Nutritionally Important Pro-Health Active Ingredients and Antioxidant Properties of Fruits and Fruit Juice of Selected Biennial Fruiting Rubus idaeus L. Cultivars
Abstract
:1. Introduction
1.1. Oxidative Stress and Its Effects and Defense Mechanisms against ROS
1.2. Rubi idaei fructus as a Source of Antioxidants
1.3. Use of R. idaeus Fruits
1.4. Selected Methods for the Determination of Oxidative Activity
2. Results
2.1. Antioxidant Activity and Content of Polyphenols in Raspberry Fruits and Juice
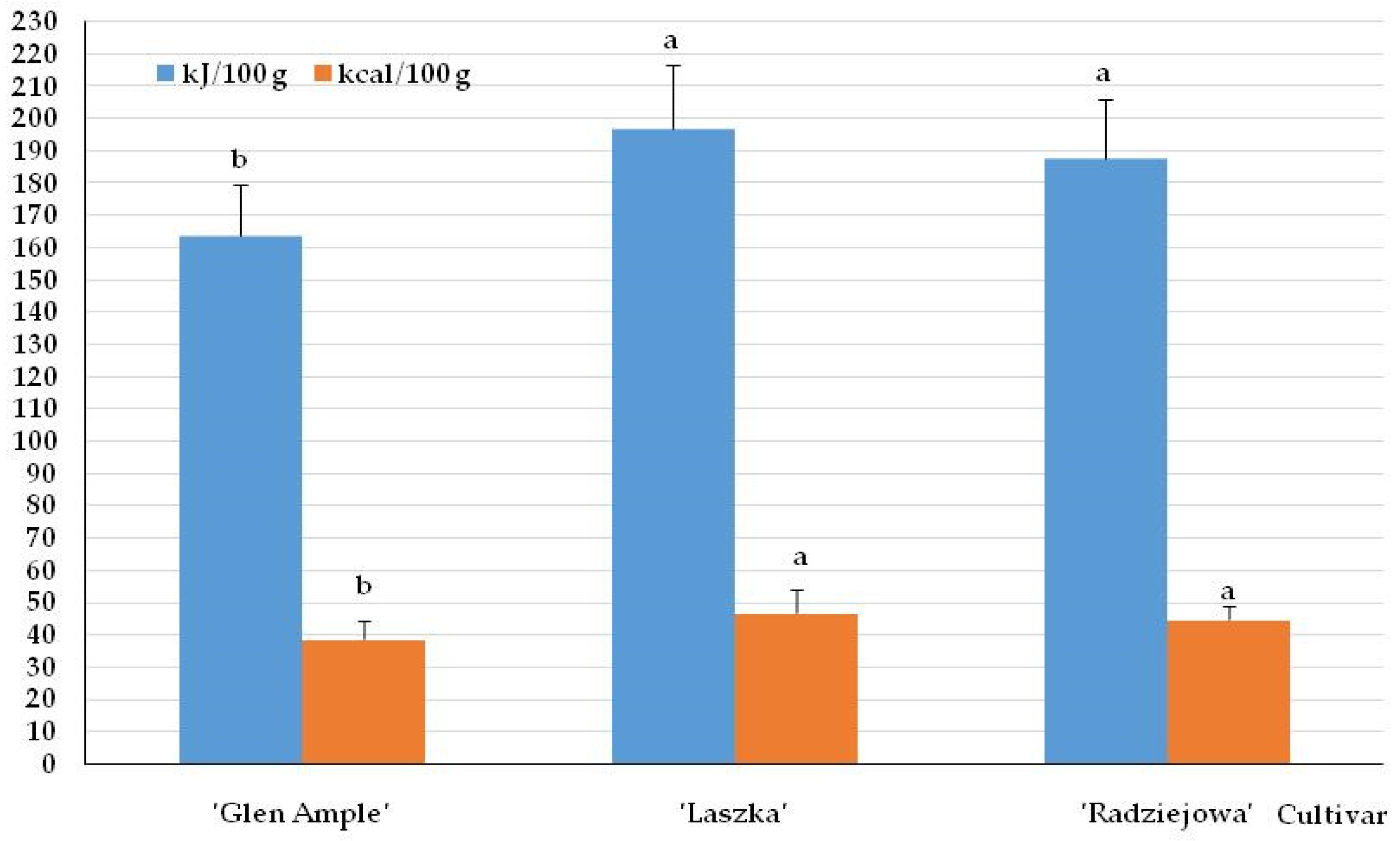
2.2. Energy Value of Fruits
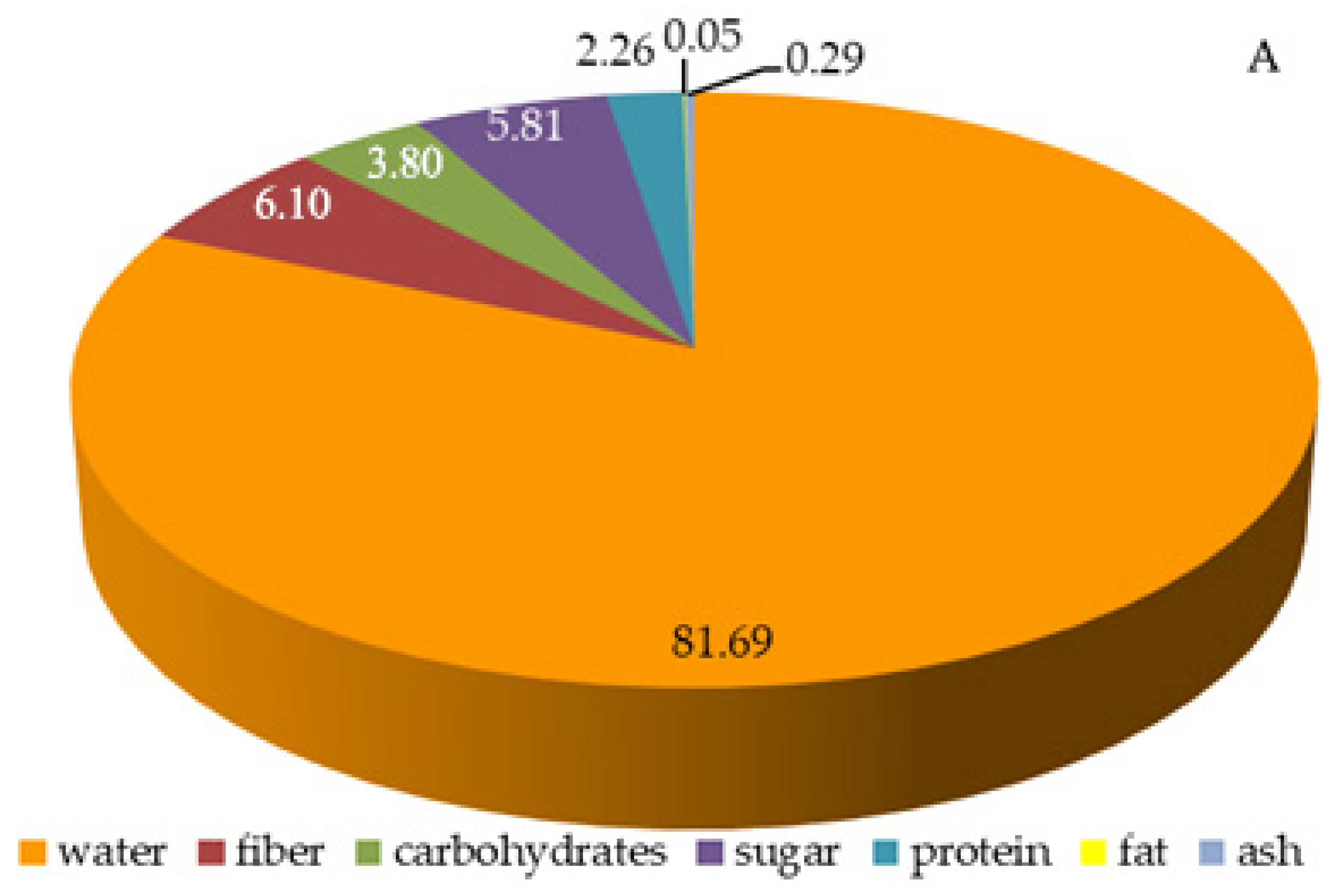
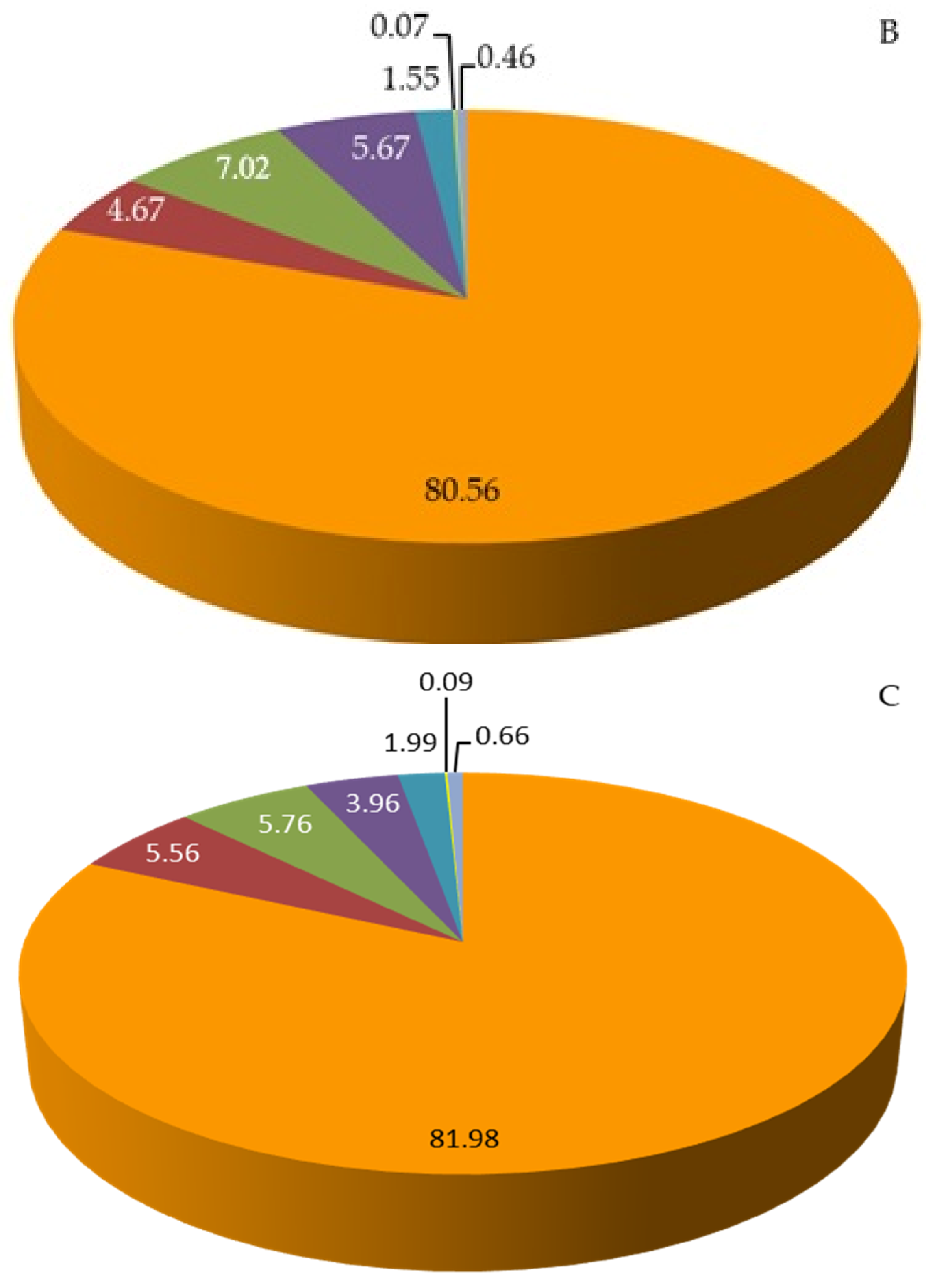
2.3. Total Content of Sugars, Carbohydrates, Fiber, Protein, and Lipids in Raspberry Fruits
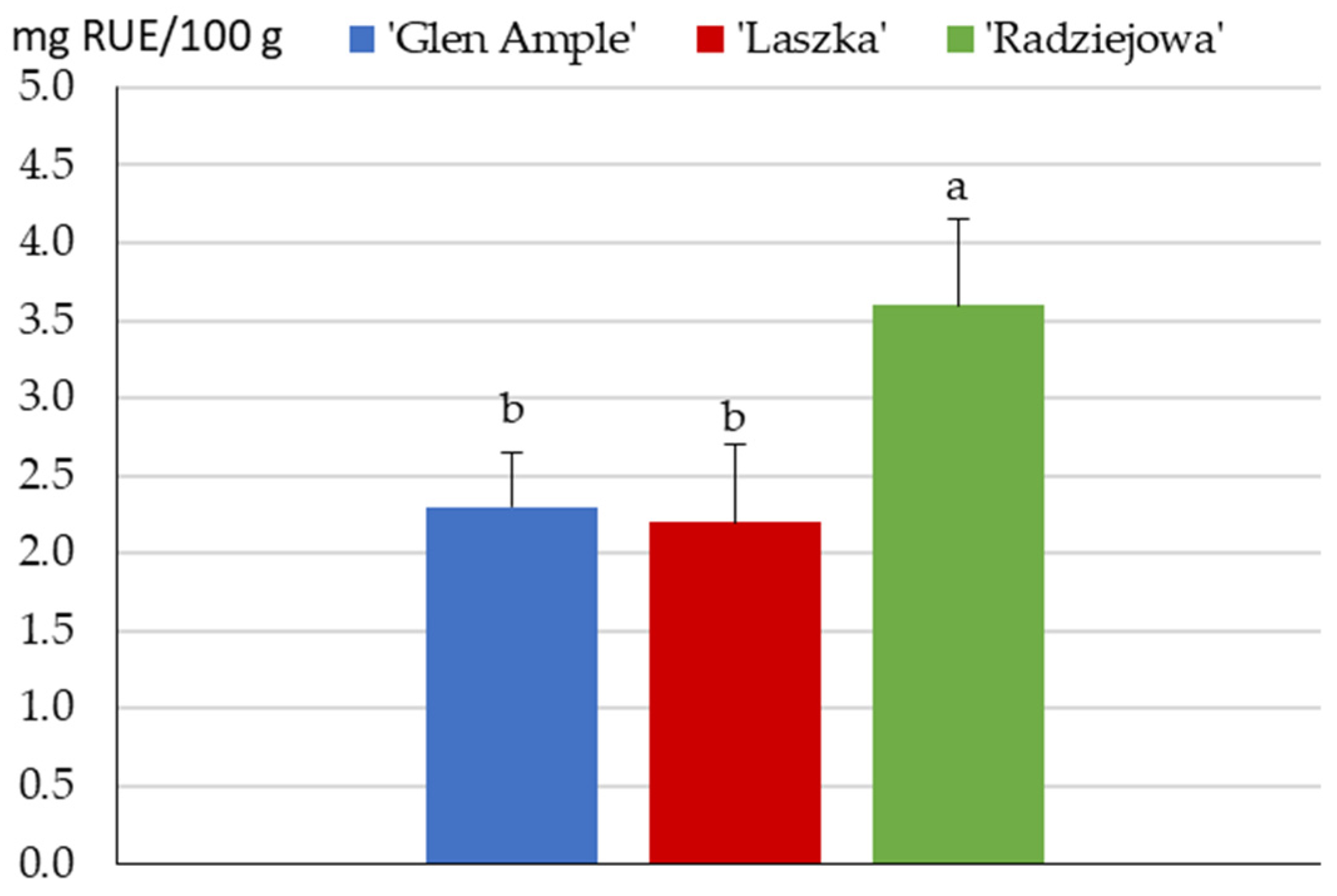
2.4. Flavonoids
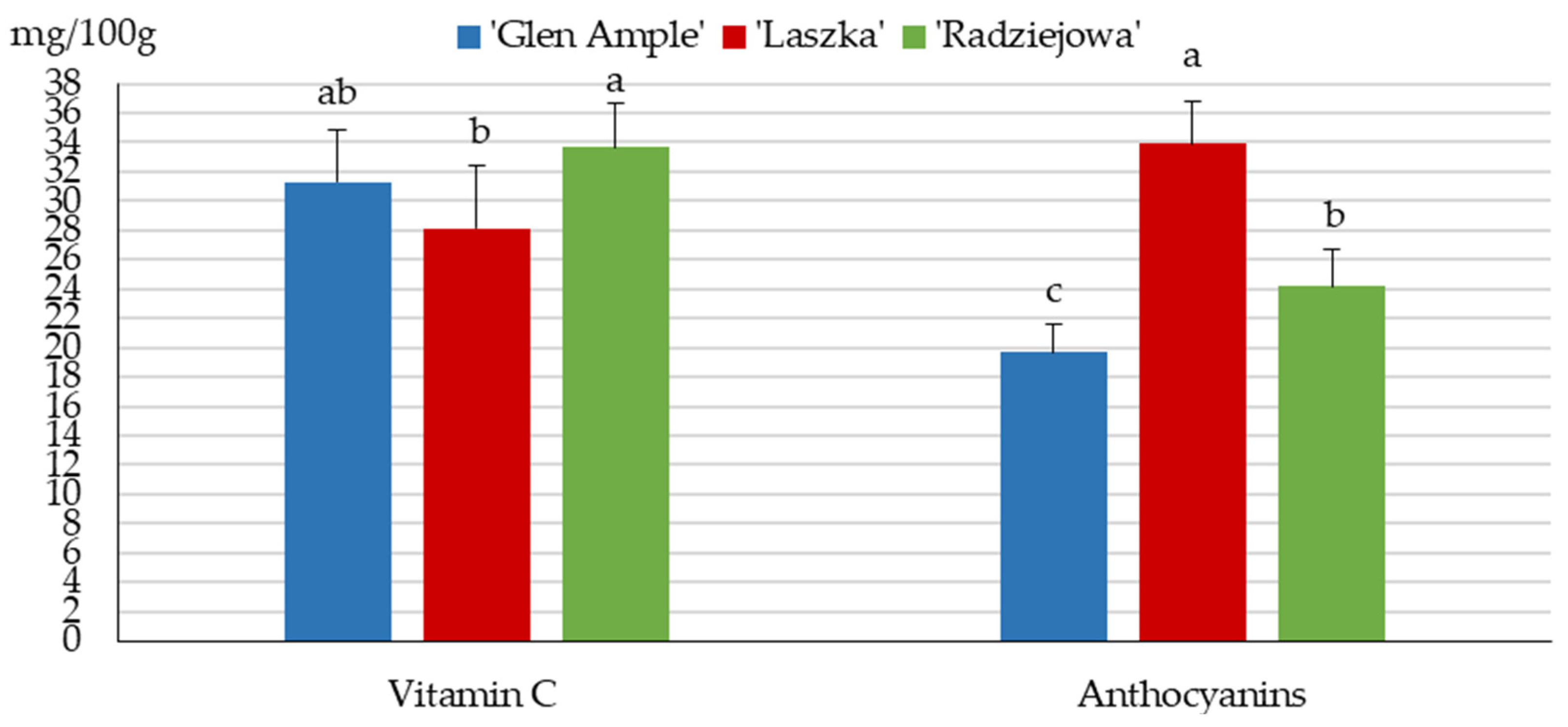
2.5. Content of Anthocyanins and Vitamin C
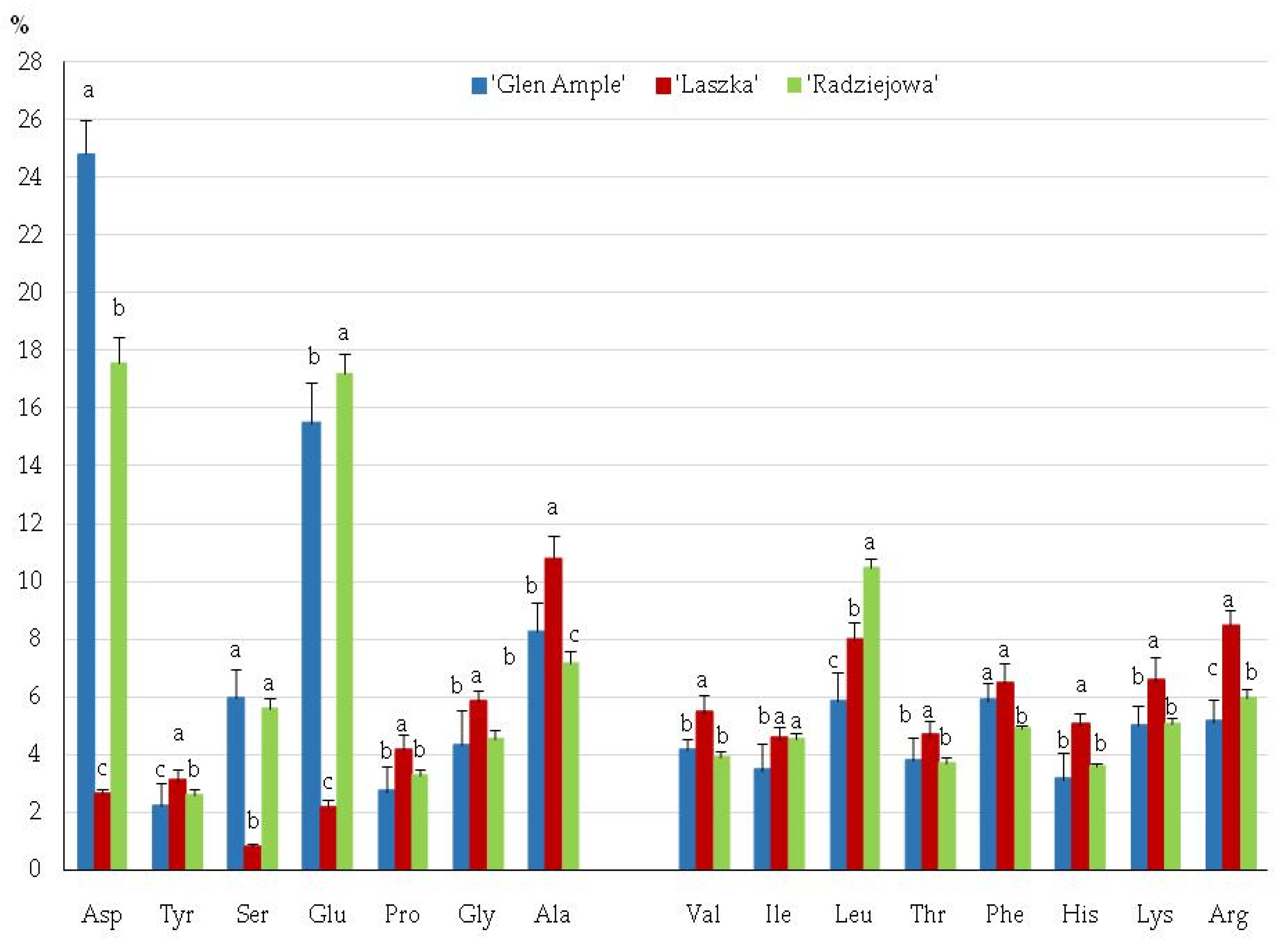
2.6. Amino Acids
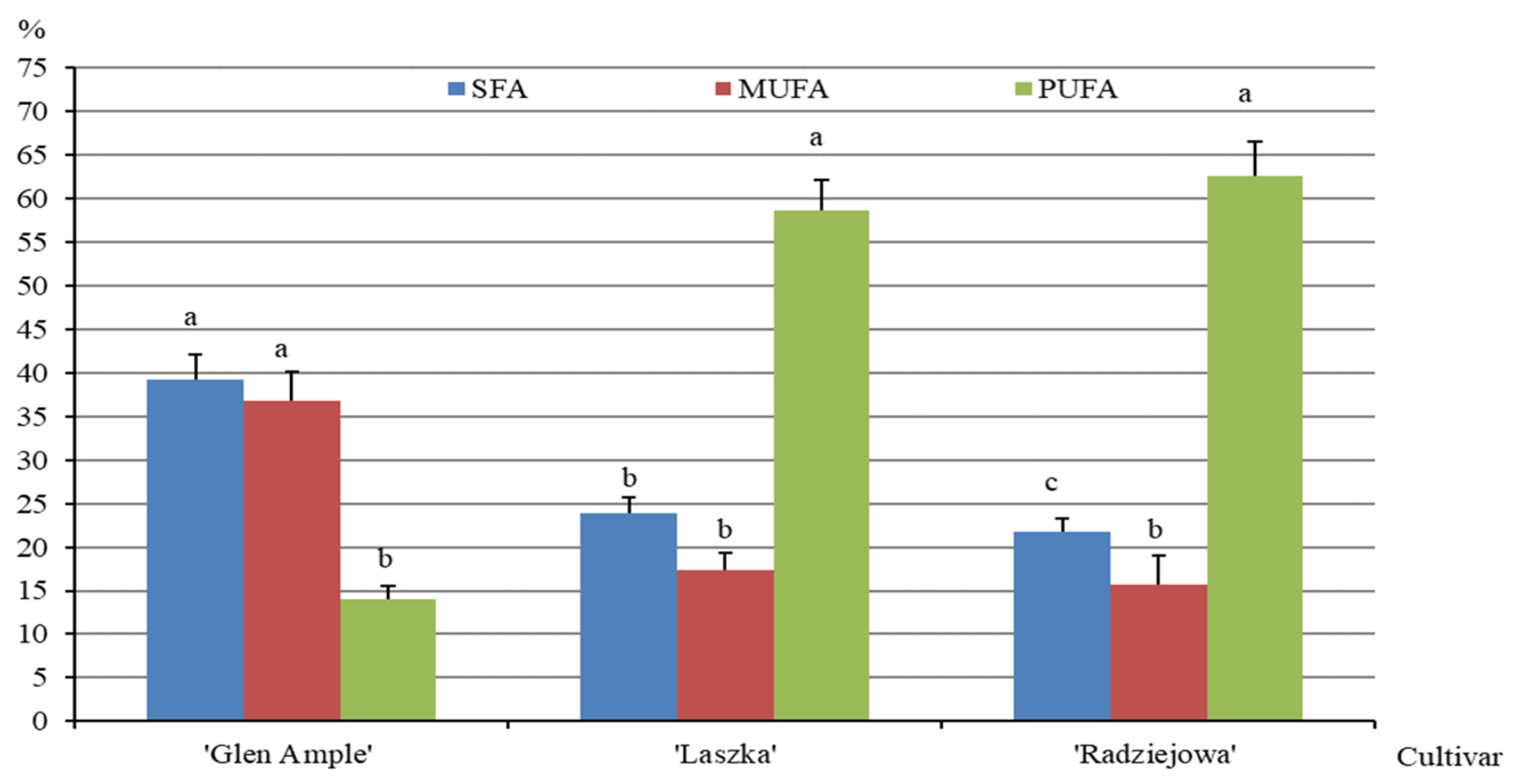
2.7. Content of Fatty Acids
3. Discussion
3.1. Polyphenolic Compounds and Antioxidant Activity
3.2. Protein
3.3. Amino Acids
3.4. Vitamin C
3.5. Flavonoids
3.6. Anthocyanins
3.7. Fatty Acids
3.8. Fiber
3.9. Application
3.10. Future Research
4. Materials and Methods
4.1. Study Material
Origin of Cultivars
4.2. Determination of Antioxidant Activity
4.2.1. FRAP Method (Ferric Reducing Antioxidant Power Assay)
4.2.2. DPPH Method
4.2.3. ABTS Method
4.2.4. OH• Radical Scavenging Method
4.2.5. Folin–Ciocalteau Method
4.3. Chemical Analyses
4.3.1. Total Sugars
4.3.2. Total Fiber
4.3.3. Total Protein
4.3.4. Amino Acids
4.3.5. Total Fat Content
4.3.6. Total Carbohydrate Content
4.3.7. Qualitative and Quantitative Composition of Fatty Acids
4.3.8. Vitamin C
4.3.9. Flavonoids
4.3.10. Anthocyanins
4.4. Energy Value
4.5. Statistical Analysis of the Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug. Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Kiran, T.R.; Otlu, O.; Karabulut, A.B. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023, 47, 1–11. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemeéle, E.J.; Fulton, D.J.R.; Fukai, T. Interplay between reactive oxygen/reactive nitrogen species and metabolism in vascular biology and disease. Antioxid. Redx. Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’Andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxid. Med. Cell Longev. 2016, 2016, e1245049. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agraval, G.K.; Srivastava, A.; Sarkar, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) in plants–maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Tripathi, D.; Nam, A.; Oldenburg, D.J.; Bendich, A.J. Reactive oxygen species, antioxidant agents, and DNA damage in developing maize mitochondria and plastids. Front. Plant Sci. 2020, 11, e596. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2011, 21, 401–409. [Google Scholar] [CrossRef]
- Xu, Q.; Huff, L.P.; Fujii, M.; Griendling, K.K. Redox regulation of the actin cytoskeleton and its role in the vascular system. Free Radic. Biol. Med. 2017, 109, 84–107. [Google Scholar] [CrossRef] [PubMed]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, e101678. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W. Role of reactive oxygen species in cell death pathways. Hanyang Med. Rev. 2013, 33, 77–82. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Irato, P.; Santovito, G. 2021. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants 2021, 10, e579. [Google Scholar] [CrossRef]
- Loudari, A.; Latique, S.; Mayane, A.; Colinet, G.; Oukarroum, A. Polyphosphate fertilizer impacts the enzymatic and non-enzymatic antioxidant capacity of wheat plants grown under salinity. Sci. Rep. 2023, 13, e11212. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Vasconcelos, M.W.; Soares, C.; Fidalgo, F.; Heuvelink, E.; Carvalho, S.M.P. Enzymatic and non-enzymatic antioxidant responses of young tomato plants (cv. Micro-Tom) to single and combined mild nitrogen and water deficit: Not the sum of the parts. Antioxidants 2023, 12, 375. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.A.; Ahmed, S.A. Nonenzymatic exogenous and endogenous antioxidants. In Free Radical Medicine and Biology, 1st ed.; Das, K., Das, S., Shivanagouda Biradar, M., Bobbarala, V., Subba Tata, S., Eds.; IntechOpen: London, UK, 2020; pp. 95–116. [Google Scholar]
- Abdulfatah, H.F. Non-enzymatic antioxidants in stressed plants: A review. J. Anbar Univ. Pure Sci. 2022, 16, 25–37. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Riaz, M.; Arif, M.S.; Rasheed, R.; Iqbal, M.; Hussain, I.; Mubarik, M.S. The role of non-enzymatic antioxidants in improving abiotic stress tolerance in plants. In Plant Tolerance to Environmental Stress: Role of Phytoprotectants, 1st ed.; Hasanuzzaman, M., Fujita, M., Oku, H., Islam, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; Volume 4, pp. 129–143. [Google Scholar]
- Piazza, S.; Fumagalli, M.; Khalilpour, S.; Martinelli, G.; Magnavacca, A.; Dell’Agli, M.; Sangiovanni, E. A review of the potential benefits of plants producing berries in skin disorders. Antioxidants 2020, 9, 542. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, e644. [Google Scholar] [CrossRef]
- Davik, J.; Røen, D.; Lysøe, E.; Buti, M.; Rossman, S.; Alsheikh, M.; Aiden, E.L.; Dudchenko, O.; Sargent, D.J. A chromosome-level genome sequence assembly of the red raspberry (Rubus idaeus L.). PLoS ONE 2022, 17, e0265096. [Google Scholar] [CrossRef] [PubMed]
- Piña-Contreras, N.; Martínez-Moreno, A.G.; Ramírez-Anaya, J.D.P.; Espinoza-Gallardo, A.C.; Valdés, E.H.M. Raspberry (Rubus idaeus L.), a promising alternative in the treatment of hyperglycemia and dyslipidemias. J. Med. Food. 2022, 25, 121–129. [Google Scholar] [CrossRef]
- Bojkovska, K.; Joshevska, F.; Tosheva, E.; Momirceski, J. Global raspberries market trends and their impact on the Macedonian raspberries market. Int. J. Res. Rev. 2021, 8, 362–369. [Google Scholar]
- Frías-Moreno, M.N.; Parra-Quezada, R.Á.; Ruíz-Carrizales, J.; González-Aguilar, G.A.; Sepulveda, D.; Molina-Corral, F.J.; Olivas, G.I. Quality, bioactive compounds and antioxidant capacity of raspberries cultivated in northern Mexico. Int. J. Food Prop. 2021, 24, 603–614. [Google Scholar] [CrossRef]
- Veljković, B.; Jakovljević, V.; Stanković, M.; Dajić-Stevanović, Z. Phytochemical and antioxidant properties of fresh fruits and some traditional products of wild grown raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 565–573. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, anti-inflammatory and cytotoxic activity of phenolic compound family extracted from raspberries (Rubus idaeus): A general review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Marić, B.; Abramović, B.; Ilić, N.; Bodroža-Solarov, M.; Pavlić, B.; Oczkowski, M.; Wilczak, J.; Četojević-Simin, D.; Šarić, L.; Teslić, N. UHPLC-Triple-TOF-MS characterization, antioxidant, antimicrobial and antiproliferative activity of raspberry (Rubus idaeus L.) seed extracts. Foods 2022, 12, 161. [Google Scholar] [CrossRef]
- Su, X.; Zhao, M.; Fu, X.; Ma, X.; Xu, W.; Hu, S. Immunomodulatory activity of purified polysaccharides from Rubus chingii Hu fruits in lymphocytes and its molecular mechanisms. J. Funct. Foods 2021, 87, 104785. [Google Scholar] [CrossRef]
- Veljković, B.; Djordjevic, N.; Dolicanin, Z.; Licina, B.; Topuzovic, M.; Stankovic, M.; Zlatić, N.; Dajic-Stevanovic, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 359–367. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Will, F.; Mesa-Marín, J.; Lewandowski, M.; Celejewska, K.; Masny, A.; Zurawicz, E.; Jennings, N.; Sønsteby, A.; et al. Exploring genotype-by-environment interactions of chemical composition of raspberry by using a metabolomics approach. Metabolites 2021, 11, e490. [Google Scholar] [CrossRef]
- Zhbanova, E.V. Fruit of raspberry Rubus idaeus L. as a source of functional ingredients (review). Food Process. Techniq. Technol. 2018, 48, 5–14. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Martìnez Garcìa, L.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Influences of postharvest storage and processing techniques on antioxidant and nutraceutical properties of Rubus idaeus L.: A mini-review. Horticulturae 2020, 6, 105. [Google Scholar] [CrossRef]
- Carey, A.N.; Pintea, G.I.; Van Leuven, S.; Gildawie, K.R.; Squiccimara, L.; Fine, E.; Rovnak, A.; Harrington, M. Red raspberry (Rubus ideaus) supplementation mitigates the effects of a high-fat diet on brain and behavior in mice. Nutr. Neurosci. 2021, 24, 406–416. [Google Scholar] [CrossRef]
- Meng, Q.; Manghwar, H.; Hu, W. Study on supergenus Rubus L.: Edible, medicinal, and phylogenetic characterization. Plants 2022, 11, 1211. [Google Scholar] [CrossRef]
- Gledovic, A.; Janosevic, L.A.; Nikolic, I.; Tasic-Kostov, M.; Antic-Stankovic, J.; Krstonosic, V.; Randjelovic, D.; Bozic, D.; Ilic, D.; Tamburic, S.; et al. Polyglycerol ester-based low energy nanoemulsions with red raspberry seed oil and fruit extracts: Formulation development toward effective In Vitro/In Vivo bioperformance. Nanomaterials 2021, 11, 217. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.H. Rubus idaeus L. (red raspberry) blocks UVB-induced MMP production and promotes type I procollagen synthesis via inhibition of MAPK/AP-1, NF-κβ and stimulation of TGF-β/Smad, Nrf2 in normal human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 185, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of dietary red raspberry consumption on pre-diabetes and type 2 diabetes mellitus parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Fan, R.; Shao, J.; Toney, A.M.; Chung, S.; Ramer-Tait, A.E. Polyphenolic fractions isolated from red raspberry whole fruit, pulp, and seed differentially alter the gut microbiota of mice with diet-induced obesity. J. Func. Foods 2021, 76, 104288. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Abo-ELmatty, D.M.; Mesbah, N.M.; Saleh, S.M.; Ali, A.M.A.; Sakr, A.T. Raspberry ketone preserved cholinergic activity and antioxidant defense in obesity induced Alzheimer disease in rats. Biomed. Pharmacother. 2018, 107, 1166–1174. [Google Scholar] [CrossRef]
- Goodman, C.; Lyon, K.N.; Scotto, A.; Smith, C.; Sebrell, T.A.; Gentry, A.B.; Bala, G.; Stoner, G.D.; Bimczok, D. A high-throughput metabolic microarray assay reveals antibacterial effects of black and red raspberries and blackberries against Helicobacter pylori infection. Antibiotics 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Bauza-Kaszewska, J.; Zary-Sikorska, E.; Gugolek, A.; Ligocka, A.; Kosmala, M.; Karlinska, E.; Fotschki, B.; Juskiewicz, J. Synergistic antimicrobial effect of raspberry (Rubus idaeus L., Rosaceae) preparations and probiotic bacteria on enteric pathogens. Pol. J. Food Nutr. Sci. 2021, 71, 51–59. [Google Scholar] [CrossRef]
- Hsin, C.H.; Huang, C.C.; Chen, P.N.; Hsieh, Y.S.; Yang, S.F.; Ho, Y.T.; Lin, C.W. Rubus idaeus inhibits migration and invasion of human nasopharyngeal carcinoma cells by suppression of MMP-2 through modulation of the ERK1/2 pathway. Am. J. Chin. Med. 2017, 45, 1557–1572. [Google Scholar] [CrossRef]
- Grabek-Lejko, D.; Wojtowicz, K. Comparison of antibacterial and antioxidant properties of fruits and leaves of blackberry (Rubus plicatus) and raspberry (Rubus idaeus). J. Microbiol. Biotechnol. Food Sci. 2014, 3, 514–518. [Google Scholar]
- Chwil, M.; Matraszek-Gawron, R.; Kostryco, M. Rubi idaei fructus as a source of bioactive chemical compounds with an important role in human health and comparison of the antioxidant potential of fruits and juice of three repeat-fruiting Rubus idaeus L. cultivars. Metabolites 2023, 13, 1124. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Changes in antioxidant substances and antioxidant enzyme activities in raspberry fruits at different developmental stages. Sci. Hortic. 2023, 321, 112314. [Google Scholar] [CrossRef]
- Shoukat, S.; Mahmudiono, T.; Al-Shawi, S.G.; Abdelbasset, W.K.; Yasin, G.; Shichiyakh, R.A.; Iswanto, A.H.; Kadhim, A.J.; Kadhim, M.M.; Al-Rekaby, H.Q. Determination of the antioxidant and mineral contents of raspberry varieties. Food Sci. Technol. Campinas 2022, 42, 118521. [Google Scholar] [CrossRef]
- Renai, L.; Scordo, C.V.A.; Chiuminatto, U.; Ulaszewska, M.; Giordani, E.; Petrucci, W.A.; Tozzi, F.; Nin, S.; Del Bubba, M. Liquid chromatographic quadrupole time-of-flight mass spectrometric untargeted profiling of (poly) phenolic compounds in Rubus idaeus L. and Rubus occidentalis L. fruits and their comparative evaluation. Antioxidants 2021, 10, 704. [Google Scholar] [CrossRef]
- Mîrza, A. Antioxidant activity of leaf and fruit extracts from Rubus fruticosus, Rubus idaeus and Rubus loganobaccus growing in the conditions of the Republic of Moldova. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2021, 21, 363–372. [Google Scholar]
- Velarde-Salcedo, A.J.; De León-Rodríguez, A.; Calva-Cruz, O.J.; Balderas-Hernández, V.E.; De Anda Torres, S.; Barba-de la Rosa, A.P. Extraction of bioactive compounds from Rubus idaeus waste by maceration and supercritical fluids extraction: The recovery of high added-value compounds. Int. J. Food Sci. Technol. 2023, 58, 5838–5854. [Google Scholar] [CrossRef]
- Szymanowska, U.; Baraniak, B.; Bogucka-Kocka, A. Antioxidant, anti-inflammatory, and postulated cytotoxic activity of phenolic and anthocyanin-rich fractions from ‘Polana’ raspberry (Rubus idaeus L.) fruit and juice—In vitro study. Molecules 2018, 23, 1812. [Google Scholar] [CrossRef]
- Schulz, M.; Chim, J.F. Nutritional and bioactive value of Rubus berries. Food Biosci. 2019, 31, 100438. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Piechowiak, T.; Szostek, M.; Kapusta, I.; Niemiec, M.; Komorowska, M.; Wróbel, M.; Mudryk, K.; Szeląg-Sikora, A.; et al. The modification of substrate in the soilless cultivation of raspberries (Rubus idaeus L.) as a factor stimulating the biosynthesis of selected bioactive compounds in fruits. Molecules 2022, 28, 118. [Google Scholar] [CrossRef] [PubMed]
- Vieira, T.M.; Alves, V.D.; Moldão Martins, M. Application of an eco-friendly antifungal active package to extend the shelf life of fresh red raspberry (Rubus idaeus L. cv. ‘Kweli’). Foods 2022, 11, 1805. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Yu, T.W.; Ong, C.N. Lag-time measurement of antioxidant capacity using myoglobin and (2,2’-Azino-bis(ethylbenzthiazoline-6-sulfnic acid): Rationale application and limitation. Anal. Biochem. 1999, 275, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D. Comparison of phenolic acids profile and antioxidant potential of six varietes of spelt (Triticum spelta L.). J. Agric Food Chem. 2012, 60, 4603–4612. [Google Scholar] [CrossRef]
- Fredes, C.; Montenegro, G.; Zoffoli, J.P.; Santander, F.; Robert, P. Comparison of the total phenolic content, total anthocyanin content and antioxidant activity of polyphenol-rich fruits grown in Chile. Cienc. Inv. Agr. 2014, 41, 49–60. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Faleva, A.V.; Ul’yanovskii, N.V.; Onuchina, A.A.; Falev, D.I.; Kosyakov, D.S. Comprehensive characterization of secondary metabolites in fruits and leaves of cloudberry (Rubus chamaemorus L.). Metabolites 2023, 13, 598. [Google Scholar] [CrossRef] [PubMed]
- Cekiç, C.; Özgen, M. Comparison of antioxidant capacity and phytochemical properties of wild and cultivated red raspberries (Rubus idaeus L.). J. Food Compost. Anal. 2010, 23, 540–544. [Google Scholar] [CrossRef]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, G.; Grieco, F. Functional properties of grape and wine polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Akimov, M.Y.; Koltsov, V.A.; Zhbanova, E.V.; Akimova, O.M. Nutritional value of promising raspberry varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 640, 022078. [Google Scholar] [CrossRef]
- Marzban, G.; Maghuly, F.; Herndl, A.; Katinger, H.; Laimer, M. Screening and identification of putative allergens in berry fruits of the Rosaceae family: Technical challenges. Biofactors 2008, 34, 37–46. [Google Scholar] [CrossRef]
- Marzban, G.; Herndl, A.; Kolarich, D.; Maghuly, F.; Mansfeld, A.; Hemmer, W.; Katinger, H.; Laimer, M. Identification of four IgE-reactive proteins in raspberry (Rubus ideaeus L.). Mol. Nutr. Food Res. 2008, 52, 1497–1506. [Google Scholar] [CrossRef]
- Hallmann, E.; Ponder, A.; Aninowski, M.; Narangerel, T.; Leszczyńska, J. The interaction between antioxidants content and allergenic potency of different raspberry cultivars. Antioxidants 2020, 9, 256. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, T.; Gomez, N.A.; Wang, B.; Zhu, M.J.; Du, M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) α1. Nutr. Diabetes 2018, 8, 39. [Google Scholar] [CrossRef]
- Zou, T.; Wang, B.; Yang, Q.; de Avila, J.M.; Zhu, M.J.; You, J.; Chen, D.; Du, M. Raspberry promotes brown and beige adipocyte development in mice fed high-fat diet through activation of AMP-activated protein kinase (AMPK) α1. J. Nutr. Biochem. 2018, 55, 157–164. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Trillos-Almanza, M.C.; Wessel, H.; Martínez-Aguilar, M.; van den Berg, E.H.; Douwes, R.M.; Moshage, H.; Connelly, M.A.; Bakker, S.J.; de Meijer, V.E.; Dullaart, R.P.F.; et al. Branched chain amino acids are associated with physical performance in patients with end-stage liver disease. Biomolecules 2023, 13, e824. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, M.; Wu, Y.; Zhang, F. Branched-chain amino acids metabolism and their roles in retinopathy: From relevance to mechanism. Nutrients 2023, 15, 2161. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Moteki, H.; Ogihara, M. Role of hepatocyte growth regulators in liver regeneration. Cells 2023, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Eichler, S.J.; Yiew, N.K.H.; Colca, J.R.; Cho, K.; Patti, G.J.; Shew, T.M.; Lutkewitte, A.J.; Mukherjee, S.; McCommis, K.S.; et al. Mitochondrial pyruvate carrier inhibition initiates metabolic crosstalk to stimulate branched chain amino acid catabolism. Mol. Metab. 2023, 70, e101694. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Townsend, J.R.; Pinzone, A.G.; Hoffman, J.R. Supplementation with nitric oxide precursors for strength performance: A review of the current literature. Nutrients 2023, 15, 660. [Google Scholar] [CrossRef]
- Kurhaluk, N. The effectiveness of L-arginine in clinical conditions associated with hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. [Google Scholar] [CrossRef]
- Pooventhiran, T.; Alzahrani, A.Y.A.; Rajimon, K.J.; Thomas, R. Solvent interaction and dynamics of neurotransmitters L-aspartic acid and L-glutamic acid with water and ethanol. J. Mol. Struct. 2023, 1273, 134347. [Google Scholar] [CrossRef]
- Qu, W.J.; Liu, T.; Chai, Y.; Ji, D.; Che, Y.X.; Hu, J.P.; Yao, H.; Lin, Q.; Wei, T.B.; Shi, B. Efficient detection of l-aspartic acid and l-glutamic acid by self-assembled fluorescent microparticles with AIE and FRET activities. Org. Biomol. Chem. 2023, 21, 4022–4027. [Google Scholar] [CrossRef] [PubMed]
- Alibabic, V.; Skender, A.; Orascanin, M.; Sertovic, E.; Bajric, E. Evaluation of morphological, chemical, and sensory characteristics of raspberry cultivars grown in Bosnia and Herzegovina. Turk. J. Agric. For. 2018, 42, 67–74. [Google Scholar] [CrossRef]
- Vara, A.L.; Pinela, J.; Dias, M.I.; Petrović, J.; Nogueira, A.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. Compositional features of the “Kweli” red raspberry and its antioxidant and antimicrobial activities. Foods 2020, 9, 1522. [Google Scholar] [CrossRef] [PubMed]
- Muniyandi, K.; George, E.; Sathyanarayanan, S.; George, B.P.; Abrahamse, H.; Thamburaj, S.; Thangaraj, P. Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats, India. Food Sci. Hum. Wellness. 2019, 8, 73–81. [Google Scholar] [CrossRef]
- Shin, D.; Chae, K.S.; Choi, H.R.; Lee, S.J.; Gim, S.W.; Kwon, G.T.; Lee, H.T.; Song, Y.C.; Kim, K.J.; Kong, H.S.; et al. Bioactive and pharmacokinetic characteristics of pre-matured black raspberry, Rubus occidentalis. Ital. J. Food Sci. 2018, 30, 428–439. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, D.; Wang, S.; Cao, X.; Ye, Y.; Suo, Y. Comparison of phenols content and antioxidant activity of fruits from different maturity stages of Ribes stenocarpum Maxim. Molecules 2018, 23, 3148. [Google Scholar] [CrossRef]
- Aaby, K.; Skaret, J.; Røen, D.; Sønsteby, A. Sensory and instrumental analysis of eight genotypes of red raspberry (Rubus idaeus L.) fruits. J. Berry Res. 2019, 9, 483–498. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Debski, H. Anthocyanins of fruits and vegetables-their occurrence, analysis and role in human nutrition. Veg. Crop. Res. Bull. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive compounds and antioxidant capacity of small berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef]
- Celik, F.; Ercisli, S. Lipid and fatty acid composition of wild and cultivated red raspberry fruits (Rubus idaeus L.). J. Med. Plant Res. 2009, 3, 583–585. [Google Scholar]
- Balta, I.; Stef, L.; Pet, I.; Iancu, T.; Stef, D.; Corcionivoschi, N. Essential fatty acids as biomedicines in cardiac health. Biomedicines 2021, 9, e1466. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P. Red raspberry (Rubus idaeus L.) seed oil: A review. Plants 2021, 10, 944. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Szyszkowska, B.; Łepecka-Klusek, C.; Kozłowicz, K.; Jazienicka, I.; Krasowska, D. The influence of selected ingredients of dietary supplements on skin condition. Postep. Derm. Alergol. 2014, 31, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Anjum, M.A.; Khaqan, K.; Hussain, S. Biodiversity in morphological and physico-chemical characteristics of wild raspberry (Rubus idaeus L.) germplasm collected from temperate region of Azad Jammu & Kashmir (Pakistan). Acta Sci. Pol. Hortorum Cultus 2014, 13, 117–134. [Google Scholar]
- Noratto, G.D.; Chew, B.P.; Atienza, L.M. Red raspberry (Rubus idaeus L.) intake decreases oxidative stress in obese diabetic (db/db) mice. Food Chem. 2017, 227, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Nuñez-Gómez, V.; Navarro-González, I.; Sánchez-Martínez, L.; García-Alonso, J.; Periago, M.J.; González-Barrio, R. Raspberry dietary fibre: Chemical properties, functional evaluation and prebiotic in vitro effect. LWT Food Sci. Technol. 2020, 134, e110140. [Google Scholar] [CrossRef]
- Żurawicz, E.; Studnicki, M.; Kubik, J.; Pruski, K. A careful choice of compatible pollinizers significantly improves the size of fruits in red raspberry (Rubus idaeus L.). Sci. Hortic. 2018, 235, 253–257. [Google Scholar] [CrossRef]
- Simlat, M.; Ptak, A.; Kula, A.; Orzeł, A. Assessment of genetic variability among raspberry accessions using molecular markers. Acta Sci. Pol. Hortorum Cultus 2018, 17, 61–72. [Google Scholar] [CrossRef]
- Fernández-Fernández, F.; Antanaviciute, L.; Govan, C.L.; Sargent, D.J. Development of a multiplexed microsatellite set for fingerprinting red raspberry (Rubus idaeus) germplasm and its transferability to other Rubus species. J. Berry Res. 2011, 1, 177–187. [Google Scholar] [CrossRef]
- Girichev, V.; Hanke, M.V.; Peil, A.; Flachowsky, H. SSR fingerprinting of a German Rubus collection and pedigree based evaluation on trueness-to-type. Genet. Resour. Crop. Evol. 2017, 64, 189–203. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of „antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berest, C. Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berest, C. Kinetics and mechanism of antioxidant activity using the DPPH• free radical method. Lebensm. Wiss. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Marshall, M.R. Ash analysis. In Food Analysis, 4th ed.; Nielsen, S.S., Ed.; Aspen Publishers: Gaithersburg, MD, USA, 2010; pp. 105–116. [Google Scholar]
- PN-90/A-75101/07; Fruit and Vegetable Products. Preparation of Samples and Testing Methods. Determination of Sugars and Sugar Free Extract. PKN, MiJ: Warsaw, Poland, 1990. (In Polish)
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljestroem, M. Rapid enzymic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef] [PubMed]
- PN-A-79011-15:1998P; Dry Food Mixes. Test Methods. Determination of Dietary Fiber Contents. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- Bradstreet, R.B. Kjeldahl method for organic nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- PN75/A-04018:1975/Az3:2002; Agricultural Food Products. Nitrogen Contents Determination with Kjeldahl’s Method and Recalculation into Protein. Polish Committee for Standardization: Warsaw, Poland, 2002. (In Polish)
- Davies, M.G.; Thomas, A.J. An investigation of hydrolytic techniques for the amino acid analysis of foodstuffs. J. Sci. Food Agric. 1973, 24, 1525–1540. [Google Scholar] [CrossRef]
- Schramm, F.; Moor, S.; Bigwood, E.J. Chromatographic determination of cystine as cysteic acid. Biochem. J. 1954, 57, 33–37. [Google Scholar] [CrossRef]
- PN-EN ISO 5508:1996; Animal and Vegetable Fats and Oils. Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. PKN: Warsaw, Poland, 1996. (In Polish)
- PN-EN ISO 12966-1:2014; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acids Methyl Esters. Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. ISO: Geneva, Switzerland, 2014.
- PN-EN 14130:2004; Foodstuffs—Determination of Vitamin C by HPLC. PKN: Warszawa, Poland, 2004.
- Lamaison, J.L.C.; Carnet, A. Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D.C) en function de la vegetation. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Martinez, A.E.; Favret, E.A. Anthocyanin synthesis and lengthening in the first leaf of barley isogenic lines. Plant Sci. 1990, 71, 35–43. [Google Scholar] [CrossRef]
- Commission Delegated Regulation UE No 78/2014. Regulation of the European Parliament and the European Council (EU) no. 1169/2011 from 25.10.2011 with Amendments. Regulation of the European Council (EU) no. 1155/2013 from 21.08.2013, Regulation of the European Council (EU) no. 78/2014 from 22.11.2013 (applied from 19 February 2014). Available online: http://data.europa.eu/eli/reg_del/2013/1155/oj (accessed on 15 March 2023).


| Cultivar | FC | FRAP | DPPH | ABTS | OH• Scavenging Assay | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/g (Caffeic Acid Equivalent) | μmol Fe(II)/g | mg TE/g | mg TE/g | mg TE/g | ||||||
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | |
| ‘Glen Ample’ | 0.99–1.10 | 0.99 ± 0.01 a | 9.51–9.58 | 9.55 ± 0.04 a | 1.06–1.08 | 1.07 ± 0.01 a | 2.3–2.31 | 2.31 ± 0.01 a | 18.75–20.19 | 19.47 ± 0.72 a |
| ‘Laszka’ | 1.15–1.22 | 1.18 ± 0.03 b | 8.18–8.38 | 8.28 ± 0.01 b | 1.26–1.28 | 1.27 ± 0.01 b | 2.62–2.67 | 2.65 ± 0.03 b | 17.53–18.33 | 17.93 ± 0.42 b |
| ‘Radziejowa’ | 1.3–1.31 | 1.31 ± 0.01 c | 9.73–9.79 | 9.76 ± 0.03 c | 1.31–1.33 | 1.32 ± 0.11 c | 3.16–3.17 | 3.17 ± 0.01 c | 19.01–19.67 | 19.34 ± 0.33 a |
| Cultivar | FC | FRAP | DPPH | ABTS | OH• Scavenging Assay | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/mL (Caffeic Acid Equivalent) | μmol Fe(II)/mL | mg TE/mL | mg TE/mL | mg TE/mL | ||||||
| Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean± SD | Min.–Max. | Mean ± SD | Min.–Max. | Mean ± SD | |
| ‘Glen Ample’ | 1.18–1.33 | 1.26 ± 0.08 b | 23.48–23.84 | 23.66 ± 0.18 a | 2.57–2.59 | 2.58 ± 0.01 b | 2.93–3.92 | 3.43 ± 0.49 a | 76.09–77.46 | 76.77 ± 0.68 a |
| ‘Laszka’ | 1.77–1.81 | 1.79 ± 0.02 a | 29.02–29.09 | 29.06 ± 0.04 b | 2.92–3.09 | 3.01 ± 0.08 a | 3.32–3.92 | 3.62 ± 0.30 a | 75.23–75.75 | 75.49 ± 0.26 b |
| ‘Radziejowa’ | 1.56–1.6 | 1.58 ± 0.02 a | 25.55–25.79 | 25.67 ± 0.12 c | 2.41–2,47 | 2.44 ±0.04 bc | 2.91–3.41 | 3.16 ± 0.25 a | 71.97–72.66 | 72.315 ± 0.35 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwil, M.; Matraszek-Gawron, R.; Kostryco, M.; Różańska-Boczula, M. Nutritionally Important Pro-Health Active Ingredients and Antioxidant Properties of Fruits and Fruit Juice of Selected Biennial Fruiting Rubus idaeus L. Cultivars. Pharmaceuticals 2023, 16, 1698. https://doi.org/10.3390/ph16121698
Chwil M, Matraszek-Gawron R, Kostryco M, Różańska-Boczula M. Nutritionally Important Pro-Health Active Ingredients and Antioxidant Properties of Fruits and Fruit Juice of Selected Biennial Fruiting Rubus idaeus L. Cultivars. Pharmaceuticals. 2023; 16(12):1698. https://doi.org/10.3390/ph16121698
Chicago/Turabian StyleChwil, Mirosława, Renata Matraszek-Gawron, Mikołaj Kostryco, and Monika Różańska-Boczula. 2023. "Nutritionally Important Pro-Health Active Ingredients and Antioxidant Properties of Fruits and Fruit Juice of Selected Biennial Fruiting Rubus idaeus L. Cultivars" Pharmaceuticals 16, no. 12: 1698. https://doi.org/10.3390/ph16121698
APA StyleChwil, M., Matraszek-Gawron, R., Kostryco, M., & Różańska-Boczula, M. (2023). Nutritionally Important Pro-Health Active Ingredients and Antioxidant Properties of Fruits and Fruit Juice of Selected Biennial Fruiting Rubus idaeus L. Cultivars. Pharmaceuticals, 16(12), 1698. https://doi.org/10.3390/ph16121698






