Synthesis, Antimalarial, Antileishmanial, and Cytotoxicity Activities and Preliminary In Silico ADMET Studies of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives
Abstract
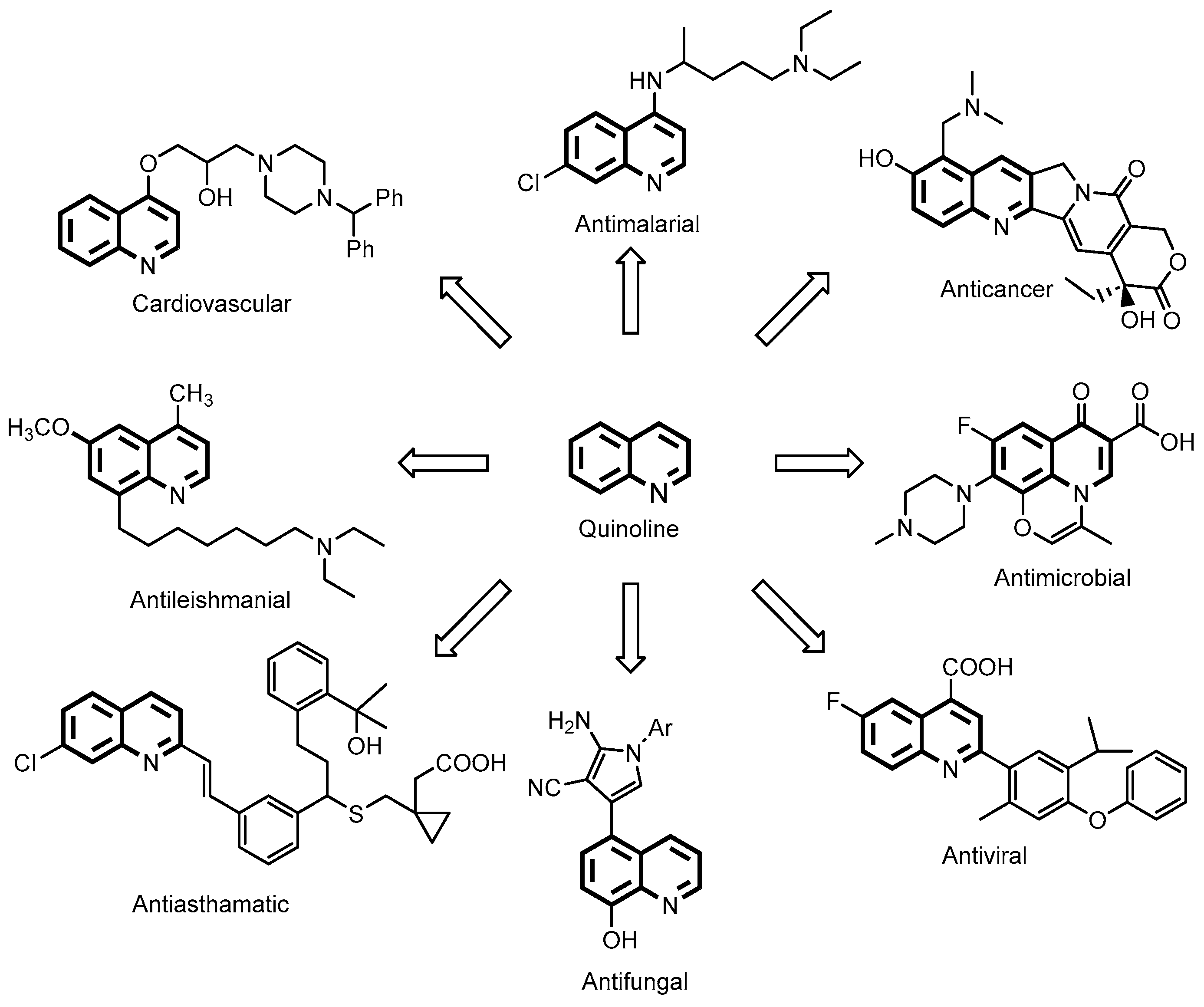
1. Introduction
2. Results and Discussion
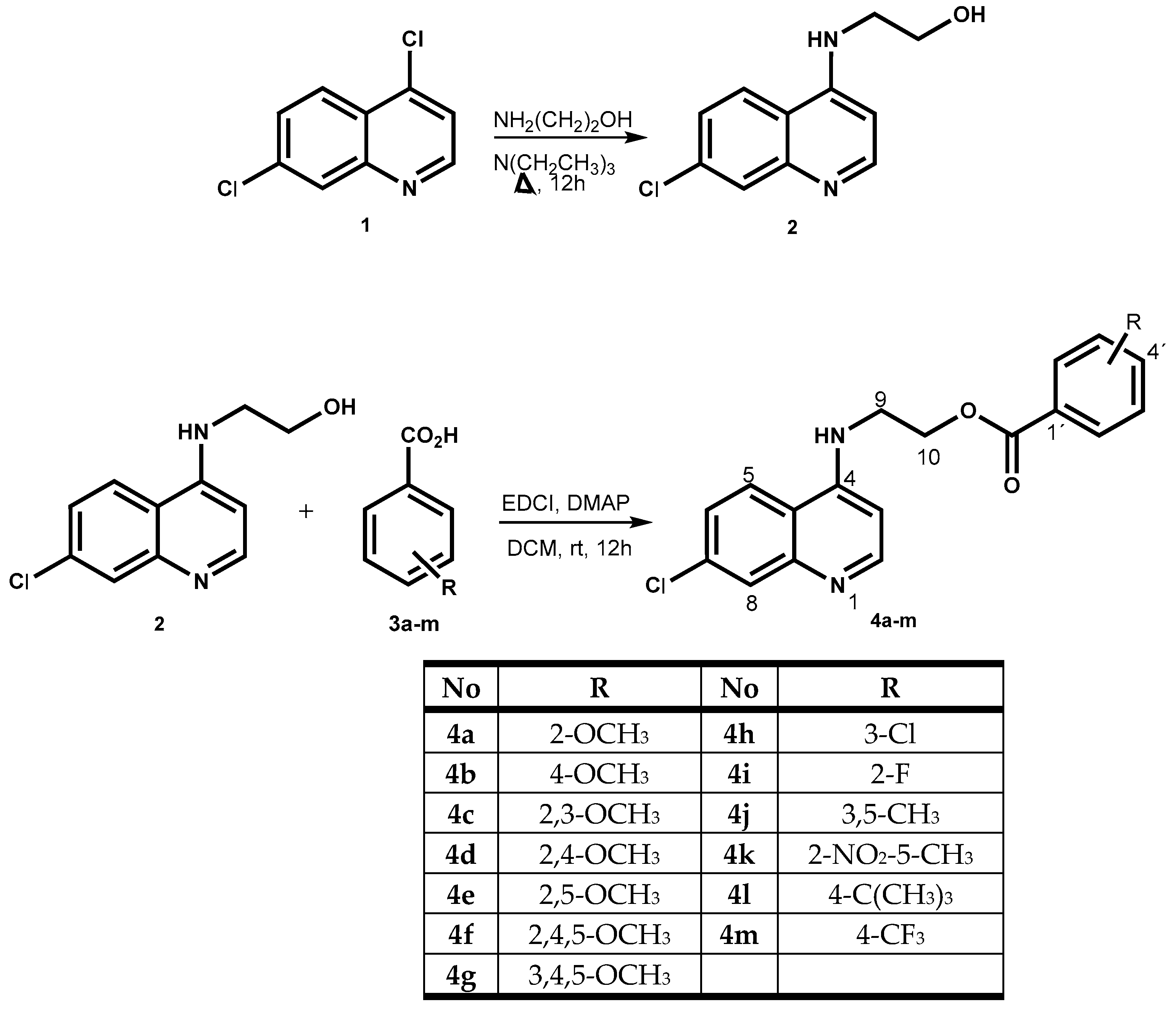
2.1. Chemistry
2.2. In Silico Analysis
2.3. Antimalarial Activity
2.4. Antileishmanial Activity
2.5. Cytotoxic Activity
2.6. Statistical Analysis
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of 2-(7-Chloroquinolin-4-ylamino)ethanol (2)
3.3. General Procedure for the Preparation of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives (4a–m)
3.3.1. 2-(7-Chloroquinolin-4-ylamino)ethyl-2-methoxybenzoate (4a)
3.3.2. 2-(7-Chloroquinolin-4-ylamino)ethyl-4-methoxybenzoate (4b)
3.3.3. 2-(7-Chloroquinolin-4-ylamino)ethyl-2,3-dimethoxybenzoate (4c)
3.3.4. 2-(7-Chloroquinolin-4-ylamino)ethyl-2,4-dimethoxybenzoate (4d)
3.3.5. 2-(7-Chloroquinolin-4-ylamino)ethyl-2,5-dimethoxybenzoate (4e)
3.3.6. 2-(7-Chloroquinolin-4-ylamino)ethyl-2,4,5-trimethoxybenzoate (4f)
3.3.7. 2-(7-Chloroquinolin-4-ylamino)ethyl-3,4,5-trimethoxybenzoate (4g)
3.3.8. 2-(7-Chloroquinolin-4-ylamino)ethyl-3-chlorobenzoate (4h)
3.3.9. 2-(7-Chloroquinolin-4-ylamino)ethyl-2-fluorobenzoate (4i)
3.3.10. 2-(7-Chloroquinolin-4-ylamino)ethyl-3,5-dimethylbenzoate (4j)
3.3.11. 2-(7-Chloroquinolin-4-ylamino)ethyl-5-methyl-2-nitrobenzoate (4k)
3.3.12. 2-(7-Chloroquinolin-4-ylamino)ethyl-4-tert-butylbenzoate (4l)
3.3.13. 2-(7-Chloroquinolin-4-ylamino)ethyl-4-(trifluoromethyl)benzoate (4m)
3.4. ADME/Tox Profile Prediction
3.5. Inhibition of β-Hematin Formation
3.6. Parasite, Experimental Host and Strain Maintenance
3.7. Four-Day Suppressive Test
3.8. In Vitro Toxicity on Mouse Red Blood Cells (RBCs)
3.9. Cell Culture
3.10. Cytotoxic Activity
3.11. Culture of L. mexicana Promastigotes and Growth Inhibition Experiments
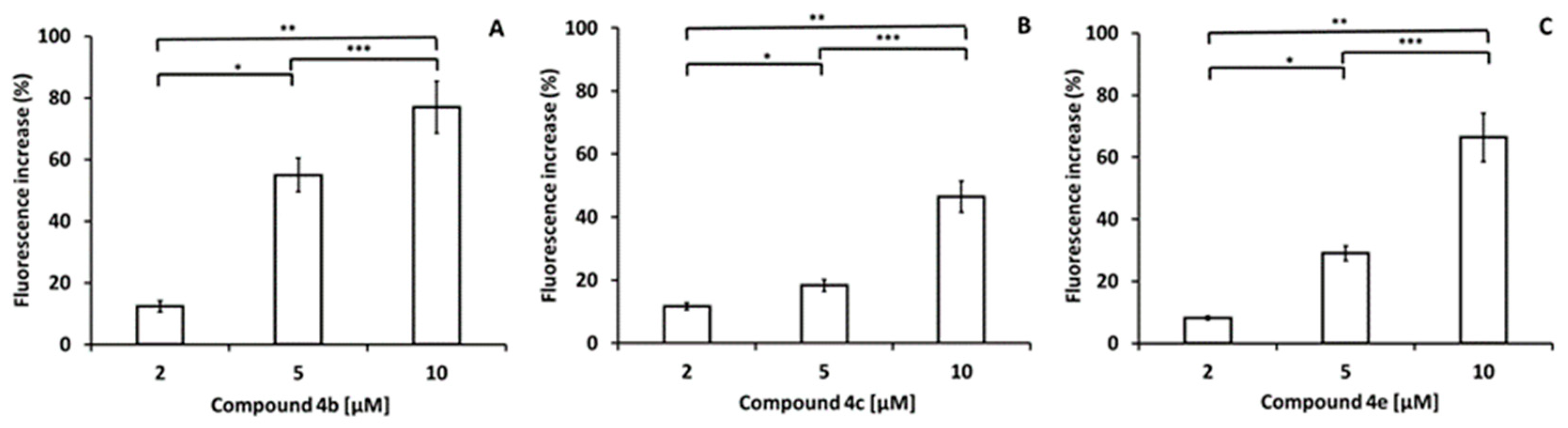
3.12. Determination of Mitochondrial Membrane Potential of Leishmania mexicana
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plewes, K.; Leopold, S.; Kingston, H.; Dondorp, A. Malaria: What’s new in the management of malaria? Infect. Dis. Clin. N. Am. 2019, 33, 39–60. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report, 6 April 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 20 June 2023).
- Short, E.E.; Caminade, C.; Bolaji, N.T. Climate change contribution to the emergence or re-emergence of parasitic diseases. Infect. Dis. Res. Treat. 2017, 10, 1178633617732296. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. 2021. Available online: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (accessed on 20 June 2023).
- Mosha, J.F.; Kulkarni, M.A.; Lukole, E.; Matowo, N.S.; Pitt, C.; Messenger, L.A.; Mallya, E.; Jumanne, M.; Aziz, T.; Kaaya, R.; et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: A four-arm, cluster-randomised trial. Lancet 2022, 399, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Ridley, R.G. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 2002, 415, 686–693. [Google Scholar] [CrossRef]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- Benaim, G.; Garcia, C.R. Targeting calcium homeostasis as the therapy of Chagas’ disease and leishmaniasis. Trop. Biomed. 2011, 28, 471–481. [Google Scholar]
- Leishmaniasis OPS/OMS. January 2022. Available online: https://www.paho.org/es/temas/leishmaniasis (accessed on 25 February 2023).
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.; Newman, S.; Ramanan, P.; Suarez, J.A. A review of Leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Rashidi, S.; Fernández-Rubio, C.; Manzano-Román, R.; Mansouri, R.; Shafiei, R.; Ali-Hassanzadeh, M.; Barazesh, A.; Karimazar, M.; Hatam, G.; Nguewa, P. Potential therapeutic targets shared between leishmaniasis and cancer. Parasitology 2021, 148, 655–671. [Google Scholar] [CrossRef]
- Croft, S.L.; Coombs, G.H. Leishmaniasis: Current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 11, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Martín, X.; García-Marchan, Y.; Fernandez, A.; Rodríguez, N.; Rojas, H.Ñ.; Visbal, G.; Benaim, G. Amiodarone destabilizes the intracellular Ca2+ homeostasis and the biosynthesis of sterols in Leishmania mexicana. Antimicrob. Agents Chemother. 2009, 53, 1403–1410. [Google Scholar] [CrossRef]
- Agrawal, V.; Singh, Z. Miltefosine: First oral drug for treatment of visceral leishmaniasis. Med. J. Armed Forces India 2006, 62, 66–67. [Google Scholar] [CrossRef] [PubMed]
- García-García, V.; Oldfield, E.; Benaim, G. Inhibition of Leishmania mexicana growth by the tuberculosis drug SQ109. Antimicrob. Agents Chemother. 2016, 60, 6386–6389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Benaim, G.; Paniz-Mondolfi, A.E.; Sordillo, E.M. Rationale for use of amiodarone and its derivatives for treatment of Chagas’ disease and leishmaniasis. Curr. Pharm. Des. 2021, 27, 1825–1833. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Gupta, H. Biological activities of quinoline derivatives. Mini Rev. Med. Chem. 2009, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar, K. Synthetic and medicinal perspective of quinolines as antiviral agents. Eur. J. Med. Chem. 2021, 215, 113220. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Stoffel, H.; Matile, H.; Bubendorf, A.; Ridley, R. Malarial haemozoin/β-haematin supports haem polymerisation in the absence of protein. Nature 1995, 374, 269–271. [Google Scholar] [CrossRef]
- De Villiers, K.; Gildenhuys, J.; Roex, T. Iron(III) protoporphyrin IX complexes of the antimalarial Cinchona alkaloids quinine and quinidine. ACS Chem. Biol. 2012, 7, 666–671. [Google Scholar] [CrossRef]
- Nordstrøm, L.; Sironi, J.; Aranda, E.; Maisonet, J.; Perez-Soler, R.; Wu, P.; Schwartz, E. Discovery of autophagy inhibitors with antiproliferative activity in lung and pancreatic cancer cells. ACS Med. Chem. Lett. 2015, 6, 134–139. [Google Scholar] [CrossRef]
- Bhat, P.; Kriel, J.; Priya, B.; Basappa; Shivananju, N.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef]
- Kapishnikov, S.; Hempelmann, E.; Elbaum, M.; Als-Nielsen, J.; Leiserowitz, L. Malaria pigment crystals: The achilles’ heel of the malaria parasite. ChemMedChem 2021, 16, 1515–1532. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Benoit-Vical, F.; Robert, A.; Meunier, B. Preparation and antimalarial activities of “trioxaquines”, new modular molecules with a trioxane skeleton linked to a 4-aminoquinoline. ChemBioChem 2000, 1, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Gemma, S.; Camodeca, C.; Coccone, S.; Joshi, B.; Bernetti, M.; Moretti, V.; Brogi, S.; de Marcos, S.M.; Savini, L.; Taramelli, D.; et al. Optimization of 4-aminoquinoline/clotrimazole-based hybrid antimalarials: Further structure-activity relationships, in vivo studies, and preliminary toxicity profiling. J. Med. Chem. 2012, 55, 6948–6967. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, S.; Raj, R.; Chowdhary, S.; Gendrot, M.; Mosnier, J.; Fonta, I.; Pradines, B.; Kumar, V. Synthesis and antiplasmodial evaluation of 1H-1,2,3-triazole grafted 4-aminoquinoline-benzoxaborole hybrids and benzoxaborole analogues. Bioorg. Chem. 2021, 109, 104733. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Sharma, A.; Legac, J.; Rosenthal, P.; Singh, P.; Kumar, V. A trio of quinoline-isoniazid-phthalimide with promising antiplasmodial potential: Synthesis, in-vitro evaluation and heme-polymerisation inhibition studies. Bioorg. Med. Chem. 2021, 39, 116159. [Google Scholar] [CrossRef]
- Rojas Ruiz, F.; García-Sánchez, R.; Villabona Estupiñan, S.; Gómez-Barrio, A.; Torres Amado, D.; Pérez-Solórzano, B.; Nogal-Ruiz, J.; Martínez-Fernández, A.; Kouznetsov, V. Synthesis and antimalarial activity of new heterocyclic hybrids based on chloroquine and thiazolidinone scaffolds. Bioorg. Med. Chem. 2011, 19, 4562–4573. [Google Scholar] [CrossRef] [PubMed]
- Tukulula, M.; Sharma, R.; Meurillon, M.; Mahajan, A.; Naran, K.; Warner, D.; Huang, J.; Mekonnen, B.; Chibale, K. Synthesis and antiplasmodial and antimycobacterial evaluation of new nitroimidazole and nitroimidazooxazine derivatives. ACS Med. Chem. Lett. 2012, 4, 128–131. [Google Scholar] [CrossRef]
- Pepe, D.; Toumpa, D.; André-Barrès, C.; Menendez, C.; Mouray, E.; Baltas, M.; Grellier, P.; Papaioannou, D.; Athanassopoulos, C. Synthesis of novel g factor or chloroquine-artemisinin hybrids and conjugates with potent antiplasmodial activity. ACS Med. Chem. Lett. 2020, 11, 921–927. [Google Scholar] [CrossRef]
- Maurya, S.S.; Khan, S.I.; Bahuguna, A.; Kumar, D.; Rawat, D.S. Synthesis, antimalarial activity, heme binding and docking studies of N-substituted 4-aminoquinoline-pyrimidine molecular hybrids. Eur. J. Med. Chem. 2017, 129, 175–185. [Google Scholar] [CrossRef]
- Marinho, J.A.; Martins Guimaraes, D.S.; Glanzmann, N.; de Almeida-Pimentel, G.; da Costa-Nunes, K.I.; Gualberto-Pereira, H.M.; Navarro, M.; de Pilla-Varotti, F.; da Silva, D.A.; Abramo, C. In vitro and in vivo antiplasmodial activity of novel quinoline derivative compounds by molecular hybridisation. Eur. J. Med. Chem. 2021, 215, 113271. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: Hybrid molecules as a new strategy in drug design. Drug Dev. Res. 2010, 71, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Molecular consortia—Various structural and synthetic concepts for more effective therapeutics synthesis. Int. J. Mol. Sci. 2018, 19, 1104–1123. [Google Scholar] [CrossRef]
- Sampath, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridisation as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar]
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Lobo, G.; Gamboa, N.; Rodrigues, J.; Abramjuk, C.; Jung, K.; Lein, M.; Charris, J.E. Synthesis of [(7-chloroquinolin-4-yl) amino] chalcones: Potential antimalarial and anticancer agents. Sci. Pharm. 2009, 77, 725–742. [Google Scholar]
- Romero, A.H.; Acosta, M.; Gamboa, N.; Charris, J.E.; Salazar, J.; López, S.E. Synthesis, β-hematin inhibition studies and antimalarial evaluation of dehydroxy isotebuquine derivatives against Plasmodium berghei. Bioorg. Med. Chem. 2015, 23, 4755–4762. [Google Scholar] [CrossRef]
- Romero, J.A.; Acosta, M.E.; Gamboa, N.; Mijares, M.R.; De Sanctis, J.B.; Charris, J.E. Optimization of antimalarial, and anticancer activities of (E)-methyl 2-(7-chloroquinolin-4-ylthio)-3-(4-hydroxyphenyl) acrylate. Bioorg. Med. Chem. 2018, 26, 815–823. [Google Scholar] [CrossRef]
- Charris, J.E.; Monasterios, M.C.; Acosta, M.E.; Rodríguez, M.A.; Gamboa, N.D.; Martínez, G.P.; Rojas, H.R.; Mijares, M.R.; De Sanctis, J.B. Antimalarial, antiproliferative, and apoptotic activity of quinoline-chalcone and quinoline-pyrazoline hybrids. A dual action. Med. Chem. Res. 2019, 28, 2050–2066. [Google Scholar] [CrossRef]
- Ramírez, H.; Fernandez-Moreira, E.; Rodrigues, J.R.; Mijares, M.R.; Ángel, J.E.; Charris, J.E. Synthesis and in silico ADME/Tox profiling studies of heterocyclic hybrids based on chloroquine scaffolds. Potential antimalarial activity. Parasitol. Res. 2022, 121, 441–451. [Google Scholar] [CrossRef]
- Kenyon, R.L.; Wiesner, J.A.; Kwartler, C.E. Chloroquine manufacture. Ind. Eng. Chem. 1949, 41, 654–662. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple method for the esterification of carboxylic acids. Angew. Chem. Int. Engl. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating aqueous solubility directly from molecular structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the general solubility equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.V. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Watanabe, R.; Esaki, T.; Kawashima, H.; Natsume-Kitatani, Y.; Nagao, C.; Ohashi, R.; Mizuguchi, K. Predicting fraction unbound in human plasma from chemical structure: Improved accuracy in the low value ranges. Mol. Pharm. 2018, 15, 5302–5311. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 structure, function and clinical significance: A review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wu, Z.; Wang, T.; Li, W.; Tang, Y.; Liu, G. In silico prediction of blood-brain barrier permeability of compounds by machine learning and resampling methods. ChemMedChem 2018, 13, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Rettie, A.E.; Fowler, D.M.; Miners, J.O. Pharmacogenomics of CYP2C9: Functional and clinical considerations. J. Pers. Med. 2017, 8, 1. [Google Scholar] [CrossRef]
- Del Tredici, A.L.; Malhotra, A.; Dedek, M.; Espin, F.; Roach, D.; Zhu, G.D.; Voland, J.; Moreno, T.A. Frequency of CYP2D6 alleles including structural variants in the United States. Front. Pharmacol. 2018, 9, 305. [Google Scholar] [CrossRef]
- Baelmans, R.; Deharo, E.; Muñoz, V.; Sauvain, M.; Ginsburg, H. Experimental conditions for testing the inhibitory activity of chloroquine on the formation of β-Hematin. Exp. Parasitol. 2000, 96, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mijoba, A.; Fernandez-Moreira, E.; Parra-Giménez, N.; Espinosa-Tapia, S.; Blanco, B.; Ramírez, H.; Charris, J.E. Synthesis of benzocycloalkanone-based Michael acceptors and biological activities as antimalarial and antitrypanosomal agents. Molecules 2023, 28, 5569. [Google Scholar] [CrossRef] [PubMed]
- Peters, W.; Robinson, B. Parasitic infection models. In Handbook of Antimalarial Models of Infection; Zak, O., Sande, M., Eds.; Academic Press: London, UK, 1999; p. 757. [Google Scholar]
- Mehta, R.; López-Berestein, G.; Hopfer, R.; Mills, K.; Juliano, R.L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim. Biophys. Acta 1984, 770, 230–234. [Google Scholar] [CrossRef]
- Benaim, G.; Bermúdez, R.; Urbina, J. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis pro-mastigotes. Mol. Biochem. Parasitol. 1990, 39, 61–68. [Google Scholar] [CrossRef]
- Benaim, G.; Paniz-Mondolfi, A.E.; Sordillo, E.M.; Martinez-Sotillo, N. Disruption of intracellular calcium homeostasis as a therapeutic target against Trypanosoma cruzi. Front. Cell. Infect. Microbiol. 2020, 10, 46. [Google Scholar] [CrossRef]
- Benaim, G.; Casanova, P.; Hernandez-Rodriguez, V.; Mujica-Gonzalez, S.; Parra-Gimenez, N.; Plaza-Rojas, L.; Concepcion, J.L.; Liu, Y.L.; Oldfield, E.; Paniz-Mondolfi, A.E.; et al. Dronedarone, an amiodarone analog with an improved anti-Leishmania mexicana efficacy. Antimicrob. Agents Chemother. 2014, 58, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- GraphPad Prism version 5.3 (GraphPad Prism Software Inc., La Jolla, CA, USA, 1992–2004). Available online: https://www.graphpad.com/features (accessed on 1 February 2023).
- Elderfield, R.C.; Gensler, W.J.; Birstein, O.; Kreysa, F.J.; Maynard, J.T.; Galbreath, J. Synthesis of certain simple 4-aminoquinoline derivatives. J. Am. Chem. Soc. 1946, 68, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Chiyanzu, I.; Clarkson, C.; Smith, P.J.; Lehman, J.; Gut, J.; Rosenthal, P.J.; Chibale, K. Design, synthesis and anti-plasmodial evaluation in vitro of new 4-aminoquinoline isatin derivatives. Bioorg. Med. Chem. 2005, 13, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Džubák, P.; Gurská, S.; Bogdanová, K.; Uhríková, D.; Kanjaková, N.; Combet, S.; Klunda, T.; Kolář, T.M.; Hajdúch, M.; Poláková, M.M. Antimicrobial and cytotoxic activity of (thio)alkyl hexopyranosides, nonionic glycolipid mimetics. Carbohydr. Res. 2020, 488, 107905. [Google Scholar] [CrossRef]
- Perlíková, P.; Rylová, G.; Naus, P.; Elbert, T.; Tloustóvá, E.; Bourderioux, A.; Postová Slavetínská, L.; Motyka, K.; Dolezal, D.; Znojek, P.; et al. 7-(2-Thienyl)-7-deazaadenosine (AB61), a new potent nucleoside cytostatic with a complex mode of action. Mol. Cancer Ther. 2016, 15, 922–937. [Google Scholar] [CrossRef]





| No | Log P | MW | Hba | Hbd | Rotb | Viol | LogSw | %HIA | FU | CLtot | LD50 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2.14 | 222.7 | 2 | 2 | 3 | 0 | −3.24 | 90.58 | 0.318 | 0.329 | 2.635 |
| 4a | 3.85 | 356.8 | 4 | 1 | 7 | 0 | −5.17 | 91.43 | 0.000 | 0.216 | 2.591 |
| 4b | 3.85 | 356.8 | 4 | 1 | 7 | 0 | −5.09 | 91.58 | 0.004 | 0.158 | 2.554 |
| 4c | 3.67 | 386.8 | 5 | 1 | 8 | 0 | −4.85 | 90.45 | 0.030 | 0.359 | 2.570 |
| 4d | 3.74 | 386.8 | 5 | 1 | 8 | 0 | −4.85 | 92.97 | 0.045 | 0.379 | 2.830 |
| 4e | 3.70 | 386.8 | 5 | 1 | 8 | 0 | −4.85 | 92.57 | 0.043 | 0.319 | 2.746 |
| 4f | 3.68 | 416.9 | 6 | 1 | 9 | 0 | −4.92 | 92.61 | 0.050 | 0.638 | 2.865 |
| 4g | 3.70 | 416.9 | 6 | 1 | 9 | 0 | −4.92 | 92.29 | 0.070 | 0.553 | 2.777 |
| 4h | 4.37 | 361.2 | 3 | 1 | 6 | 0 | −5.70 | 91.00 | 0.013 | 0.063 | 2.376 |
| 4i | 4.00 | 344.8 | 4 | 1 | 6 | 0 | −4.88 | 90.59 | 0.018 | 0.103 | 2.538 |
| 4j | 4.40 | 354.8 | 3 | 1 | 6 | 0 | −5.32 | 91.83 | 0.012 | 0.016 | 2.618 |
| 4k | 3.47 | 385.8 | 5 | 1 | 7 | 0 | −5.07 | 95.38 | 0.000 | 0.041 | 2.877 |
| 4l | 5.05 | 382.9 | 3 | 1 | 7 | 0 | −6.37 | 90.72 | 0.000 | 0.015 | 2.649 |
| 4m | 4.91 | 394.7 | 6 | 1 | 7 | 0 | −5.94 | 88.32 | 0.000 | 0.050 | 2.787 |
| CQ* | 1.30 | 515.9 | 10 | 7 | 8 | 3 | −1.59 | 31.34 | 0.424 | 0.314 | 2.775 |
| Solubility Predictor | 2 | 4c | 4e | CQ(2DPh) |
|---|---|---|---|---|
| Log S (ESOL) | −3.24 | −4.85 | −4.85 | −1.59 |
| Solubility | 5.81 × 10−4 M | 1.41 × 10−5 M | 1.41 × 10−5 M | 2.58 × 10−2 M |
| Class | Soluble | Moderately soluble | Moderately soluble | Very soluble |
| Log S (Ali) | −3.33 | −5.47 | −5.47 | −1.92 |
| Solubility | 4.65 × 10−4 M | 3.42 × 10−6 M | 3.42 × 10−6 M | 1.20 × 10−2 M |
| Class | Soluble | Moderately soluble | Moderately soluble | Very soluble |
| Log S (SILICOS-IT) | −4.61 | −7.57 | −7.57 | −6.92 |
| Solubility | 2.45 × 10−5 M | 2.67 × 10−8 M | 2.67 × 10−8 M | 1.21 × 10−7 M |
| Class | Moderately soluble | Poorly soluble | Poorly soluble | Poorly soluble |
| No | CYP2D6 Substrate | CYP3A4 Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor |
|---|---|---|---|---|---|---|---|
| 2 | No | No | Yes | No | No | No | No |
| 4a | No | Yes | Yes | Yes | Yes | No | Yes |
| 4b | No | Yes | Yes | Yes | Yes | No | Yes |
| 4c | No | Yes | Yes | Yes | Yes | No | Yes |
| 4d | No | Yes | Yes | Yes | Yes | No | Yes |
| 4e | No | Yes | Yes | Yes | Yes | No | Yes |
| 4f | No | Yes | No | Yes | Yes | No | Yes |
| 4g | No | Yes | No | Yes | Yes | No | Yes |
| 4h | No | Yes | Yes | Yes | Yes | No | Yes |
| 4i | No | Yes | Yes | Yes | Yes | No | No |
| 4j | No | Yes | Yes | Yes | Yes | No | Yes |
| 4k | No | Yes | Yes | Yes | Yes | No | Yes |
| 4l | No | Yes | Yes | Yes | Yes | No | Yes |
| 4m | No | Yes | Yes | Yes | Yes | No | Yes |
| CQ* | No | Yes | No | No | No | No | No |
| No | IC50 a (µM) | (%) Hemolysis | Sd b (± SEM) d | %P c (± SEM) d | Survival e |
|---|---|---|---|---|---|
| 2 | 5.06 ± 0.31 | ND | ND | ND | ND |
| 4a | 4.16 ± 0.61 | ND | ND | ND | ND |
| 4b | 3.09 ± 0.27 | ND | ND | ND | ND |
| 4c | 2.10 ± 0.48 † | 4.73 ± 0.12 | 16.71 ± 2.16 * | 9.86 ± 1.55 ** | 0/6 |
| 4d | 8.59 ± 0.01 | ND | ND | ND | ND |
| 4e | 1.81 ± 0.83 † | 4.17 ± 0.21 | 14.43 ± 1.20 * | 12.05 ± 2.36 ** | 0/6 |
| 4f | 3.29 ± 0.23 | ND | ND | ND | ND |
| 4g | 2.98 ± 0.17 | ND | ND | ND | ND |
| 4h | 6.85 ± 0.94 | ND | ND | ND | ND |
| 4i | 4.01 ± 0.09 | ND | ND | ND | ND |
| 4j | 3.16 ± 0.38 | ND | ND | ND | ND |
| 4k | 4.27 ± 0.67 | ND | ND | ND | ND |
| 4l | 3.57 ± 0.22 | ND | ND | ND | ND |
| 4m | 3.24 ± 0.15 | ND | ND | ND | ND |
| CQ | 1.50 ± 0.01 | 2.07 ± 0.17 | 30 | 0.28 ± 0.15 | 6/6 |
| CiSS | - | - | 8.12 ± 1.13 | 27.2 ± 2.25 | 0/6 |
| No | IC50 (µM) |
|---|---|
| 4b | 8.09 ± 1.47 |
| 4c | 8.46 ± 1.86 |
| 4e | 5.67 ± 2.15 |
| SQ109 | 0.53 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.E.; Ramírez, H.; Fernandez-Moreira, E.; Acosta, M.E.; Mijares, M.R.; De Sanctis, J.B.; Gurská, S.; Džubák, P.; Hajdúch, M.; Labrador-Fagúndez, L.; et al. Synthesis, Antimalarial, Antileishmanial, and Cytotoxicity Activities and Preliminary In Silico ADMET Studies of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives. Pharmaceuticals 2023, 16, 1709. https://doi.org/10.3390/ph16121709
Gutiérrez JE, Ramírez H, Fernandez-Moreira E, Acosta ME, Mijares MR, De Sanctis JB, Gurská S, Džubák P, Hajdúch M, Labrador-Fagúndez L, et al. Synthesis, Antimalarial, Antileishmanial, and Cytotoxicity Activities and Preliminary In Silico ADMET Studies of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives. Pharmaceuticals. 2023; 16(12):1709. https://doi.org/10.3390/ph16121709
Chicago/Turabian StyleGutiérrez, Joyce E., Hegira Ramírez, Esteban Fernandez-Moreira, María E. Acosta, Michael R. Mijares, Juan Bautista De Sanctis, Soňa Gurská, Petr Džubák, Marián Hajdúch, Liesangerli Labrador-Fagúndez, and et al. 2023. "Synthesis, Antimalarial, Antileishmanial, and Cytotoxicity Activities and Preliminary In Silico ADMET Studies of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives" Pharmaceuticals 16, no. 12: 1709. https://doi.org/10.3390/ph16121709
APA StyleGutiérrez, J. E., Ramírez, H., Fernandez-Moreira, E., Acosta, M. E., Mijares, M. R., De Sanctis, J. B., Gurská, S., Džubák, P., Hajdúch, M., Labrador-Fagúndez, L., Stella, B. G., Díaz-Pérez, L. J., Benaim, G., & Charris, J. E. (2023). Synthesis, Antimalarial, Antileishmanial, and Cytotoxicity Activities and Preliminary In Silico ADMET Studies of 2-(7-Chloroquinolin-4-ylamino)ethyl Benzoate Derivatives. Pharmaceuticals, 16(12), 1709. https://doi.org/10.3390/ph16121709






