Preclinical Studies of Chiauranib Show It Inhibits Transformed Follicular Lymphoma through the VEGFR2/ERK/STAT3 Signaling Pathway
Abstract
1. Introduction
2. Results
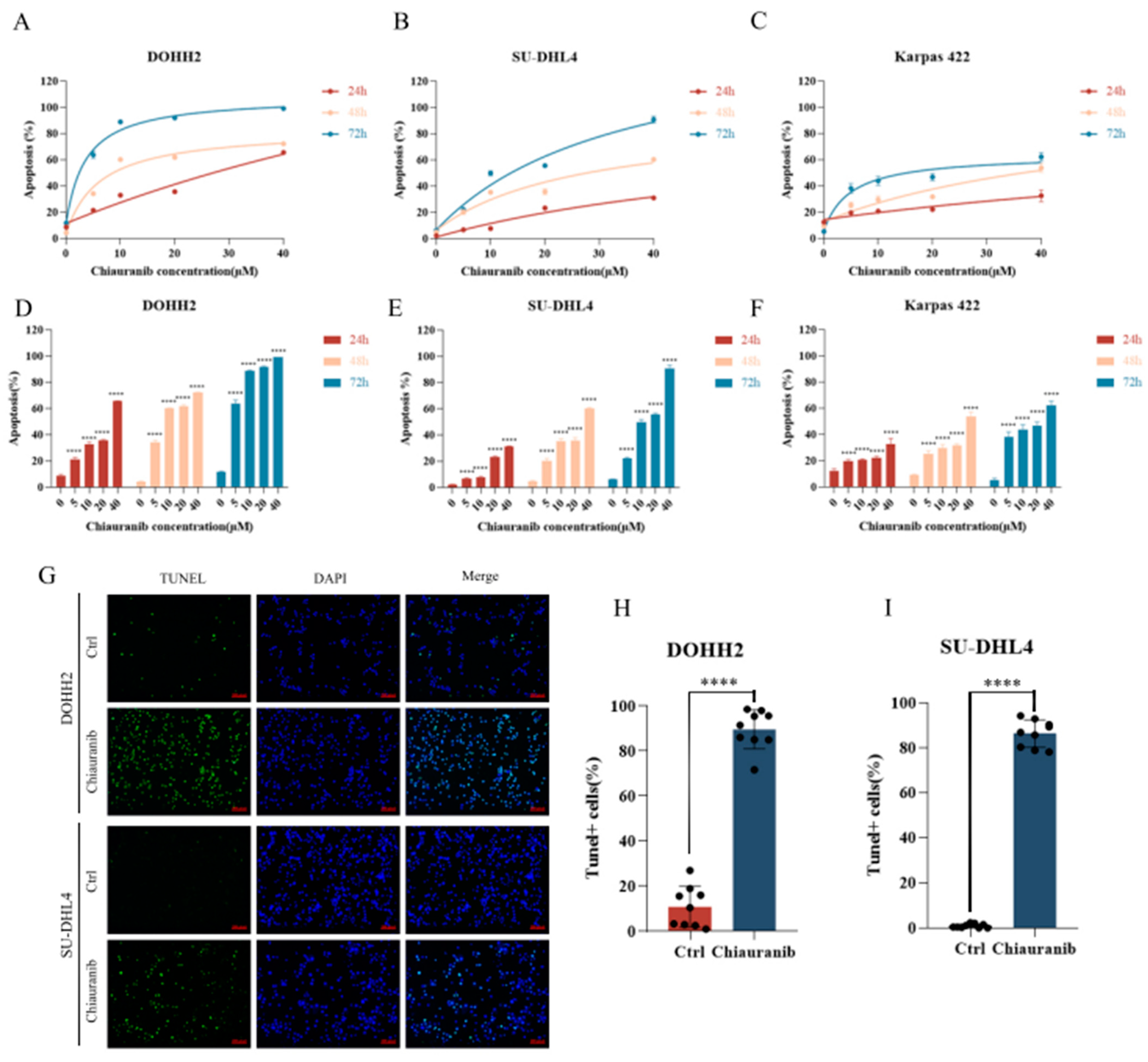
2.1. Chiauranib Exhibits Dose- and Time-Dependent Cytotoxicity in t-FL Cells
2.2. Chiauranib Induces Apoptosis in t-FL Cells
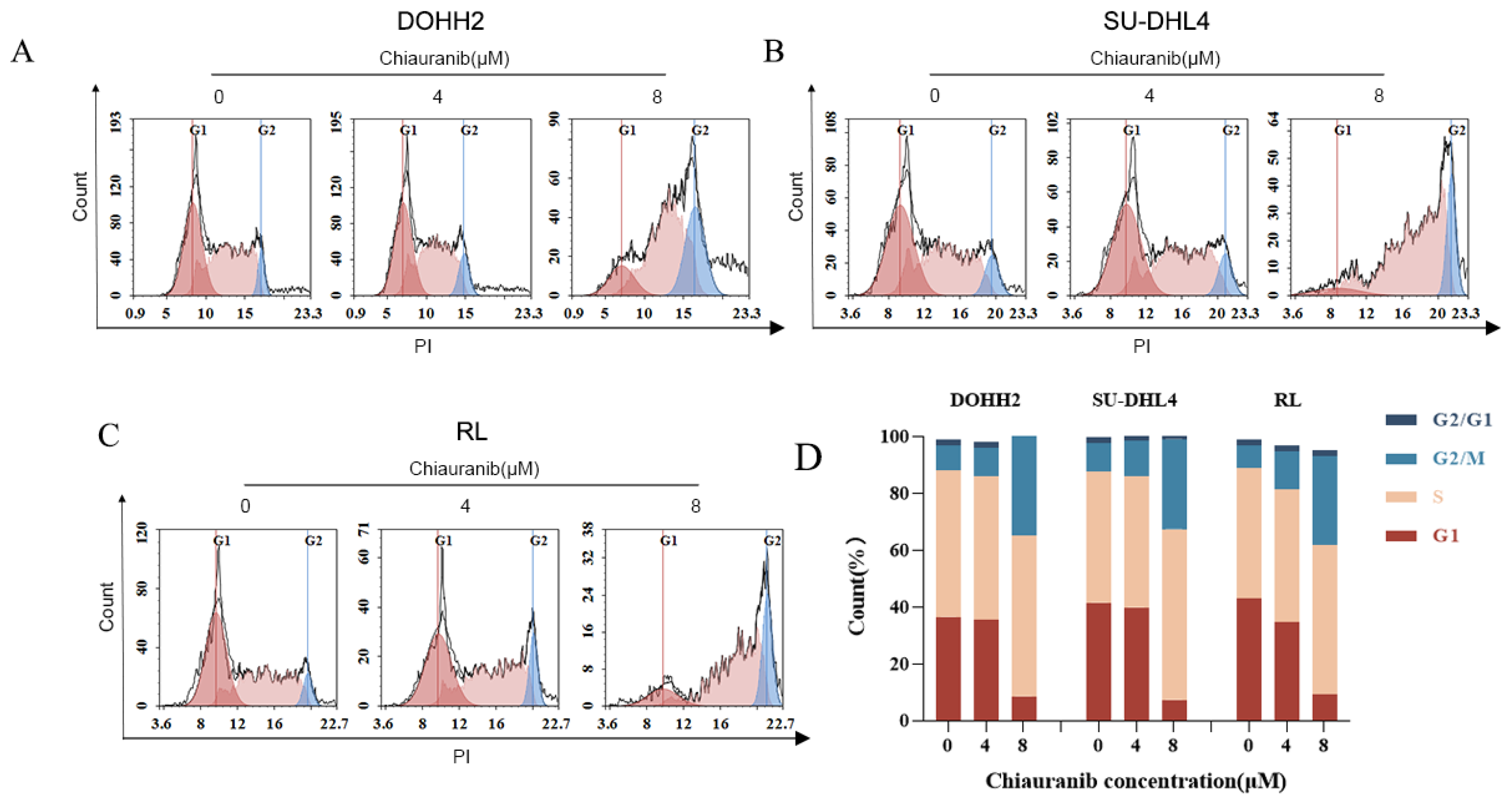
2.3. Chiauranib Causes G2/M Phase Cell Cycle Arrest
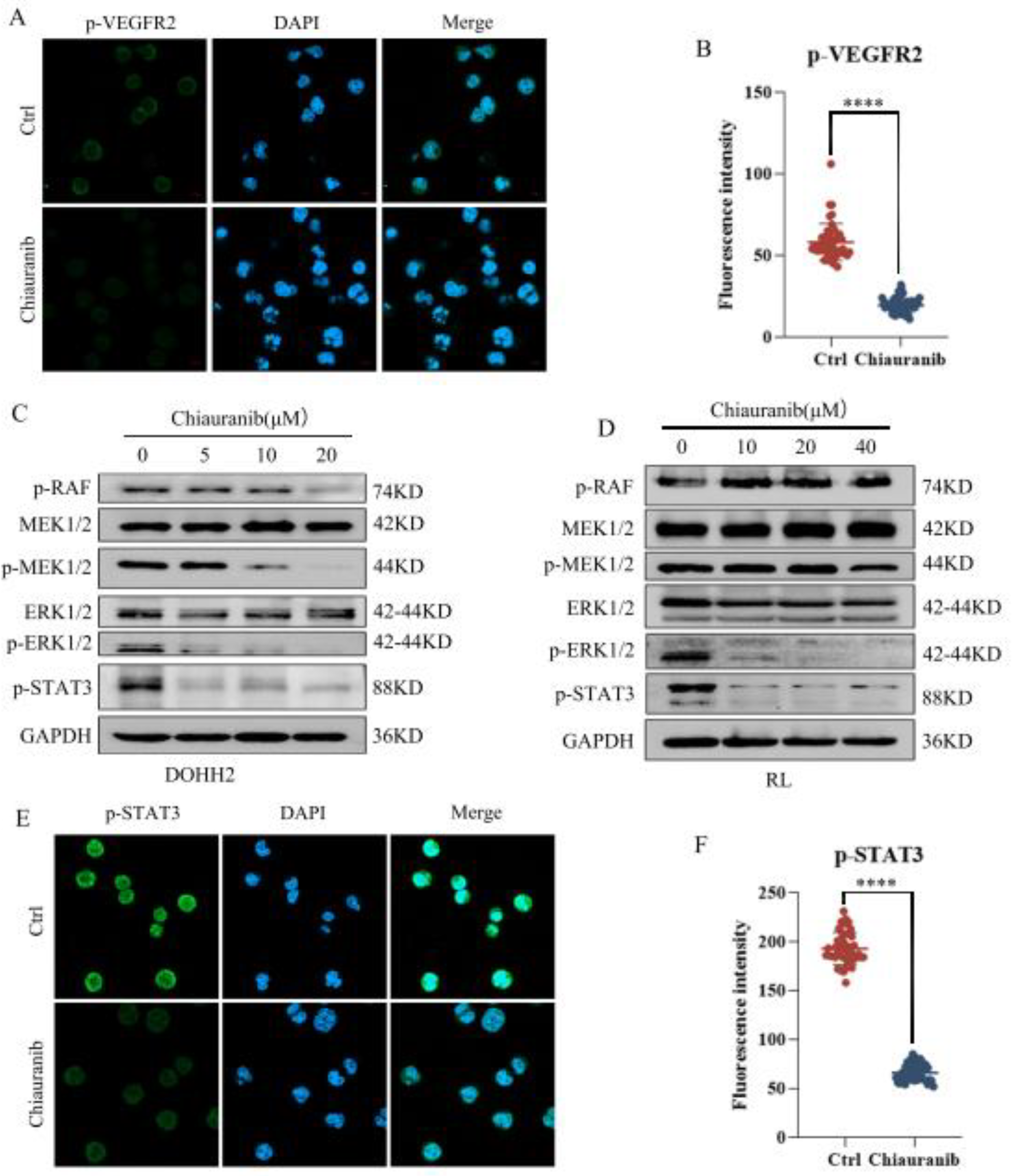
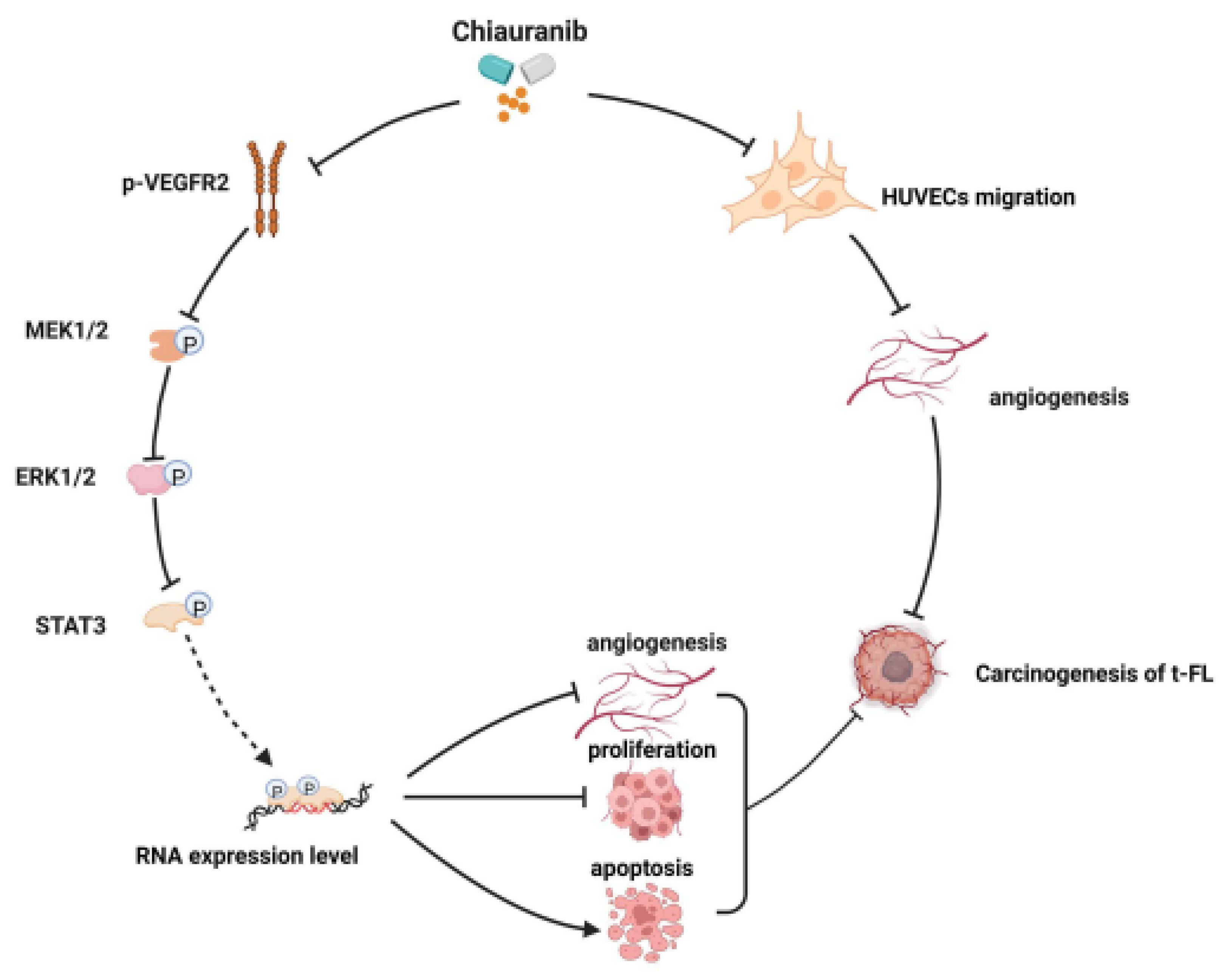
2.4. Chiauranib Inhibits the Phosphorylation of the VEGFR2/MEK/ERK/STAT3 Signaling Pathway in t-FL Cells
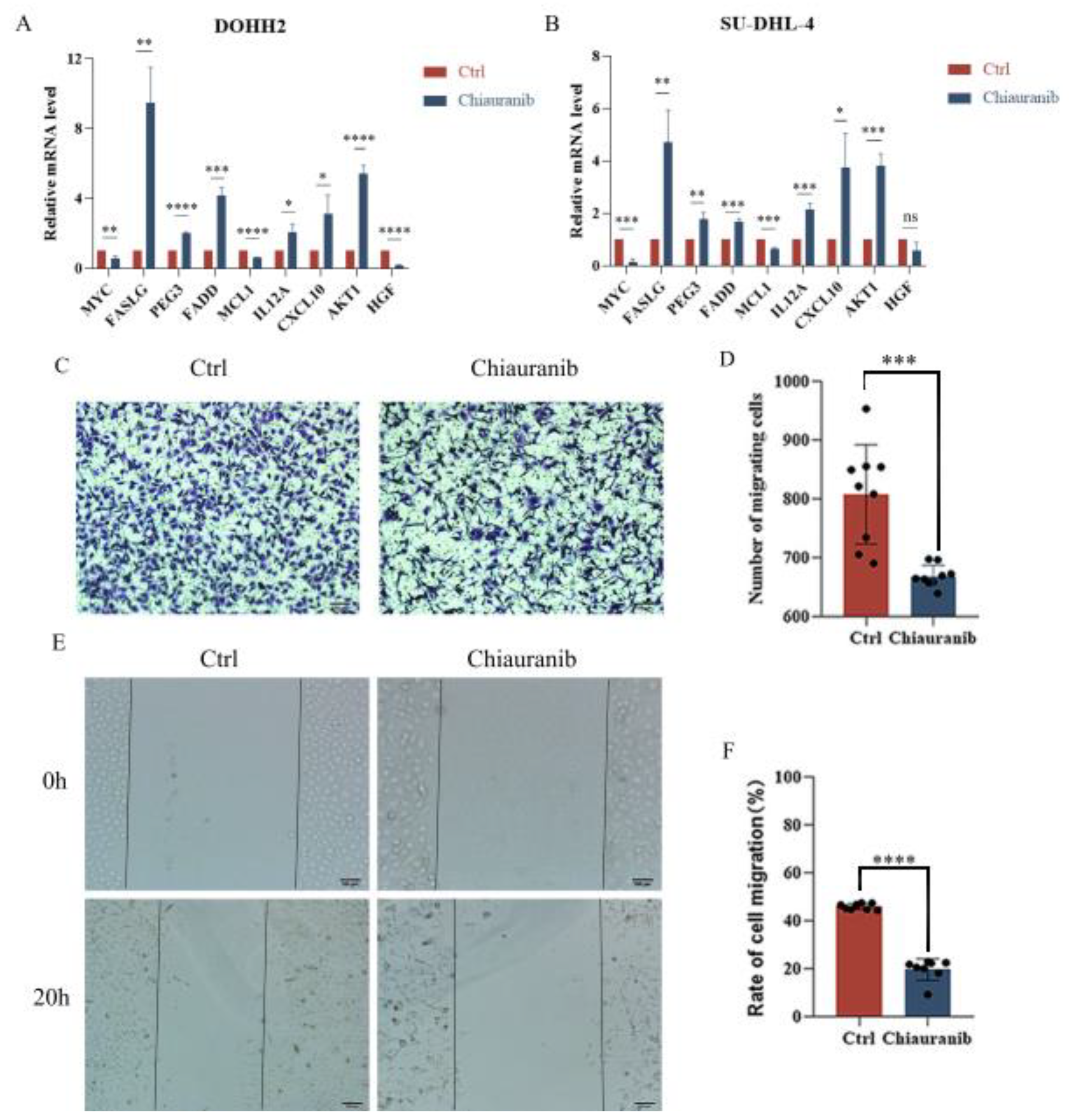
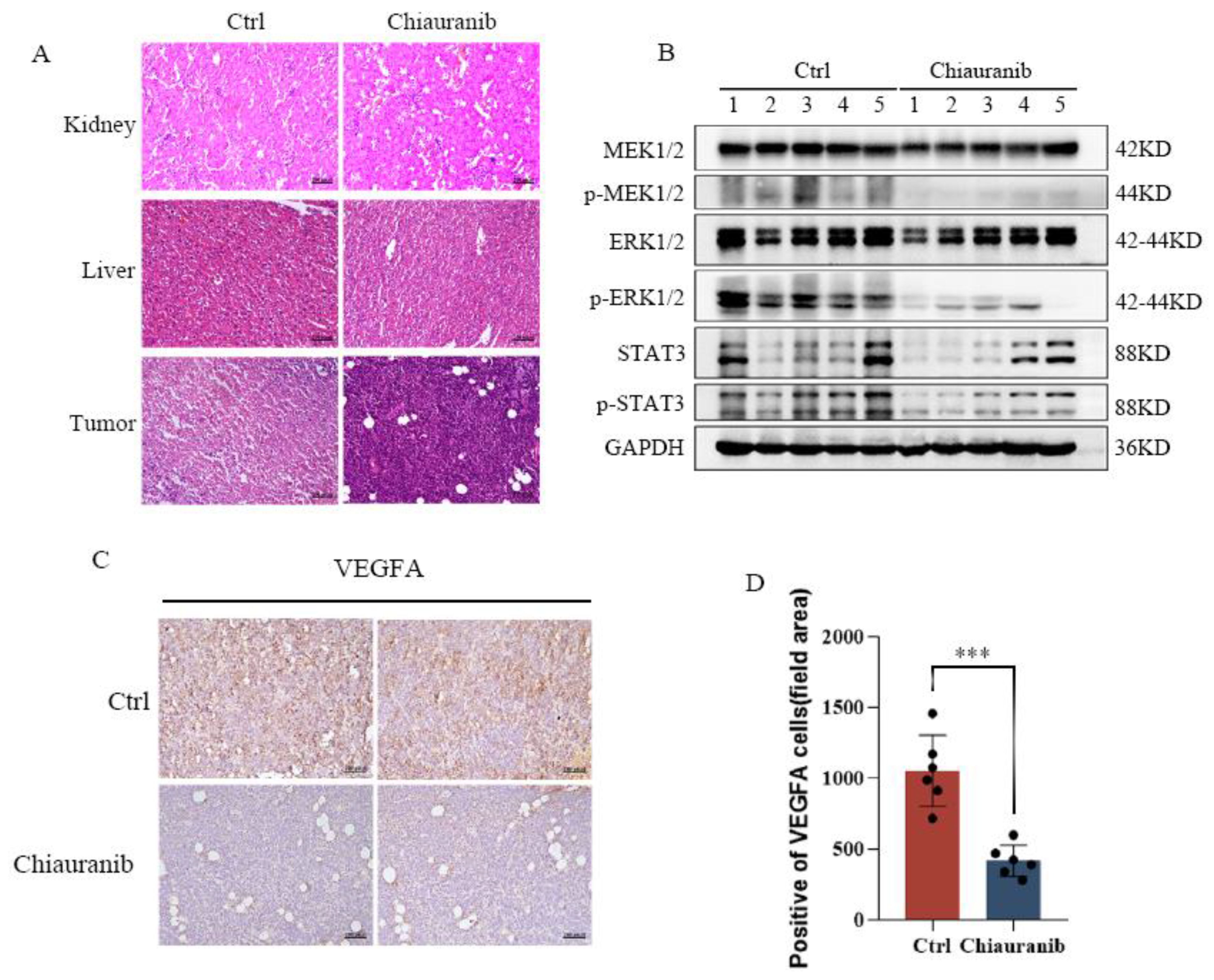
2.5. Chiauranib Alters the Levels of STAT3-Downstream Genes and Suppresses Angiogenesis in t-FL
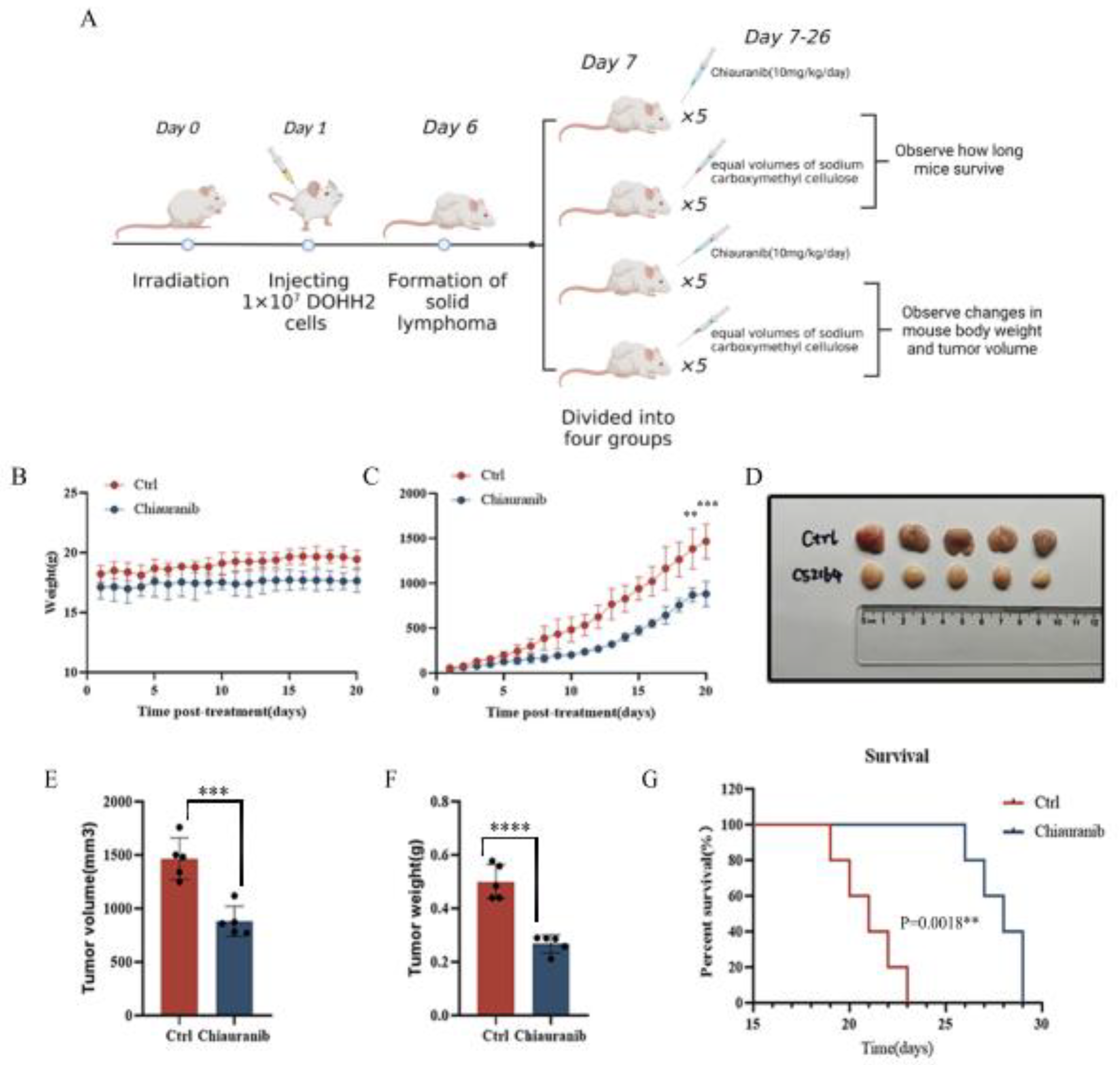
2.6. In Vivo Efficacy of Chiauranib against t-FL Xenograft Mouse Models
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Chemical Reagents
4.2. Cell Viability
4.3. Cell Apoptosis and Cycle Arrest
4.4. EdU Cell Proliferation Staining
4.5. Wound Healing Assay
4.6. Transwell Assay
4.7. Western Blotting
4.8. Quantitative RT-PCR
4.9. Immunofluorescence
4.10. Tumor Xenograft Models
4.11. Statistical Analysis
4.12. Ethics Approval and Consent to Participate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carbone, A.; Roulland, S.; Gloghini, A.; Younes, A.; von Keudell, G.; Lopez-Guillermo, A.; Fitzgibbon, J. Follicular lymphoma. Nat. Rev. Dis. Prim. 2019, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Zing, N.P.C.; Chiattone, C.S.; Federico, M.; Luminari, S. Transformed follicular lymphoma. Ann. Hematol. 2018, 97, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.A.; Shadman, M.; Gopal, A.K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood 2018, 132, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Casulo, C.; Burack, W.R.; Friedberg, J.W. Transformed follicular non-Hodgkin lymphoma. Blood 2015, 125, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Junlen, H.R.; Peterson, S.; Kimby, E.; Lockmer, S.; Linden, O.; Nilsson-Ehle, H.; Erlanson, M.; Hagberg, H.; Radlund, A.; Hagberg, O.; et al. Follicular lymphoma in Sweden: Nationwide improved survival in the rituximab era, particularly in elderly women: A Swedish Lymphoma Registry study. Leukemia 2015, 29, 668–676. [Google Scholar] [CrossRef]
- Casulo, C.; Friedberg, J.W.; Ahn, K.W.; Flowers, C.; DiGilio, A.; Smith, S.M.; Ahmed, S.; Inwards, D.; Aljurf, M.; Chen, A.I.; et al. Autologous Transplantation in Follicular Lymphoma with Early Therapy Failure: A National LymphoCare Study and Center for International Blood and Marrow Transplant Research Analysis. Biol. Blood Marrow Transplant. 2018, 24, 1163–1171. [Google Scholar] [CrossRef]
- Zhou, Y.; Shan, S.; Li, Z.B.; Xin, L.J.; Pan, D.S.; Yang, Q.J.; Liu, Y.P.; Yue, X.P.; Liu, X.R.; Gao, J.Z.; et al. CS2164, a novel multi-target inhibitor against tumor angiogenesis, mitosis and chronic inflammation with anti-tumor potency. Cancer Sci. 2017, 108, 469–477. [Google Scholar] [CrossRef]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, C.; Kong, Y.; Pan, D.; Wang, Y.; Huang, S.; Li, Z.; Ning, Z.; Lu, X.; Shan, S.; et al. Antitumor and immunomodulatory effects of a novel multitarget inhibitor, CS2164, in mouse hepatocellular carcinoma models. Anticancer Drugs 2019, 30, 909–916. [Google Scholar] [CrossRef]
- Yin, H.; Xie, J.; Jiang, P.; Jiang, X.; Duan, D.; Qi, J.; Luo, Z.; Ma, C.; Hong, H. Chiauranib selectively inhibits colorectal cancer with KRAS wild-type by modulation of ROS through activating the p53 signaling pathway. Am. J. Cancer Res. 2020, 10, 3666–3685. [Google Scholar]
- Deng, M.; Shi, Y.; Chen, K.; Zhao, H.; Wang, Y.; Xie, S.; Zhao, J.; Luo, Y.; Fang, Z.; Fan, Y.; et al. CS2164 exerts an antitumor effect against human Non-Hodgkin’s lymphomas in vitro and in vivo. Exp. Cell Res. 2018, 369, 356–362. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, L.; Hao, X.; Liu, Y.; Zhang, J.; Ning, Z.; Shi, Y. Phase I dose-escalation study of chiauranib, a novel angiogenic, mitotic, and chronic inflammation inhibitor, in patients with advanced solid tumors. J. Hematol. Oncol. 2019, 12, 9. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, H.; Chen, Q.; Zhao, J.; Shi, Y.; Yu, L.; Fang, Z.; Xu, B. CS2164 suppresses acute myeloid leukemia cell growth via inhibiting VEGFR2 signaling in preclinical models. Eur. J. Pharmacol. 2019, 853, 193–200. [Google Scholar] [CrossRef]
- Ma, J.; Hu, X.; Liao, C.; Xiao, H.; Zhu, Q.; Li, Y.; Liu, Z.; Tao, A.; He, Z.; Xu, C.; et al. Gypenoside L Inhibits Proliferation of Liver and Esophageal Cancer Cells by Inducing Senescence. Molecules 2019, 24, 1054. [Google Scholar] [CrossRef]
- Wu, P.K.; Becker, A.; Park, J.I. Growth Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int. J. Mol. Sci. 2020, 21, 5436. [Google Scholar] [CrossRef]
- Lin, W.H.; Chang, Y.W.; Hong, M.X.; Hsu, T.C.; Lee, K.C.; Lin, C.; Lee, J.L. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT-MET switch and cancer metastasis. Oncogene 2021, 40, 791–805. [Google Scholar] [CrossRef]
- Huiliang, Z.; Mengzhe, Y.; Xiaochuan, W.; Hui, W.; Min, D.; Mengqi, W.; Jianzhi, W.; Zhongshan, C.; Caixia, P.; Rong, L. Zinc induces reactive astrogliosis through ERK-dependent activation of Stat3 and promotes synaptic degeneration. J. Neurochem. 2021, 159, 1016–1027. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- Snyder, M.; Huang, X.Y.; Zhang, J.J. Identification of novel direct Stat3 target genes for control of growth and differentiation. J. Biol. Chem. 2008, 283, 3791–3798. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Ribatti, D.; Nico, B.; Ranieri, G.; Specchia, G.; Vacca, A. The role of angiogenesis in human non-Hodgkin lymphomas. Neoplasia 2013, 15, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.M.; Penson, R.T.; Matulonis, U.; Horowitz, N.S.; Whalen, C.; Pereira, L.; Tyburski, K.; Roche, M.; Szymonifka, J.; Berlin, S. A phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol. Oncol. 2013, 128, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Li, X.L.; Xu, M.D.; Li, X.M.; Wu, M.Y.; Zhang, Y.; Tao, M.; Li, W.; Shen, X.M.; Zhou, C.; et al. VEGFR2 promotes tumorigenesis and metastasis in a pro-angiogenic-independent way in gastric cancer. BMC Cancer 2019, 19, 183. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Ruan, J.; Hajjar, K.; Rafii, S.; Leonard, J.P. Angiogenesis and antiangiogenic therapy in non-Hodgkin’s lymphoma. Ann. Oncol. 2009, 20, 413–424. [Google Scholar] [CrossRef]

| Cell Lines | IC50 (μmol/L) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| DOHH2 | 9.57 ± 1.27 | 1.75 ± 0.24 | 1.06 ± 0.19 |
| SU-DHL4 | 28.72 ± 2.62 | 12.85 ± 0.51 | 5.84 ± 0.44 |
| RL | 68.19 ± 8.94 | 13.89 ± 2.13 | 10.37 ± 0.61 |
| SC-1 | 61.33 ± 9.08 | 8.03 ± 2.05 | 3.68 ± 0.40 |
| Karpas422 | 42.63 ± 5.14 | 13.31 ± 2.99 | 4.26 ± 1.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zhong, M.; Pan, G.; Tan, J.; Xie, C.; Jiang, Y.; Yao, J.; Shan, W.; Lin, J.; Huang, J.; et al. Preclinical Studies of Chiauranib Show It Inhibits Transformed Follicular Lymphoma through the VEGFR2/ERK/STAT3 Signaling Pathway. Pharmaceuticals 2023, 16, 15. https://doi.org/10.3390/ph16010015
Tang Y, Zhong M, Pan G, Tan J, Xie C, Jiang Y, Yao J, Shan W, Lin J, Huang J, et al. Preclinical Studies of Chiauranib Show It Inhibits Transformed Follicular Lymphoma through the VEGFR2/ERK/STAT3 Signaling Pathway. Pharmaceuticals. 2023; 16(1):15. https://doi.org/10.3390/ph16010015
Chicago/Turabian StyleTang, Yuanfang, Mengya Zhong, Guangchao Pan, Jinshui Tan, Chendi Xie, Yuelong Jiang, Jingwei Yao, Weihang Shan, Jiaqi Lin, Jiewen Huang, and et al. 2023. "Preclinical Studies of Chiauranib Show It Inhibits Transformed Follicular Lymphoma through the VEGFR2/ERK/STAT3 Signaling Pathway" Pharmaceuticals 16, no. 1: 15. https://doi.org/10.3390/ph16010015
APA StyleTang, Y., Zhong, M., Pan, G., Tan, J., Xie, C., Jiang, Y., Yao, J., Shan, W., Lin, J., Huang, J., Liu, Y., Li, Z., Xu, B., & Zha, J. (2023). Preclinical Studies of Chiauranib Show It Inhibits Transformed Follicular Lymphoma through the VEGFR2/ERK/STAT3 Signaling Pathway. Pharmaceuticals, 16(1), 15. https://doi.org/10.3390/ph16010015






