P.; Colpaert, C.; Blyweert, B.; Kui, B.; Vermeulen, P.; Ferguson, M.; Hendriks, J.; Weyler, J.; Pezzella, F.; Van Marck, E.; et al. Prognostic Value of Nonangiogenic and Angiogenic Growth Patterns in Non-Small-Cell Lung Cancer. Br. J. Cancer 2004, 91, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.L.; Gomes, M.P.; Catarino, R.J.; Rolfo, C.; Lopes, A.M.; Medeiros, R.M.; Araújo, A.M. Angiogenesis in NSCLC: Is Vessel Co-Option the Trunk That Sustains the Branches? Oncotarget 2017, 8, 39795–39804. [Google Scholar] [CrossRef] [PubMed]
- Sardari Nia, P.; Colpaert, C.; Vermeulen, P.; Weyler, J.; Pezzella, F.; Van Schil, P.; Van Marck, E. Different Growth Patterns of Non-Small Cell Lung Cancer Represent Distinct Biologic Subtypes. Ann. Thorac. Surg. 2008, 85, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Truong, A.K.; Becker, L.M.; Saavedra-garc, P. Tumor Vessel Co-Option: The Past & the Future. Front. Oncol. 2022, 1–20. [Google Scholar] [CrossRef]
- Liao, Y.; Wu, X.; Wu, M.; Fang, Y.; Li, J.; Tang, W. Non-Coding RNAs in Lung Cancer: Emerging Regulators of Angiogenesis. J. Transl. Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Boehm, T.; Shing, Y.; Flynn, E.; Birkhead, J.; Bjor, R.; Folkman, J. Endostatin, a Endogenous Inhibitor of Angiogenesis and Tumor Growth. Cell Press 1997, 88, 277–285. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Konduri, S.; Basha, R.; Philip, P.A.; Baker, C.H. Angiogenesis: An Update and Potential Drug Approaches (Review). Int. J. Oncol. 2010, 36, 5–18. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a Therapeutic Target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Berry, M.R.; Fan, T.M. Target-Based Radiosensitization Strategies: Concepts and Companion Animal Model Outlook. Front. Oncol. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, H.; Liu, X.; Wang, Z.; Zhang, Q.; Wei, N. Vascular Normalization: A New Window Opened for Cancer Therapies. Front. Oncol. 2021, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Goedegebuure, R.S.A.; de Klerk, L.K.; Bass, A.J.; Derks, S.; Thijssen, V.L.J.L. Combining Radiotherapy With Anti-Angiogenic Therapy and Immunotherapy; A Therapeutic Triad for Cancer? Front. Immunol. 2018, 9, 3107. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Guo, F.; Cui, J. Anti-Angiogenesis: Opening a New Window for Immunotherapy. Life Sci. 2020, 258, 118163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhai, Y.; Hui, Z. Application Basis of Combining Antiangiogenic Therapy with Radiotherapy and Immunotherapy in Cancer Treatment. Front. Oncol. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Fontanini, G.; Lucchi, M.; Vignati, S.; Mussi, A.; Ciardiello, F.; De Laurentiis, M.; De Placido, S.; Basolo, F.; Angeletti, C.A.; Bevilacqua, G. Angiogenesis as a Prognostic Indicator of Survival in Non-Small-Cell Lung Carcinoma: A Prospective Study. J. Natl. Cancer Inst. 1997, 89, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Onn, A.; Sandler, A. Angiogenesis and Lung Cancer: Prognostic and Therapeutic Implications. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3243–3256. [Google Scholar] [CrossRef]
- Daum, S.; Hagen, H.; Naismith, E.; Wolf, D.; Pircher, A. The Role of Anti-Angiogenesis in the Treatment Landscape of Non-Small Cell Lung Cancer – New Combinational Approaches and Strategies of Neovessel Inhibition. Front. Cell Dev. Biol. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Takeda, M.; Nakagawa, K. First- and Second-Generation EGFR-TKIs Are All Replaced to Osimertinib in Chemo-Naive EGFR Mutation-Positive Non-Small Cell Lung Cancer? Int. J. Mol. Sci. 2019, 20, 146. [Google Scholar] [CrossRef]
- Teuwen, L.-A.; De Rooij, L.P.M.H.; Cuypers, A.; Rohlenova, K.; Dumas, S.J.; García-Caballero, M.; Meta, E.; Amersfoort, J.; Taverna, F.; Becker, L.M.; et al. Tumor Vessel Co-Option Probed by Single-Cell Analysis. Cell Rep. 2021, 35, 109253. [Google Scholar] [CrossRef]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Javier Oliver, F. Vasculogenic Mimicry: Become an Endothelial Cell “But Not so Much”. Front. Oncol. 2019, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of Resistance to Anti-Angiogenic Therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tang, H.; Huang, Y.; Song, N.; Luo, Y. Critical Review Unraveling the Mysteries of Endostatin. IUBMB Life 2009, 61, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Antiangiogenesis in Cancer Therapy—Endostatin and Its Mechanisms of Action. Exp. Cell Res. 2006, 312, 594–607. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, J.S. Canstatin, a endogenous inhibitor of angiogenesis and tumor growth. Chin. J. Cancer Res. 2004, 16, 229–234. [Google Scholar] [CrossRef]
- Li, K.; Shi, M.; Qin, S. Current Status and Study Progress of Recombinant Human Endostatin in Cancer Treatment. Oncol. Ther. 2018, 6, 21–43. [Google Scholar] [CrossRef]
- Shu, H.; Dong, Y.; Xu, Z.; Luo, W.; Xu, L.; Zhu, H.; Cheng, L.; Lv, Y. The Efficacy and Safety of Continuous Intravenous Endostar Treatment Combined With Concurrent Chemoradiotherapy in Patients With Locally Advanced Cervical Squamous Cell Carcinoma: A Randomized Controlled Trial. Front. Oncol. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Lv, W.; Pei, X.; Zhao, W.; Cong, Y.; Wei, Y.; Li, T.; Zhang, H.; Lin, Z.; Saito, Y.; Kim, J.J.; et al. Safety and Efficacy of Nivolumab plus Recombinant Human Endostatin in Previously Treated Advanced Non-Small-Cell Lung Cancer. Transl. Lung Cancer Res. 2022, 11, 201–212. [Google Scholar] [CrossRef]
- Ma, H.; Peng, F.; Xu, Y.; Bao, Y.; Hu, X.; Wang, J.; Fang, M.; Kong, Y.; Dong, B.; Chen, M. Five-Year Survival Rate Analysis: The Combination of Fortnightly-Administration of Endostar and Concurrent Chemoradiotherapy versus Concurrent Chemoradiotherapy in the Treatment of Inoperable Locally Advanced Non-Small Cell Lung Cancer. Ann. Palliat. Med. 2021, 10, 7560–7570. [Google Scholar] [CrossRef]
- Bodzioch, M.; Bajger, P.; Foryś, U. Angiogenesis and Chemotherapy Resistance: Optimizing Chemotherapy Scheduling Using Mathematical Modeling. J. Cancer Res. Clin. Oncol. 2021, 147, 2281–2299. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Yu, J.; Zheng, J. Intraperitoneal Infusion of Recombinant Human Endostatin Improves Prognosis in Gastric Cancer Ascites. Futur. Oncol. 2022, 18, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, F.; Jiang, S.; Cao, J.; Meng, Y.; Xu, Y.; Yong, C. Rh-Endostatin Combined with Chemotherapy in Patients with Advanced or Recurrent Mucosal Melanoma: Retrospective Analysis of Real - World Data. Inverstigational New Drugs 2022, 40, 453–460. [Google Scholar] [CrossRef]
- Zhang, S.L.; Han, C.B.; Sun, L.; Huang, L.T.; Ma, J.T. Efficacy and Safety of Recombinant Human Endostatin Combined with Radiotherapy or Chemoradiotherapy in Patients with Locally Advanced Non-Small Cell Lung Cancer: A Pooled Analysis. Radiat. Oncol. 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Dreier, L.; Bryant, R.A.R.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted Cathepsin L Generates Endostatin from Collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef]
- Yoon, S.S.; Eto, H.; Lin, C.M.; Nakamura, H.; Pawlik, T.M.; Song, S.U.; Tanabe, K.K. Mouse Endostatin Inhibits the Formation of Lung and Liver Metastases. Cancer Res. 1999, 59, 6251–6256. [Google Scholar]
- Kisker, O.; Becker, C.M.; Prox, D.; Fannon, M.; D’Amato, R.; Flynn, E.; Fogler, W.E.; Sim, B.K.; Allred, E.N.; Pirie-Shepherd, S.R.; et al. Continuous Administration of Endostatin by Intraperitoneally Implanted Osmotic Pump Improves the Efficacy and Potency of Therapy in a Mouse Xenograft Tumor Model. Cancer Res. 2001, 61, 7669–7674. [Google Scholar] [PubMed]
- Fu, Y.; Chen, Y.; Luo, X.; Liang, Y.; Shi, H.; Gao, L.; Zhan, S.; Zhou, D.; Luo, Y. The Heparin Binding Motif of Endostatin Mediates Its Interaction with Receptor. Biochemistry 2009, 11655–11663. [Google Scholar] [CrossRef]
- Shi, H.; Huang, Y.; Zhou, H.; Song, X.; Yuan, S.; Fu, Y.; Luo, Y. Nucleolin Is a Receptor That Mediates Antiangiogenic and Antitumor Activity of Endostatin. Blood 2007, 110, 2899–2906. [Google Scholar] [CrossRef]
- Bager, C.L.; Karsdal, M.A. Type XVIII Collagen; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128098998. [Google Scholar]
- Poluzzi, C.; Iozzo, R.V.; Schaefer, L. Endostatin and Endorepellin: A Common Route of Action for Similar Angiostatic Cancer Avengers. Adv. Drug Deliv. Rev. 2016, 97, 156–173. [Google Scholar] [CrossRef]
- Mutgan, A.C.; Jandl, K.; Kwapiszewska, G. Endothelial Basement Membrane Components and Their Products, Matrikines: Active Drivers of Pulmonary Hypertension? Cells 2020, 9, 2029. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, A.; Sugimoto, H.; Yang, C.; Lively, J.; Zeisberg, M.; Kalluri, R. Human Tumstatin and Human Endostatin Exhibit Distinct Antiangiogenic Activities Mediated by Avβ and A5β1 Integrins. Proc. Natl. Acad. Sci. USA 2003, 100, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Yang, Y.; Lu, N.; You, Q.; Wang, S.; Gao, Y.; Chen, Y.; Guo, Q. Endostar, a Novel Recombinant Human Endostatin, Exerts Antiangiogenic Effect via Blocking VEGF-Induced Tyrosine Phosphorylation of KDR/Flk-1 of Endothelial Cells. Biochem. Biophys. Res. Commun. 2007, 361, 79–84. [Google Scholar] [CrossRef]
- Moreau, C.; Chautard, E.; Jetne, R.; Fukai, N.; Ruggiero, F.; Humphries, M.J.; Olsen, B.R.; Ricard-blum, S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol. Chem. 2009, 284, 22029–22040. [Google Scholar] [CrossRef]
- Wickström, S.A.; Veikkola, T.; Rehn, M.; Pihlajaniemi, T.; Alitalo, K.; Keski-Oja, J. Endostatin-Induced Modulation of Plasminogen Activation with Concomitant Loss of Focal Adhesions and Actin Stress Fibers in Cultured Human Endothelial Cells. Cancer Res. 2001, 61, 6511–6516. [Google Scholar] [PubMed]
- Dixelius, J.; Cross, M.; Matsumoto, T.; Sasaki, T.; Timpl, R.; Claesson-Welsh, L. Endostatin Regulates Endothelial Cell Adhesion and Cytoskeletal Organization. Cancer Res. 2002, 62, 1944–1947. [Google Scholar]
- Wang, S.; Lu, X.-A.; Liu, P.; Fu, Y.; Jia, L.; Zhan, S.; Luo, Y. Endostatin Has ATPase Activity, Which Mediates Its Antiangiogenic and Antitumor Activities. Mol. Cancer Ther. 2015, 14, 1192–1201. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, S.; Jia, L.; Wang, S.; Liu, J.; Ma, X.; Wang, C.; Fu, Y.; Luo, Y. E-M, an Engineered Endostatin with High ATPase Activity, Inhibits the Recruitment and Alternative Activation of Macrophages in Non-Small Cell Lung Cancer. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Lee, S.; Jeung, I.C.; Park, T.W.; Lee, K.; Lee, D.G.; Cho, Y.; Lee, T.S.; Na, H.; Lee, H.G.; Jeong, M.S.; et al. Extension of the in Vivo Half-Life of Endostatin and Its Improved Anti-Tumor Activities upon Fusion to a Humanized Antibody against Tumor-Associated Glycoprotein 72 in a Mouse Model of Human Colorectal Carcinoma. Oncotarget 2015, 6. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Tjin Tham Sjin, R.M.; Movahedi, S.; Ahmed, B.; Pravda, E.A.; Lo, K.-M.; Gillies, S.D.; Folkman, J.; Javaherian, K. Linking Antibody Fc Domain to Endostatin Significantly Improves Endostatin Half-Life and Efficacy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 1487–1493. [Google Scholar] [CrossRef]
- Hai-Tao, Z.; Hui-Cheng, L.; Zheng-Wu, L.; Chang-Hong, G. A Tumor-Penetrating Peptide Modification Enhances the Antitumor Activity of Endostatin in Vivo. Anticancer. Drugs 2011, 22, 409–415. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Ramakrishnan, S. Addition of Integrin Binding Sequence to a Mutant Human Endostatin Improves Inhibition of Tumor Growth. Int. J. Cancer 2004, 111, 839–848. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, X.; Wang, X.; Chen, J. Preparation and Stability of N-Terminal Mono-PEGylated Recombinant Human Endostatin. Bioconjug. Chem. 2006, 17, 995–999. [Google Scholar] [CrossRef]
- Li, H.L.; Li, S.; Shao, J.Y.; Lin, X.B.; Cao, Y.; Jiang, W.Q.; Liu, R.Y.; Zhao, P.; Zhu, X.F.; Zeng, M.S.; et al. Pharmacokinetic and Pharmacodynamic Study of Intratumoral Injection of an Adenovirus Encoding Endostatin in Patients with Advanced Tumors. Gene Ther. 2008, 15, 247–256. [Google Scholar] [CrossRef]
- Jin, X.; Bookstein, R.; Wills, K.; Avanzini, J.; Tsai, V.; LaFace, D.; Terracina, G.; Shi, B.; Nielsen, L.L. Evaluation of Endostatin Antiangiogenesis Gene Therapy in Vitro and in Vivo. Cancer Gene Ther. 2001, 8, 982–989. [Google Scholar] [CrossRef][Green Version]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Pillay, V. Design and Characterization of Endostatin-Loaded Nanoparticles for in Vitro Antiangiogenesis in Squamous Cell Carcinoma. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Marimuthu, T.; Kondiah, P.P.D.; Pillay, V. Folate-Decorated, Endostatin-Loaded, Nanoparticles for Anti-Proliferative Chemotherapy in Esophaegeal Squamous Cell Carcinoma. Biomed. Pharmacother. 2019, 119, 109450. [Google Scholar] [CrossRef]
- Adeyemi, S.A.; Choonara, Y.E. In Vitro and In Vivo Evaluation of a Cyclic LyP-1-Modified Nanosystem for Targeted Endostatin Delivery in a KYSE-30 Cell Xenograft Athymic Nude Mice Model. Pharmaceuticals 2022, 15, 353. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Wang, L.; Zhao, L.; Cheng, Y.; Wang, A.; Wang, F.; Zhang, X. A Dual Receptor Targeting-and Bbb Penetrating-Peptide Functionalized Polyethyleneimine Nanocomplex for Secretory Endostatin Gene Delivery to Malignant Glioma. Int. J. Nanomedicine 2020, 15, 8875–8892. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Du, B.; Li, X.; Liu, S.; Yang, X.-Y.; Ding, H.; Yang, W.; Pan, F.; Wu, X.; et al. Gold Nanoparticle–Mediated Targeted Delivery of Recombinant Human Endostatin Normalizes Tumour Vasculature and Improves Cancer Therapy. Sci. Rep. 2016, 6, 30619. [Google Scholar] [CrossRef]
- Rezaei, N.; Mehrnejad, F.; Vaezi, Z.; Sedghi, M.; Asghari, S.M.; Naderi-Manesh, H. Encapsulation of an Endostatin Peptide in Liposomes: Stability, Release, and Cytotoxicity Study. Colloids Surf. B Biointerfaces 2020, 185, 110552. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, H.; Zheng, B.; Liu, J.; Huang, Y.; Wang, H.; Zheng, D.; Mao, N.; Meng, J.; Zhou, S.; Zhong, L.; et al. Efficient Targeted Tumor Imaging and Secreted Endostatin Gene Delivery by Anti-CD105 Immunoliposomes. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, Q.; Tang, J.; Jiang, Y.Q.; Yang, L.S.; Shi, X.X.; Chen, Y.; Zhang, Y.; Fu, S.Z.; Lin, S. Anti-Tumor Effect of Local Injectable Hydrogel-Loaded Endostatin Alone and in Combination with Radiotherapy for Lung Cancer. Drug Deliv. 2021, 28, 183–194. [Google Scholar] [CrossRef]
- de la Torre, P.; Pérez-Lorenzo, M.J.; Alcázar-Garrido, Á.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef] [PubMed]
- Bouleftour, W.; Rowinski, E.; Louati, S.; Sotton, S.; Wozny, A.-S.; Moreno-Acosta, P.; Mery, B.; Rodriguez-Lafrasse, C.; Magne, N. A Review of the Role of Hypoxia in Radioresistance in Cancer Therapy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e934116. [Google Scholar] [CrossRef] [PubMed]
- Rakotomalala, A.; Escande, A.; Furlan, A.; Meignan, S.; Lartigau, E. Hypoxia in Solid Tumors: How Low Oxygenation Impacts the “Six Rs” of Radiotherapy. Front. Endocrinol. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Steel, G.G.; McMillan, T.J.; Peacock, J.H. The 5Rs of Radiobiology. Int. J. Radiat. Biol. 1989, 56, 1045–1048. [Google Scholar] [CrossRef]
- Van Den Heuvel, F.; Vella, A.; Fiorini, F.; Brooke, M.; Hill, M.A.; Maughan, T. Incorporating Oxygenation Levels in Analytical DNA-Damage Models - Quantifying the Oxygen Fixation Mechanism. Phys. Med. Biol. 2021, 66. [Google Scholar] [CrossRef]
- Wu, J.; Tang, Y.; Liang, X. Targeting VEGF Pathway to Normalize the Vasculature: An Emerging Insight in Cancer Therapy. Onco. Targets. Ther. 2018, 11, 6901–6909. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef]
- Li, W.; Quan, Y.-Y.; Yong, L.; Lu, L.; Cui, M. Monitoring of Tumor Vascular Normalization: The Key Points from Basic Research to Clinical Application. Cancer Manag. Res. 2018, 10, 4163–4172. [Google Scholar] [CrossRef]
- Alaoui-lasmaili, K.E.; Faivre, B. Critical Reviews in Oncology/Hematology Antiangiogenic Therapy: Markers of Response, “Normalization” and Resistance. Crit. Rev. Oncol./Hematol. 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Lee, C.G.; Heijn, M.; di Tomaso, E.; Griffon-Etienne, G.; Ancukiewicz, M.; Koike, C.; Park, K.R.; Ferrara, N.; Jain, R.K.; Suit, H.D.; et al. Anti-Vascular Endothelial Growth Factor Treatment Augments Tumor Radiation Response under Normoxic or Hypoxic Conditions. Cancer Res. 2000, 60, 5565–5570. [Google Scholar]
- Park, J.-S.; Park, I.; Koh, G.Y. Benefits and Pitfalls of Tumor Vessel Normalization. In Tumor Angiogenesis: A Key Target for Cancer Therapy; Marmé, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 51–71. ISBN 978-3-319-33673-2. [Google Scholar]
- Lupo, G.; Caporarello, N.; Olivieri, M.; Cristaldi, M.; Motta, C.; Bramanti, V.; Avola, R.; Salmeri, M.; Nicoletti, F.; Anfuso, C.D. Anti-Angiogenic Therapy in Cancer: Downsides and New Pivots for Precision Medicine. Front. Pharmacol. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Q.; Hong, Y. Tumor Vessel Normalization: A Window to Enhancing Cancer Immunotherapy. Technol. Cancer Res. Treat. 2020, 19, 1533033820980116. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, A.L.; Mills, I.G. Vascular Normalisation as the Stepping Stone into Tumour Microenvironment Transformation. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Durante, M.; Orecchia, R.; Loeffler, J.S. Charged-Particle Therapy in Cancer: Clinical Uses and Future Perspectives. Nat. Rev. Clin. Oncol. 2017, 14, 483–495. [Google Scholar] [CrossRef]
- Grabham, P.; Sharma, P. The Effects of Radiation on Angiogenesis. Vasc. Cell 2013, 5, 19. [Google Scholar] [CrossRef]
- Durante, M.; Debus, J.; Loeffler, J.S. Physics and Biomedical Challenges of Cancer Therapy with Accelerated Heavy Ions. Nat. Rev. Phys. 2021, 3, 777–790. [Google Scholar] [CrossRef]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological Effects of Proton Radiation: An Update. Radiat. Prot. Dosimetry 2015, 166. [Google Scholar] [CrossRef]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef]
- Girdhani, S.; Lamont, C.; Hahnfeldt, P.; Abdollahi, A.; Hlatky, L. Proton Irradiation Suppresses Angiogenic Genes and Impairs Cell Invasion and Tumor Growth. Radiat. Res. 2012, 178, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kamlah, F.; Hanze, J.; Arenz, A.; Seay, U.; Hasan, D.; Juricko, J.; Bischoff, B.; Gottschald, O.; Fournier, C.; Taucher-Scholz, G.; et al. Comparison of the Effects of Carbon Ion and Photon Irradiation on the Angiogenic Response in Human Lung Adenocarcinoma Cells. Int. J. Radiation Oncol. Biol. Phys. 2011, 80, 1541–1549. [Google Scholar] [CrossRef]
- Takahashi, Y.; Teshima, T.; Kawaguchi, N.; Hamada, Y.; Mori, S.; Madachi, A.; Ikeda, S.; Mizuno, H.; Ogata, T.; Nojima, K.; et al. Heavy Ion Irradiation Inhibits in Vitro Angiogenesis Even at Sublethal Dose. Cancer Res. 2003, 63, 4253–4257. [Google Scholar]
- Girdhani, S.; Lamont, C.; Peluso, M.; Sun, M.; Hlatky, L. 56Fe Ion Irradiation Enhances Angiogenesis and Other Inter-Cellular Determinants of Carcinogenesis Risk. J. Radiat. Res. 2014, 55, i124–i126. [Google Scholar] [CrossRef][Green Version]
- He, L. Normalization Time Window of Recombinant Endostatin: An Overview. Cancer Cell Res. 2019, 21, 558–564. [Google Scholar]
- Guo, L.; Chen, Y.; He, T.; Qi, F.; Liu, G.; Fu, Y.A.N. Nuclear-Translocated Endostatin Downregulates Hypoxia Inducible Factor-1 α Activation through Interfering with Zn ( II ) Homeostasis. Mol. Med. Rep. 2015, 3473–3480. [Google Scholar] [CrossRef]
- Li, N.; Zheng, D.; Wei, X.; Jin, Z. Effects of Recombinant Human Endostatin and Its Synergy with Cisplatin on Circulating Endothelial Cells and Tumor Vascular Normalization in A549 Xenograft Murine Model. J. Cancer Res. Clin. Oncol. 2012, 1131–1144. [Google Scholar] [CrossRef]
- Peng, Q.; Li, M.; Wang, Z.; Jiang, M.; Yan, X.; Lei, S.; Zhang, H.; Zhang, W.; Liu, Y.Y.; Luo, F. Polarization of Tumor-Associated Macrophage Is Associated with Tumor Vascular Normalization by Endostatin. Thorac. Cancer 2013, 4, 295–305. [Google Scholar] [CrossRef]
- Meng, M.-B.; Jiang, X.-D.; Deng, L.; Na, F.-F.; He, J.-Z.; Xue, J.-X.; Guo, W.-H.; Wen, Q.-L.; Lan, J.; Mo, X.-M.; et al. Enhanced Radioresponse with a Novel Recombinant Human Endostatin Protein via Tumor Vasculature Remodeling: Experimental and Clinical Evidence. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2013, 106, 130–137. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Fei, S.; Wei, C.; Tong, F.; Wu, G.; Ma, H.; Dong, X. The Effect of Combining Endostar with Radiotherapy on Blood Vessels, Tumor-Associated Macrophages, and T Cells in Brain Metastases of Lewis Lung Cancer. Transl. Lung Cancer Res. 2020, 9, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, S.; Huang, J.; Liang, J.; Zhang, D.; Zhao, X.; Ding, H.; Qin, L.; Shi, C.; Luo, L.; et al. Monitoring the Process of Endostar-Induced Tumor Vascular Normalization by Non-Contrast Intravoxel Incoherent Motion. Front. Oncol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xu, Z.; Wang, J.; Chen, Y.; Li, Q.; Zuo, Y.; Chen, J.; Hu, X.; Zhou, Q.; Wang, Y.; et al. Recombinant Human Endostatin Normalizes Tumor Vasculature and Enhances Radiation Response in Xenografted Human Nasopharyngeal Carcinoma Models. PLoS ONE 2012, 7, 1–10. [Google Scholar] [CrossRef]
- Chu, X.-D.; Bao, H.; Lin, Y.-J.; Chen, R.-X.; Zhang, Y.-R.; Huang, T.; He, J.-S.; Huangfu, S.-C.; Pan, Y.-L.; Ding, H. Endostatin Induces Normalization of Blood Vessels in Colorectal Cancer and Promotes Infiltration of CD8+ T Cells to Improve Anti-PD-L1 Immunotherapy. Front. Immunol. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Jiang, X.D.; Qiao, Y.; Dai, P.; Chen, Q.; Wu, J.; Song, D.A.; Li, S.Q. Enhancement of Recombinant Human Endostatin on the Radiosensitivity of Human Pulmonary Adenocarcinoma A549 Cells and Its Mechanism. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- You, Z.Y.; Zhao, Y.; Liu, F.; Zhang, Y.D.; Wang, J.J. The Radiosensitization Effects of Endostar on Human Lung Squamous Cancer Cells H-520. Cancer Cell Int. 2010, 10, 1–10. [Google Scholar]
- Chen, X.; Zhang, H.; Zhu, H.; Yang, X.; Yang, Y.; Yang, Y.; Min, H.; Chen, G.; Lu, J.; Cheng, H.; et al. Endostatin Combined with Radiotherapy Suppresses Vasculogenic Mimicry Formation through Inhibition of Epithelial – Mesenchymal Transition in Esophageal Cancer. Tumor Biol. 2016, 37, 4679–4688. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, Y.; Hu, C.; Liu, Y.; Xia, Y.; Wang, L.; Liu, B.; Chen, H.; Jiang, X. Endostatin Exerts Radiosensitizing Effect in Non-Small Cell Lung Cancer Cells by Inhibiting VEGFR2 Expression. Clin. Transl. Oncol. 2016, 18–26. [Google Scholar] [CrossRef]
- Ling, C.; Ji, C.; Chen, Y.; Fu, J.; Zhou, J.; Chen, W.; Yang, J.; Su, L. Combined effects of endostatin gene transfer and ionizing radiation on lung adenocarcinoma model of A549-cells. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua Jiehe He Huxi Zazhi = Chin. J. Tuberc. Respir. Dis. 2004, 27, 683–686. [Google Scholar]
- Wu, D.S.; Wu, C.M.; Huang, T.H.; Xie, Q.D. Combined Effects of Radiotherapy and Endostatin Gene Therapy in Melanoma Tumor Model. Radiat. Environ. Biophys. 2008, 47, 285–291. [Google Scholar] [CrossRef]
- Itasaka, S.; Komaki, R.; Herbst, R.S.; Shibuya, K.; Shintani, T.; Hunter, N.R.; Onn, A.; Bucan, C.D.; Milas, L.; Kian Ang, K.; et al. Endostatin Improves Radioresponse and Blocks Tumor Revascularization after Radiation Therapy for A431 Xenografts in Mice. Int. J. Radiation Oncol. Biol. Phys. 2007, 67, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Xu, X.; Tu, Y.; Qin, S.; Yin, Y. Antitumor Activity of Endostar Combined with Radiation against Human Nasopharyngeal Carcinoma in Mouse Xenograft Models. Oncol. Lett. 2012, 4, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pan, L.K.; Qi, D.; Xin, L.; Cui, Y.; An, G. Recombinant Human Endostatin Combined with TP Regimen as Postoperative Adjuvant Treatment for Non-Small-Cell Lung Cancer: Efficacy Analysis. Med. J. Chinese People’s Lib. Army 2012, 37, 49–53. [Google Scholar]
- Aydemir, E.A.; Oz, E.C.E.S.; Korcum, A.F.; Fiskin, K. Endostatin Enhances Radioresponse in Breast Cancer Cells via Alteration of Substance P Levels. Oncol. Lett. 2011, 879–886. [Google Scholar] [CrossRef]
- Aydemir, E.A.; Şimşek, E.C.E.; Korcum, A.F.; Fişkin, K. Endostatin and Irradiation Modifies the Activity of ADAM10 and Neprilysin in Breast Cancer Cells. Mol. Med. Rep. 2016, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chang, H.; Li, B.; Zhang, Y.; Li, D.; Liu, Y.; Yang, Y. Effect of Recombinant Human Endostatin Onradiotherapy for Esophagus Cancer. Asian Pac. J. Trop. Med. 2016, 9, 86–90. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Ding, Y.; Liu, J.; Lu, J.; Zhan, L.; Qin, Q.; Zhang, H.; Chen, X.; Yang, Y.; et al. Recombinant Human Endostatin Enhances the Radioresponse in Esophageal Squamous Cell Carcinoma by Normalizing Tumor Vasculature and Reducing Hypoxia. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Feng, J.; Luo, W.; Qin, S.; Wu, Q.; Wang, X.; Yin, X.; Sun, X.; Qu, W.; Ye, Q. Synergistic Effects of the Combination of Endostar and Radiotherapy against Hepatocellular Carcinoma in a Mouse Model. Int. J. Clin. Exp. Med. 2017, 10, 10066–10078. [Google Scholar]
- Zhang, K.; Wang, Y.; Yu, X.; Shi, Y.; Yao, Y.; Wei, X.; Ma, X. Recombinant Human Endostatin Combined with Radiotherapy Inhibits Colorectal Cancer Growth. BMC Cancer 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Jiang, X.; Dai, P.; Wu, J.; Song, D.; Yu, J. Lung Cancer Inhibitory Effect of Radiotherapy Combined with Weekly Recombinant Human Endostatin on the Human Pulmonary Adenocarcinoma A549 Xenografts in Nude Mice. Lung Cancer 2011, 72, 165–171. [Google Scholar] [CrossRef]
- Yin, L.; He, J.; Xue, J.; Na, F.; Tong, R.; Wang, J.; Gao, H.; Tang, F.; Mo, X.; Deng, L.; et al. PDGFR- β Inhibitor Slows Tumor Growth but Increases Metastasis in Combined Radiotherapy and Endostar Therapy. Biomed. Pharmacother. 2018, 99, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.N.; Seetharam, S.; Mauceri, H.J.; Beckett, M.A.; Jaskowiak, N.T.; Salloum, R.M.; Hari, D.; Dhanabal, M.; Ramchandran, R.; Kalluri, R.; et al. Antitumor Interaction of Short-Course Endostatin and Ionizing Radiation. Cancer J. 2000, 6, 287–293. [Google Scholar]
- Wen, Q.L.; Meng, M.B.; Yang, B.; Tu, L.L.; Jia, L.; Zhou, L.; Xu, Y.; Lu, Y. Endostar, a Recombined Humanized Endostatin, Enhances the Radioresponse for Human Nasopharyngeal Carcinoma and Human Lung Adenocarcinoma Xenografts in Mice. Cancer Sci. 2009, 100, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.W.; Liu, Y.Y.; Yu, Q.T.; Zhang, Y.P.; Li, K.; Xu, L.Y.; Luo, S.X.; Qin, F.Z.; Chen, Z.T.; et al. Long-Term Results of a Randomized, Double-Blind, and Placebo-Controlled Phase III Trial: Endostar (Rh-Endostatin) versus Placebo in Combination with Vinorelbine and Cisplatin in Advanced Non-Small Cell Lung Cancer. Thorac. Cancer 2013, 4, 440–448. [Google Scholar] [CrossRef]
- Yuan, M.; Zhai, Y.; Men, Y.; Wang, J.; Deng, L.; Wang, W.; Bao, Y.; Yang, X.; Sun, S.; Ma, Z.; et al. Endostar (Rh-Endostatin) Improves Efficacy of Concurrent Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Thorac. Cancer 2021, 12, 3208–3215. [Google Scholar] [CrossRef]
- Jiang, X.; Guan, W.; Li, M.; Liang, W.; Qing, Y.; Dai, N.; Zhang, S.; Deng, Y.; Meng, H.; Yang, Y.; et al. Endostatin Combined with Platinum-Based Chemo-Radiotherapy for Advanced Non-Small Cell Lung Cancer. Cell Biochem. Biophys. 2015, 71, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, F.; Wei, Q.; Zou, L.; Qin, B.; Peng, X. Phase II Study of Cisplatin/Etoposide and Endostar for Extensive-Stage Small-Cell Lung Cancer. Cancer Chemother. Pharmacol. 2011, 68, 1027–1032. [Google Scholar] [CrossRef]
- Jianhua, C.; Yongzhong, L.U.O.; Wenwei, Z.; Hui, Z.; Wei, W. Clinical Observation of Recombinant Human Endostatin Combined with Carboplatin and Etoposide for Advanced Small-Cell Lung Cancer. J. Clin. Med. Pract. 2013, 26–28. [Google Scholar] [CrossRef]
- Li, N.; Jin, Z.; Liu, Z.; Wang, J.; Li, K. Efficacy of endostar combined with chemotherapy in multi-cycle treatment of patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2011, 33, 937–942. [Google Scholar]
- Wang, J.; Sun, Y.; Liu, Y.; Yu, Q.; Zhang, Y.; Li, K.; Zhu, Y.; Zhou, Q.; Hou, M.; Guan, Z.; et al. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi 2005, 8, 283–290. [Google Scholar] [CrossRef]
- Ge, W.; Cao, D.; Wang, H.; Jie, F.; Zheng, Y.; Chen, Y. Endostar Combined with Chemotherapy versus Chemotherapy Alone for Advanced NSCLCs: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2011, 12, 2705–2711. [Google Scholar] [PubMed]
- Rong, B.; Yang, S.; Li, W.; Zhang, W.; Ming, Z. Systematic Review and Meta-Analysis of Endostar (Rh-Endostatin) Combined with Chemotherapy versus Chemotherapy Alone for Treating Advanced Non-Small Cell Lung Cancer. World J. Surg. Oncol. 2012, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Z.-Y.; Li, Y.-H.; Lu, Y.-H.; Wang, S.; Yu, W.-X.; Zhao, H. Usefulness of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for Predicting Treatment Response to Vinorelbine-Cisplatin with or without Recombinant Human Endostatin in Bone Metastasis of Non-Small Cell Lung Cancer. Am. J. Cancer Res. 2016, 6, 2890–2900. [Google Scholar] [CrossRef]
- Han, B.; Xiu, Q.; Wang, H.; Shen, J.; Gu, A.; Luo, Y.; Bai, C.; Guo, S.; Liu, W.; Zhuang, Z.; et al. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy of Paclitaxel-Carboplatin Alone or with Endostar for Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Ma, H.; Hui, Z.; Zhao, L.; Li, D.; Liang, J.; Wang, X.; Xu, L.; Chen, B.; Tang, Y.; et al. HELPER Study: A Phase II Trial of Continuous Infusion of Endostar Combined with Concurrent Etoposide plus Cisplatin and Radiotherapy for Treatment of Unresectable Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 131, 27–34. [Google Scholar] [CrossRef]
- Jiang, X.-D.; Dai, P.; Wu, J.; Song, D.-A.; Yu, J.-M. Effect of Recombinant Human Endostatin on Radiosensitivity in Patients with Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 1272–1277. [Google Scholar] [CrossRef]
- Sun, X.-J.; Deng, Q.-H.; Yu, X.-M.; Ji, Y.-L.; Zheng, Y.-D.; Jiang, H.; Xu, Y.-P.; Ma, S.-L. A Phase II Study of Endostatin in Combination with Paclitaxel, Carboplatin, and Radiotherapy in Patients with Unresectable Locally Advanced Non-Small Cell Lung Cancer. BMC Cancer 2016, 16, 266. [Google Scholar] [CrossRef]
- Honglian, M.; Zhouguang, H.; Fang, P.; Lujun, Z.; Dongming, L.; Yujin, X.; Yong, B.; Liming, X.; Yirui, Z.; Xiao, H.; et al. Different Administration Routes of Recombinant Human Endostatin Combined with Concurrent Chemoradiotherapy Might Lead to Different Efficacy and Safety Profile in Unresectable Stage III Non-Small Cell Lung Cancer: Updated Follow-up Results from Two Phase. Thorac. Cancer 2020, 11, 898–906. [Google Scholar] [CrossRef]
- Wang, B.; Xu, L.; Li, Q.; Man, S.; Jin, C.; Liu, L.; Zhan, S.; Ning, Y. Endostar Continuous versus Intermittent Intravenous Infusion Combined with Chemotherapy for Advanced NSCLC: A Systematic Review and Meta-Analysis Including Non-Randomized Studies. BMC Cancer 2020, 20, 1021. [Google Scholar] [CrossRef]
- Chen, L.; Tong, F.; Peng, L.; Huang, Y.; Yin, P.; Feng, Y.; Cheng, S.; Wang, J.; Dong, X. Efficacy and Safety of Recombinant Human Endostatin Combined with Whole-Brain Radiation Therapy in Patients with Brain Metastases from Non-Small Cell Lung Cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 174, 44–51. [Google Scholar] [CrossRef]
- Jiang, X.D.; Ding, M.H.; Qiao, Y.; Liu, Y.; Liu, L. Study on Lung Cancer Cells Expressing Vegfr2 and the Impact on the Effect of RHES Combined with Radiotherapy in the Treatment of Brain Metastases. Clin. Lung Cancer 2014, 15, e23–e29. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Peng, F.; Zhou, Q.C.; Yu, Z.H.; Li, J.C.; Cheng, Z.B.; Chen, L.; Hu, X.; Chen, Y.Y.; Wang, J.; et al. Phase II Trial of Recombinant Human Endostatin in Combination with Concurrent Chemoradiotherapy in Patients with Stage III Non-Small-Cell Lung Cancer. Radiother. Oncol. 2015, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, H.; Peng, F.; Bao, Y.; Hu, X.; Wang, J.; Xu, Y.; Chen, M. Prognostic Performance of Inflammation-Based Prognostic Indices in Locally Advanced Non-Small-Lung Cancer Treated with Endostar and Concurrent Chemoradiotherapy. Mol. Clin. Oncol. 2016, 4, 801–806. [Google Scholar] [CrossRef]
- Xu, H.; Lv, D.; Meng, Y.; Wang, M.; Wang, W.; Zhou, C.; Zhou, S.; Chen, X.; Yang, H. Endostar Improved Efficacy of Concurrent Chemoradiotherapy with Vinorelbine plus Carboplatin in Locally Advanced Lung Squamous Cell Carcinoma Patients with High Serum Lp(a) Concentration. Ann. Palliat. Med. 2020, 9, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Valable, S.; Gérault, A.N.; Lambert, G.; Leblond, M.M.; Anfray, C.; Toutain, J.; Bordji, K.; Petit, E.; Bernaudin, M.; Pérès, E.A. Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers 2020, 12, 2019. [Google Scholar] [CrossRef]
- Césaire, M.; Montanari, J.; Curcio, H.; Lerouge, D.; Gervais, R.; Demontrond, P.; Balosso, J.; Chevalier, F. Radioresistance of Non-Small Cell Lung Cancers and Therapeutic Perspectives. Cancers 2022, 14, 2829. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Ruggieri, S.; Tamma, R.; Crivellato, E. Limitations of Anti-Angiogenic Treatment of Tumors. Transl. Oncol. 2019, 12, 981–986. [Google Scholar] [CrossRef]
- Ribatti, D. Antiangiogenic Therapy Accelerates Tumor Metastasis. Leuk. Res. 2011, 35, 24–26. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Iwamoto, H.; Hosaka, K.; Seki, T.; Andersson, P.; Lim, S.; Fischer, C.; Nakamura, M.; Abe, M.; et al. Discontinuation of Anti-VEGF Cancer Therapy Promotes Metastasis through a Liver Revascularization Mechanism. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Mukwaya, A.; Jensen, L.; Lagali, N. Relapse of Pathological Angiogenesis: Functional Role of the Basement Membrane and Potential Treatment Strategies. Exp. Mol. Med. 2021, 53, 189–201. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Nowak-Sliwinska, P.; Huijbers, E.J.M.; Thijssen, V.L.; Griffioen, A.W. The Great Escape; the Hallmarks of Resistance to Antiangiogenic Therapy. Pharmacol. Rev. 2015, 67, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Paganetti, H. Relative Biological Effectiveness (RBE) Values for Proton Beam Therapy. Variations as a Function of Biological Endpoint, Dose, and Linear Energy Transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Vanderwaeren, L.; Dok, R.; Verstrepen, K.; Nuyts, S. Clinical Progress in Proton Radiotherapy: Biological Unknowns. Cancers 2021, 13, 604. [Google Scholar] [CrossRef]
- Mohan, R. A Review of Proton Therapy – Current Status and Future Directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef]
- Kim, H.; Pyo, H.; Noh, J.M.; Lee, W.; Park, B.; Park, H.Y.; Yoo, H. Preliminary Result of Definitive Radiotherapy in Patients with Non-Small Cell Lung Cancer Who Have Underlying Idiopathic Pulmonary Fibrosis: Comparison between X-Ray and Proton Therapy. Radiat. Oncol. 2019, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Lee, J.J.; Komaki, R.; Gomez, D.R.; O’Reilly, M.S.; Fossella, F.V.; Blumenschein, G.R.J.; Heymach, J.V.; Vaporciyan, A.A.; Swisher, S.G.; et al. Bayesian Adaptive Randomization Trial of Passive Scattering Proton Therapy and Intensity-Modulated Photon Radiotherapy for Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Current Status of Proton Therapy Techniques for Lung Cancer. Radiat. Oncol. J. 2019, 37, 232–248. [Google Scholar] [CrossRef]
- Gjyshi, O.; Liao, Z. Proton Therapy for Locally Advanced Non-Small Cell Lung Cancer. Br. J. Radiol. 2020, 93, 20190378. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Doi, R.; Obata, T.; Hatachi, G.; Nagayasu, T. Lung Microvascular Niche, Repair, and Engineering. Front. Bioeng. Biotechnol. 2020, 8, 1–19. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of Anti-Angiogenic Therapy and Immune Checkpoint Blockade Normalizes Vascular-Immune Crosstalk to Potentiate Cancer Immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Wang, J.; Wei, X.-Y.; Chen, P.; Wang, L.-C.; Lin, L.; Sun, B.-C.; Li, K. Predictive Value of Circulating Endothelial Cells for Efficacy of Chemotherapy with Rh-Endostatin in Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2012, 138, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Su, Y.; You, J.; Gong, L.; Zhang, Z.; Wang, M.; Zhao, Z.; Zhang, Z.; Li, X.; Wang, C. Combining Antiangiogenic Therapy with Neoadjuvant Chemotherapy Increases Treatment Efficacy in Stage IIIA (N2) Non-Small Cell Lung Cancer without Increasing Adverse Effects. Oncotarget 2016, 7, 62619–62626. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.W.; Cui, C.X.; Hang, J.; Zang, H.P.; Li, S.T.; Sun, Y. Rh-Endostatin (YH-16) in Combination with Vinorelbine and Cisplatin for Advanced Non-Small Cell Lung Cancer: A Multicenter Phase II Trial. Chin. J. New Drugs 2005, 14, 204–207. [Google Scholar]
- Zhao, X.; Mei, K.; Cai, X.; Chen, J.; Yu, J.; Zhou, C.; Li, Q. A Randomized Phase II Study of Recombinant Human Endostatin plus Gemcitabine/Cisplatin Compared with Gemcitabine/Cisplatin Alone as First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Investig. New Drugs 2012, 30, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, K.; Sun, T.; Zhang, M.; Li, W.; Yao, Q.; Liu, W.; Ding, C.; He, Z.; Mao, W.; et al. Efficacy and safety of rh-endostatin combined with docetaxel in second-line or intolerant toxicity for first-line treatment in patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2013, 35, 618–622. [Google Scholar]
- Zhang, F.-L.; Gao, E.-Y.; Shu, R.-B.; Wang, H.; Zhang, Y.; Sun, P.; Li, M.; Tang, W.; Jiang, B.-Q.; Chen, S.-Q.; et al. Human Recombinant Endostatin Combined with Cisplatin Based Doublets in Treating Patients with Advanced NSCLC and Evaluation by CT Perfusion Imaging. Asian Pac. J. Cancer Prev. 2015, 16, 6765–6768. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, H.; Han, T.; Wang, W.; Tong, W.; Zhu, X. A Study on the Efficacy of Recombinant Human Endostatin Combined with Apatinib Mesylate in Patients with Middle and Advanced Stage Non-Small Cell Lung Cancer. J. BUON. 2019, 24, 2267–2272. [Google Scholar]
- Yu, X.; Zhang, L.; Chen, J. Effectiveness of Treatment with Endostatin in Combination with Emcitabine, Carboplatin, and Gemcitabine in Patients with Advanced Non-Small Cell Lung Cancer: A Retrospective Study. Open Med. 2018, 13, 142–147. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Zhou, C.; Long, X.; Guan, R.; Yang, N.; Zhang, Y. Real-World Outcomes of Various Regimens of Recombinant Human Endostatin Combined with Chemotherapy in Non-Driver Gene Mutation Advanced Non-Small Cell Lung Cancer. Cancer Med. 2019, 8, 1434–1441. [Google Scholar] [CrossRef]
- Lu, S.; Li, L.; Luo, Y.; Zhang, L.; Wu, G.; Chen, Z.; Huang, C.; Guo, S.; Zhang, Y.; Song, X.; et al. A Multicenter, Open-Label, Randomized Phase II Controlled Study of Rh-Endostatin (Endostar) in Combination with Chemotherapy in Previously Untreated Extensive-Stage Small-Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 206–211. [Google Scholar] [CrossRef]
- Zhou, S.; Zuo, L.; He, X.; Pi, J.; Jin, J.; Shi, Y. Efficacy and Safety of Rh-Endostatin (Endostar) Combined with Pemetrexed/Cisplatin Followed by Rh-Endostatin plus Pemetrexed Maintenance in Non-Small Cell Lung Cancer: A Retrospective Comparison with Standard Chemotherapy. Thorac. Cancer 2018, 9, 1354–1360. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).